Abstract
Transition metal-transporting P1B-type CPx ATPases play crucial roles in mediating metal homeostasis and resistance in all cells. The degree to which N-terminal metal binding domains (MBDs) confer metal specificity to the transporter is unclear. We show that the two MBDs of the Zn/Cd/Pb effluxing pump Anabaena AztA are functionally nonequivalent, but only with respect to zinc resistance. Inactivation of the a-MBD largely abrogates resistance to high intracellular Zn(II) levels, whereas inactivation of the b-MBD is not as deleterious. In contrast, inactivation of either the a- or b-MBD has little measurable impact on Cd(II) and Pb(II) resistance. The membrane proximal b-MBD binds Zn(II) with a higher affinity than the distal N-terminal a-MBD. Facile Zn(II)-specific intermolecular transfer from the a-MBD to the higher-affinity b-MBD is readily observed by 1H–15N HSQC spectroscopy. Unlike Zn(II), Cd(II) and Pb(II) form saturated 1:1 S4 or S3(O/N) complexes with AztAaHbH, where a single metal ion bridges the two MBDs. We propose that the tandem MBDs enhance Zn(II)-specific transport, while stabilizing a non-native inter-MBD Cd/Pb cross-linked structure that is a poor substrate and/or regulator for the transporter.
Complex metal homeostasis and trafficking systems control the bioavailability of essential transition metal ions while ensuring that these and other abiological xenobiotics, including Cd, Pb, Hg, and As, do not accumulate inside cells. The heavy metal ion-transporting CPx-ATPases represent a large subfamily of P-type ATPases (P1B-type) found in both prokaryotes and eukaryotes that play important roles in metal homeostasis (1–5). All are characterized by eight transmembrane (TM)1 helices that likely form the channel for transport. TM6 bears the CPx signature sequence, which is thought, in conjunction with other residues in the membrane helices, to coordinate the metal during transport (6). Large cytoplasmic loops are folded into structurally characterized actuator (7) [A-domain (Figure 1A)] and ATP binding domains (8, 9) (N- and P-domains), with metal translocation coupled to phosphorylation of an aspartate residue in the P-domain.
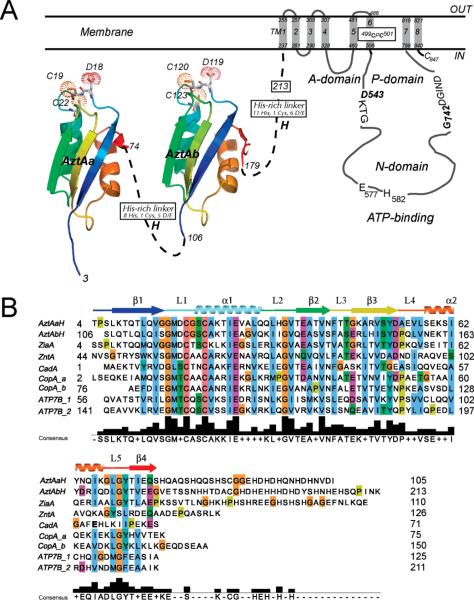
Figure 1.
(A) Schematic representation of the domain structure of AztA showing the two tandem MBDs followed by an ≈30-residue hydrophilic His-rich linker. The predicted eight transmembrane helices, cytosolic ATP binding domains (N and P), and actuator domain (A), and putative CPC intramembrane zinc transport site are shown. The homology models of the AztA a-MBD and b-MBD were generated using B. subtilis CopAa (PDB entry 1OPZ) as a template using Swiss-Model (http://swissmodel.expasy.org//SWISS-MODEL.html). (B) Sequence alignment of the N-terminal region of AztA with analogous domains associated with other divalent or monovalent CPx-ATPases. The sequences are the first and second MBDs of Anabaena PCC 7120 AztA, ZiaA from Synechocystis spp. PCC 6803, ZntA from E. coli, CopA_a and CopA_b from B. subtilis, and the first and second MBDs from the Wilson disease ATPase, ATP7B. The deduced secondary structure derived for AztAaH is also shown.
Another significant feature of CPx-ATPases is that most have N-terminal and/or C-terminal cytosolic extensions, often containing one or more tandemly linked ferredoxin fold-like βαββαβ metal binding domains (MBDs) (Figure 1A) (10). For example, the Wilson's and Menkes disease Cu/Ag-specific ATPases ATP7A and ATP7B, respectively, have six tandemly linked MBDs, while those from lower eukaryotes and most prokaryotes have zero, one, or two MBDs. These MBDs are known to provide docking sites for Cu chaperones that allow Cu to be handed off, via intermolecular metal–ligand exchange reactions, to partner MBDs without dissociation of the metal into bulk solution (11–14). This provides strong support for the central tenet of the Cu-trafficking hypothesis (15, 16). Mechanistic studies with Archaeglobus fulgidis CopA suggest that metal binding to the single N-terminal MBD plays a regulatory role in enhancing the rate of dephosphorylation of the phosphoaspartate residue in the E2 state; this increases the rate of metal ion release which is rate-limiting in multiple-turnover experiments (17).
Some divalent metal ion (Zn/Cd/Pb)-specific P1B-type ATPases (2) are also known to possess an MBD, but the functional role that this domain plays is a topic of ongoing investigation. Since there are no known zinc chaperones, the significance of protein–protein docking and intermolecular transfer is unclear. However, there is some evidence in support of the idea that the specific structural features of individual MBDs might provide some metal selectivity to the transporter itself, despite the fact that they all adopt essentially the same βαββαβ fold (10) and all metal complexes employ the two conserved Cys residues of the CXXC sequence as metal donor atoms (18–21). For example, in both copper chaperones and MBDs derived from Cu/Ag transporters, the Cu(I) is often coordinated via a linear bis-thiolate complex (22–24); in another case, a distorted trigonal S2N complex is found, where a His derived from loop 5 between helix α2 and strand β4 is a ligand (25). For the Zn/Cd/Pb transporter Escherichia coli ZntA, a conserved Asp just N-terminal to the first Cys (DCXXC) was proposed to drive 3- or 4-coordination of Zn(II) (19); in contrast, for the Cd/Pb-selective transporter Listeria monocytogenes CadA, a conserved Glu in loop 5 (E61 in Figure 1B) appears to form a coordination bond to the Cd(II) in a binuclear homodimeric subunit bridging structure (20).
Anabaena AztA (alr7622) is a P1B-type ATPase efflux pump encoded by the azt (Anabaena zinc transport) operon whose expression is transcriptionally induced upon direct binding of Zn, Pb, or Cd by the ArsR (or ArsR/SmtB) family (26) regulator AztR (27). AztA possesses the unique functional property of conferring significant Zn(II) resistance to a transformed Zn/Cd/Pb-hypersensitive E. coli strain (GG48), relative to Cd(II) and Pb(II) (27). In fact, cadmium and lead resistance is barely detectable relative to that conferred by other known Pb/Cd/Zn-transporting ATPases, including E. coli ZntA (28) and CadAs from Streptomyces aureus, Ralstonia metallodurans, and L. monocytogenes (29). AztA is also distinguished from other characterized Zn/Cd/ Pb transporters on the basis of harboring two N-terminal MBDs, each of which is followed by an ≈30-residue hydrophilic His-rich linker (Figure 1). The two MBDs of AztA are 44% identical, pairwise, in amino acid sequence (71% similar), and each contains a DCXXC sequence and lacks the Glu in loop 5 found in CadAs. Since AztA is unique in incorporating tandem MBDs followed by His-rich segments, this led us to hypothesize that some aspect of these specific features might confer higher selectivity for Zn(II) relative to Cd(II) and Pb(II).
In this report, we show that the two MBDs of Anabaena AztA play nonredundant structural and functional roles in metal binding and heavy metal resistance in vivo, but only with respect to zinc resistance. We show that the membrane proximal b-MBD has a higher affinity for Zn(II) than the distal a-MBD, and consistent with this, Zn(II) prebound to the a-MBD facilely moves to a metal-free b-MBD. However, the low-affinity a-MBD plays a critical role in mediating zinc resistance under conditions of high zinc toxicity in a manner that does not require the b-MBD. This suggests that the a-MBD is capable of functioning in a manner independent of the b-MBD in maximally stimulating transport under high intracellular zinc loads. In contrast, both Cd(II) and Pb(II) form stable 1:1 S4 or S3(N/O) complexes in AztAaHbH, where a single metal ion bridges the two Cys-X2-Cys sites from aand b-MBDs or forms intermolecular a–a′ and b–b′ cross-linked structures. We hypothesize that these structures represent kinetically trapped intermediates that are poor substrates or regulators for metal transport by AztA.
MATERIALS AND METHODS
Plasmid Construction of Wild-Type and Mutant azt Operons and Metal Sensitivity Assays
The complete azt operon (27) was amplified from genomic DNA from the cyanobacterium Anabaena strain PCC 7120 and subcloned into pET3a (Novagen) between the BglII and EcoRI sites to create pETazt. A PCR-based quick-change method was employed for simultaneous substitution of Cys19 and Cys22 or Cys120 and Cys123 with Ser using pETazt as a template to create pETazt-C19S/C22S or pETazt-C120S/C123S, respectively. pETazt-C19S/C22S/C120S/C123S was prepared in an analogous fashion using pETazt-C19S/C22S as the PCR template. pETazt-Δ(1–105) was constructed by looping out the coding region for AztAaH using a PCR-based quick-change mutagenesis strategy with pETazt as the template. All plasmids were fully sequenced to verify their integrity. pETazt, pETazt-C19S/C22S, pETazt-C120S/C123S, pETazt-C19S/ C22S/C120S/C123S, and pETazt-Δ(1–105) were transformed into E. coli GG48 (ΔzitB::Cm ΔzntA::Km) (30) and grown overnight at 37 °C in LB medium supplemented with 100 μg/mL ampicillin. Cultures were then diluted 1:50 into 10 mL of fresh LB/ampicillin medium supplemented with the indicated concentrations of metal salts, and the OD600 was recorded from duplicate or triplicate cultures as a function of time. For the metal concentration dependence, the OD600 was recorded following an 8–10 h incubation.
Purification of Recombinant AztAaH and AztAaHbH from E. coli
The regions encoding AztAaH (residues 1–105) and AztAaHbH (residues 1–213) were amplified by PCR from Anabaena PCC 7120 genomic DNA and cloned into pET3a (Novagen) between Ndel and EcoRI restriction sites to create pET3a-AztAaH and pET3a-AztAaHbH, respectively. Plasmids encoding C19S/C22S AztAaHbH and C120S/C123S AztAaHbH were constructed from pET3a-AztAaHbH using PCR-based quick-change mutagenesis. All plasmids were transformed into E. coli BL21(DE3) and grown on LB to midlog phase and induced via addition of 0.4 mM IPTG. Freshly harvested cells were pelleted by low-speed centrifugation and suspended in 100 mL of buffer A [25 mM Tris-HCl, 3 mM DTT, 1 mM EDTA, and 80 mM imidazole (pH 6.0)] and lysed by sonication. The sonicated supernatant was subjected to HisTrapHP (Amersham Biosciences) chromatography (20 mL bed volume) on anÄkta-10 purifier, with elution achieved with a linear imidazole gradient (from 50 to 400 mM) in buffer B [25 mM Tris-HCl, 3 mM DTT, and 1 mM EDTA (pH 8.0)]. AztA MBD-containing fractions typically eluted between 125 and 175 mM imidazole, were pooled conservatively, and were further purified using Superdex 75 size-exclusion chromatography (27). Highly purified AztA MBD-containing fractions were pooled, concentrated, and dialyzed against 3 L of buffer C [5 mM MES-HCl and 0.20 M NaCl (pH 6.5)] in an anaerobic Vacuum Atmospheres glovebox. The purity of the final products was estimated by visualization of Coomassie-stained 18% Tricine–SDS–PAGE gels to be ≥90%. Uniformly 15N-labeled AztAaH and AztAaHbH were purified in exactly the same way except that cells were grown on an M9 minimal medium, with (15NH4)2SO4 as the sole nitrogen source (31). The concentrations of wild-type AztAaHbH, C19S/C22S (or C120C/C123S) AztAaHbH, and AztAaH were determined using ∊280 values of 11 180, 10 930, and 4845 M−1 cm−1, respectively. All Cys residues in AztA domains were reduced as revealed by an anaerobic DTNB assay (32).
Cd(II) and Pb(II) Binding Experiments
Metal ion binding experiments with Cd(II) and Pb(II) were carried out anaerobically at ambient temperature (≈25 °C) as described previously (27) in either buffer C (for Cd) or buffer H [10 mM bis-Tris and 0.4 M NaCl (pH 7.0)] (for Pb). Metal binding isotherms were fit using DynaFit (33) as described previously (34) to 1:1 binding models with the total concentration of metal binding sites on AztA derivatives being an adjustable parameter.
Zn(II) Binding Experiments
Mag-fura-2 (Invitrogen M1290) (KZn = 5.0 × 107 M−1) (35) was employed in a Zn(II) competition assay with various AztA MBD fragments. Magfura-2 (7–10 μM) was mixed anaerobically with a known concentration of apoprotein (18–20 μM) in buffer C (34). The metal concentrations of each titrant were verified by atomic absorption spectroscopy, and the data were fit using a competitive binding model (DynaFit) (33, 34) with KZn (mag-fura-2) and [mag-fura-2] as fixed parameters, and the total concentration of Zn(II) binding sites on AztA derivatives as an adjustable parameter, to account for errors in pipetting, partial inactivation due to cysteine oxidation, or heterogeneity of metal sites. For AztAaHbH, a two-site sequential binding model was used and defined by KZn1 and KZn2, with the best-fit values of [AztAaHbH] being 19.4 μM (20.0 μM input). For AztAaH, a 1:1 binding model (KZn) was used with a best-fit [AztAaH] of 9.1 μM (18.2 μM added). For C19S/C22S AztAaHbH, a model assuming a mixture of two metal binding species with apparent affinities KZnA and KZnB was used, with best-fit[C19S/C22S AztAaHbH–A]and[C19S/C22S AztAaHbH–B] values of 9.9 μM each (20.0 μM total input), respectively. KZnA reports on a 1:1 complex with the b-MBD, while KZnB likely reports on an intermolecular b–b′ bridging species, analogous to the a–a′ bridging species that is observed for AztAaH (see Figure S1 of the Supporting Information).
NMR Spectroscopy
All NMR spectra were acquired on a Varian Unity Inova 600 MHz spectrometer in the Biomolecular NMR Laboratory at Texas A&M University. Sample preparation and subsequent metal additions were conducted in an anaerobic Vacuum Atmospheres glovebox at ambient temperature and incubated at least 2 h before the spectra were recorded. 1H–15N HSQC spectra were typically acquired using a 400 μM solution of uniformly 15N-labeled AztAaHbH or AztAaH in buffer C [5 mM Mes and 0.20 M NaCl (pH 6.5)] at 25 °C. Sequential 1HN, 15N, 13Cα, and 13Cβ resonance assignments of the a-MBD region were obtained using a 0.35 mM uniformly 15N- and 13C-labeled apo-AztAaH sample from analysis of 1H–15N HSQC, CBCA-(CO)NH, and HNCACB experiments in buffer C containing 90% H2O and 10% D2O. NMR data were processed using NMRPipe and analyzed using Sparky, essentially as previously described (31). PREDICTOR (36) was used to analyze backbone 1HN, 15N, 13Cα and 13Cβ chemical shifts and sequence analysis to define the secondary structural segments in AztAaH. The torsion angles (Ψ, Φ, and χ1) of AztAaH predicted by PREDICTOR were found to be comparable to those experimentally determined in Bacillus subtilis CopZ (37).
NMR Analysis of Mixtures of [15N]AztAaH and Unlabeled C19S/C22S AztAaHbH
Zn1-bound 15N-labeled AztAaH (0.4 mM) incubated for 12 h in an anaerobic chamber was mixed with unlabeled C19S/C22S AztAaHbH at molar ratios of 1:1 and 1:1.5, and 1H–15N HSQC spectra were recorded within 2 h as described above.
X-ray Absorption Spectroscopy of Zn– and Cd–AztAaH Complexes
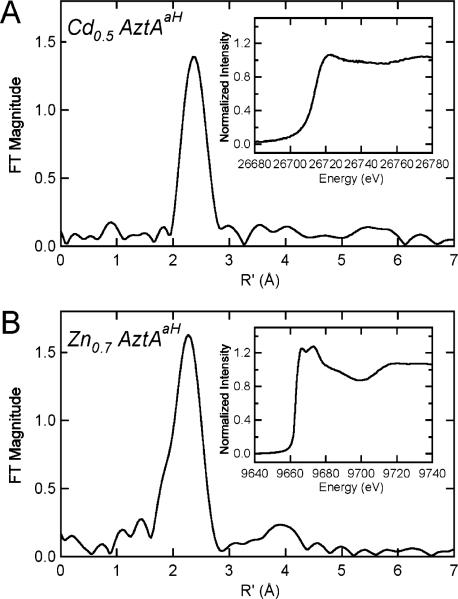
Samples of ≈1 mM AztAaH containing 0.7 molar equiv of Zn(II) or 0.5 molar equiv of Cd(II) were loaded into 10 μL wells of Lexan cuvets with 0.001 in. thick Kapton windows. Zn and Cd K edge X-ray absorption spectroscopic data were collected at the Stanford Synchrotron Radiation Laboratory on beamline 9–3 with the SPEAR3 ring operating at 3.0 GeV and 85–100 mA, with a fully tuned Si (220) LN2-cooled monochromator, using a vertical aperture of 1 mm, and the upstream mirror set for harmonic rejection. Fluorescence excitation spectra were collected using a 30-element intrinsic Ge detector (Canberra) windowed to either Zn or Cd Kα emission. For Zn, a 6 μm Cu fluorescence filter backed by Soller slits was employed. The spectra shown in Figure 5 were averaged from six (Zn) and three (Cd) 21 min scans, and the data were reduced and analyzed using EXAFSPAK (http://www-ssrl.slac.stanford.edu/exafspak.html). Internal energy calibration defined the first inflection of Zn and Cd elemental standards as 9660.7 and 26714.0 eV, respectively. k values were calculated using threshold (k = 0) energies of 9670 and 26 720 eV, respectively.
Figure 5.
EXAFS analysis of Zn(II)- and Cd(II)-bound AztAaH. (A) Fourier transform (k = 2–12 Å−1; k3 weighting) of the Cd K-edge EXAFS of the Cd(II)0.5–AztAaH complex. (B) Fourier transform (k = 2–12 Å−1; k3 weighting) of the Zn K-edge EXAFS of the Zn(II)0.7–AztAaH complex. Cadmium and zinc K-edge X-ray absorption near-edge spectra are shown in the insets of panels A and B, respectively. Parameters that define the best fits to these data are compiled in Table SII.
RESULTS
The a- and b-MBDs Play Functionally Nonequivalent Roles in Zinc Resistance in E. coli
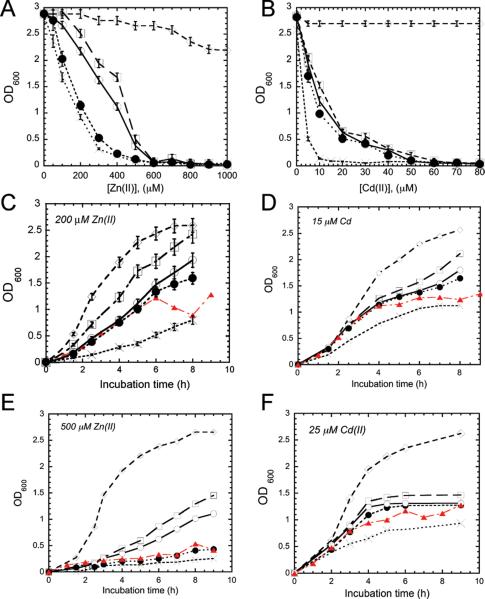
Previous studies of metal-sensing cyanobacterial operons regulated by ArsR family repressors suggest that these operons function similarly in E. coli and their original cyanobacterial hosts (27, 38, 39). To evaluate the degree to which individual MBDs are required to confer metal resistance in cell culture, we transformed the well-characterized Zn/Pb/Cd hypersensitive strain E. coli GG48 (ΔzitB, ΔzntA) (30), which lacks both known low- and high-affinity Zn/Cd efflux systems, with plasmids encoding the complete Anabaena azt operon in which one MBD or the other was inactivated by mutation. Figure 2A reveals that E. coli GG48 transformed with an azt operon in which the a-MBD was inactivated (C19S/C22S azt) is more sensitive to zinc toxicity at long incubation times (8–10 h) relative to GG48 transformed with an azt operon in which the b-MBD was inactivated (C120S/C123S azt). Analysis of the full growth curves for these strains measured in the presence of an intermediate concentration of Zn(II) (200 μM) reveals that this differential effect manifests itself at long incubation times (Figure 2C). In contrast, growth curves measured at highly toxic levels of Zn(II) (≥500 μM) (Figure 2E) reveal that while the b-MBD mutant retains a level of zinc resistance just below that of the wild-type operon, inactivation of the a-MBD confers a level of resistance only just above that of nontransformed cells. A similar level of resistance is obtained when both the a- and b-MBDs are inactivated compared to the a-MBD alone; this suggests that the a-MBD is largely capable of functioning independently of the b-MBD at high intracellular zinc loads. Unlike the case with Zn(II), inactivation of either the a- or the b-MBDs has only a small, but roughly equivalent, influence on Cd(II) resistance relative to the wild-type azt operon, with the double mutant only slightly more sensitive to high Cd(II) loads (25 μM) with long incubation times (Figure 2B,D,F). Thus, the presence of at least one functional MBD is sufficient to confer nearly wild-type, albeit modest (27), levels of Cd(II) resistance in these cells, a situation that appears to contrast with that of Zn(II).
Figure 2.
Zinc and cadmium resistance of Zn/Cd hypersensitive E. coli GG48 transformed with azt operons containing aztA metal binding missense mutations. Representative data were derived from duplicate or triplicate measurements for E. coli GG48 transformed with pET3a vector (X), pETazt-C19S/C22S/C120S/C123S (red triangles), pETazt-C19S/C22S (black circles), pETazt-C120S/C123S (white circles), and pETazt (white squares) relative to an isogenic wild-type W3100 strain (white diamonds).
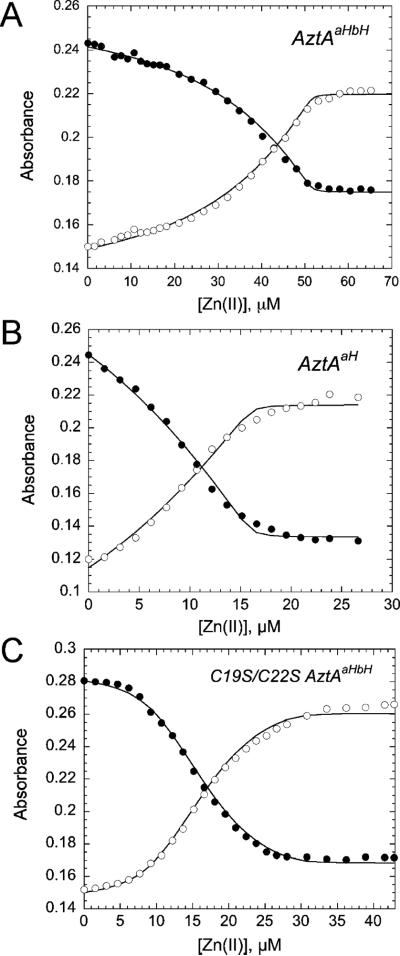
The Two MBDs of AztA Have Different Zn(II) Binding Affinities
The in vivo metal resistance experiments described above make the prediction that individual MBDs might have distinct Zn(II) binding properties and that complexes formed with Zn(II) might be structurally different from those formed by Cd(II) and Pb(II). We first determined the stoichiometry and affinity of Zn(II) binding to various MBD-containing AztAs using a metal chelator competition assay. Here, the Zn(II) chelator mag-fura-2 (34) with a KZn of 5.0 107 M−1 (35) is mixed with purified AztA MBD-containing fragments and titrated with Zn(II) in an anaerobic atmosphere. The binding of Zn(II) to mag-fura-2 leads to a shift in the absorption maximum (from 366 to 325 nm). As shown in Figure 3A, the total concentration of Zn(II) required to saturate AztAaHbH (20 μM) and mag-fura-2 (9.7 μM) is ≈50 μM. This experiment reveals that AztAaHbH binds 2 molar equiv of Zn(II) with a KZn of ≥ 105 M−1. Quantitative curve fitting to a two-site binding model reveals that one Zn(II) binds to AztAaHbH with an affinity far greater than that of mag-fura-2 [KZn1 = (9.4 ± 1.0) × 108 M−1], while the second binds with an affinity only slightly greater than that of mag-fura-2 [KZn2 = 8.0 ± 0.6) × 107 M−1]. The simplest interpretation of this experiments is that each MBD of AztAaHbH binds 1 molar equiv of Zn(II) with measurably different affinities. If the His-rich linker segments in AztAaHbH bind Zn(II), these sites are characterized by an affinity KZn of ≤ 105 M−1 or far weaker than that of magfura-2, since they are not observed in this assay.
Figure 3.
Representative anaerobic titrations of Zn(II) into a mixture of mag-fura-2 and purified AztAaHbH (A), AztAaH (B), or C19S/C22S AztAaHbH (C) in buffer C [5 mM Mes and 0.2 M NaCl (pH 6.5)]. For panel A, mag-fura-2 (10.0 μM) and AztAaHbH (20.0 μM) were used. For panel B, 7.0 μM mag-fura-2 was mixed with 18.2 μM AztaH. For panel C, 10.0 μM mag-fura-2 was mixed with 20.0 μM C19S/C22S AztAaHbH. The absorbance changes at 366 (●) and 325 nm (○) were monitored and plotted as a function of total Zn(II) concentration. The solid lines represent a simultaneous, nonlinear, least-squares fitting curve generated by Dynafit to 2:1 stepwise binding models (defined by KZn1 and KZn2; see the text) for AztAaHbH, a 1:1 model for AztAaH [KZn ) (9.3 ± 1.5) × 107 M−1], and an equimolar mixture of sites model for C19S/C22S AztAaHbH [see Materials and Methods; KZnA) (8.4 ± 2.8) × 108 M−1, and KZnB = (7.4 ± 1.2) × 106 M−1] with KZn (mag-fura-2) fixed at 5.0 × 107 M−1.
To determine which MBD (a or b) binds Zn(II) more tightly, we performed parallel competition experiments with AztAaH (Figure 3B), which contains only the a-MBD and the His-rich region, and C19S/C22S AztAaHbH (Figure 3C), which contains a wild-type b-MBD and both His-rich domains. As one can see, the added Zn(II) binds to AztAaH and mag-fura-2 with approximately equal affinity until saturation of the available sites occurs, with a KZn of (9.3 ± 1.5) × 107 M−1, or comparable to that of the low-affinity binding site in intact AztAaHbH (Figure 3B). In contrast, there is a clear plateau in the early region of the titration curve for C19S/C22S AztAaHbH (Figure 3C) like that for intact AztAaHbH, with a KZn of (8.4 ± 2.8) × 108 M−1 or comparable to that of the high-affinity site in wild-type AztAaHbH (Figure 3A). These data reveal that each of the two MBDs of AztAaHbH harbors a Zn(II) binding site and that the affinity of Zn(II) for the N-terminal a-MBD is lower by a factor of 5–10 than that for the C-terminal b-MBD.
Cd(II) and Pb(II) Form Inter- and Intramolecularly Bridging Structures
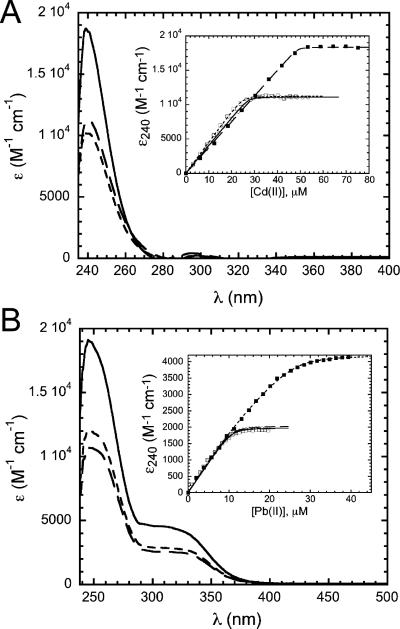
Cd(II) was shown to be a strong inducer of aztA expression in Anabaena but confers little resistance to Cd(II) salts in E. coli (27). The UV–visible absorption spectra of AztAaHbH titrated with Cd(II) show the formation of a stoichiometric 1:1 Cd(II)–AztAaHbH complex (Figure 4A, inset), in contrast to that obtained with Zn(II) (Figure 3). The intense absorption at ≈240 nm can be attributed to S− → Cd(II) ligand-to-metal charge transfer transitions with an ∊240 of ≈5000–6000 M−1 cm−1 expected per Cd–S bond (40, 41). The molar absorptivity of the 1:1 Cd(II)–AztAaHbH complex is 19 000 MCd−1 cm−1 (Figure 4A), a value consistent with three to four cysteine thiolate ligands. This result suggests that a single Cd(II) ion bridges both MBDs in intact AztAaHbH, an interpretation supported by sedimentation equilibrium experiments (see below).
Figure 4.
(A) Optical absorption spectra of 1:1 Cd(II)-saturated AztAaHbH (—), 0.5:1 Cd(II)-saturated AztAaH (- - -), and 0.5:1 Cd-(II) saturated C19S/C22S AztAaHbH (– – –). The inset shows anaerobic Cd(II)–AztA binding isotherms {∊240 vs total [Cd(II)], 50 μM protein} generated from the optical spectra of AztAaHbH (■), AztAaH (□), and C19S/C22S AztAaHbH (◯). The smooth curve drawn through the data points reflects a KCd of >5 × 107 M−1, a lower limit given the high protein concentration that was used. (B) Optical absorption spectra of 1:1 Pb(II)-saturated AztAaHbH (—), 0.5:1 Pb(II)-saturated AztAaH (- - -), and 0.5:1 Pb(II)-saturated C19S/C22S AztAaHbH (– – –). The inset shows anaerobic binding isotherms {∊320 vs total [Pb(II)], 20 μM protein} generated from the optical spectra of AztAaHbH (■), AztAaH (□), and C19S/C22S AztAaHbH (◊) obtained upon titration of Pb(II). For AztAaHbH, the solid line represents a least-squares fitted curve with a KPb of 8.0 × 107 M−1. For AztAaH and C19S/C22S AztAaHbH, the smooth curve reflects a lower limit for a KPb of ≥ 1 × 108 M−1.
In contrast, the single a-MBD-containing AztAaH binds only 0.5 molar equiv of Cd(II) at saturation (Figure 4A, inset), with a molar absorptivity ∊240 of 11 000 MCd−1 cm−1, or approximately one-half of that of AztAaHbH; this suggests that a single Cd(II) ion forms an intermolecular S4 complex between two AztAaH domains. The same stoichiometry and molar absorptivity characterize the C19S/C22S AztAaHbH complex (Figure 4A). Similar trends are observed for Pb(II) (Figure 4B). Two S− → Pb(II) LMCT transitions are observed for Pb(II)-saturated AztAaHbH with the molar absorptivities and energies of these absorption bands most consistent with approximately three thiolate ligands to the Pb(II) ion (27, 42–44).
The assembly states of Zn1-, Zn2-, Cd1-, and Pb1-substituted AztAaHbH are all described well by a single-ideal species molecular mass of ≈24 kDa in all cases, consistent with monomeric AztAaHbH (Table SI of the Supporting Information). Thus, the Cys-thiolate-rich Cd1 and Pb1 complexes are best described as intramolecularly bridging chelates that cross-link the a- and b-MBDs. In contrast, size exclusion chromatography analysis of apo versus Zn1 and Cd0.5 forms of AztAaH (Figure S1) reveals that while the apparent molecular masses of apo-AztAaH and Zn1-bound AztAaH are mostly monomeric (≈12 kDa), addition of 0.5 molar equiv of Cd(II) gives rise to an ≈23 kDa dimeric species. These results are consistent with parallel dynamic light scattering experiments (Figure S1). Thus, Cd(II) readily mediates intermolecular a–a′ cross-linking of a-MBDs between AztAaH molecules, in contrast to Zn(II).
X-ray Absorption Spectroscopy of Zn(II)- and Cd(II)-Bound AztAaH
K-Edge X-ray absorption spectroscopy (XAS) was employed to further investigate the Zn(II) and Cd(II) metal–ligand donor sets. Cd K-edge X-ray absorption spectroscopic analysis of the Cd(II)–AztAaH complex indicates a coordination environment consisting primarily of sulfur scatterers at 2.50 Å (Figure 5A). The data are most consistent with an average Cd(II)S3(O/N) coordination complex (Table SII of the Supporting Information). In contrast, curve fitting of EXAFS obtained for equilibrium Zn(II)0.7–AztAaH complexes reveals a Zn(II)S2(N/O)2 coordination environment, a finding consistent with coordination by Cys19 and Cys22 and possibly Asp18, as reported in E. coli ZntA (19), with a water molecule completing the tetrahedral chelate structure (Figure 5B and Table SII).
NMR Studies of Zn(II)-, Cd(II)-, and Pb(II)-Bound AztAaHbH
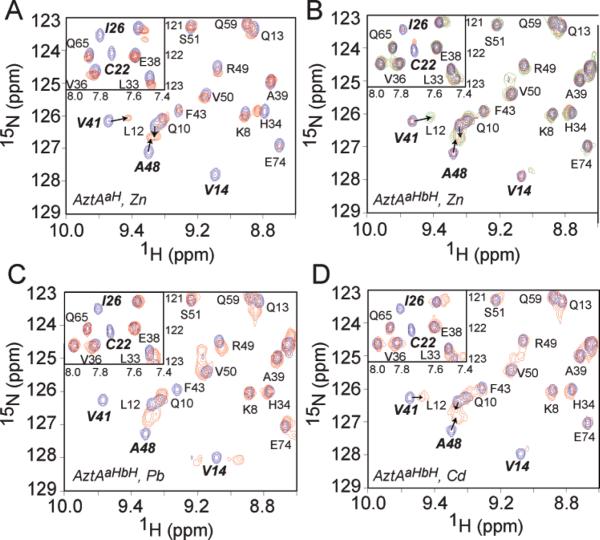
Since Zn(II) and Cd(II)/Pb(II) bind to AztAaHbH with different stoichiometries and coordination structures, we next examined the nature of the structural changes by NMR spectroscopy in an attempt to correlate these changes to the specificity of metal transport by AztA. We recorded two-dimensional 1H–15N HSQC spectra of AztAaHbH in the absence and presence of 1 and 2 molar equiv of Zn(II) versus saturating 1:1 Cd(II) and Pb(II) and compared these spectra to those of apo and Zn1-bound AztAaH (Figure 6). Inspection of the 1H–15N HSQC spectra acquired for apo-AztAaHbH (Figure 6B) versus those for apo-AztAaH (Figure 6A, blue contours) reveals that of the two MBDs of AztAaHbH, only the N-terminal a-MBD gives well-resolved resonances of relatively uniform intensity; in contrast, the expected resonances for the C-terminal b-MBD are of significantly lower intensity and, in some cases, missing altogether (Figure S2 of the Supporting Information). Intermediate (microsecond to millisecond) exchange on the 1H NMR time scale between two or more conformations seems a likely origin of resonance broadening, a characteristic not unprecedented from NMR studies of other Atx1-like MBDs (45).
Figure 6.
Superposition of selected regions of the 1H–15N HSQC spectra of (A) apo-AztAaH (blue cross-peaks) and apo-AztAaH with 1 molar equiv of Zn(II) (red contours), (B) apo-AztAaHbH (blue contours) mixed with 1 molar equiv of Zn(II) (red contours) and 2 molar equiv of Zn(II) (green contours), (C) apo-AztAaHbH (blue contours) mixed with 1 molar equiv of saturating Cd(II) (red contours), and (D) apo-AztAaHbH (blue contours) mixed with 1 molar equiv of saturating Pb(II) (red contours). Note that the movement of the Val41 and Ala48 cross-peaks in the β2–β3 loop in the AztAaHbH spectrum (panel B) is maximal only when both the a- and b-MBDs are filled with Zn; in contrast, this perturbation appears to be maximal with only 1 molar equiv of Cd and Pb (panels C and D). In the insets, extensive line broadening of both Cys22 and Ile26 is also reporting on the metal occupancy of the DCXXC region in the a-MBD.
Complete 1HN and 15N, 13Cα, and 13Cβ assignments were obtained for apo-AztAaH from Ser6 to Gln75, with the exception of Leu30, and Asp18, Cys19, and Ser21 in the metal binding loop (Table SIII of the Supporting Information). A comparison of the amide chemical shifts of the a-MBD in the context of AztAaH versus AztAaHbH shows that they are virtually identical in the apo state (Figures S2 and S3), consistent with little interaction between the a- and b-MBDs in the two-domain AztAaHbH molecule. Chemical shift indexing using PREDICTOR (36) is consistent with the expected βαββαβ topology (10), with secondary structural segments encompassing approximately residues 7–15 (β1), 22–31 (α1), 37–42 (β2), 47–53 (β3), 59–67 (α2), and 71–74 (β4) (see Figure 1B). Binding of Zn(II) to AztAaH induces significant chemical shift perturbations in the metal binding loop region, flanking residues in the β1 strand and α1 helix, and the β2–β3 loop in the proximity of the metal binding loop (Figure 6A and Figure S3). In the spectral region that is shown, Val14, Cys22, and Ile26 in the metal binding loop and Val41 and Ala48 in the β2–β3 loop region are good reporters of metal binding by the a-MBD.
A comparison of the changes in the spectra of apo-AztAaHbH upon addition of 1 (red contours) versus 2 (green contours) molar equiv of Zn(II) reveals that the addition of the first molar equivalent of Zn(II) induces very little perturbation in the amide groups of the a-MBD, consistent with the relative affinities of the two MBDs for Zn(II) (Figure 3). Upon addition of the second molar equivalent of Zn(II), we see significant perturbations in the a-MBD within AztAaHbH (Figure 6B), analogous to those changes observed upon addition of Zn(II) to AztAaH (Figure 6A). In contrast, addition of a stoichiometric amount of Cd(II) (Figure 6D) or Pb(II) (Figure 6C) induces perturbations of the a-MBD resonances qualitatively analogous to those observed upon addition of Zn(II) to AztAaH, with Cys22, Ile26, and Val41 reporting most strongly on these changes (Figure 6A). Note that these spectral changes do not allow us to easily discriminate between monomolecular a-MBD and interdo-main a–b MBD metal-cross-linked species since we can observe changes in only a-MBD resonances. Nonetheless, these data are consistent with formation of an a-MBD–b-MBD site-bridging complex in Cd1– and Pb1–AztAaHbH complexes that is structurally distinct from that in the Zn1 and Zn2 complexes.
Facile Transfer of Zn(II) from the a-MBD to the b-MBD
Since the b-MBD has a higher equilibrium affinity for Zn-(II) relative to the N-terminal a-MBD, this finding makes the prediction that Zn(II) bound to the a-MBD should be capable of transferring to the b-MBD on thermodynamic grounds, provided the energy barrier between the two states is small. To test this idea, we incubated Zn(II)-complexed, 15N-labeled AztAaH (red contours) with unlabeled apo-C19S/C22S AztAaHbH at a ratio of 1:1 (blue contours) and monitored metal occupancy of the a-MBD by 1H–15N HSQC spectroscopy (Figure 7). These data show that the apo b-MBD is capable of stripping the Zn(II) prebound to the a-MBD, given the appearance of apo state resonance frequencies for Cys22, Ile26, and Val41 in 15N-labeled AztAaH, among others. The transfer efficiency estimated by the relative peak intensities of Zn(II) versus apo state cross-peaks for Val41 suggests that ≥80% of the Zn(II) moved from the a-MBD in AztAaH to the b-MBD in C19S/C22S AztAaHbH under these conditions, a finding roughly consistent with their relative affinities.
Figure 7.
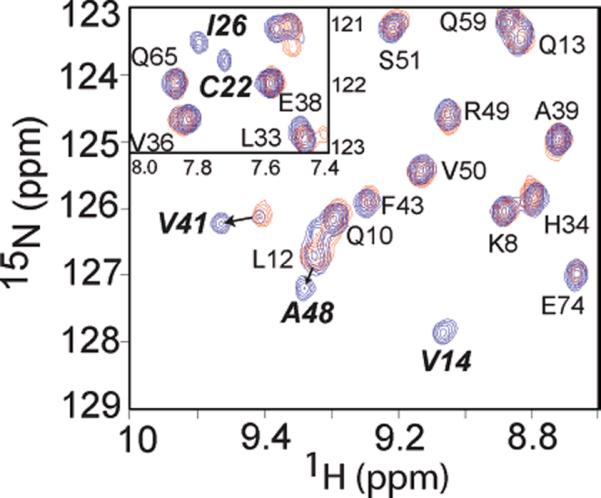
Transfer of Zn(II) from Zn(II)-loaded uniformly 15N-labeled AztAaH to unlabeled apo-C19S/C22S AztAaHbH. Superposition of 1H–15N HSQC spectra of a 1:1 complex of Zn(II) and AztAaH alone (red contours) or in the presence of unlabeled apo-C19S/C22S AztAaHbH at a 1:1 molar ratio (blue contours). Note that resonances corresponding to Val14, Cys22, Ile26, Val41, and Ala48 return to their characteristic positions in apo-AztAaH upon addition of a stoichiometric amount of apo-b-MBD, indicative of Zn(II) transfer. In the inset, extensive line broadening of both Cys22 and Ile26 also reports on the metal occupancy of the DCXXC region in the a-MBD.
DISCUSSION
How N-terminal MBDs influence metal transport by the divalent metal ion-specific P1B-type ATPases is not fully understood. In simple Zn/Cd/Pb-specific P1B-type ATPases, e.g., E. coli ZntA, kinetic studies reveal that the single N-terminal MBD enhances the steady state ATPase activity (46) and overall turnover of the enzyme, either by increasing the rate of metal binding, which is fast (≈108 M−1 s−1) (47), or by stimulating the release of metal ion from the transmembrane site, which is rate-limiting for transport (17). Our studies with Anabaena AztA provide new insights into how tandemly linked MBDs in a divalent metal transporter might bias the metal specificity of the efflux pump more toward Zn(II), relative to abiological Cd(II) and Pb(II) ions, in a way that is not achievable with a single MBD. They further suggest that the trend toward increasing numbers of MBDs found in mammalian copper-specific transporters might have been used to fine-tune the intrinsic metal selectivity and transport efficiency of the pump beyond that which was possible with no or one MBD.
Consistent with previous studies, neither MBD is absolutely essential for mediating metal resistance and, by extension, metal efflux through the AztA transporter (48). However, they do suggest that the low-affinity a-MBD serves a specialized regulatory role under high intracellular zinc loads in a manner that appears to be largely independent of the b-MBD (Figure 2). Perhaps only under these conditions would the low-affinity N-terminal a-MBD be capable of binding Zn(II) in the cell, which would then quickly move via simple mass action (dissociation and/or reassociation) or direct transfer via metal–ligand exchange to the membrane proximal b-MBD (Figure 7) or the transmembrane site itself (see Figure 8A) on thermodynamic grounds, given the higher affinity of each for Zn(II) relative to the a-MBD (Figure 3) (49). However, it is important to emphasize that it has not yet been shown that Zn(II) is capable of moving from the a-MBD to either site directly within the same polypeptide chain, nor has the affinity of the transmembrane site for Zn(II) in AztA been experimentally determined. Although inter-MBD copper transfer has also been observed among the MBDs in ATP7B, as well as intermolecular transfer between Hah1 and select MBDs in both ATP7A and ATP7B (12, 50, 51), our data do not support a model of an obligatory transfer from the a-MBD to the b-MBD since inactivation of the b-MBD alone mediates a level of Zn(II) resistance that is nearly as effective as that of the wild-type MBD.
Figure 8.
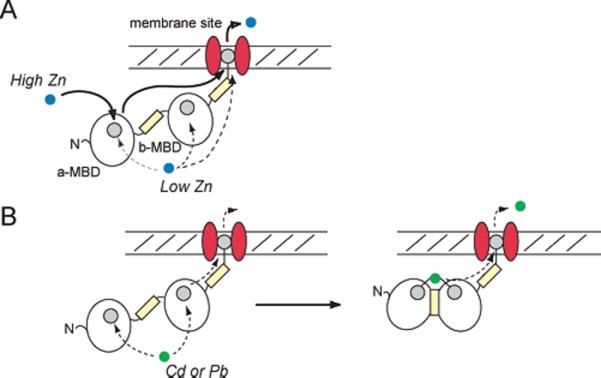
Hypothetical model that illustrates plausible pathways for the transfer of (A) Zn or (B) Cd/Pb from the cytosolic MBD region of AztA to the transmembrane site, and on to the other side of the membrane, consistent with the data presented here. At low cytosolic Zn loads, the metal can interact with the a-MBD, b-MBD, or transmembrane site in a manner dictated by their relative affinities. At high intracellular Zn loads, metal interacts with the low-affinity a-MBD to maximally stimulate the transporter. For Cd/ Pb, these ions form a trapped inter-MBD complex. Metal binding sites are indicated by the filled gray circles, within the a-MBD, b-MBD, and the transmembrane sites indicated (49). The contribution of the His-rich domains (yellow rectangles) to Zn(II) transport is not yet known; however, they likely bind Zn(II) (61) and may well influence rates of transport of metal through the membrane.
In contrast to the situation with Zn(II), the more thiophilic Cd(II) and Pb(II) ions appear to form kinetically stable inter- and intramolecularly bridged metal complexes in AztA (Figures 3–5), analogous to those observed previously for Cd(II)-bound CadA from L. monocytogenes (20) and metalated copper chaperones (14, 25, 52) (Figure 8B). Furthermore, this intramolecularly bridged metal complex in AztAaHbH may be electrostatically stabilized as well, due to the anticipated complementary surface potentials of the a- and b-MBDs, with the a-MBD characterized by a small positive patch contributed by the β3 strand with the b-MBD exhibiting a strongly negative surface potential on the α3–β4 face of the domain (Figure S5 of the Supporting Information). The Cd(II) and Pb(II) complexes thus formed may not be a strong allosteric modulator of the ATPase activity relative to the Zn(II) complex, thus providing a means of biasing the metal selectivity of the efflux pump toward Zn(II).
Anabaena AztA, like the closely related Synechocystis ZiaA (53), likely transports Zn(II) from the cytoplasm to the periplasm. The periplasm is known to be important for metal ion partitioning and homeostasis, and this is particularly so for cyanobacteria which have unusual requirements for iron, copper, and manganese to supply the photosynthetic machinery with metal cofactors, as well as for other metal ions such as Zn, Co, and Mo (54). This compartment contains several metal-specific solute binding proteins, including Synechocystis ZnuA (Zn-specific) (55) and FutA2 (FeIII-specific) (56) and Anabaena AzuA (likely Zn-specific) (27) that could function in metal storage and homeostasis of essential transition ions, and at high intracellular loads, in detoxification. Anabaena, like other cyanobacteria such as Oscillatoria brevis (57) and Synechococcus (58), is known to encode a metallothionein that efficiently sequesters divalent metals in the cytoplasm (59). Cd binds to Synechococcus SmtA avidly and is readily capable of displacing three of its four bound Zn ions (60). Anabaena and other cyanobacteria may have evolved partially overlapping or redundant detoxification and storage systems for zinc in a manner that maintains an optimal response to abiological Cd and Pb.
Finally, although similar His-rich sequences have been identified in other Zn/Cd-specific P1B-type ATPases (53, 57), the functional role of these regions C-terminal to both the a- and b-MBDs in AztA requires further study. Blast analysis against the complete plant and bacterial genome databases suggests that an AztA-like domain organization of a canonical MBD followed by a His-rich segment in predicted metal-transporting ATPases is not unique and can be found in most classes of bacteria, including Proteobacteria, Firmicutes, Bacteroidetes, and Cyanobacteria, in addition to Arabidopsis. Clearly, the His-rich region between residue ≈77 and 105 in AztAaH is unstructured and/or conformationally dynamic since sequential assignments for most of these resonances could not be obtained due to resonance overlap or conformation exchange broadening (Figure S4). However, addition of a stoichiometric amount of Zn(II) to AztAaH results in movement and chemical exchange broadening of at least a subset of these resonances which is largely paralleled by chemical shift perturbations in the metal binding and β2–β3 loops within the folded domain (Figure 6). The simplest interpretation of these spectral changes is that the bound Zn(II) is in intermediate exchange between sites, despite the fact that the His-rich linker binds Zn(II) with an apparent affinity that is weaker than even that of the a-MBD, since mutagenesis of the Cys pair in individual MBDs abrogates high-affinity (KZn ≥ 5 × 105 M−1) metal binding (Figure 3). Binding of metal to these His-rich domains may enhance the on-rate and/or facilitate the transfer of metal between MBDs and the b-MBD and intramembrane sites. Consistent with this, preliminary findings reveal that incubation with antisera raised against the His-rich sequence in Bxa1, a closely related CPx-ATPase from the cyanobacterium O. brevis (39), significantly decreases the ATPase activity of Bxa1 in vitro (T. Liu, unpublished results). Detailed kinetic studies of metal binding will be required to understand the mechanistic role that these domains play in stimulation of ATPase activity and metal transport across membranes.
Supplementary Material
ACKNOWLEDGMENT
We thank Dr. Xiangming Kong for help in acquiring the NMR data, Dr. Xiaohua Chen and Mr. Zhen Ma for assistance in analyzing the metal titration data, and Dr. Xiaohua Chen for constructing plasmids used in this work.
Footnotes
This work was supported by grants from the National Institutes of Health (NIH) (GM042569 to D.P.G. and GM042025 to R.A.S.) and the Robert A. Welch Foundation (A-1295 to D.P.G.). Portions of this research were carried out at the Stanford Synchrotron Radiation Laboratory (SSRL), operated by Stanford University on behalf of the U.S. Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the NIH National Center for Research Resources, Biomedical Technology Program.
Abbreviations: ArsR, arsenic repressor; azt, Anabaena zinc transport; AztAaH, N-terminal a-MBD with a His-rich tail (residues 1–105); AztaHbH, complete MBD of AztA (residues 1–213); mag-fura-2, 2-{6-[bis(carboxymethyl)amino]-5-(carboxymethoxy)-2-benzofuranyl}-5-oxazolecarboxylic acid; MBD, metal binding domain; TM, transmembrane.
SUPPORTING INFORMATION AVAILABLE Tables of sedimentation equilibrium, EXAFS curve fitting results, and resonance assignments; HPLC size exclusion chromatographic analysis, HSQC spectra, chemical shift difference plots, and electrostatic surface potential renderings of homology models; and supplementary methods. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Lutsenko S, Kaplan JH. Organization of P-type ATPases: Significance of structural diversity. Biochemistry. 1995;34:15607–15613. doi: 10.1021/bi00048a001. [DOI] [PubMed] [Google Scholar]
- 2.Arguello JM. Identification of ion-selectivity determinants in heavy-metal transport P1B-type ATPases. J. Membr. Biol. 2003;195:93–108. doi: 10.1007/s00232-003-2048-2. [DOI] [PubMed] [Google Scholar]
- 3.Arnesano F, Banci L, Bertini I, Ciofi-Baffoni S, Molteni E, Huffman DL, O'Halloran TV. Metallochaperones and metal-transporting ATPases: A comparative analysis of sequences and structures. Genome Res. 2002;12:255–271. doi: 10.1101/gr.196802. [DOI] [PubMed] [Google Scholar]
- 4.Bartee MY, Lutsenko S. Hepatic copper-transporting ATPase ATP7B: Function and inactivation at the molecular and cellular level. Biometals. 2007;20:627–637. doi: 10.1007/s10534-006-9074-3. [DOI] [PubMed] [Google Scholar]
- 5.Williams LE, Mills RF. P(1B)-ATPases: An ancient family of transition metal pumps with diverse functions in plants. Trends Plant Sci. 2005;10:491–502. doi: 10.1016/j.tplants.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Dutta SJ, Liu J, Hou Z, Mitra B. Conserved aspartic acid 714 in transmembrane segment 8 of the ZntA subgroup of P1B-type ATPases is a metal-binding residue. Biochemistry. 2006;45:5923–5931. doi: 10.1021/bi0523456. [DOI] [PubMed] [Google Scholar]
- 7.Sazinsky MH, Agarwal S, Arguello JM, Rosenzweig AC. Structure of the Actuator Domain from the Archaeoglobus fulgidus Cu+-ATPase. Biochemistry. 2006;45:9949–9955. doi: 10.1021/bi0610045. [DOI] [PubMed] [Google Scholar]
- 8.Sazinsky MH, Mandal AK, Arguello JM, Rosenzweig AC. Structure of the ATP binding domain from the Archaeoglobus fulgidus Cu+-ATPase. J. Biol. Chem. 2006;281:11161–11166. doi: 10.1074/jbc.M510708200. [DOI] [PubMed] [Google Scholar]
- 9.Dmitriev O, Tsivkovskii R, Abildgaard F, Morgan CT, Markley JL, Lutsenko S. Solution structure of the N-domain of Wilson disease protein: Distinct nucleotide-binding environment and effects of disease mutations. Proc. Natl. Acad. Sci. U.S.A. 2006;103:5302–5307. doi: 10.1073/pnas.0507416103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenzweig AC, O'Halloran TV. Structure and chemistry of the copper chaperone proteins. Curr. Opin. Chem. Biol. 2000;4:140–147. doi: 10.1016/s1367-5931(99)00066-6. [DOI] [PubMed] [Google Scholar]
- 11.Walker JM, Huster D, Ralle M, Morgan CT, Blackburn NJ, Lutsenko S. The N-terminal metal-binding site 2 of the Wilson's Disease Protein plays a key role in the transfer of copper from Atox1. J. Biol. Chem. 2004;279:15376–15384. doi: 10.1074/jbc.M400053200. [DOI] [PubMed] [Google Scholar]
- 12.Achila D, Banci L, Bertini I, Bunce J, Ciofi-Baffoni S, Huffman DL. Structure of human Wilson protein domains 5 and 6 and their interplay with domain 4 and the copper chaperone HAH1 in copper uptake. Proc. Natl. Acad. Sci. U.S.A. 2006;103:5729–5734. doi: 10.1073/pnas.0504472103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banci L, Bertini I, Cantini F, Felli IC, Gonnelli L, Hadjiliadis N, Pierattelli R, Rosato A, Voulgaris P. The Atx1-Ccc2 complex is a metal-mediated protein-protein interaction. Nat. Chem. Biol. 2006;2:367–368. doi: 10.1038/nchembio797. [DOI] [PubMed] [Google Scholar]
- 14.Banci L, Bertini I, Ciofi-Baffoni S, Kandias NG, Robinson NJ, Spyroulias GA, Su XC, Tottey S, Vanarotti M. The delivery of copper for thylakoid import observed by NMR. Proc. Natl. Acad. Sci. U.S.A. 2006;103:8320–8325. doi: 10.1073/pnas.0600142103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pufahl RA, Singer CP, Peariso KL, Lin SJ, Schmidt PJ, Fahrni CJ, Culotta VC, Penner-Hahn JE, O'Halloran TV. Metal ion chaperone function of the soluble Cu(I) receptor Atx1. Science. 1997;278:853–856. doi: 10.1126/science.278.5339.853. [DOI] [PubMed] [Google Scholar]
- 16.Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O'Halloran TV. Undetectable intracellular free copper: The requirement of a copper chaperone for superoxide dismutase. Science. 1999;284:805–808. doi: 10.1126/science.284.5415.805. [DOI] [PubMed] [Google Scholar]
- 17.Mandal AK, Arguello JM. Functional roles of metal binding domains of the Archaeoglobus fulgidus Cu+-ATPase CopA. Biochemistry. 2003;42:11040–11047. doi: 10.1021/bi034806y. [DOI] [PubMed] [Google Scholar]
- 18.Borrelly GP, Rondet SA, Tottey S, Robinsn NJ. Chimeras of P-type ATPases and their transcriptional regulators: Contributions of a cytosolic amino-terminal domain to metal specificity. Mol. Microbiol. 2004;53:217–227. doi: 10.1111/j.1365-2958.2004.04106.x. [DOI] [PubMed] [Google Scholar]
- 19.Banci L, Bertini I, Ciofi-Baffoni S, Finney LA, Outten CE, O'Halloran TV. A new zinc-protein coordination site in intracellular metal trafficking: Solution structure of the Apo and Zn(II) forms of ZntA(46–118) J. Mol. Biol. 2002;323:883–897. doi: 10.1016/s0022-2836(02)01007-0. [DOI] [PubMed] [Google Scholar]
- 20.Banci L, Bertini I, Ciofi-Baffoni S, Su XC, Miras R, Bal N, Mintz E, Catty P, Shokes JE, Scott RA. Structural basis for metal binding specificity: The N-terminal cadmium binding domain of the P1-type ATPase CadA. J. Mol. Biol. 2006;356:638–650. doi: 10.1016/j.jmb.2005.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finney LA, O'Halloran TV. Transition metal speciation in the cell: Insights from the chemistry of metal ion receptors. Science. 2003;300:931–936. doi: 10.1126/science.1085049. [DOI] [PubMed] [Google Scholar]
- 22.Chen K, Yuldasheva S, Penner-Hahn JE, O'Halloran TV. An atypical linear Cu(I)-S2 center constitutes the high-affinity metal-sensing site in the CueR metalloregulatory protein. J. Am. Chem. Soc. 2003;125:12088–12089. doi: 10.1021/ja036070y. [DOI] [PubMed] [Google Scholar]
- 23.Ralle M, Lutsenko S, Blackburn NJ. X-ray absorption spectroscopy of the copper chaperone HAH1 reveals a linear two-coordinate Cu(I) center capable of adduct formation with exogenous thiols and phosphines. J. Biol. Chem. 2003;278:23163–23170. doi: 10.1074/jbc.M303474200. [DOI] [PubMed] [Google Scholar]
- 24.Banci L, Bertini I, Ciofi-Baffoni S, Su XC, Borrelly GP, Robinson NJ. Solution structures of a cyanobacterial metallochaperone: Insight into an atypical copper-binding motif. J. Biol. Chem. 2004;279:27502–27510. doi: 10.1074/jbc.M402005200. [DOI] [PubMed] [Google Scholar]
- 25.Borrelly GP, Blindauer CA, Schmid R, Butler CS, Cooper CE, Harvey I, Sadler PJ, Robinson NJ. A novel copper site in a cyanobacterial metallochaperone. Biochem. J. 2004;378:293–297. doi: 10.1042/BJ20031669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Busenlehner LS, Pennella MA, Giedroc DP. The SmtB/ArsR family of metalloregulatory transcriptional repressors: Structural insights into prokaryotic metal resistance. FEMS Microbiol. Rev. 2003;27:131–143. doi: 10.1016/S0168-6445(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 27.Liu T, Golden JW, Giedroc DP. A zinc(II)/lead-(II)/cadmium(II)-inducible operon from the cyanobacterium Anabaena is regulated by AztR, an R3N ArsR/SmtB metalloregulator. Biochemistry. 2005;44:8673–8683. doi: 10.1021/bi050450+. [DOI] [PubMed] [Google Scholar]
- 28.Rensing C, Mitra B, Rosen BP. The zntA gene of Escherichia coli encodes a Zn(II)-translocating P-type ATPase. Proc. Natl. Acad. Sci. U.S.A. 1997;94:14326–14331. doi: 10.1073/pnas.94.26.14326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rensing C, Sun Y, Mitra B, Rosen BP. Pb(II)-translocating P-type ATPases. J. Biol. Chem. 1998);273:32614–32617. doi: 10.1074/jbc.273.49.32614. [DOI] [PubMed] [Google Scholar]
- 30.Grass G, Fan B, Rosen BP, Franke S, Nies DH, Rensing C. ZitB (YbgR), a member of the cation diffusion facilitator family, is an additional zinc transporter in Escherichia coli. J. Bacteriol. 2001;183:4664–4667. doi: 10.1128/JB.183.15.4664-4667.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pennella MA, Arunkumar AI, Giedroc DP. Individual metal ligands play distinct functional roles in the zinc sensor Staphylococcus aureus CzrA. J. Mol. Biol. 2006;356:1124–1136. doi: 10.1016/j.jmb.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 32.VanZile ML, Cosper NJ, Scott RA, Giedroc DP. The zinc metalloregulatory protein Synechococcus PCC7942 SmtB binds a single zinc ion per monomer with high affinity in a tetrahedral coordination geometry. Biochemistry. 2000;39:11818–11829. doi: 10.1021/bi001140o. [DOI] [PubMed] [Google Scholar]
- 33.Kuzmic P. Program DYNAFIT for the analysis of enzyme kinetic data: Application to HIV proteinase. Anal. Biochem. 1996;237:260–273. doi: 10.1006/abio.1996.0238. [DOI] [PubMed] [Google Scholar]
- 34.VanZile ML, Chen X, Giedroc DP. Structural characterization of distinct α3N and α5 metal sites in the cyanobacterial zinc sensor SmtB. Biochemistry. 2002;41:9765–9775. doi: 10.1021/bi0201771. [DOI] [PubMed] [Google Scholar]
- 35.Walkup GK, Imperiali B. Fluorescent chemosensors fr divalent zinc based on zinc finger domains. Enhanced oxidative stability, metal binding affinity, and structural and functional characterization. J. Am. Chem. Soc. 1997;119:3443–3450. [Google Scholar]
- 36.Berjanskii MV, Neal S, Wishart DS. PREDITOR: A web server for predicting protein torsion angle restraints. Nucleic Acids Res. 2006;34:W63–W69. doi: 10.1093/nar/gkl341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banci L, Bertini I, Del Conte R, Markey J, Ruiz-Duenas FJ. Copper trafficking: the solution structure of Bacillus subtilis CopZ. Biochemistry. 2001;40:15660–15668. doi: 10.1021/bi0112715. [DOI] [PubMed] [Google Scholar]
- 38.De Marac NT, Houmard J. The Cyanobacteria. Elsevier Science Publishing Co., Inc.; New York; 1987. [Google Scholar]
- 39.Tong L, Nakashima S, Shibasaka M, Katsuhara M, Kasamo K. A novel histidine-rich CPx-ATPase from the filamentous cyanobacterium Oscillatoria brevis related to multiple-heavy-metal cotolerance. J. Bacteriol. 2002;184:5027–5035. doi: 10.1128/JB.184.18.5027-5035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pountney DL, Tiwari RP, Egan JB. Metal- and DNA-binding properties and mutational analysis of the transcription activating factor, B, of coliphage 186: A prokaryotic C4 zinc-finger protein. Protein Sci. 1997;6:892–902. doi: 10.1002/pro.5560060416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Busenlehner LS, Weng TC, Penner-Hahn JE, Giedroc DP. Elucidation of primary (α(3)N) and vestigial (α(5)) heavy metal-binding sites in Staphylococcus aureus pI258 CadC: Evolutionary implications for metal ion selectivity of ArsR/SmtB metal sensor proteins. J. Mol. Biol. 2002;319:685–701. doi: 10.1016/S0022-2836(02)00299-1. [DOI] [PubMed] [Google Scholar]
- 42.Busenlehner LS, Cosper NJ, Scott RA, Rosen BP, Wong MD, Giedroc DP. Spectroscopic properties of the metalloregulatory Cd(II) and Pb(II) sites of S. aureus pI258 CadC. Biochemistry. 2001;40:4426–4436. doi: 10.1021/bi010006g. [DOI] [PubMed] [Google Scholar]
- 43.Payne JC, ter Horst MA, Godwin HA. Lead Fingers: Pb2+ Binding to Structural Zinc-Binding Domains Determined Directly by Monitoring Lead-Thiolate Charge-Transfer Bands. J. Am. Chem. Soc. 1999;121:6850–6855. [Google Scholar]
- 44.Magyar JS, Weng TC, Stern CM, Dye DF, Rous BW, Payne JC, Bridgewater BM, Mijovilovich A, Parkin G, Zaleski JM, Penner-Hahn JE, Godwin HA. Reexamination of lead(II) coordination preferences in sulfur-rich sites: Implications for a critical mechanism of lead poisoning. J. Am. Chem. Soc. 2005;127:9495–9505. doi: 10.1021/ja0424530. [DOI] [PubMed] [Google Scholar]
- 45.Banci L, Bertini I, Ciofi-Baffoni S, Gonnelli L, Su XC. A core mutation affecting the folding properties of a soluble domain of the ATPase protein CopA from Bacillus subtilis. J. Mol. Biol. 2003;331:473–484. doi: 10.1016/s0022-2836(03)00769-1. [DOI] [PubMed] [Google Scholar]
- 46.Okkeri J, Haltia T. The metal-binding sites of the zinc-transporting P-type ATPase of Escherichia coli. Lys(693) and Asp(714) in the seventh and eighth transmembrane segments of ZntA contribute to the coupling of metal binding and ATPase activity. Biochim. Biophys. Acta. 2006;1757:1485–1495. doi: 10.1016/j.bbabio.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 47.Dutta SJ, Liu J, Mitra B. Kinetic analysis of metal binding to the amino-terminal domain of ZntA by monitoring metal-thiolate charge-transfer complexes. Biochemistry. 2005;44:14268–14274. doi: 10.1021/bi050761k. [DOI] [PubMed] [Google Scholar]
- 48.Mitra B, Sharma R. The cysteine-rich amino-terminal domain of ZntA, a Pb(II)/Zn(II)/Cd(II)-translocating ATPase from Escherichia coli, is not essential for its function. Biochemistry. 2001;40:7694–7699. doi: 10.1021/bi010576g. [DOI] [PubMed] [Google Scholar]
- 49.Liu J, Dutta SJ, Stemmler AJ, Mitra B. Metal-binding affinity of the transmembrane site in ZntA: Implications for metal selectivity. Biochemistry. 2006;45:763–772. doi: 10.1021/bi051836n. [DOI] [PubMed] [Google Scholar]
- 50.Bunce J, Achila D, Hetrick E, Lesley L, Huffman DL. Copper transfer studies between the N-terminal copper binding domains one and four of human Wilson protein. Biochim. Biophys. Acta. 2006;1760:907–912. doi: 10.1016/j.bbagen.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Banci L, Bertini I, Ciofi-Baffoni S, Chasapis CT, Hadjiliadis N, Rosato A. An NMR study of the interaction between the human copper(I) chaperone and the second and fifth metal-binding domains of the Menkes protein. FEBS J. 2005;272:865–871. doi: 10.1111/j.1742-4658.2004.04526.x. [DOI] [PubMed] [Google Scholar]
- 52.Wernimont AK, Huffman DL, Lamb AL, O'Halloran TV, Rosenzweig AC. Structural basis for copper transfer by the metallochaperone for the Menkes/Wilson disease proteins. Nat. Struct. Biol. 2000;7:766–771. doi: 10.1038/78999. [DOI] [PubMed] [Google Scholar]
- 53.Thelwell C, Robinson NJ, Turner-Cavet JS. An SmtB-like repressor from Synechocystis PCC 6803 regulates a zinc exporter. Proc. Natl. Acad. Sci. U.S.A. 1998;95:10728–10733. doi: 10.1073/pnas.95.18.10728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cavet JS, Borrelly GP, Robinson NJ. Zn, Cu and Co in cyanobacteria: Selective control of metal availability. FEMS Microbiol. Rev. 2003;27:165–181. doi: 10.1016/S0168-6445(03)00050-0. [DOI] [PubMed] [Google Scholar]
- 55.Banerjee S, Wei B, Bhattacharyya-Pakrasi M, Pakrasi HB, Smith TJ. Structural determinants of metal specificity in the zinc transport protein ZnuA from Synechocystis 6803. J. Mol. Biol. 2003;333:1061–1069. doi: 10.1016/j.jmb.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 56.Waldron KJ, Tottey S, Yanagisawa S, Dennison C, Robinson NJ. A periplasmic iron binding protein contributes towards inward copper supply. J. Biol. Chem. 2007;282:3837–3846. doi: 10.1074/jbc.M609916200. [DOI] [PubMed] [Google Scholar]
- 57.Liu T, Nakashima S, Hirose K, Uemura Y, Shibasaka M, Katsuhara M, Kasamo K. A metallothionein and CPx-ATPase handle heavy-metal tolerance in the filamentous cyanobacterium Oscillatoria brevis. FEBS Lett. 2003;542:159–163. doi: 10.1016/s0014-5793(03)00370-3. [DOI] [PubMed] [Google Scholar]
- 58.Huckle JW, Morby AP, Turner JS, Robinson NJ. Isolation of a prokaryotic metallothionein locus and analysis of transcriptional control by trace metal ions. Mol. Microbiol. 1993;7:177–187. doi: 10.1111/j.1365-2958.1993.tb01109.x. [DOI] [PubMed] [Google Scholar]
- 59.Blindauer CA, Harrison MD, Robinson AK, Parkinson JA, Bowness PW, Sadler PJ, Robinson NJ. Multiple bacteria encode metallothioneins and SmtA-like zinc fingers. Mol. Microbiol. 2002;45:1421–1432. doi: 10.1046/j.1365-2958.2002.03109.x. [DOI] [PubMed] [Google Scholar]
- 60.Blindauer CA, Harrison MD, Parkinson JA, Robinson AK, Cavet JS, Robinson NJ, Sadler PJ. A metallothionein containing a zinc finger within a four-metal cluster protects a bacterium from zinc toxicity. Proc. Natl. Acad. Sci. U.S.A. 2001;98:9593–9598. doi: 10.1073/pnas.171120098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grossoehme NE, Akilesh S, Guerinot ML, Wilcox DE. Metal-binding thermodynamics of the histidine-rich sequence from the metal-transport protein IRT1 of Arabidopsis thaliana. Inorg. Chem. 2006;45:8500–8508. doi: 10.1021/ic0606431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.