Abstract
Background
Identifying pancreatic cancer patients at high risk of early mortality following pancreaticoduodenectomy (PD) is important for treatment decisions in a multidisciplinary setting. This study examines the preoperative predictors of early mortality following PD and combines these variables into an early mortality risk score (EMRS).
Methods
Medical records of patients who underwent PD for pancreatic adenocarcinoma at the Johns Hopkins Hospital between 30 August 1993 and 28 February 2005 were reviewed. Cox proportional hazards analysis was performed to identify predictors of early mortality, defined as death at 9 and 12 months. EMRS was constructed from univariate associated risk factors (age >75 years, tumor size ≥3cm, poor differentiation, co-morbid diseases) with each factor assigned 1 point (range of 0–4). EMRS was evaluated as an independent predictor of death at 9 and 12 months.
Results
On univariate analysis, risk factors for death at 9 months included age ≥75 years (RR, 1.6; p=.009), comorbid disease (RR, 1.5; p=0.020), tumor ≥3 cm (RR, 1.4; P=0.050), and poor differentiation (RR, 2.1; P<0.001). EMRS was associated with early mortality among those who did (p=0.038) and did not receive adjuvant treatment (p<0.001). A modified EMRS without tumor differentiation was also associated with early mortality (p<0.001). Results persisted when reanalyzed using death at 12 months.
Conclusions
EMRS may identify patients at risk of early mortality following PD who may be candidates for alternatively sequenced treatment protocols. Prospective validation of this EMRS is needed.
Keywords: Early mortality, Risk score, Resectable, Pancreatic adenocarcinoma
Introduction
In 2010, there were an estimated 43,140 cases of pancreatic adenocarcinoma diagnosed in the USA.1 While surgical resection is the only curative modality for those with localized disease, only 10–15% of pancreatic adenocarcinoma patients are resectable at presentation.2–5 For those who undergo a potentially curative resection, prognosis remains poor, with 5-year survival rate of <20%.4–6 Additionally, up to 30% of patients who undergo a pancreaticoduodenectomy (PD) die within 1 year.7 Both systemic and local relapses are common following PD or total pancreatectomy, suggesting that systemic and local adjuvant therapies are also needed.8 The identification of patients at risk of early failure can be used to determine those patients in whom it may be appropriate to explore alternative sequencing of treatment.
Neoadjuvant chemoradiation (CRT) for pancreatic adenocarcinoma provides several theoretical benefits over upfront surgery, including an increased likelihood that patients receive planned therapy, delivery of systemic therapy without delay, improved resectability of marginally unresectable disease, and better prospects of attaining negative surgical margins. Perhaps most importantly, clinically aggressive occult metastatic disease may manifest during neoadjuvant treatment, reducing the likelihood of an ineffective PD and its associated morbidity. Several nonrandomized studies have demonstrated favorable median survival (range, 21–23.9 months) in borderline resectable patients given neoadjuvant therapy.9–14 However, in the absence of phase III evidence comparing neoadjuvant CRT with adjuvant CRT, there is no clear method to identify which patients could benefit from preoperative therapy.15 It would, therefore, be useful to identify specific subpopulations with poor outcomes from PD in order to avoid aggressive surgical management in patients who are unlikely to benefit from it and to investigate the role of other approaches such as neoadjuvant treatment in these patients. Our goal was to create a tool that can help define patients at risk of early mortality after PD with curative intent. The study proposes to (1) determine which factors predict early mortality, (2) develop a preoperatively obtainable early mortality risk score (EMRS), and (3) assess for EMRS associations with margin and node positivity.
Materials and Methods
Patients and Methods
Between 30 August 1993 and 28 February 2005, data were prospectively collected on all patients undergoing elective PD or total pancreatectomy for pancreatic adenocarcinoma at the Johns Hopkins Hospital (JHH). Bile duct, ampullary, and duodenal adenocarcinomas and distal pancreatic cancers were excluded. Patients underwent a pylorus-preserving, standard (hemigastrectomy), or total PD. The standard surgical approaches at JHH are described elsewhere.16 Johns Hopkins University institutional review board approval was granted prior to chart review. Nine hundred eight patients underwent surgical resection for ductal adenocarcinoma at JHH during the period of the study. Patients were excluded if they were found to have T4 or M1 disease at the time of surgery or if they were missing data for pathologic tumor size, node status, pathologic tumor histology grade, or comorbid disease (n= 168). Patients who experienced perioperative mortality defined as in-hospital mortality or death ≤60 days after PD were also excluded. Information regarding borderline resectable status based on preoperative imaging was unavailable. The final study population included 740 patients who underwent resection with curative intent for pancreatic adenocarcinoma. Of these 740 patients, 392 (53.0%) were treated with adjuvant therapy, while 348 (47.0%) received surgery alone. Adjuvant therapy most commonly consisted of 5-fluorouracil (5-FU) based CRT (n=367, 93.6%), with the remaining patients receiving chemotherapy alone, radiation alone, or CRT with a different systemic agent. Patient follow-up information was obtained from paper and electronic hospital charts. Survival was determined and cross-checked by review of clinical follow-up information, cancer center abstracting services, and the social security death index.
Preoperative information was prospectively collected. Comorbid conditions included diabetes mellitus (DM), hypertension, cardiovascular disease (CVD), and chronic obstructive pulmonary disease (COPD). Patients were classified as having DM if they were diagnosed prior to surgical resection. Data describing postoperative complications were collected and described elsewhere.17
Following surgery, most patients were seen by a medical and radiation oncologist and offered standard therapy at our institution, which consisted of continuous 5-FU infusion with radiation therapy followed by maintenance 5-FU for an additional 2–6 months. Patients with a satisfactory recovery from PD were encouraged to accept either standard or protocol therapy. Patients treated elsewhere were given the same recommendations prior to discharge as those patients treated at our institution. These recommendations were often communicated to referring physicians in a dictated consultation. Patients who were offered adjuvant treatment and elected to receive no therapy did so after being informed fully about the potential risks and benefits of such therapy.
Of the 367 patients who received either adjuvant radiation or CRT therapy, 51.2% received treatment at JHH, while 48.8% were treated at other facilities. The median daily fraction size and total dose of radiation for those patients treated at our institution was 180 and 5,000 cGy, respectively. The majority of patients treated at JHH received a continuous course of radiation therapy without a planned break (79%). The details of therapy and toxicities could not be fully assessed for patients treated elsewhere.
Statistical Analysis
Statistical analyses were performed using STATA, version 9 (Stata, College Station, TX, USA). Summary statistics for continuous and dichotomous variables are provided. Tests of differences were performed using t tests and χ2 tests. For characteristics with individuals missing data, χ2 tests were performed, including only those with known status, as indicated. The primary outcome was early mortality. In defining early mortality, it was important to choose a survival time that reflected a significantly worse outcome when compared to expected survival in our population (~18 months) but that also allowed for sufficient follow-up to examine associations with preoperative variables.16 We, therefore, used death within both 9 and 12 months from surgery as appropriate definitions of early mortality and attempted to construct a risk score that would predict early mortality within these time intervals. Survival curves were estimated using the Kaplan–Meier technique.18 Comparisons of survival between groups were made using the log-rank test. Median overall survival (OS; in months) with 95% confidence intervals was estimated within each risk group and by adjuvant treatment.
Proportional hazards models were used to examine the association with early mortality of preoperative and postoperative patient characteristics.19 Univariate analyses were used to examine individual risk factors and associations with early mortality at 9 months (n=154) and at 12 months (n=236). The EMRS consisted of dichotomous preoperatively obtainable risk factors associated with early mortality. Each risk factor was assigned 1 point if positive: age >75 years, tumor size ≥3 cm, histologic grade of poor differentiation or worse, and comorbid disease (hypertension, DM, CVD, or COPD). Tumor size and histology were based on operative pathologic specimens, and it was assumed that preoperative assessment of these characteristics would not significantly differ for the purposes of this study. Each risk factor was assigned 1 point and summed, with the EMRS ranging 0–4 for each patient. Furthermore, we examined a secondary version of the EMRS which did not include histologic grade as a factor so that the predictive score would be applicable to patients for whom tumor grade is not available preoperatively. Additionally, χ2 tests and logistic regression were used to assess associations between EMRS and postoperative margin positive status and node positive status.
EMRS was evaluated as a predictor of early mortality in both unadjusted and adjusted analyses in the total patient population, as well as in analyses stratified by adjuvant treatment status. Multivariate analyses were adjusted for sex, race, surgery type, node status, margin status, and postoperative complications.
Results
Characteristics and Associations with Early Mortality
Median follow-up for our study population was 18.3 months. Overall median survival was 18.2 months (95% CI, 16.9–19.6). Of the 740 patients who underwent resection with curative intent, 154 (20.8%) had early mortality when defined as death within 9 months of surgery, while 236 (31.9%) had early mortality when defined as death within 12 months of surgery. Table 1 describes the preoperatively and postoperatively obtainable characteristics and their associations with early mortality at 9 months. Among characteristics that could be preoperatively obtained, age >75 years (RR, 1.56; 95% CI, 1.11–2.20; p=0.009), tumor size ≥3 cm (RR, 1.37; 95% CI, 1.00–1.89; p=0.050; log-rank p-value=0.049), comorbid disease (RR, 1.49; 95% CI, 1.06–2.08; p=0.020), and histologic grade of at least poor differentiation (RR, 2.12; 95% CI, 1.53–2.92; p<0.001) were each associated with early mortality. Amongst postoperative characteristics, node positive status (RR, 1.58; 95% CI, 1.01–2.49; p=0.046), margin status (RR, 1.36; 95% CI, 1.15–1.61; p<0.001), and postoperative complications (RR, 1.88; 95% CI, 1.37–2.57; p<0.001) were associated with early mortality, whereas adjuvant treatment was inversely associated with early mortality (unadjusted RR, 0.33; 95% CI, 0.23–0.47; p<0.001). Table 2 summarizes these data for death at 12 months. Note that the preoperative characteristics associated with early mortality remained the same for the 12-month analysis.
Table 1.
Association of baseline patient, disease, and treatment characteristics with early mortality within 9 months following surgery
| Survival ≥9 months after resection (n=586) | Survival <9 months after resection (n=154) | Log-rank test p value | |
|---|---|---|---|
| Preoperative | |||
| Age >75 | 128 (22%) | 49 (32%) | 0.009 |
| Comorbid disease | 331 (56%) | 103 (67%) | 0.020 |
| Tumor size ≥3 cm | 210 (36%) | 68 (44%) | 0.049 |
| Poor differentiation | 231 (39%) | 92 (60%) | <0.001 |
| Male sex | 307 (52%) | 77 (50%) | 0.45 |
| White race | 529 (90%) | 143 (93%) | 0.32 |
| Tobacco use | 148 (25%) | 44 (29%) | 0.46 |
| Weight loss | 254 (49%) | 80 (57%) | 0.11 |
| Postoperative | |||
| Adjuvant CRT | 324 (57%) | 43 (29%) | <0.001 |
| Classic Whipple | 147 (25%) | 48 (31%) | 0.27 |
| Node positive | 460 (79%) | 132 (86%) | 0.044 |
| Margin positive | 238 (41%) | 86 (56%) | <0.001 |
| Perineural invasiona | 424 (92%) | 115 (93%) | 0.89 |
| Vascular invasiona | 212 (49%) | 63 (56%) | 0.13 |
| Postoperative complications | 189 (32%) | 75 (49%) | <0.001 |
CRT chemoradiation
Number missing from analyses: vascular invasion (n=191), perineural invasion (n=157)
Table 2.
Association of baseline patient, disease, and treatment characteristics with early mortality within 12 months following surgery
| Survival ≥12 months after resection (n=504) | Survival <12 months after resection (n=236) | Log-rank test p value | |
|---|---|---|---|
| Preoperative | |||
| Age >75 | 109 (22%) | 68 (29%) | 0.023 |
| Comorbid disease | 281 (56%) | 153 (65%) | 0.017 |
| Tumor size ≥3 cm | 172 (34%) | 106 (45%) | 0.005 |
| Poor differentiation | 188 (37%) | 135 (57%) | <0.001 |
| Male sex | 270 (54%) | 114 (48%) | 0.148 |
| White race | 452 (90%) | 220 (93%) | 0.163 |
| Tobacco use | 133 (27%) | 59 (25%) | 0.730 |
| Weight loss | 220 (50%) | 114 (52%) | 0.535 |
| Postoperative | |||
| Adjuvant CRT | 296 (61%) | 71 (31%) | <0.001 |
| Classic Whipple | 136 (27%) | 59 (25%) | 0.112 |
| Node positive | 394 (78%) | 198 (84%) | 0.059 |
| Margin positive | 201 (40%) | 123 (52%) | 0.001 |
| Perineural invasiona | 354 (91%) | 185 (94%) | 0.281 |
| Vascular invasiona | 175 (47%) | 100 (57%) | 0.030 |
| Postoperative complications | 162 (32%) | 102 (43%) | <0.001 |
CRT chemoradiation
Number missing from analyses: vascular invasion (n=191), perineural invasion (n=157)
Evaluation of the Early Mortality Risk Score
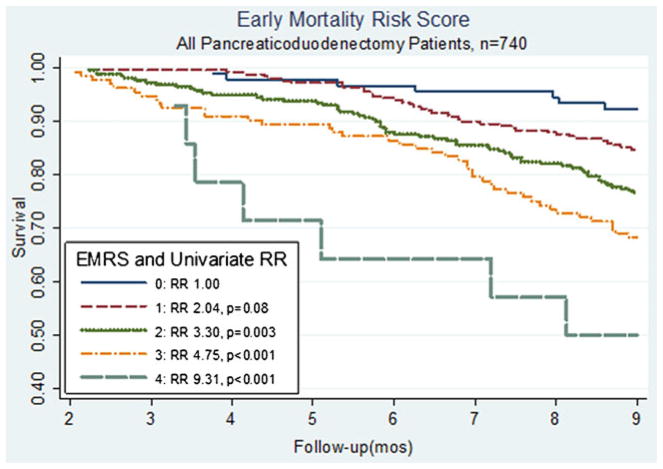
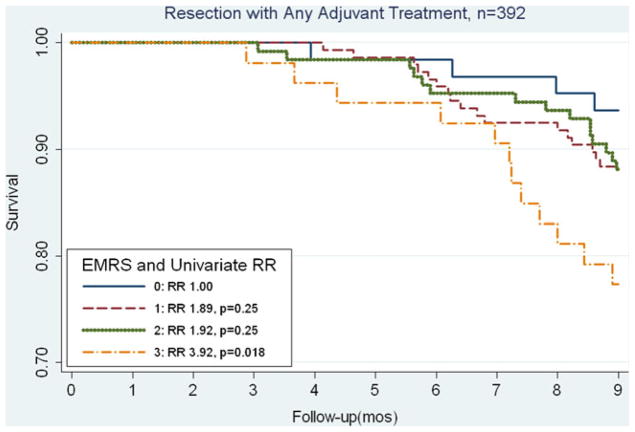
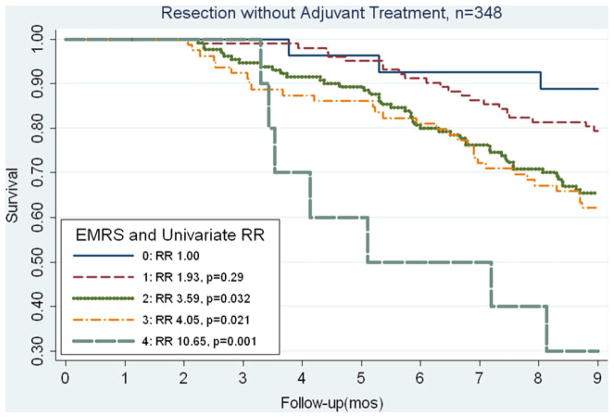
Based on the associations with early mortality of those characteristics which could be preoperatively obtained, the EMRS was formulated using the characteristics: age >75 years, tumor size ≥3 cm, comorbid disease, and histologic grade of at least poor differentiation, with each characteristic contributing 1 point (based on the similarity among relative risk values). The distribution of scores among our patient population was: score 0 (n=90, 12%), score 1 (n=248, 33%), score 2 (n=256, 35%), score 3 (n= 132, 18%), and score 4 (n=14, 2%). As seen in Fig. 1, the EMRS predicts for early mortality at 9 months (p trend< 0.001). Compared to an EMRS of 0, a score of 1 was associated with an increased risk of mortality (RR, 2.04) that was borderline statistically significant in our total population (p=0.08). For those with EMRS of at least 2, each increase of 1 point in score was associated significantly with early mortality (EMRS 2: RR=3.30, p=0.003; EMRS 3: RR=4.75, p<0.001; and EMRS 4: RR=9.31, p<0.001). In our study, when we stratified by adjuvant CRT status, we saw similar associations between the EMRS and mortality (Figs. 2 and 3). In Fig. 2, among patients who underwent adjuvant treatment, the EMRS score was associated with increased 9-month mortality (p trend=0.038). Additionally, an EMRS score of 3 was associated with increased early mortality at 9 months compared to score 0 (RR, 3.92; p= 0.018; Fig. 2), and adjustment for location of adjuvant radiation treatment did not significantly alter this risk estimate (RR, 3.65; p=0.026). As shown in Fig. 3, among those without adjuvant CRT, EMRS was associated with 9-month early mortality (p trend<0.001), and approximately 70% of patients with an EMRS of 4 did not survive 9 months postoperatively. Similarly, for those with an EMRS of 2 or 3, approximately one third died within 9 months of surgery. When early mortality was defined as death at 12 months, increases in the EMRS remained significantly associated with early mortality (EMRS 1: RR=2.11, p=0.018; EMRS 2: RR=2.97, p<0.001, EMRS 3: RR=4.55, p<0.001, EMRS 4: RR=6.75, p<0.001). These associations also persisted after stratifying by adjuvant CRT status.
Fig. 1.
Kaplan–Meier curves comparing OS in all patients, stratified by EMRS. EMRS above 1 was significantly associated with an increased risk of early mortality
Fig. 2.
Kaplan–Meier curves comparing OS in patients who received any form of adjuvant therapy following resection, stratified by EMRS. EMRS above 2 was significantly associated with an increased risk of early mortality
Fig. 3.
Kaplan–Meier curves comparing OS in patients who did not receive adjuvant therapy following resection, stratified by EMRS. EMRS above 1 was significantly associated with an increased risk of early mortality
Since it has been demonstrated that adjuvant CRT is associated with improved survival within our cohort, we focused on further validation of the EMRS among those who did not receive adjuvant treatment.16,17 Table 3 details the univariate and multivariate associations of the EMRS with early mortality at 9 months among patients who underwent PD without adjuvant treatment. The p trend was statistically significant in both models (p<0.001). After adjustment for potential confounders, compared to score 0, each increase in the score above 1 significantly predicted early mortality. In fact, having a risk score of 4 resulted in an approximately nine times greater risk of early mortality. Table 4 indicates similar results when early mortality was defined as death within 12 months.
Table 3.
EMRS associations with early mortality within 9 months following surgery among PD patients without adjuvant treatment
| EMRS | Univariate RR (95% CI) of early mortality | Multivariate RR (95% CI) of early mortality |
|---|---|---|
| Score 0 | Ref=1.00 | Ref=1.00 |
| Score 1 | 1.93 (0.58–6.47), p=0.287 | 1.80 (0.53–6.06), p=0.344 |
| Score 2 | 3.59 (1.12–11.55), p=0.032 | 3.26 (1.00–10.59), p=0.049 |
| Score 3 | 4.05 (1.24–13.29), p=0.021 | 3.62 (1.10–11.94), p=0.035 |
| Score 4 | 10.65 (2.75–41.24), p=0.001 | 9.18 (2.25–37.56), p=0.002 |
EMRS early mortality risk score
Table 4.
EMRS associations with early mortality within 12 months following surgery among PD patients without adjuvant treatment
| EMRS | Univariate RR (95% CI) of early mortality | Multivariate RR (95% CI) of early mortality |
|---|---|---|
| Score 0 | Ref=1.00 | Ref=1.00 |
| Score 1 | 1.50 (0.61–3.36), p=0.326 | 1.44 (0.64–3.25), p=0.379 |
| Score 2 | 2.23 (1.02–4.87), p=0.045 | 2.09 (0.95–4.61), p=0.068 |
| Score 3 | 2.70 (1.21–6.01), p=0.015 | 2.49 (1.11–5.57), p=0.027 |
| Score 4 | 5.27 (1.85–15.05), p=0.002 | 5.08 (1.70–15.22), p=0.004 |
EMRS early mortality risk score
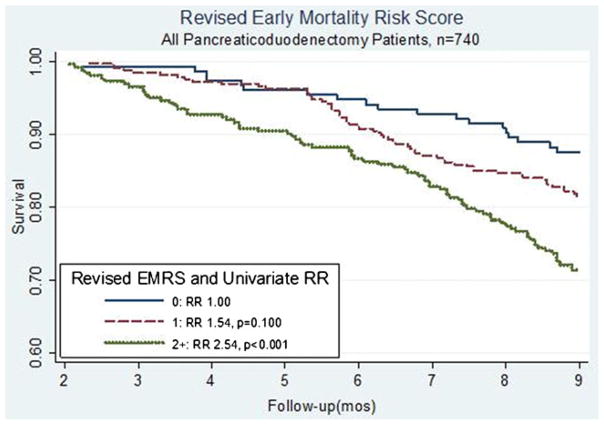
At certain institutions, preoperative pancreatic biopsies are infrequently performed when patients have clearly resectable pancreatic masses. Therefore, we evaluated a revised EMRS that did not incorporate histologic grade to ensure that the score would remain applicable to those patients in whom tissue was not preoperatively sampled. The revised EMRS, therefore, included age ≥75 years, comorbid disease, and tumor size ≥3 cm (range, 0–3 points). The revised EMRS had a distribution among our patients of score 0 (n=153, 21%), score 1 (n=325, 44%), and scores 2–3 (n=262, 35%) and also predicted early mortality. As shown in Fig. 4, patients with a revised EMRS score of 2 or greater had an RR of 2.54 (p<0.001) when compared to those with a score of 0, with roughly 40% not surviving for 9 months following surgery. This relationship persisted when early mortality was defined as death within 12 months. With early mortality at 12 months, those patients with an EMRS of 2 or greater had an RR of 2.22 (p<0.001) when compared to patients with a score of 0.
Fig. 4.
Kaplan–Meier curves comparing OS in all patients, stratified by the revised EMRS. EMRS above 1 was significantly associated with an increased risk of early mortality
Finally, we determined that EMRS was associated with pathologic margin positive status (p=0.003) but not with node positivity (p=0.364). Compared to those with an EMRS of 0, patients with scores of 2 to 4 had increased relative odds of margin positive disease of 2.08 (p=0.005) to 3.99 (p=0.022), respectively. More than 50% of those with an EMRS of 3 or more had margin positive disease at resection.
Discussion
Utilizing the experience of a high-volume center for the treatment of pancreatic adenocarcinoma, we established that age, tumor size, tumor differentiation, and comorbid disease are preoperatively obtainable characteristics which are associated with clinically significant early mortality following resection with curative intent. The EMRS, a simple scoring system composed of these variables, was demonstrated to strongly predict for death within 9 months of surgery. Additionally, the EMRS remained a strong predictor of death within 12 months of surgery. Furthermore, the EMRS was a significant predictor of early mortality regardless of whether patients received adjuvant therapy. Notably, among patients who underwent surgery without adjuvant CRT, approximately 70% with an EMRS of 4 died before surviving 9 months postoperatively and approximately one third with an EMRS of 2 or 3 died within the same period. While the EMRS must be validated in a prospective study before being incorporated clinically, it may help clinicians select patients for protocols that examine alternatives to upfront surgery, such as neoadjuvant chemotherapy, CRT, or supportive care. By identifying those who may rapidly progress, the EMRS may also allow clinicians to better counsel patients regarding the risks and benefits of various treatment options.
Considerable controversy exists regarding the appropriate choice and timing of local and systemic therapies for resectable pancreatic cancer. In the USA, CRT is typically given postoperatively based on several trials and retrospective studies which have demonstrated the utility of adjuvant CRT.16,17,20–23 However, within the adjuvant setting, a large percentage of patients may not actually receive or complete adjuvant therapy because of treatment biases, referral patterns, and/or complications from surgery or treatment-related toxicity. For example, various series have shown that prolonged recovery following PD prevents the delivery of planned adjuvant CRT in 25–30% of cases.17,21,24
Offering preoperative neoadjuvant therapy instead of upfront surgery in patients at a very high risk of early mortality following PD may be advantageous for a number of reasons.9,15,25,26 First, it would necessitate that these patients receive chemotherapy and/or CRT prior to surgery, allowing them to attain the potential benefits of multimodality therapy.25 Second, there would be no delay in the delivery of systemic treatment as there would be with adjuvant therapy.10 Third, neoadjuvant therapy may improve resectability of marginally unresectable tumors and decrease the likelihood of margin positive resections, resulting in improved local control.15,27 Fourth, and perhaps the most important rationale for neoadjuvant treatment, is that patients with aggressive, occult metastatic disease could be identified prior to surgery and spared the potential morbidity of a PD, as they are unlikely to benefit from surgery.10 Indeed, several major academic centers have achieved favorable results with neoadjuvant therapy in patients with technically resectable pancreatic cancer, although it should be noted that the existing literature largely consists of single-institution experiences.9–15,20,21,26
There are several limitations to our current study. Given the study’s retrospective design, there are potential confounders which were not accounted for in our analysis, such as performance status and reasons for withholding adjuvant CRT. These potential confounders are particularly relevant when examining absolute differences in the predictive ability of EMRS with respect to adjuvant therapy. Nonetheless, the fact that all patients in our cohort were deemed fit for PD suggests that preoperative performance status was not grossly dissimilar. Other relevant variables that were unavailable for the majority of our patients include preoperative CA 19–9 level and duration of symptoms prior to treatment, two factors shown to predict early mortality in another series.28 Also, preoperative assessment of borderline resectability by CT imaging was not taken into account, as these were all patients who underwent PD with curative intent. Borderline resectability is considered an indication for neoadjuvant treatment.15
Furthermore, although the EMRS consists of characteristics which are ideally obtainable preoperatively, tumor size in our study was based on pathologic assessment of operative specimens. While improvements in CT imaging have allowed a high degree of accuracy in the prediction of tumor size, a recent study with pancreatic protocol multidetector CT imaging did suggest that imaging underestimates maximum tumor diameter (MTD) by a median of 7 mm.29 A study from our institution also found that conventional CT underestimated MTD as assessed by final pathology (p= 0.08), but no significant difference was found between 3D-CT MTD and pathology (p=0.54) (manuscript submitted). Therefore, future studies of the EMRS should ideally use the MTD obtained from preoperative 3D-CT.
Moreover, tumor differentiation was also obtained from patients’ operative specimens, raising the question of whether such information would be available preoperatively. Currently, the decision to biopsy pancreatic masses differs by institution. While guidelines from the NCCN and AHPBA recommend routine biopsy in patients being considered for neoadjuvant treatment, they acknowledge that routine sampling for resectable patients is debatable, as there is a small risk of biopsy-related complications and negative biopsies may not change patient management.30,31 Proponents of biopsy in the resectable setting, however, point towards improvements in EUS-obtained biopsies with recently reported accuracy, sensitivity, and specificity for diagnosing malignant neoplasm of 97.6%, 96.6%, and 99.0%, respectively.32 Moreover, increased interest in incorporating endoscopic core biopsies into the workup of pancreatic masses may allow for better preoperative assessment of tumor differentiation when compared to cytology. Regardless, recognizing that tumor differentiation may not be available to many clinicians, we were able to demonstrate that, even without tumor differentiation data, the modified EMRS is still able to predict early mortality.
We believe that the EMRS may serve as a tool to help identify patients for consideration of alternatively sequenced management of resectable and borderline resectable pancreatic adenocarcinoma patients. However, we also believe that the EMRS first needs to be validated as a predictor of early mortality in a separate cohort of PD patients. While the EMRS identifies those who may not have a significant survival benefit from traditional management, it does not predict which patients would benefit from other approaches such as neoadjuvant CRT. Yet, by helping to determine which patients will be less likely to benefit from traditionally sequenced treatment, the EMRS can help ascertain those populations in which to explore alternate sequencing of treatment.
Acknowledgments
We are indebted to the staff and patients of the Johns Hopkins Hospital for their important contributions. This work was supported in part by the Claudio X. Gonzalez Family Foundation and the DeSanti Family Foundation.
Footnotes
This study was presented at the 49th annual meeting of the American Society for Therapeutic Radiology and Oncology (ASTRO), Los Angeles, California, 2007.
Conflicts of Interest No conflicts of interest or financial disclosures exist for any of the authors. No specific funding was provided.
Contributor Information
Charles C. Hsu, The Department of Radiation Oncology and Molecular Radiation, Sciences, Johns Hopkins University School of Medicine, 410 North Broadway/Suite 1440, Baltimore, MD 21231-2410, USA. The Department of Radiation Oncology, University of California, San Francisco, San Francisco, CA, USA
Christopher L. Wolfgang, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA. The Sol Goldman Pancreatic Cancer Research Center, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Daniel A. Laheru, Department of Medical Oncology, Johns Hopkins University School of Medicine, Baltimore, MD, USA. The Sol Goldman Pancreatic Cancer Research Center, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Timothy M. Pawlik, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA. The Sol Goldman Pancreatic Cancer Research Center, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Michael J. Swartz, The Department of Radiation Oncology and Molecular Radiation, Sciences, Johns Hopkins University School of Medicine, 410 North Broadway/Suite 1440, Baltimore, MD 21231-2410, USA
Jordan M. Winter, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Raymond Robinson, The Department of Radiation Oncology and Molecular Radiation, Sciences, Johns Hopkins University School of Medicine, 410 North Broadway/Suite 1440, Baltimore, MD 21231-2410, USA.
Barish H. Edil, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA. The Sol Goldman Pancreatic Cancer Research Center, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Amol K. Narang, The Department of Radiation Oncology and Molecular Radiation, Sciences, Johns Hopkins University School of Medicine, 410 North Broadway/Suite 1440, Baltimore, MD 21231-2410, USA
Michael A. Choti, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Ralph H. Hruban, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, USA. The Sol Goldman Pancreatic Cancer Research Center, Johns Hopkins University School of Medicine, Baltimore, MD, USA
John L. Cameron, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA. The Sol Goldman Pancreatic Cancer Research Center, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Richard D. Schulick, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA. The Sol Goldman Pancreatic Cancer Research Center, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Joseph M. Herman, Email: jherma15@jhmi.edu, The Department of Radiation Oncology and Molecular Radiation, Sciences, Johns Hopkins University School of Medicine, 410 North Broadway/Suite 1440, Baltimore, MD 21231-2410, USA. The Sol Goldman Pancreatic Cancer Research Center, Johns Hopkins University School of Medicine, Baltimore, MD, USA
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population based, linked database analysis of 396 patients. Ann Surg. 2003;237:74–85. doi: 10.1097/00000658-200301000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Ann Surg. 1990;211:447–458. doi: 10.1097/00000658-199004000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg. 1993;165:68–72. doi: 10.1016/s0002-9610(05)80406-4. [DOI] [PubMed] [Google Scholar]
- 6.Crist DW, Sitzmann JV, Cameron JL. Improved hospital morbidity, mortality, and survival after the Whipple procedure. Ann Surg. 1987;206:358–365. doi: 10.1097/00000658-198709000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy EP, Yeo CJ. The case for routine use of adjuvant therapy in pancreatic cancer. J Surg Oncol. 2007;95:597–603. doi: 10.1002/jso.20719. [DOI] [PubMed] [Google Scholar]
- 8.Foo ML, Gunderson LL, Nagorney DM, McLlrath DC, van Heerden JA, Robinow JS, Kvols LK, Garton GR, Martenson JA, Cha SS. Patterns of failure in grossly resected pancreatic ductal adenocarcinoma treated with adjuvant irradiation +/− 5 fluorouracil. Int J Radiat Oncol Biol Phys. 1993;26:483–489. doi: 10.1016/0360-3016(93)90967-z. [DOI] [PubMed] [Google Scholar]
- 9.Katz MH, Wang H, Fleming JB, Sun CC, Hwang RF, Wolff RA, Varadhachary G, Abbruzzese JL, Crane CH, Krishnan S, Vauthey JN, Abdalla EK, Lee JE, Pisters PW, Evans DB. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16:836–847. doi: 10.1245/s10434-008-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breslin TM, Hess KR, Harbison DB, Jean ME, Cleary KR, Dackiw AP, Wolff RA, Abbruzzese JL, Janjan NA, Crane CH, Vauthey JN, Lee JE, Pisters PW, Evans DB. Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: treatment variables and survival duration. Ann Surg Oncol. 2001;8:123–132. doi: 10.1007/s10434-001-0123-4. [DOI] [PubMed] [Google Scholar]
- 11.Sasson AR, Wetherington RW, Hoffman JP, Ross EA, Cooper H, Meropol NJ, Freedman G, Pingpank JF, Eisenberg BL. Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: analysis of histopathology and outcome. Int J Gastrointest Cancer. 2003;34:121–128. doi: 10.1385/IJGC:34:2-3:121. [DOI] [PubMed] [Google Scholar]
- 12.White RR, Xie HB, Gottfried MR, Czito BG, Hurwitz HI, Morse MA, Blobe GC, Paulson EK, Baillie J, Branch MS, Jowell PS, Clary BM, Pappas TN, Tyler DS. Significance of histological response to preoperative chemoradiotherapy for pancreatic cancer. Ann Surg Oncol. 2005;12:214–221. doi: 10.1245/ASO.2005.03.105. [DOI] [PubMed] [Google Scholar]
- 13.Snady H, Bruckner H, Cooperman A, Paradiso J, Kiefer L. Survival advantage of combined chemoradiotherapy compared with resection as the initial treatment of patients with regional pancreatic carcinoma. An outcomes trial Cancer. 2000;89:314–327. doi: 10.1002/1097-0142(20000715)89:2<314::aid-cncr16>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 14.Evans DB, Varadhachary GR, Crane CH, Sun CC, Lee JE, Pisters PW, Vauthey JN, Wang H, Cleary KR, Staerkel GA, Charnsangavej C, Lano EA, Ho L, Lenzi R, Abbruzzese JL, Wolff RA. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496–3502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 15.Katz MH, Pisters PW, Evans DB, Sun CC, Lee JE, Fleming JB, Vauthey JN, Abdalla EK, Crane CH, Wolff RA, Varadhachary GR, Hwang RF. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833–846. doi: 10.1016/j.jamcollsurg.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herman JM, Swartz MJ, Hsu CC, Winter J, Pawlik TM, Sugar E, Robinson R, Laheru DA, Jaffee E, Hruban RH, Campbell KA, Wolfgang CL, Asrari F, Donehower R, Hidalgo M, Diaz LA, Jr, Yeo C, Cameron JL, Schulick RD, Abrams R. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: results of a large, prospectively collected database at the Johns Hopkins Hospital. J Clin Oncol. 2008;26:3503–3510. doi: 10.1200/JCO.2007.15.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeo CJ, Abrams RA, Grochow LB, Sohn TA, Ord SE, Hruban RH, Zahurak ML, Dooley WC, Coleman J, Sauter PK, Pitt HA, Lillemoe KD, Cameron JL. Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival. A prospective, single-institution experience. Ann Surg. 1997;225:621–633. doi: 10.1097/00000658-199705000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein JP, Moeschberger ML. Survival analysis: techniques for censored and truncated data. New York, New York: Springer-Verlag; 2003. [Google Scholar]
- 19.Cox DR, Oakes D. Analysis of Survival Data. London: Chapman and Hall; 1984. [Google Scholar]
- 20.Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 21.Klinkenbijl JH, Jeekel J, Sahmoud T, van Pel R, Couvreur ML, Veenhof CH, Arnaud JP, Gonzalez DG, de Wit LT, Hennipman A, Wils J. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230:776–782. doi: 10.1097/00000658-199912000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garofalo MC, Regine WF, Tan MT. On statistical reanalysis, the EORTC trial is a positive trial for adjuvant chemoradiation in pancreatic cancer. Ann Surg. 2006;244:332–333. doi: 10.1097/01.sla.0000229980.81505.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corsini MM, Miller RC, Haddock MG, Donohue JH, Farnell MB, Nagorney DM, Jatoi A, McWilliams RR, Kim GP, Bhatia S, Iott MJ, Gunderson LL. Adjuvant radiotherapy and chemotherapy for pancreatic carcinoma: the Mayo Clinic experience (1975–2005) J Clin Oncol. 2008;26:3511–3516. doi: 10.1200/JCO.2007.15.8782. [DOI] [PubMed] [Google Scholar]
- 24.Staley CA, Lee JE, Cleary KR, Abbruzzese JL, Fenoglio CJ, Rich TA, Evans DB. Preoperative chemoradiation, pancreaticoduode< nectomy, and intraoperative radiation therapy for adenocarcinoma of the pancreatic head. Am J Surg. 1996;171:118–124. doi: 10.1016/S0002-9610(99)80085-3. [DOI] [PubMed] [Google Scholar]
- 25.Crane CH, Varadhachary G, Wolff RA, Pisters PW, Evans DB. The argument for preoperative chemoradiation for localized, radiographically resectable pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20:365–382. doi: 10.1016/j.bpg.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Varadhachary GR, Wolff RA, Crane CH, Sun CC, Lee JE, Pisters PW, Vauthey JN, Abdalla E, Wang H, Staerkel GA, Lee JH, Ross WA, Tamm EP, Bhosale PR, Krishnan S, Das P, Ho L, Xiong H, Abbruzzese JL, Evans DB. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3487–3495. doi: 10.1200/JCO.2007.15.8642. [DOI] [PubMed] [Google Scholar]
- 27.Allendorf JD, Lauerman M, Bill A, DiGiorgi M, Goetz N, Vakiani E, Remotti H, Schrope B, Sherman W, Hall M, Fine RL, Chabot JA. Neoadjuvant chemotherapy and radiation for patients with locally unresectable pancreatic adenocarcinoma: feasibility, efficacy, and survival. J Gastrointest Surg. 2008;12:91–100. doi: 10.1007/s11605-007-0296-7. [DOI] [PubMed] [Google Scholar]
- 28.Barugola G, Partelli S, Marcucci S, Sartori N, Capelli P, Bassi C, Pederzoli P, Falconi M. Resectable Pancreatic Cancer: Who Really Benefits From Resection? Ann Surg Oncol. 2009;16:3316–3322. doi: 10.1245/s10434-009-0670-7. [DOI] [PubMed] [Google Scholar]
- 29.Arvold ND, Niemierko A, Mamon HJ, Fernandez-del Castillo C, Hong TS. Pancreatic cancer tumor size on CT scan versus pathologic specimen: Implications for radiation treatment planning. Int J Radiat Oncol Bios Phys. 2010 doi: 10.1016/j.ijrobp.2010.04.058. epub accessed November 1, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Callery MP, Chang KJ, Fishman EK, Talamonti MS, William Traverso L, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1727–1733. doi: 10.1245/s10434-009-0408-6. [DOI] [PubMed] [Google Scholar]
- 31.NCCN Practice Guidelins in Oncology (NCCN Guidelines™) Pancreatic Adenocarcinoma. 2010;1 www.nccn.org. [Google Scholar]
- 32.Krishna NB, LaBundy JL, Saripalli S, Safdar R, Agarwal B. Diagnostic value of EUS-FNA in patients suspected of having pancreatic cancer with a focal lesion on CT scan/MRI but without obstructive jaundice. Pancreas. 2009;38:625–630. doi: 10.1097/MPA.0b013e3181ac35d2. [DOI] [PubMed] [Google Scholar]