Abstract
Background
Sleep spindles are thought to induce synaptic changes and thereby contribute to memory consolidation during sleep. Patients with schizophrenia show dramatic reductions of both spindles and sleep-dependent memory consolidation, which may be causally related.
Methods
To examine the relations of sleep spindle activity to sleep-dependent consolidation of motor procedural memory, 21 chronic, medicated schizophrenia outpatients and 17 healthy volunteers underwent polysomnography on two consecutive nights. On the second night, participants were trained on the finger tapping Motor Sequence Task (MST) at bedtime and tested the following morning. The number, density, frequency, duration, amplitude, spectral content, and coherence of stage 2 sleep spindles were compared between groups and examined in relation to overnight changes in MST performance.
Results
Patients failed to show overnight improvement on the MST and differed significantly from controls who did improve. Patients also exhibited marked reductions in the density (reduced 38% relative to controls), number (reduced 36%), and coherence (reduced 19%) of sleep spindles, but showed no abnormalities in the morphology of individual spindles or of sleep architecture. In patients, reduced spindle number and density predicted less overnight improvement on the MST. In addition, reduced amplitude and sigma power of individual spindles correlated with greater severity of positive symptoms.
Conclusions
The observed sleep spindle abnormalities implicate thalamocortical network dysfunction in schizophrenia. In addition, our findings suggest that abnormal spindle generation impairs sleep-dependent memory consolidation in schizophrenia, contributes to positive symptoms, and is a promising novel target for the treatment of cognitive deficits in schizophrenia.
Keywords: sleep, schizophrenia, procedural learning, motor skill, sleep spindles, memory consolidation
Sleep disturbances in schizophrenia have been described since Kraepelin (1) and are a common complaint throughout its course (2), including in the prodrome (3), but the nature of the abnormality and its relations to the pathophysiology, cognitive deficits, and symptoms of schizophrenia remain poorly understood (4). Several recent studies have reported markedly reduced sleep spindle activity in schizophrenia (5–8). Mediated by thalamocortical circuits, spindles are a defining feature of stage 2 NREM (non-rapid eye movement) sleep, evident in the electroencephalogram (EEG) as brief bursts of 12–15Hz synchronous activity. Animal studies point to spindles as a key mechanism of synaptic plasticity, mediating memory consolidation during sleep (9–12). In humans, spindles correlate with overnight memory consolidation across diverse learning paradigms (13). In schizophrenia, memory deficits are a key neurocognitive abnormality and there is evidence of impaired consolidation across multiple domains (14–16). In particular, schizophrenia patients have marked impairments of sleep-dependent motor procedural memory consolidation (17) in the context of reduced spindle activity (5, 8). We conducted a new study to more completely specify the nature of sleep spindle abnormalities in schizophrenia and their relation to deficient sleep-dependent memory consolidation.
The most widely reported sleep architecture abnormalities in schizophrenia – reduced slow wave sleep (SWS) (18–20) and abnormal rapid eye movement (REM) sleep (20–22) are not consistently observed and have not withstood meta-analyses (23, 24). Relatively few studies have gone beyond sleep architecture to examine the EEG spectral characteristics of sleep. Three older studies of unmedicated patients that identified NREM spindles with visual detection at a single EEG channel had contradictory findings. Poulin et al. (20) examined spindles in the 12–14 Hz range and found no differences between patients (n=11) and controls. Van Cauter also reported no differences in patients (n=9) compared to controls. Finally, Hiatt et al. (25) found that patients (n=5) showed increased spindle density during a 10 minute NREM segment, but did not distinguish between Stage 2 and SWS sleep across participants. In contrast, several recent studies that used automated spindle detection algorithms, a larger number of EEG channels, and larger samples comprised of chronic medicated schizophrenia patients report a dramatic reduction of sleep spindles (5–8). In one of these studies, the spindle deficit was associated with greater severity of positive symptoms and was absent in a non-schizophrenic psychiatric control group treated with antipsychotic medications (7) suggesting that the spindle deficit influences symptom presentation and is not due to treatment with antipsychotic medications. To characterize the functional significance of the sleep spindle deficit in schizophrenia, we examined the relations of spindle activity to overnight consolidation of motor memory and to symptoms.
We employed the same simple, well-characterized test of motor procedural learning used in our previous studies of schizophrenia, the finger tapping motor sequence task (MST) (26, 27). After training on the MST, healthy young participants show significant improvement in speed after a night of sleep, but not after an equivalent period of wake (27). In young healthy individuals, overnight improvement on the MST and other simple motor skill tasks correlates with the amount of stage 2 sleep (27) and with the number and density of sleep spindles (28–31). In two studies, we have reported that this overnight improvement is absent in schizophrenia (5, 17). Our primary hypothesis was that reduced stage 2 sleep spindle activity would correlate with impaired overnight memory consolidation in schizophrenia. We also expected patients to show reduced spindle coherence during sleep based on evidence of reduced EEG coherence during quiet awake (32–35), and dysconnectivity in thalamocortical networks in schizophrenia (36–38).
Methods
Participants
Twenty-five schizophrenia outpatients were recruited from an urban mental health center and 21 completed the study. Patients had been maintained on stable doses of atypical antipsychotic medications for at least six weeks and 12 took diverse adjunctive medications for anxiety, agitation, and/or concurrent mood disturbance (Supplemental Table 1). Diagnoses were confirmed with Structured Clinical Interviews for DSM-IV (39) and symptoms were rated with the Positive and Negative Syndrome Scale (40).
Twenty-one healthy control participants, screened to exclude a personal history of mental illness, family history of schizophrenia spectrum disorder, and psychoactive medication use, were recruited from the community by poster and website advertisements and 18 completed the study.
All participants were screened to exclude diagnosed sleep disorders, treatment with sleep medications, a history of significant head injury, neurological illness, and substance abuse or dependence within the past six months. One control participant was excluded for sleeping less than four hours on two of the five nights preceding the laboratory visit based on wrist actigraphy and sleep log data. The final sample of 21 schizophrenia and 17 control participants did not differ in age, sex, or parental education (Supplemental Table 2). All participants gave written informed consent. The study was approved by the Institutional Review Boards of Massachusetts General Hospital, the Massachusetts Department of Mental Health, and Beth Israel Deaconess Medical Center.
Procedures
In the week prior to their stay in the Clinical Research Center (CRC), participants completed informed consent, demographic questionnaires, and rating scales, toured the CRC, and received an actigraph to wear until study completion. Participants also completed home sleep logs.
Participants spent two consecutive weeknights in the CRC with polysomnography (PSG) and engaged in their usual activities during the day. On the second night, participants were trained on the MST one hour prior to their usual bedtime, wired for PSG, and allowed to sleep for up to 10 hours. They were tested on the MST one hour after awakening.
Polysomngraphy (PSG)
Data were digitally acquired at 100Hz using an Embla N7000 system (Medcare Systems, Buffalo NY), with a standard montage including five to eight channels of EEG (electroencephalography; F3, F4, C3, Cz, C4, Pz, O1, O2) referenced to the linked mastoids, EMG (electromyography), and EOG (electrooculography). Data from all available electrodes were analyzed for each participant.
Finger Tapping Motor Sequence Task (MST)
The MST involves pressing four numerically labeled keys on a standard computer keyboard with the fingers of the left hand, repeating a five element sequence (4-1-3-2-4) "as quickly and accurately as possible" for 30s. The numeric sequence was displayed at the top of the screen, and dots appeared beneath it with each keystroke. During both training and test sessions, participants alternated tapping and resting for 30s for a total of 12 tapping trials. The primary outcome measure was the number of correct sequences per trial, which reflects both the speed and accuracy of performance. Practice-dependent improvement was defined as the percent increase in correct sequences from the first training trial to the average of the last three training trials. Overnight improvement was calculated as the percent increase in correct sequences from the last three training trials to the first three test trials the following morning (27).
Actigraphy
The Mini-Mitter Actiwatch®-64 records wrist movement in 15s epochs to provide estimates of sleep time based on periods of wrist immobility. Standard scoring algorithms were used to determine time in bed (TIB), total sleep time (TST), and sleep efficiency (TST/TIB).
Data Analysis
As none of the sleep architecture or spindle measures differed significantly across the two laboratory nights in either group, data are presented as an average across both nights. Unless otherwise specified, EEG data are averaged across all electrode sites.
Sleep Architecture
Each 30s epoch of PSG sleep was visually classified into stages (Wake, 1, 2, 3, 4, and REM) according to standard criteria (41) by raters blind to diagnosis and night. Awakenings were scored when one or more 30s epoch was classified as “Wake” following initial sleep onset. Sleep stages are reported as the percentage of TST.
Spindle Analyses
We analyzed spindles during stage 2 sleep, given our primary hypothesis of a relation of stage 2 sleep spindles with memory consolidation. PSG data were preprocessed and analyzed using BrainVision Analyzer 2.0 (BrainProducts, Munich Germany) and MatLab R2009b (The MathWorks, Natick MA). Artifacts were automatically detected and removed and EEG data were filtered at 0.5–35Hz. Artifact rejection was confirmed by visual inspection.
Power spectral density (µV2/Hz) was calculated by Fast Fourier Transform (FFT), applying a Hanning window to successive 3s epochs of stage 2 sleep with 50% overlap. Sigma band (12–15Hz) spectral power, which correlates with sleep spindle activity, was divided into low (12–13.5Hz) and high (13.5–15Hz) sigma band power (42, 43).
Discrete sleep spindle events were automatically detected using a wavelet-based algorithm. The raw EEG signal was subjected to a time-frequency transformation using an 8-parameter complex Morlet wavelet (44). Spindles were detected at each EEG channel by applying a thresholding algorithm to the extracted wavelet scale corresponding approximately to the 10–16Hz frequency range. For thresholding, the rectified moving average of the signal was first calculated, using a 100ms sliding window. A spindle event was identified whenever this wavelet signal exceeded threshold (defined as 4.5 times the mean signal amplitude of all artifact-free epochs) for a minimum of 400ms. This method was validated by examining the correlation of algorithmically detected spindle density with hand-counted spindles in ten controls (r=0.87; slope=1.2 automated/hand scored) and ten patients (r=0.95; slope=1.8). The ratio of algorithm-detected to hand-counted spindles was not significantly different between groups. As spindle density is known to correlate with sigma power, albeit moderately since background sigma-frequency EEG activity weakens the correlation (42), we also correlated algorithm-detected spindle density with 12–15Hz sigma power in all controls (r=0.50, p=.04) and patients: (r=.52, p=.02).
For each spindle, measures of amplitude, sigma power, duration, and peak frequency were based on analysis of 2s EEG epochs centered on the point of spindle detection (Figure 1). Within the sigma range (12–15Hz), amplitude was the maximal voltage following 12–15Hz band pass filtering, peak frequency was defined as the spectral peak of the spindle following FFT decomposition, and sigma power was defined as the mean FFT-derived power spectral density across the 12–15Hz range (µV2/Hz; Figure 1, middle).
Figure 1.
Frequency and time-frequency decomposition of individual spindle events used to define amplitude, frequency, and duration variables. A) EEG trace of a sleep spindle waveform filtered at 12–15Hz, B) FFT transform of the same sleep spindle C) Wavelet decomposition of the spindle, representing spectral power across time. Spindle duration defined as half-height width of wavelet energy.
To examine the time-frequency characteristics of individual spindles, wavelet analysis was conducted at Cz, where detected spindles were most numerous in both control and patient samples. A complex Morlet wavelet was applied separately to each spindle epoch (Figure 1, right). The duration of each spindle was calculated as the half-height width of wavelet energy within the spindle frequency range. Finally, we assessed the cross-spectrum/auto-spectrum sigma coherence (45) of spindles across EEG channels. Coherence (Coh) of spindles across EEG channels (c) was assessed for each 2s epoch during which a spindle was auto-detected in any channel. Coherence values range from 0–1, calculated as the cross-spectrum (CS)/autospectrum coherence within the full 12–15Hz sigma frequency band (f), according to the formula:
Coh(c1, c2)(f) = | CS(c1, c2)(f) |2 / (| CS(c1, c1)(f) | | CS(c2, c2)(f) |)
Results
Sleep Architecture
Patients and controls showed relatively normal sleep and did not differ significantly on any measure of sleep quality or architecture measured either as the total number of minutes or the percentage of TST spent in any sleep stage during the experimental nights (Table 1). Actigraphy indicated that patients spent significantly more TIB and had more TST during the pre-study nights.
Table 1.
Sleep data in patients and controls reported as means ± SD.
| Patients (n=21) | Controls (n=17) | p | |
|---|---|---|---|
| Sleep Architecture | |||
| Total Sleep Time (TST, min) | 443 ± 79 | 413 ± 55 | .19 |
| Stage 1 % | 9 ± 6 | 9 ± 1 | .76 |
| Stage 2 % | 54 ± 10 | 56 ± 7 | .40 |
| SWS % | 17 ± 10 | 13 ± 5 | .12 |
| REM % | 19 ± 4 | 22 ± 7 | .17 |
| WASO (min) | 70 ± 60 | 61 ± 46 | .62 |
| Awakenings/Hr | 4.1± 2.5 | 4.2± 1.5 | .86 |
| Spindle Measures | |||
| Spindle Number | 308 ± 144 | 485 ± 122 | .0003** |
| Spindle Density/min | 1.3 ± .5 | 2.1 ± .6 | .0001** |
| Low Sigma Power (µV2/Hz) | .043 ± .02 | .074 ± .06 | .03* |
| High Sigma (µV2/Hz) | .034 ± .02 | .043 ± .03 | .27 |
| Characteristics of Individual Spindles | |||
| Amplitude (µV) | 16.8 ± 3.4 | 17.3 ± 5.0 | .67 |
| Frequency (Hz) | 12.9 ± 1.5 | 13.0 ± 1.8 | .50 |
| Duration (sec) | 1.01 ± .16 | 1.04 ± .05 | .47 |
| Sigma Power (µV2/Hz) | 0.31 ± .13 | 0.35 ± .19 | .51 |
| Sigma Coherence (0–1) | 0.35 ± .09 | 0.43 ± .10 | .02* |
| Pre-Study Actigraphy | |||
| TIB (min) | 589 ± 93 | 454 ± 73 | .0003** |
| TST (min) | 521 ± 99 | 402 ± 70.6 | .0004** |
| Sleep Efficiency % | 88 ± 5 | 88 ± 5 | .80 |
WASO=Wake after sleep onset (min). TIB = Time in bed.
Spindle Number, Density, and Sigma Power
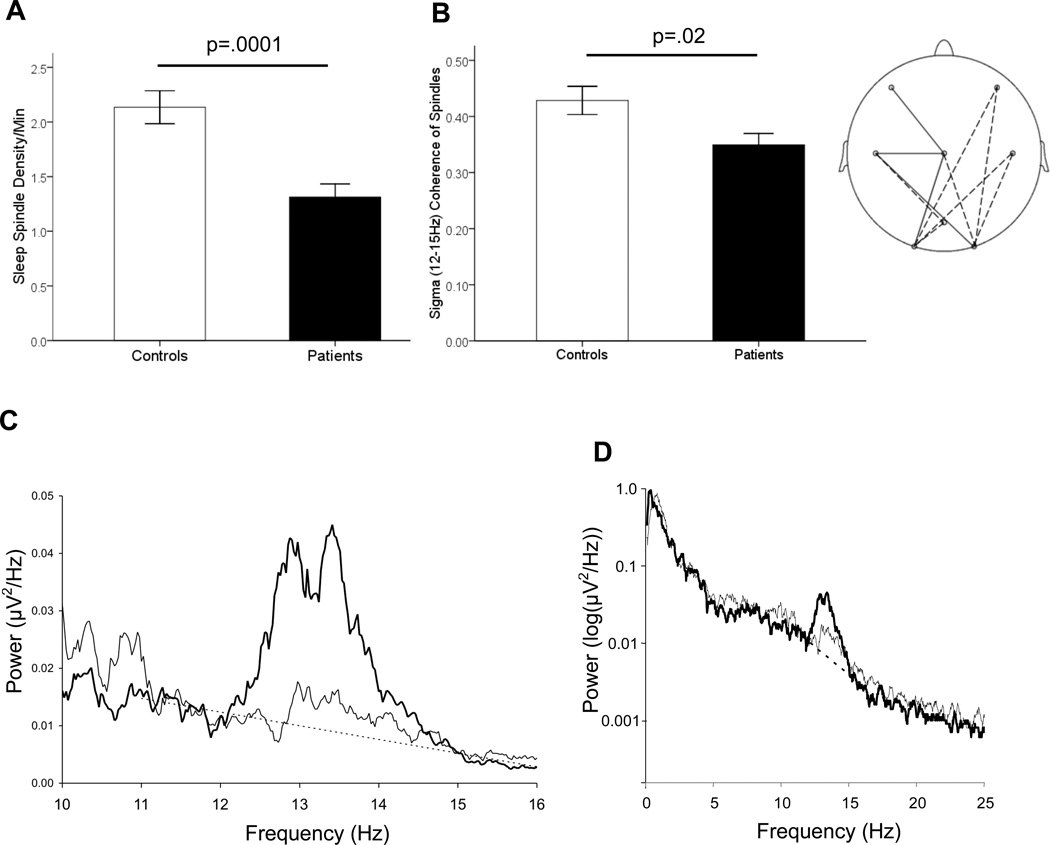
Relative to controls, schizophrenia patients exhibited markedly reduced sleep spindle number (36% reduction) and density (38%) (Table 1; Figure 2A, Left) (p-≤.01 for all electrodes). While controls showed a typical spindle-frequency peak in the stage 2 power spectrum, this peak was dramatically reduced in the patient group (Figure 2C). Sigma power was significantly reduced in patients in the low but not high-frequency sigma band (Table 1). There were no group differences in the delta (1–4Hz), theta (4–7Hz), alpha (8–12Hz), or beta (13–25Hz) frequency bands (Figure 2D). The reductions in sigma power were more pronounced when calculated relative to the overall EEG power baseline (Figure 2C, dashed line). At Cz, for example, power in patients was only 10% of that seen in controls in the low sigma band, and 32% in the high sigma band. Group differences were similar across electrode sites and when study nights were analyzed separately.
Figure 2.
Spindle activity. A) Bar graph of sleep spindle density during stage 2 sleep averaged across electrode sites with standard error bars. B) Left: Bar graph of sigma-band coherence during spindles averaged across electrode sites with standard error bars. Right: Topographical map of reduced sigma coherence of spindles in schizophrenia across electrodes. Solid lines indicate electrode pairs showing a significant reduction in schizophrenia following correction for multiple comparisons (p<.0006); dashed lines indicate significance at the uncorrected p<.05 level. C) Spectral power in controls (heavy line) and patients (light line) at the Cz electrode, where sigma power was maximal. The sigma band baseline (12–15Hz; dashed line) is a best fit to the 11–12Hz and 15–16Hz data. D) Logarithm of spectral power across the broader 0–25Hz range. There were no group differences in the delta (1–4Hz), theta (4–7Hz), alpha (8–12Hz), or beta (13–25Hz) frequency bands. Spectral profiles were similar across all recording sites.
Morphology of Individual Spindles
There were no significant differences in the characteristics of individual spindles between groups. The average amplitude, duration, and peak frequency of individual spindles differed by less than 3% between groups, and the average sigma power in individual spindles differed by 12% (Table 1). The distribution of spindles across the scalp was also similar in patients and controls, with the greatest number of spindles detected at central recording sites for both groups.
Spindle Coherence
Patients showed reduced cross-electrode coherence of individual spindles in the sigma band (19% reduction relative to controls, Table 1, Figure 2B), with several individual electrode pairs surviving Bonferroni correction based on eight electrode sites (Cz-C3, Cz-O1, Cz-F3 and C3-O2; mean 33% reduction for these sites, all p’s <.006).
Motor Sequence Task (MST) Overnight Improvement
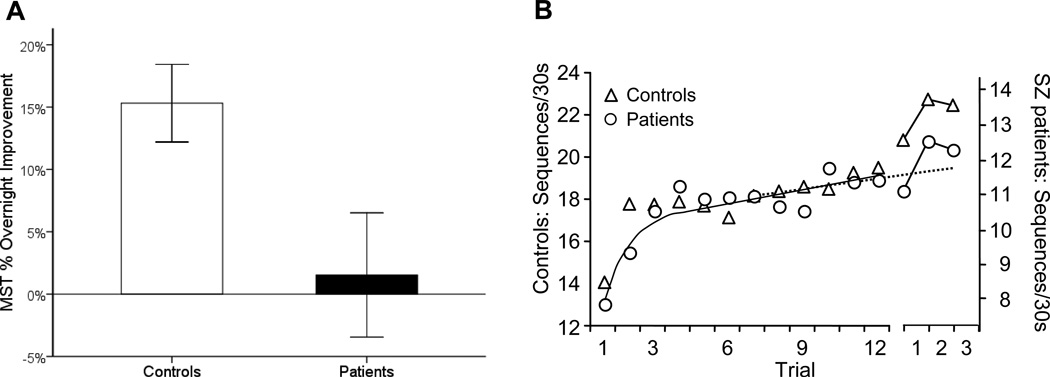
Unlike controls, who showed robust overnight improvement (15.3±12.8% s.d; t16=4.92, p=.0002), schizophrenia patients failed to show significant improvement (1.5±22.8%; t20=0.31, p=.76) and differed significantly from controls in this regard (t36=2.20, p=.03; Figure 3, left). This occurred in the context of comparable practice-dependent improvement during training in patients (37.6±37.3%) and controls (22.5±32.0%; t36=1.32, p=.20).
Figure 3.
Overnight improvement on the Motor Sequence Task (MST) in patients and controls. A) Bar graphs of overnight improvement with standard error bars. B) Motor skill learning across training and the first three test trials for controls (triangles) and schizophrenia patients (circles). The y-axis is scaled separately for controls (left) and patients (right) to highlight the qualitative similarity of learning curves on Day 1 and the failure of overnight improvement in the schizophrenia group only. The dashed line is the regression line fit to data from the last 6 training trials. The discontinuity in the x-axis represents a night of sleep. Patients and controls did not differ in the amount of learning during training (63% vs 58%, p=.77), but only controls showed significant overnight improvement.
Relations of overnight MST improvement to spindle parameters
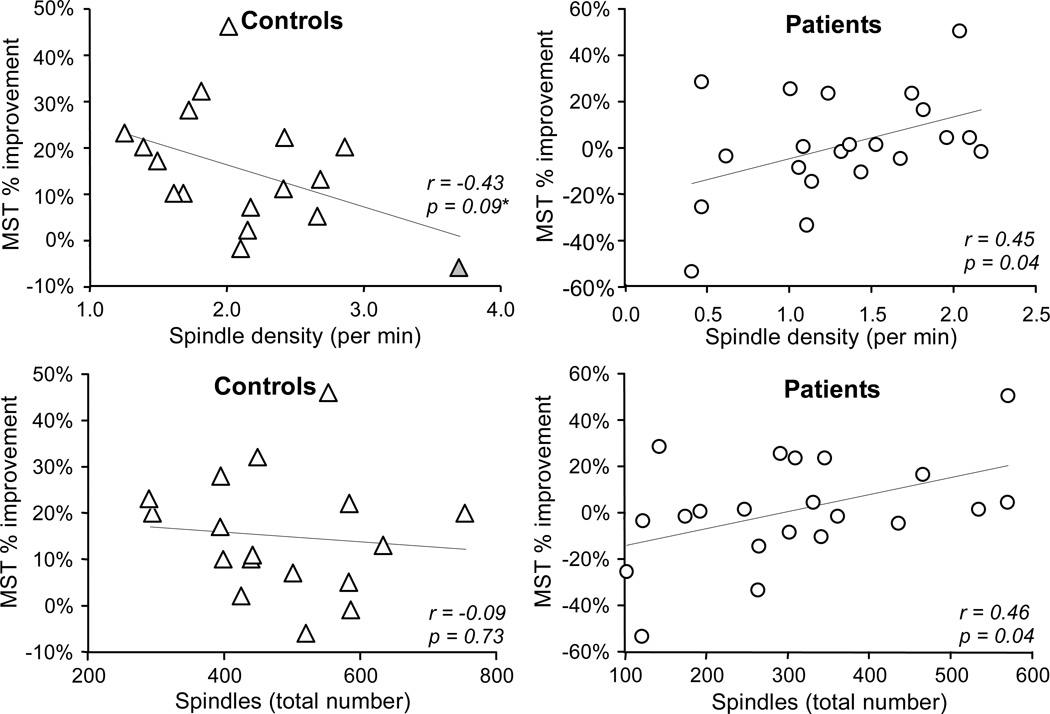
In schizophrenia, overnight improvement correlated with both spindle number (Figure 4, r21=.46, p=.04) and density (r21=.45, p=.04). In contrast, controls showed no correlation with spindle number (r17=−.09, p=.73) and a trend toward a negative correlation between spindle density and overnight improvement (r17=−.43, p=.09) that was primarily driven by a single outlier whose value exceeded the 75th percentile by greater than 1.5 times the inter-quartile range in a boxplot analysis (outlier excluded: r16=−.23, p=.40). The correlations of spindle number and density with overnight MST improvement were significantly stronger in patients (Group by Spindle: number F(2,35)=4.00, p=.03; density without outlier F(2,34)=3.50, p=.04). MST improvement was not related to sigma coherence of spindles in either group. Correlations of MST improvement with spindle parameters were similar on both nights, and the change in spindle parameters from the baseline to the testing night did not correlate with MST improvement.
Figure 4.
Overnight improvement as a function of spindle density and number in patients and controls. Regression includes one outlier (gray triangle), with outlier excluded, r = −0.23, p = 0.40.
Relations of Spindles to Symptoms
Lower peak amplitude (r21=−.47, p=.03) and sigma power (r21=−.45, p=.04) of individual spindles correlated with greater severity of positive but not negative symptoms. No other measure of spindle activity correlated with positive or negative symptoms.
Medication Effects
Neither chlorpromazine (CPZ) equivalent nor illness duration correlated with any measure of spindle activity. Spindle densities in participants divided by medication type are presented in Supplemental Table 1. Participants taking clozapine (n=9) exhibited significantly lower spindle densities than other patients (t19=2.60, p=.02). When these patients were excluded from analysis, the remaining patients still exhibited lower spindle number (t27=2.86, p=.008) and density (t27=2.89, p=.008) than controls. No other medication type significantly affected spindle parameters.
Discussion
The present study is the first to demonstrate a relationship between sleep spindle activity and sleep-dependent memory consolidation in schizophrenia. Relative to controls, schizophrenia patients showed fewer sleep spindles, a lower density of sleep spindles, and less overnight improvement on the MST than controls. Within the schizophrenia group, spindle number and density both predicted overnight MST improvement. Although the present study cannot establish causality, these findings are consistent with the hypothesis that reduced spindle activity impairs memory consolidation in schizophrenia. We also report, for the first time, reduced spindle coherence across the cortex in patients compared to controls, suggesting that there is reduced synchrony in thalamocortical spindle-generating networks in schizophrenia. This resonates with the large body of literature showing reduced EEG coherence, particularly in the beta and gamma bands, during wake in schizophrenia (for review see, 46) and extends these observations to sleep. Finally, the amplitude and power of individual sleep spindles were inversely related to positive symptoms in schizophrenia. These findings suggest that abnormal sleep spindles contribute to cognitive deficits and symptoms in schizophrenia and offer a novel biomarker and target for treatment development.
The hypothesis that sleep spindle abnormalities contribute to cognitive deficits in schizophrenia is consistent with mounting evidence concerning the function of spindles (13). In healthy individuals spindles are thought to promote synaptic plasticity critical to memory consolidation during sleep (12) thus mediating the overnight consolidation of both declarative and procedural memory (28, 47–50), and to index learning potential and general intelligence (51, 52). In this context, we note that spindles are also reported to be abnormal in several other neurodevelopmental disorders affecting cognition, including mental retardation (53), phenylketonuria (54), and autism (55).
Sleep spindles are generated in the thalamic reticular nucleus (TRN) (56), which is comprised entirely of γ-aminobutyric acid (GABA)ergic neurons (57). TRN neurons primarily project to glutamatergic thalamic neurons that then project to the cortex. Cortical neurons, in turn, send glutamatergic inputs back to N-methyl-D-aspartate acid (NMDA) receptors on TRN neurons. Thus, spindles are mediated by a thalamocortical feedback loop that is regulated by both GABAergic and NMDA-receptor mediated glutamatergic neurotransmission (58). The TRN is thought to be the spindle pacemaker, as TRN neurons exhibit spindle frequency burst firing in vivo (59, 60), which is believed to rhythmically entrain thalamocortical relay neurons and ultimately the cortex (61). While the TRN can generate spindles in isolation (62, 63), glutamatergic feedback from the cortex is critical for the synchronization of spindles (42, 64). As sleep spindles are temporally correlated with hippocampal sharp-wave ripples (10, 11), coherent expression of spindles across wide areas of cortex could reflect the synchronous “reactivation” of recent memory traces across cortical regions. Such reactivation is thought to mediate the consolidation of newly acquired information into more permanent forms for long-term storage and integration (65, 66). In schizophrenia, reduced and less synchronous spindle activity due to thalamocortical dysfunction could interfere with sleep-dependent memory processing by preventing the simultaneous reactivation of memory components stored across visual, spatial, emotional, and goal-representation networks, thus leading to a fragmentation of memory and cognition.
While exact mechanism of spindle deficits in schizophrenia is unknown, the reduction in spindle activity may reflect thalamic dysfunction, while decreased spindle synchrony may reflect abnormal NMDA-receptor mediated thalamic responses to cortical inputs. This hypothesis is consistent with evidence of GABA deficits (67) and abnormal expression of NMDA receptors and glutamate transporters in the thalamus in schizophrenia (68, 69). It is striking that current models of schizophrenia posit dysfunction in thalamocortical circuitry and in both GABA and the NMDA glutamatergic systems. Future studies using GABA and/or NMDA-receptor agonists could provide greater insight into the mechanisms underlying spindle abnormalities in schizophrenia and their contribution to impaired sleep-dependent memory.
A limitation to the interpretation of our findings is that the patients were treated with a variety of second generation antipsychotics and adjunctive agents and we did not have adequate power to examine the effects of different medication types on our outcome measures. For example, the four patients who took benzodiazapines, which are known to increase sleep spindles and sigma power (e.g., 70, 71), did not differ from other patients on spindle parameters. While a prior study of schizophrenia reported decreased sleep spindle activity following a single dose of olanzapine (72), the effects of chronic atypical antipsychotic treatment on spindle activity are unknown. In the present study, the subset of patients taking clozapine showed significantly fewer spindles, but the spindle reduction remained significant in those patients not taking clozapine and it is unclear whether the more profound reduction reflects the medication itself or illness factors that lead to treatment with clozapine. Moreover, antipsychotic dosage was not related to any measure of spindle activity. The recent finding of a spindle deficit in schizophrenia, but not in a non-schizophrenic psychiatric control group treated with antipsychotic medications suggests that the deficit is unlikely to be merely a side-effect of antipsychotics (7). While the present study cannot exclude a contribution from medications, even if the spindle reductions were affected by medications, illness progression, or epiphenomena of long-term illness, since the vast majority of patients with schizophrenia are chronic and medicated, the findings remain clinically important.
There are discrepancies with prior work that merit consideration. While our findings of reduced spindle number and density in schizophrenia agree with those of Ferrarelli et al. (6, 7), we observed these reductions across all electrode sites and did not see reductions in spindle amplitude and duration, suggesting that spindles are morphologically similar in patients and controls. Differences in spindle detection algorithms may account for these discrepancies. We also note that in the present study the spindle parameters that were inversely correlated with positive symptoms, the amplitude and sigma power of individual spindles, were not abnormal in schizophrenia and that our findings differ from Ferrarelli et al. (7) who found inverse correlations of integrated spindle activity and spindle number with positive symptoms. The present sample had only mild symptoms (PANSS total=55 ± 7) and was less symptomatic than the sample of Ferrarelli et al. (7) (PANSS total=89, variability not provided). The restricted range and mild symptoms of the present sample may limit the generalizability of our findings. Larger, more heterogenous samples may be necessary to determine which positive symptoms correlate with specific spindle parameters as this may provide clues to the mechanisms of these relations.
Unlike some previous studies (e.g., 31, 73), we did not detect any effects of motor task training on spindle characteristics in either group. Spindle parameters did not differ significantly on the baseline and testing nights and the changes in spindle activity did not correlate with overnight MST improvement. These findings are more consistent with evidence that the EEG power spectrum during NREM sleep in humans is relatively stable over time, resistant to experimental perturbations, and genetically-mediated (74, 75).
Although our prior study failed to detect a significant correlation between spindle density and overnight MST improvement in schizophrenia (Manoach et al 2010), the effect size was remarkably similar (r14=.46, p=.10 prior study; r21=.45, p=.04 here) and the difference in significance reflects that the sample was smaller by a third. Related to this, unlike some previous studies of the MST in young healthy controls (28, 31), spindle activity did not correlate with overnight improvement in the middle-aged healthy controls of either the present or prior study. We are not aware of any studies that show correlations in middle-aged participants and a recent study of older adults (mean age 68) found significant overnight MST improvement, but no correlation of improvement with any measure of spindle power (r’s range from −.07 to .06, 76). In addition, the more restricted range of both spindle activity and overnight improvement in controls compared to patients in the present study may have limited our ability to detect a correlation. When controls and patients are plotted together, the relations between spindle density and number with overnight improvement are best described by saturating non-linear inverse functions (Figure 5; density: R236=.20, p=.005; number: R236=.24, p=.002) suggesting that spindle activity improves MST performance, but only up to a point beyond which further increases do not lead to additional benefit.
Figure 5.
Overnight improvement as a function of spindle density and number in all participants combined, with the best fit non-linear saturating function lines.
In summary, we report that reduced sleep spindle activity predicts impaired consolidation of motor procedural memory and increased severity of positive symptoms in schizophrenia, suggesting that disrupted thalamocortical activity during stage 2 sleep contributes to both cognitive deficits and symptoms. Reduced and less synchronized spindle activity may impair memory consolidation by interfering with normal processes of coordinated memory reactivation and consolidation during sleep. We therefore propose that abnormal sleep spindles be considered as a potentially treatable contributor to cognitive deficits in schizophrenia.
Supplementary Material
Although sleep plays a critical role in learning and memory, abnormal sleep has generally been overlooked as a potential contributor to cognitive deficits in schizophrenia. Sleep spindles are a characteristic of sleep that has been linked to cognitive function. This study is the first to demonstrate a relationship between abnormal sleep spindle activity and impaired memory in schizophrenia. The findings suggest that sleep spindle abnormalities are a potentially treatable contributor to cognitive deficits in schizophrenia.
Acknowledgements
We are grateful to Todd Grinnell and to the staff of the MGH Clinical Research Center, particularly Mary Sullivan, RN, for their support and to Michael Halassa, MD, PhD, for his comments on the manuscript. This work was funded by research grant #30675 from Sepracor, Inc. to DSM; the National Institutes of Health R01-MH48832 and T32-HL07901; and the National Center for Research Resources UL1-RR025758 and M01-RR-01066, Harvard Clinical and Translational Science Center.
RS consults to Actelion Pharmaceuticals, has received speaking honoraria from Eli Lilly, and has received research support from Merck & Co. DSM has received a consulting fee from Sepracor, Inc and a speaking honorarium from Eli Lilly. AKS’s salary is supported in part by an unrestricted educational grant from the Harvard-MIT Division of Health Sciences and Technology/Beth Israel Deaconess Medical Center (BIDMC), in collaboration with Merck & Co. and Pfizer, Inc. DCG reports having served as a consultant or advisor to: Xytis, Forest Labs, Pfizer, Indevus Pharmaceuticals, H. Lundbeck, Schering-Plough, Eli Lilly, Takeda, Biovail, Solvay, Hoffman- La Roche, Cypress, and Dianippon Sumitomo. He served on a DSMB for Otsuka and Wyeth. He received research funding from Pfizer, Janssen, Novartis, and GlaxoSmithKline.
Footnotes
Previous presentation: Association for Psychological Science Conference, May 27–30, 2010, Boston, MA
Some of the findings were presented in preliminary form as a poster at the Association for Psychological Science Conference, May 27–30, 2010, in Boston, MA.
Financial Disclosures: None of the other authors have any conflicts of interest to disclose.
References
- 1.Kraepelin E. Dementia praecox and paraphrenia. Edinburgh, Scotland: E.S. Livingston; 1919. [Google Scholar]
- 2.Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 3.Miller TJ, Zipursky RB, Perkins D, Addington J, Woods SW, Hawkins KA, et al. The PRIME North America randomized double-blind clinical trial of olanzapine versus placebo in patients at risk of being prodromally symptomatic for psychosis. II. Baseline characteristics of the "prodromal" sample. Schizophr Res. 2003;61:19–30. doi: 10.1016/s0920-9964(02)00440-1. [DOI] [PubMed] [Google Scholar]
- 4.Manoach DS, Stickgold R. Does abnormal sleep impair memory consolidation in schizophrenia? Frontiers in Human Neuroscience. 2009;3:21. doi: 10.3389/neuro.09.021.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manoach DS, Thakkar KN, Stroynowski E, Ely A, McKinley S, Wamsley E, et al. Reduced overnight consolidation of procedural learning in chronic medicated schizophrenia is related to specific sleep stages. J Psychiatr Res. 2010;44:112–120. doi: 10.1016/j.jpsychires.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrarelli F, Huber R, Peterson MJ, Massimini M, Murphy M, Riedner BA, et al. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164:483–492. doi: 10.1176/ajp.2007.164.3.483. [DOI] [PubMed] [Google Scholar]
- 7.Ferrarelli F, Peterson MJ, Sarasso S, Riedner BA, Murphy MJ, Benca RM, et al. Thalamic Dysfunction in Schizophrenia Suggested by Whole-Night Deficits in Slow and Fast Spindles. Am J Psychiatry. 2010 doi: 10.1176/appi.ajp.2010.09121731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seeck-Hirschner M, Baier PC, Sever S, Buschbacher A, Aldenhoff JB, Goder R. Effects of daytime naps on procedural and declarative memory in patients with schizophrenia. J Psychiatr Res. 2011;44:42–47. doi: 10.1016/j.jpsychires.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Werk CM, Harbour VL, Chapman CA. Induction of long-term potentiation leads to increased reliability of evoked neocortical spindles in vivo. Neuroscience. 2005;131:793–800. doi: 10.1016/j.neuroscience.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 10.Sirota A, Csicsvari J, Buhl D, Buzsaki G. Communication between neocortex and hippocampus during sleep in rodents. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2065–2069. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- 12.Rosanova M, Ulrich D. Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. Journal of Neuroscience. 2005;25:9398–9405. doi: 10.1523/JNEUROSCI.2149-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fogel SM, Smith CT. The function of the sleep spindle: A physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehav Rev. 2011 doi: 10.1016/j.neubiorev.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry. 2004;56:301–307. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 15.Holt DJ, Lebron-Milad K, Milad MR, Rauch SL, Pitman RK, Orr SP, et al. Extinction memory is impaired in schizophrenia. Biol Psychiatry. 2009;65:455–463. doi: 10.1016/j.biopsych.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herbener ES. Impairment in long-term retention of preference conditioning in schizophrenia. Biol Psychiatry. 2009;65:1086–1090. doi: 10.1016/j.biopsych.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manoach DS, Cain MS, Vangel MG, Khurana A, Goff DC, Stickgold R. A failure of sleep-dependent procedural learning in chronic, medicated schizophrenia. Biol Psychiatry. 2004;56:951–956. doi: 10.1016/j.biopsych.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Jus K, Kiljan A, Wilczak H, Kubacki A, Rzepecki J, Jus A. Polygraphic study of night sleep in schizophrenia before and during treatment with phenothiazine derivatives. Ann Med Psychol (Paris) 1968;126:713–725. [PubMed] [Google Scholar]
- 19.Feinberg I, Braun M, Koresko RL, Gottlieb F. Stage 4 sleep in schizophrenia. Arch Gen Psychiatry. 1969;21:262–266. doi: 10.1001/archpsyc.1969.01740210006002. [DOI] [PubMed] [Google Scholar]
- 20.Poulin J, Daoust AM, Forest G, Stip E, Godbout R. Sleep architecture and its clinical correlates in first episode and neuroleptic-naive patients with schizophrenia. Schizophr Res. 2003;62:147–153. doi: 10.1016/s0920-9964(02)00346-8. [DOI] [PubMed] [Google Scholar]
- 21.Tandon R, Shipley JE, Taylor S, Greden JF, Eiser A, DeQuardo J, et al. Electroencephalographic sleep abnormalities in schizophrenia. Relationship to positive/negative symptoms and prior neuroleptic treatment. Arch Gen Psychiatry. 1992;49:185–194. doi: 10.1001/archpsyc.1992.01820030017003. [DOI] [PubMed] [Google Scholar]
- 22.Yang C, Winkelman JW. Clinical significance of sleep EEG abnormalities in chronic schizophrenia. Schizophr Res. 2006;82:251–260. doi: 10.1016/j.schres.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 23.Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders. A meta-analysis. Arch Gen Psychiatry. 1992;49:651–668. doi: 10.1001/archpsyc.1992.01820080059010. discussion 669–670. [DOI] [PubMed] [Google Scholar]
- 24.Chouinard S, Poulin J, Stip E, Godbout R. Sleep in untreated patients with schizophrenia: a meta-analysis. Schizophr Bull. 2004;30:957–967. doi: 10.1093/oxfordjournals.schbul.a007145. [DOI] [PubMed] [Google Scholar]
- 25.Hiatt JF, Floyd TC, Katz PH, Feinberg I. Further evidence of abnormal non-rapid-eye-movement sleep in schizophrenia. Arch Gen Psychiatry. 1985;42:797–802. doi: 10.1001/archpsyc.1985.01790310059007. [DOI] [PubMed] [Google Scholar]
- 26.Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, et al. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci U S A. 1998;95:861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35:205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 28.Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS ONE. 2007;2:e341. doi: 10.1371/journal.pone.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamaki M, Matsuoka T, Nittono H, Hori T. Fast sleep spindle (13–15 hz) activity correlates with sleep-dependent improvement in visuomotor performance. Sleep. 2008;31:204–211. doi: 10.1093/sleep/31.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamaki M, Matsuoka T, Nittono H, Hori T. Activation of fast sleep spindles at the premotor cortex and parietal areas contributes to motor learning: a study using sLORETA. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2009;120:878–886. doi: 10.1016/j.clinph.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Barakat M, Doyon J, Debas K, Vandewalle G, Morin A, Poirier G, et al. Fast and slow spindle involvement in the consolidation of a new motor sequence. Behav Brain Res. 2011;217:117–121. doi: 10.1016/j.bbr.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 32.Medkour T, Walden AT, Burgess AP, Strelets VB. Brain connectivity in positive and negative syndrome schizophrenia. Neuroscience. 2010;169:1779–1788. doi: 10.1016/j.neuroscience.2010.05.060. [DOI] [PubMed] [Google Scholar]
- 33.Wada Y, Nanbu Y, Kikuchi M, Koshino Y, Hashimoto T. Aberrant functional organization in schizophrenia: analysis of EEG coherence during rest and photic stimulation in drug-naive patients. Neuropsychobiology. 1998;38:63–69. doi: 10.1159/000026518. [DOI] [PubMed] [Google Scholar]
- 34.Winterer G, Egan MF, Radler T, Hyde T, Coppola R, Weinberger DR. An association between reduced interhemispheric EEG coherence in the temporal lobe and genetic risk for schizophrenia. Schizophrenia Research. 2001;49:129–143. doi: 10.1016/s0920-9964(00)00128-6. [DOI] [PubMed] [Google Scholar]
- 35.Yeragani VK, Cashmere D, Miewald J, Tancer M, Keshavan MS. Decreased coherence in higher frequency ranges (beta and gamma) between central and frontal EEG in patients with schizophrenia: A preliminary report. Psychiatry Res. 2006;141:53–60. doi: 10.1016/j.psychres.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 36.Bunney WE, Bunney BG. Evidence for a compromised dorsolateral prefrontal cortical parallel circuit in schizophrenia. Brain Res Brain Res Rev. 2000;31:138–146. doi: 10.1016/s0165-0173(99)00031-4. [DOI] [PubMed] [Google Scholar]
- 37.Andreasen NC, Paradiso S, O'Leary DS. "Cognitive dysmetria" as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- 38.Oh JS, Kubicki M, Rosenberger G, Bouix S, Levitt JJ, McCarley RW, et al. Thalamo-frontal white matter alterations in chronic schizophrenia: a quantitative diffusion tractography study. Hum Brain Mapp. 2009;30:3812–3825. doi: 10.1002/hbm.20809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Patient Edition with Psychotic Screen (SCID-I/P W/PSY SCREEN) New York: Biometrics Research, New York State Psychiatric Institute; 1997. [Google Scholar]
- 40.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 41.Rechtschaffen A, Kales A. In: A manual standardized terminology, techniques and scoring system for sleep stages of human subjects. Health UDo, editor. Bethesda, MD: 1968. [DOI] [PubMed] [Google Scholar]
- 42.De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev. 2003;7:423–440. doi: 10.1053/smrv.2002.0252. [DOI] [PubMed] [Google Scholar]
- 43.Schabus M, Dang-Vu TT, Albouy G, Balteau E, Boly M, Carrier J, et al. Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. Proc Natl Acad Sci U S A. 2007;104:13164–13169. doi: 10.1073/pnas.0703084104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akay M. Time frequency and wavelets in biomedical signal processing. Piscataway, NJ: Wiley-IEEE Press; 1997. [Google Scholar]
- 45.Nunez P. Electric Fields of the Brain: The Neurophysics of EEG. USA: Oxford University Press; 2005. [Google Scholar]
- 46.Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 47.Clemens Z, Fabo D, Halasz P. Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience. 2005;132:529–535. doi: 10.1016/j.neuroscience.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 48.Clemens Z, Fabo D, Halasz P. Twenty-four hours retention of visuospatial memory correlates with the number of parietal sleep spindles. Neurosci Lett. 2006;403:52–56. doi: 10.1016/j.neulet.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 49.Schabus M, Gruber G, Parapatics S, Sauter C, Klosch G, Anderer P, et al. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004;27:1479–1485. doi: 10.1093/sleep/27.7.1479. [DOI] [PubMed] [Google Scholar]
- 50.Tamminen J, Payne JD, Stickgold R, Wamsley EJ, Gaskell MG. Sleep spindle activity is associated with the integration of new memories and existing knowledge. J Neurosci. 2010;30:14356–14360. doi: 10.1523/JNEUROSCI.3028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fogel SM, Nader R, Cote KA, Smith CT. Sleep spindles and learning potential. Behavioral Neuroscience. 2007;121:1–10. doi: 10.1037/0735-7044.121.1.1. [DOI] [PubMed] [Google Scholar]
- 52.Schabus M, Hodlmoser K, Gruber G, Sauter C, Anderer P, Klosch G, et al. Sleep spindle-related activity in the human EEG and its relation to general cognitive and learning abilities. European Journal of Neuroscience. 2006;23:1738–1746. doi: 10.1111/j.1460-9568.2006.04694.x. [DOI] [PubMed] [Google Scholar]
- 53.Shibagaki M, Kiyono S, Watanabe K. Spindle evolution in normal and mentally retarded children: a review. Sleep. 1982;5:47–57. doi: 10.1093/sleep/5.1.47. [DOI] [PubMed] [Google Scholar]
- 54.De Giorgis GF, Nonnis E, Crocioni F, Gregori P, Rosini MP, Leuzzi V, et al. Evolution of daytime quiet sleep components in early treated phenylketonuric infants. Brain Dev. 1996;18:201–206. doi: 10.1016/0387-7604(96)00005-8. [DOI] [PubMed] [Google Scholar]
- 55.Limoges E, Mottron L, Bolduc C, Berthiaume C, Godbout R. Atypical sleep architecture and the autism phenotype. Brain. 2005;128:1049–1061. doi: 10.1093/brain/awh425. [DOI] [PubMed] [Google Scholar]
- 56.Guillery RW, Harting JK. Structure and connections of the thalamic reticular nucleus: Advancing views over half a century. J Comp Neurol. 2003;463:360–371. doi: 10.1002/cne.10738. [DOI] [PubMed] [Google Scholar]
- 57.Houser CR, Vaughn JE, Barber RP, Roberts E. GABA neurons are the major cell type of the nucleus reticularis thalami. Brain Res. 1980;200:341–354. doi: 10.1016/0006-8993(80)90925-7. [DOI] [PubMed] [Google Scholar]
- 58.Jacobsen RB, Ulrich D, Huguenard JR. GABA(B) and NMDA receptors contribute to spindle-like oscillations in rat thalamus in vitro. Journal of Neurophysiology. 2001;86:1365–1375. doi: 10.1152/jn.2001.86.3.1365. [DOI] [PubMed] [Google Scholar]
- 59.Contreras D, Steriade M. Spindle oscillation in cats: the role of corticothalamic feedback in a thalamically generated rhythm. J Physiol. 1996;490(Pt 1):159–179. doi: 10.1113/jphysiol.1996.sp021133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Llinas RR, Steriade M. Bursting of thalamic neurons and states of vigilance. J Neurophysiol. 2006;95:3297–3308. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- 61.Beenhakker MP, Huguenard JR. Neurons that fire together also conspire together: is normal sleep circuitry hijacked to generate epilepsy? Neuron. 2009;62:612–632. doi: 10.1016/j.neuron.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fuentealba P, Steriade M. The reticular nucleus revisited: intrinsic and network properties of a thalamic pacemaker. Prog Neurobiol. 2005;75:125–141. doi: 10.1016/j.pneurobio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 63.Steriade M, Domich L, Oakson G, Deschenes M. The deafferented reticular thalamic nucleus generates spindle rhythmicity. J Neurophysiol. 1987;57:260–273. doi: 10.1152/jn.1987.57.1.260. [DOI] [PubMed] [Google Scholar]
- 64.Contreras D, Destexhe A, Sejnowski TJ, Steriade M. Control of spatiotemporal coherence of a thalamic oscillation by corticothalamic feedback. Science. 1996;274:771–774. doi: 10.1126/science.274.5288.771. [DOI] [PubMed] [Google Scholar]
- 65.O'Neill J, Pleydell-Bouverie B, Dupret D, Csicsvari J. Play it again: reactivation of waking experience and memory. Trends Neurosci. 2010;33:220–229. doi: 10.1016/j.tins.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 66.Buzsaki G. Memory consolidation during sleep: a neurophysiological perspective. J Sleep Res. 1998;7(Suppl 1):17–23. doi: 10.1046/j.1365-2869.7.s1.3.x. [DOI] [PubMed] [Google Scholar]
- 67.Thompson M, Weickert CS, Wyatt E, Webster MJ. Decreased glutamic acid decarboxylase(67) mRNA expression in multiple brain areas of patients with schizophrenia and mood disorders. J Psychiatr Res. 2009;43:970–977. doi: 10.1016/j.jpsychires.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 68.Smith RE, Haroutunian V, Davis KL, Meador-Woodruff JH. Expression of excitatory amino acid transporter transcripts in the thalamus of subjects with schizophrenia. Am J Psychiatry. 2001;158:1393–1399. doi: 10.1176/appi.ajp.158.9.1393. [DOI] [PubMed] [Google Scholar]
- 69.Ibrahim HM, Hogg AJ, Jr, Healy DJ, Haroutunian V, Davis KL, Meador-Woodruff JH. Ionotropic glutamate receptor binding and subunit mRNA expression in thalamic nuclei in schizophrenia. Am J Psychiatry. 2000;157:1811–1823. doi: 10.1176/appi.ajp.157.11.1811. [DOI] [PubMed] [Google Scholar]
- 70.Borbely AA, Mattmann P, Loepfe M, Strauch I, Lehmann D. Effect of benzodiazepine hypnotics on all-night sleep EEG spectra. Hum Neurobiol. 1985;4:189–194. [PubMed] [Google Scholar]
- 71.Kubicki S, Herrmann WM, Holler L, Haag C. On the distribution of REM and NREM sleep under two benzodiazepines with comparable receptor affinity but different kinetic properties. Pharmacopsychiatry. 1987;20:270–277. doi: 10.1055/s-2007-1017120. [DOI] [PubMed] [Google Scholar]
- 72.Goder R, Fritzer G, Gottwald B, Lippmann B, Seeck-Hirschner M, Serafin I, et al. Effects of olanzapine on slow wave sleep, sleep spindles and sleep-related memory consolidation in schizophrenia. Pharmacopsychiatry. 2008;41:92–99. doi: 10.1055/s-2007-1004592. [DOI] [PubMed] [Google Scholar]
- 73.Morin A, Doyon J, Dostie V, Barakat M, Hadj Tahar A, Korman M, et al. Motor sequence learning increases sleep spindles and fast frequencies in post-training sleep. Sleep. 2008;31:1149–1156. [PMC free article] [PubMed] [Google Scholar]
- 74.Finelli LA, Achermann P, Borbely AA. Individual 'fingerprints' in human sleep EEG topography. Neuropsychopharmacology. 2001;25:S57–S62. doi: 10.1016/S0893-133X(01)00320-7. [DOI] [PubMed] [Google Scholar]
- 75.De Gennaro L, Marzano C, Fratello F, Moroni F, Pellicciari MC, Ferlazzo F, et al. The electroencephalographic fingerprint of sleep is genetically determined: a twin study. Ann Neurol. 2008;64:455–460. doi: 10.1002/ana.21434. [DOI] [PubMed] [Google Scholar]
- 76.Tucker M, McKinley S, Stickgold R. Sleep optimizes motor skill in older adults. J Am Geriatr Soc. 2011;59:603–609. doi: 10.1111/j.1532-5415.2011.03324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.