Abstract
Galectin-1 (Gal-1), an endogenous β-galactoside-binding protein, binds to laminins, which are highly expressed in the nucleus pulposus (NP) of the intervertebral disc (IVD). The objective of this study is to evaluate the expression of Gal-1 protein in IVD tissues during aging and the effect of Gal-1 on IVD cell adhesion to laminins. Tissues from rat, porcine and human (scoliosis or disc degeneration) IVDs were used to evaluate Gal-1 expression via immunostaining, RT-PCR and Western Blot analysis. Attachment of isolated IVD cells (porcine and human) on select laminin isoforms (LM-111 and LM-511) was compared with/without pre-incubation with exogenous Gal-1. A biotinylated Gal-1(B-Gal-1) was used to evaluate for binding to IVD cells and to select for IVD cells by magnetic activated cell sorting (MACS). NP cells expressed high levels of Gal-1 protein as compared to anulus fibrosus (AF) cells in immature tissues, while exogenous Gal-1 increased both NP and AF cell attachment to laminins and exhibited a similar binding to both cell types in vitro. With aging, Gal-1 levels in NP tissue appeared to decrease. In addition, incubation with B-Gal-1 was able to promote the retention of more than 50% of IVD cells via MACS. Our results provide new findings for the presence and functional role of Gal-1 within IVDs. Similar staining patterns for Gal-1 and LM-511 in IVD tissue suggest that Gal-1 may serve as an adhesion molecule to interact with both cells and laminins. This MACS protocol may be useful for selecting pure IVD cells from mixed cells of pathological tissue.
Keywords: galectin-1, laminin, intervertebral disc, extracellular matrix, cell adhesion
Introduction
Pain and disability associated with degenerated and herniated intervertebral disc (IVD) disorders are some of the most common medical and social problems in today's adult population 1-3. IVD disorders are believed to be linked to age-related or pathology-related cell loss and phenotypic changes, as well as biochemical and structural changes in the extracellular matrix of the nucleus pulposus (NP), a central region of IVD, derived from notochord 4, 5. NP cells are responsible for extracellular matrix synthesis and the maintenance of functional IVD, but experience major challenges to cell survival that result in decreased cell density and matrix synthesis with age 6. There exists great interest in understanding the interactions between IVD cells and their extracellular matrix, and in the study of cell-mediated approaches to promote tissue regeneration and arrest or slow the progression of disc degeneration.
Autologous cellular therapies have been studied over many years, with at least one published clinical study (112 patients over two years), which demonstrated that autologous disc cell transplantation plus discectomy may be associated with a reduction in lower back pain (primary outcomes of patient-reported pain and disability scales) as compared to discectomy alone 7. A challenge to the use of autologous IVD cells is that few IVD cells can be isolated from the very small amounts of available human discectomy tissue. Also, IVD cells isolated from autologous disc tissue are frequently heterogeneous and contain NP and anulus fibrosus (AF) cells, as well as multiple inflammatory cell types, including macrophages, lymphocytes, endothelium, and fibroblasts 8, 9, which can interfere with the potential matrix regeneration function of native IVD cells. In addition, the transplanted cells may survive for only a short period of time or lose their intrinsic regenerative potential over time in culture due to a non-suitable matrix environment. A means to enrich pure IVD cells from IVD tissue from pathological sources and promote cell-matrix interaction could advance the study of their regenerative potential. Laminins are key cell-adhesive ligands in a variety of tissues, formed as heterotrimeric proteins composed of α, β, and γ chains that comprise 15 known laminin isotypes 10. Specific laminin isoforms are known to modulate many biological functions, including cell adhesion, migration, proliferation, differentiation and survival 10. Previously, our group has shown that immature NP cells reside in an extracellular matrix (ECM) environment rich in laminin (LM) and that they express high levels of laminin-binding receptors as compared to the surrounding AF cells 11-14. In particular, immature NP cells (rat, porcine and human) expressed higher levels of the α1, α6, and β1 integrin subunits, and higher levels of the extracellular matrix proteins, LM-511 and LM322 12, 14, 15. Immature porcine NP cells were also shown to interact with laminins predominantly through α6 and β1 integrin subunits 12. In addition, NP cells spread significantly more on and adhered with greater strength to laminins (LM-511 and LM-111) as compared to collagen II or fibronectin 15. These findings demonstrate that the interactions between NP cells and multiple laminin isoforms may be important for understanding NP cellular function.
Galectin-1 (Gal-1), an endogenous β-galactoside-binding protein, binds to various ECM proteins such as laminins and to the cell surface through the β1 integrin subunit 16, 17. Gal-1 is multifunctional and has been shown to play roles in regulating cell growth, adhesion, migration, apoptosis, immunoregulation and in modulating differentiation and tissue development 18. Previously, Gal-1 mRNA was found in the anlagen of the IVD during the prenatal development of mice 19, and transcriptional profiling of mRNA in rat NP tissues revealed higher levels of Gal-1 mRNA in immature NP as compared to mature NP 20. These observations suggest that the interactions between Gal-1 and laminins may be important for regulating IVD cell-matrix interaction during growth and development. Additional observations motivate interests in Gal-1, including the finding that both Gal-1 21 and laminin 22 are specific to the notochordal region of zebrafish and the notochord is the confirmed tissue of NP origin 23. Another carbohydrate-binding protein, galectin-3 (Gal-3) is present in human notochord, IVD and chordoma 24. Gal-3 is not expressed in IVD tissues in a tissue-specific or aging-specific pattern, however, since both the NP and AF regions of neonatal, young and mature rat IVD tissues expressed Gal-3 25. For these reasons, this current study is focused on Gal-1 with the motivating hypothesis that Gal-1 may function as an adhesive molecule that binds both NP cells and laminin matrix and regulates cell-matrix interactions. Furthermore, this study aims to determine if Gal-1 can selectively promote NP cell attachment to specific ECM proteins. Protein expression of Gal-1 was first evaluated in IVD tissues of varying ages in three species (rat, porcine and human) to determine the potential for a role of endogenous Gal-1 in the IVD. In addition, cell attachment and magnetic activated cell sorting (MACS) protocols were conducted to test the ability of exogenous Gal-1 to bind IVD cells and to mediate IVD cell adhesion to laminins.
Materials and Methods
Tissue harvesting
Porcine IVDs were obtained from the lumbar spines of skeletally immature (3 month-old, n=3) and mature (24 month-old, n=3) pigs within 8 hours of sacrifice (Duke University Vivarium or local abattoir). Rat IVDs were similarly harvested from the lumbar and coccygeal spines of immature (Fisher 344, 1 month-old, n=3) and mature (12 month-old, n=3) rats within 1 hour of sacrifice (Duke University Vivarium). All procedures were approved by the Institutional Animal Care & Use Committee of Duke University. Human IVD samples were obtained as to-be-discarded surgical waste tissues from the lumbar spines of juvenile patients undergoing anterior fusion for scoliosis only (2-19 year-old, n=4) and from the lumbar spines of adult patients undergoing posterior fusion for disc degeneration (41-66 year-old, n=6), with approval of the Duke University IRB. Disc tissues were provided as intact pieces that allowed for easy separation of NP and AF regions according to their gross morphological appearance (i.e. with or without collagen fiber orientation). The surgical tissues were kept at room temperature for no more than 2 hours until further processing was performed. IVDs were also obtained from the lumbar spine of an adult normal donor with no described spinal pathology (35 year-old, n=1) through in the procurement services of the Duke Fresh Human Tissue Laboratory. The gross appearance of human IVD tissues from the pediatric scoliosis patients and normal donor were consistent with grades 1 of the Thompson grading scheme 26, and so were considered to be non-degenerate tissues. Tissues from the NP and AF regions were procured and processed for the experimental assays as shown in Table 1 and as described in more detail below.
Table 1.
Summary of tissue and cell sources for the different assays in the experimental design (m: month-old, y: year-old, --: assay not performed).
| Assays | Rat | Pig | Human |
|---|---|---|---|
| tissue immunostaining | 1, 12m | 3, 24m | 2, 35y |
| Tissue/cell RT-PCR | -- | 3m | -- |
| Western blot analysis | -- | 3m | -- |
| immuno-cytochemistry | -- | 3m | 9y |
| cell attachment | -- | 3m | 17, 41, 54, 66y |
| cell binding | -- | 3m | -- |
| cell sorting with MACS | -- | -- | 19, 50, 55, 63y |
IVD cell isolation and culture
Cells were isolated from the AF and NP regions of porcine (3-month old, n=3) and human (9-66 years old, n=9) IVDs using a sequential pronase-collagenase digestion as described previously 27. Isolated cells were subcultured in monolayers (50,000 cells/cm2) on 0.1% gelatin-coated (Sigma, St. Louis, MO) tissue culture flasks for 4-7 days in culture media (F-12 media supplement (Gibco, Invitrogen, Carlsbad, CA) with 10% FBS (Hyclone, Thermo Scientific, Rockford, IL), 10mM HEPES, 100 U/ml penicillin, 100 ug/ml streptomycin, and 1.25ug/ml Fungizone/Amphotericin B (Gibco)) at 37°C and 5% CO2. Confluent cells were used for the experiments described below.
Immunohistochemical detection of Gal-1 in IVD tissues and cells
AF and NP tissues from rat (1 and 12m), porcine (3 and 24m) and human (2 and 35y) IVDs were embed in OCT medium then immediately flash-frozen in liquid nitrogen and stored at −80°C until cryosectioning. Frozen tissue sections (7-8μm) were fixed in 4% formaldehyde (10 min at room temperature), blocked in blocking solution (3.75% BSA/5% goat serum, Zymed, Carlsbad, CA) for 30 min and then incubated for 60 min with a human Gal-1 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Samples were washed twice in PBS and then incubated for 30 minutes with appropriate secondary antibodies (AlexaFluor 488, Molecular Probes, Eugene, OR). Control samples were incubated with blocking solution instead of primary antibody. All sections were counterstained with propidium iodide (0.2 mg/ml, Sigma) to label cell nuclei, and imaged using confocal laser scanning microscopy (Zeiss LSM 510; 20× NA 0.5 and 63× water immersion NA 1.2 objectives; Carl Zeiss, Thornwood, NY).
For detection of Gal-1 expression in cells cultured under monolayer conditions, human (9y) and porcine (3m) cells were removed from the culture surface (0.025% trypsin/EDTA, Cambrex, East Rutherford, NJ), re-suspended in culture media and then plated on 8-well chamber slides (Nalge Nunc, Rochester, NY, 20,000 cells/well) coated with 0.1% gelatin. The cells were incubated overnight at 37°C to allow for attachment and then fixed in methanol (5 min). Subsequently, the samples were incubated in blocking solution for 30 min, washed with PBS and then incubated with appropriate primary and secondary antibodies. Imaging via confocal microscopy was performed as described above.
Gal-1 mRNA expression in immature porcine IVD
Porcine IVDs (3m) were dissected into zones corresponding to AF and NP tissues as described above, then immediately flash-frozen in liquid nitrogen followed by pulverizing. Tissue powder was homogenized in TRIzol reagent and total RNA extracted with the RNeasy mini kit plus DNase I digestion (Qiagen, Valencia, CA). To detect the effect of monolayer cell culture conditions on Gal-1 mRNA expression, total RNA was also isolated from freshly isolated NP and AF cells (0 day) and cells after 4-7 days of monolayer culture as described above. A total of three RNA samples (n=3) for each tissue or cell type (AF, NP) was analyzed to quantify the expression of Gal-1 mRNA via RT-PCR as described previously (iCycler iQ system, BioRad, Hercules, CA) 27. Two porcine-specific PCR primers and one fluorescently-labeled intron-spanning probe were used (Gal-1 # SS03388270_m1, Applied Biosystems, Foster City, CA). Relative mRNA for Gal-1 was quantified in tissues and cells of NP and AF using the comparative Ct method with 18S rRNA as an internal control 27. A one-factor ANOVA was performed to test for a difference in ΔCt (Ct of target - Ct of 18S rRNA) values between NP and AF tissues (StatView, SAS Institute, Cary, NC). Similarly, a two-factor ANOVA was performed to test for differences between NP and AF cells as well as culture conditions (Day 0 = freshly isolated vs. Day 4-7 = monolayer cultured). Fold-differences in relative mRNA levels (2−ΔΔCt) between compared samples were reported if greater than or equal to 2, and where ANOVA detected a difference at p<0.05 27.
Western blot analysis for Gal-1 expression in immature porcine IVD
Frozen porcine (3m) AF and NP tissues, and porcine cells harvested from monolayer culture for 7 days were homogenized in protein extraction buffer (50mM Tris/150mM NaCl2, pH 7.4) containing a protease inhibitor cocktail (AEBSF 104 mM, Aprotinin 80 μM, Leupeptin 2.1 mM, Bestatin 3.6 mM, Pepstatin A 1.5 mM and E-64 1.4 mM, Sigma) at 4°C. After centrifugation (13,000g for 15 minutes at 4°C), the supernatant (soluble fraction) was collected and stored, while the pellet (insoluble fraction) was further extracted by boiling in SDS-PAGE sample buffer. The total protein concentration was measured by RC DC™ protein assay kit (Biorad, Hercules, CA). An equal amount (10μg) of total protein from each sample were fractionated on 4-12% SDS-PAGE, transferred to PVDF membranes and blocked with 5% dry milk in PBST (10 mM sodium phosphate buffer pH 7.2, 150 mM NaCl and 0.05% Tween) for 1 hour. The membranes were then incubated in a human Gal-1 polyclonal antibody (Santa Cruz Biotechnology) with PBST for 1 hour, washed and incubated with HRP-conjugated secondary antibody for 1 hour followed by washing in PBST. The transferred proteins then were detected with HRP-conjugated secondary antibody and an enhanced chemiluminescent substrate (Pierce, Rockford, IL). In addition, 10μg of total protein from 3T3 cells was used as the positive control for the anti-Gal-1 antibody according to the manufacturer's suggestion.
Cell attachment assay
Ninety-six-well culture plates (Corning Costar, Acton, MA) were coated with laminins (mouse LM-111 and human placenta LM-511, 5μg/ml, Sigma) via overnight incubation at 4°C. Coated wells were then blocked with 3.75% BSA (Gibco) for 3 h at 37°C. Wells without laminin and without BSA blocking were used as blank substrate controls (termed “no-coat”, tissue-culture plastic only). Primary AF and NP cells from both porcine (3m, n=3) and human (17-66y, n=4) tissues were cultured in monolayer for 4-7 days and then in serum-free media (F12 + Insulin-Transferrin-Selenium (ITS, Gibco)) for 48 h (human) or overnight (porcine). Cells were detached from the culture surface using 0.025% trypsin/EDTA (Cambrex) and immediately washed in soybean trypsin inhibitor (Sigma). Cells were then preincubated with/ without human recombinant Gal-1 protein (0.5μg/ml, R&D Systems, Minneapolis, MN) in serum-free media for 1 h at 37°C, then seeded on the laminin-coated culture wells or “no coat” control wells (4,000 cells per well, 4 replicates per substrate, 37°C) to allow for attachment. Preliminary studies showed that 1hour of attachment for porcine cells, and 2 hours of attachment of human cells, was sufficient to insure a minimum of 50% of cells attaching under these conditions. The concentrations chosen for Gal-1 (0.5μg/ml) and laminin substrates (5μg/ml) in the current study were based on an initial dosing study performed for Gal-1(0.1, 0.5 and 1μg/ml) and laminin (5, 10, and 20μg/ml) to ensure consistent cell attachment. Unattached cells were rinsed away and the remaining attached cells per well were counted via the CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison, WI). Cell attachment with Gal-1 incubation was reported as a percentage increase of cell attachment numbers relative to without Gal-1 incubation (control) for each laminin substrate and “no coat” control substrate conditions (% increase of attached cells = {(# Gal-1+) − (# control)} / {# control} × 100). Differences in the % increase of cell attachment between groups incubated with and without Gal-1 were detected via one-factor ANOVA for each substrate (a significance level of p< 0.05).
Gal-1 binding to IVD cells
Gal-1 was conjugated with Sulfo-NHS-LC biotin using a biotinylation kit (Pierce, Rockford, IL) according to the manufacturer's instructions and the method described previously28. Briefly, human recombinant Gal-1 (1 mg/ml, ProSpec, Rehovot, Israel) in 2mM PBS at pH 8.0 was dialyzed for 12 h against the same buffer containing 20 mM lactose at 4°C to protect the carbohydrate-binding site during the biotinylation. Sulfo-NHS-LC biotin reagent was then added to the Gal-1 solution with molar ratio of 50:1 (Biotin: Gal-1). The mixture was incubated at room temperature for 1 hour and then further dialyzed against 2mM PBS at pH 7.2 for 12 hours at 4°C to remove excess biotin and lactose. The final protein concentration of biotinylated Gal-1 (B-gal-1) was measured by a BCA protein assay reagent (Pierce).
To measure the binding of Gal-1 to cells, porcine AF and NP cells (3m) were incubated with different concentrations of B-Gal-1 (2.5, 5, 12.5 and 25 μg/ml) at room temperature for 1 hour, and labeled with Alexa Fluor 488 conjugated streptavidin, and then analyzed via a FACScan flow cytometer (Becton Dickinson, San Jose, CA) to quantify the percentage of labeled cells (in duplicate). Statistical tests of differences amongst cell binding for different B-Gal-1 levels were not performed as the experiments were performed in duplicate only.
MACS of IVD cells using Gal-1
An objective of this study was to determine the ability of Gal-1 to uniquely mediate binding to IVD cells. Magnetic activated cell sorting was performed to test for Gal-1 binding to IVD cells compared against other cell populations. Preparations of human AF and NP cells alone and human cells mixed with two inflammatory cell lines (U937 for macrophages and Jurkat for lymphocytes) were obtained for sorting via magnetic activated cell sorting (MACS®, Miltenyi Biotec, Auburn, CA). First, monolayer-cultured human NP and AF cells (19-63y, n=4) and suspension-cultured U937 and Jurkat cells (1-5×106) were separately re-suspended in binding buffer (DPBS and 0.5% BSA) with/without B-Gal-1 (50 μg/ml) and then incubated for 1 hour at room temperature. Subsequently, the cell mixture was incubated for 30 min with streptavidin-conjugated magnetic microbeads (Miltenyi Biotec). The cell-bead mixture was loaded on a MACS mini column (Miltenyi Biotec) for positive selection of B-Gal-1 bound cells. The number of cells in the effluent and the retained fraction after MACS was counted as a measurement of Gal-1 binding in NP, AF U937 and Jurkat cells. Cells from both fractions were evaluated for cell viability by the trypan blue assay immediately after sorting and one week after culture in monolayer. A difference in % of cells retained after B-Gal-1 incubation was detected among cell types via one-factor ANOVA (n=4, p<0.05).
Second, U937 and Jurkat cells were prelabeled with a fluorescence dye (Vybrant CFDA SE cell tracer kit, Invitrogen) according to the manufacturer's instruction, then mixed at fixed ratios (0-100%) with unlabeled AF and NP cells (Human, 55y) for FACScan analysis. The percentage of cells with fluorescence measured by FACScan was found to be comparable to the actual mixing ratio (correlation coefficient R2=0.99). A mixture of 50% AF cells and 50% CFDA SE labeled-U937 cells, and a mixture of 50% NP cells and 50% CFDA SE labeled-Jurkat cells, were incubated with B-Gal-1 (50 μg/ml), then subjected to the MACS protocol as described above. The percentage of cells with fluorescence in the effluent (flow-through) and the retained fraction after MACS was measured by FACScan. These values were used to calculate the percentage of AF or NP cells in the mixed populations before and after MACS selection.
Results
Expression of Gal-1 in NP cells
Immature NP tissue from human (2y), porcine (3m) and rat (1m) IVDs stained intensely positive for Gal-1 as compared to adjacent AF tissue (Fig.1 A). Only rat AF tissue stained positively but slightly in the pericellular region (Fig.1 A, top right), while NP tissue from all three species showed a staining pattern that appeared as a dense network connected to the cells (Fig.1 A, bottom). This expression pattern for Gal-1 in NP was similar to that of LM-511 reported previously by our group in immature IVDs 14. Gal-1 staining intensity appeared to drastically decrease with age since there was less staining in mature NP tissues of human (35y), porcine (24m) and rat (12m) IVDs (Fig.1 B, bottom) and no staining in mature AF tissues in all three species (Fig.1 B, top), although quantitative methods for the staining would be required to support this statement. In addition, RT-PCR revealed that the relative mRNA levels of Gal-1 were also higher in NP tissue as compared to AF tissue of immature porcine IVDs (3m) (∼7-fold higher in NP than AF, n=3, p<0.05, ANOVA). Similarly, significantly higher levels for Gal-1 mRNA were detected in freshly isolated NP cells of immature porcine IVDs (3m) (Fig.2 C, ∼250-fold higher in NP cells than AF cells, n=3, p<0.05, ANOVA). After cell isolation and in vitro culture, primary AF and NP cells from porcine (3m) and human (9y) IVDs both stained positively for Gal-1 protein (Fig.2 A). Unlike in IVD tissue, there was no difference in both mRNA and protein expression pattern of Gal-1 between AF and NP cells when they were cultured in vitro. Higher protein expression for Gal-1 in immature porcine NP tissues as compared to AF was further confirmed by Western blot analysis. The Gal-1 protein was approximately 100-fold more abundant in NP tissue than in AF tissue (as estimated by band intensity), while Gal-1 protein was almost equivalent for protein from in vitro cultured AF and NP cells (Fig.2 B). In addition, Gal-1 was present in both the soluble and insoluble fractions of total protein from the tissue, which indicates its production to be both intracellular and membrane-bound in situ. Furthermore, RT-PCR analysis revealed that monolayer culture condition (4-7 days) led to significant up-regulation of Gal-1 mRNA levels in both NP and AF cells as compared to freshly isolated cells (Fig.2 C, AF: ∼250-fold higher than freshly isolated AF cells; NP: ∼2-fold higher than freshly isolated NP cells, n=3, p<0.05, ANOVA).
Figure 1.

Immunostaining demonstrated higher expression of galectin-1(Gal-1) in nucleus pulposus (NP) as compared anulus fibrosus (AF) of intervertebral disc (IVD) tissue: A. immature IVDs tissue from human (2y), porcine (3m) and rat (1m); B. mature IVDs tissue from human (35y), porcine (24m) and rat (12m). scale bar = 20μm. green = Gal-1; red = propidium iodide counterstain.
Figure 2.
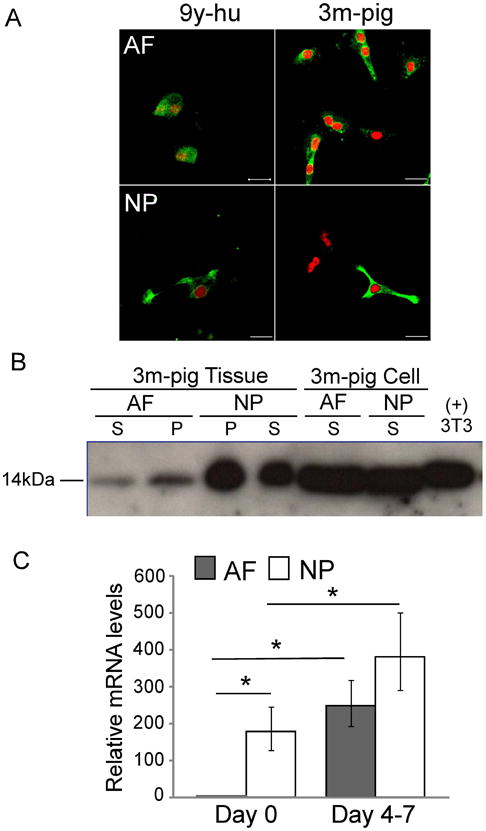
A. Immunostaining for galectin-1 (Gal-1) expression in monolayer cultured anulus fibrosus (AF) and nucleus pulposus (NP) cells of human (9y) and porcine (3m) intervertebral disc (IVD) in vitro (scale bar = 20μm. green = Gal-1; red = propidium iodide counterstain). B. Western blot of Gal-1 expression in AF and NP tissues and monolayer cultured cells of porcine IVDs (3m). The soluble fractions of protein are labeled as supernatant (S) and the insoluble fractions of protein are labeled as pellet (P). For cells, only supernatant was used in this blot because Gal-1 level was detected at the similar level in both supernatant and pellet in a preliminary study. Protein from 3T3 cells was used as positive control. The size of Gal-1 protein was labeled as 14kDa at the left. C. RT-PCR for relative mRNA levels of Gal-1 in freshly isolated cells (day 0) and monolayer cultured cells (day 4-7) of porcine IVDs (3m) (relative fold changes normalized by AF=1.0 at day 0, * p<0.05, two-factor ANOVA between tissues and culture conditions, n=3).
Gal-1 promotes cell adhesion to laminin substrates
Initial studies of cell attachment dependence on Gal-1 dose (0.1, 0.5 and 1μg/ml) were performed by seeding porcine NP cells (3m) on different concentrations of laminin substrates (LM-511 or LM-111, 5, 10, and 20μg/ml). Following pre-incubation of cells with each Gal-1 concentration, 0.5 and 1μg/ml of Gal-1 were found to promote optimal attachment of NP cells to LM-511 and LM-111 at laminin coating concentrations above 5μg/ml (Fig.3). A Gal-1 concentration of 0.5μg/ml and a laminin concentration of 5μg/ml were thus selected for all subsequent experiments. Pre-incubation of cells with Gal-1 significantly increased the numbers of attached cells to laminin substrates for both human (17-66y, n=4, Fig.4) and porcine (3m, n=3, Fig.4) NP and AF cells compared to incubation without Gal-1 protein (p<0.05, ANOVA, Fig.4). A more modest effect of Gal-1 on increasing cell attachment to tissue culture plastic (no-coat control) was also observed for both porcine and human cells (Fig.4 bottom). However, no statistically significant difference was detected for cell attachment between NP and AF cells for either human and porcine samples on all substrate conditions.
Figure 3.

Dose response curves for the effect of galectin-1(Gal-1) on porcine nucleus pulposus (NP) cell attachment on different concentrations of laminin substrates (LM-511, LM-111). Data represents an example from three independent experiments.
Figure 4.

Cell attachment assay for nucleus pulposus (NP) and anulus fibrosus (AF) cells of porcine (3m, n=3) and human (17-66y, n=4) IVDs on laminins (top: LM-511, middle: LM-111) with galectin-1 (Gal-1) pre-incubation (y axis: % increase in cell number normalized to no Gal-1 treatment, mean ± SD). The tissue-culture plastic surface only was used as no protein substrate controls (bottom: no-coat). All Gal-1 treatments under each substrate and no substrate conditions were statistically different from control groups without any Gal-1 incubation (one factor ANOVA, P<0.05).
Gal-1 binding to IVD cells
B-Gal-1 levels were quantified following incubation with porcine NP and AF cells via flow cytometry. B-Gal-1 levels increased for both porcine NP and AF cells (3m) in a dose-dependent manner (Fig.5). Also, B-Gal-1 binding was generally similar for NP and AF cells except at the lower B-Gal-1 concentration (5μg/ml).
Figure 5.

Dose response curve of biotinylated galectin-1 (B-Gal-1) binding to cultured porcine anulus fibrosus (AF) and nucleus pulposus (NP) cells analyzed by flow cytometry (Alexa Fluor 488 conjugated streptavidin).
Gal-1 mediated selection IVD cells via MACS
Human IVD cells were retained in the magnetic column by B-Gal-1 binding. In general, more than 50% of NP and AF cells were detected in the retained fraction following incubation with B-Gal-1 (Fig.6). No statistically significant difference was detected for % cells retained between NP and AF cells after B-Gal-1 incubation. In the absence of B-Gal-1, there was some residual binding of cells (less than ∼5%) to the column (Fig.6). Interestingly, both NP and AF cells had statistically higher binding efficiencies than that of U937 and Jurkat cells (> 2-fold higher, p<0.05 ANOVA, Fig.6). All selected NP and AF cells were confirmed to be viable immediately after sorting and after a week of culture for both retained and eluted fractions.
Figure 6.

% of cells in the retained fraction for cultured human anulus fibrosus (AF) or nucleus pulposus (NP) cells (n=4, 19-63y) and for the two inflammatory cell types (U937 and Jurkat) after MACS positive selection with biotinylated galectin-1(B-Gal-1) pre-incubation (50 μg/ml Gal-1 pre-incubation, control = cells without B-Gal-1; * P<0.05, one factor ANOVA).
B-Gal-1 binding was able to assist in selecting both NP and AF cells from impure mixtures of IVD cells with U937 and Jurkat cells. The percentage of NP and AF cells was increased in the retained fraction when the mixture was pre-incubated with B-Gal-1 as compared to no pre-incubation period (AF: 15% vs 74%; NP: 68% vs 96%, no-incubation vs. B-Gal-1 pre-incubation, Table 2). These results suggest that MACS with B-Gal-1 binding may be useful for selecting IVD cells from mixed cell populations in pathological tissue.
Table 2.
Percentage of cells before and after magnetic activated cell sorting (MACS). Anulus fibrosus (AF) or nucleus pulposus (NP) cells from surgical samples of patient (55y) were pre-mixed with (*) CFDA SE labeled-U937 cells or labeled-Jurkat cells before incubation with biotinylated galectin-1 (B-Gal-1), or an equivalent incubation period without B-Gal-1, then followed by MACS. The percentage of cells with fluorescence (labeled cells U937 or Jurkat) in both the effluent (flow-through) and the retained fractions after MACS was measured by FACScan. These values were used for calculating the percentage of AF or NP cells enriched by B-Gal-1 from mixed population after MACS selection.
| AF (%) | U937* (%) | NP (%) | Jurkat* (%) | |
|---|---|---|---|---|
|
|
||||
| starting values | 50 | 50 | 50 | 50 |
| MACS without B-Gal-1 | 15 | 85 | 68 | 32 |
| MACS with B-Gal-1 | 74 | 26 | 96 | 4 |
Discussion
This study provides new findings for the presence and functional role of Gal-1 within rat, porcine and human intervertebral discs. The expression pattern for Gal-1 was found to be similar to that previously reported for LM-511 in IVD tissue 14, which suggests that Gal-1 may serve as an adhesion molecule that may interact with both IVD cells and laminins. The higher expression of Gal-1 in immature NP tissue corroborates with findings from our cDNA microarray data which show that Gal-1 mRNA levels are expressed higher in immature NP (1m) compared to mature NP (12m) rat tissue (2.5-fold higher levels) 20. Similarly, the higher expression of Gal-1 in NP tissue compared to AF as reported here is corroborated by our prior findings of higher Gal-1 mRNA in NP (1 m) compared to AF rat tissue (3.1-fold higher levels) 20. In our current studies, these observations were confirmed with both RT-PCR and Western blot analyses which showed higher Gal-1 mRNA and protein expression in NP tissue than in AF tissue in porcine IVD. During the embryonic development of zebrafish, the expression of both Gal-1 21 and laminin 22 protein was found to be specific to the region of notochord, a known origin of NP tissue. Our previous studies have found a NP tissue-specific expression of laminin in rat, porcine and human IVDs 12, 14, 15 consistent with this observation in zebrafish. Previous reports for Gal-1 expression in IVD tissue are limited, however, to one study showing Gal-1 mRNA in the anlagen of IVD during mouse prenatal development 19. Our findings show that Gal-1 is expressed in NP tissue and cells for several species and that the expression level of Gal-1 in NP tissue declines with age in a pattern similar to that of LM-511 in IVDs 14. These results suggest that Gal-1 may play an important role in mediating cell-matrix interactions during disc development and aging-related degeneration.
Of interest was the finding that both Gal-1 mRNA and protein were expressed in AF cells when cultured in monolayer, and at levels similar to that of cultured NP cells. These results indicate that both NP and AF cells are capable of expressing Gal-1 when exposed to common culture media and conditions in vitro, unlike the distinct regional difference that existed for NP and AF tissues in situ. These differences in Gal-1 expression for cells in tissue or isolated from the tissue may also indicate some phenotype changes related to in vitro culture conditions. While Gal-1 immuno-cytochemistry confirmed the intracellular localization of Gal-1 protein in both NP and AF cells in culture, we did not detect cell surface expression of endogenous Gal-1 in cultured IVD cells by flow cytometry except in the positive cell line (3T3) of mouse fibroblasts in our preliminary studies. This may indicate that IVD cells are not able to secrete Gal-1 to the cell surface during in vitro culture. The mechanism that contributes to this apparent lack of Gal-1 secretion in cultured IVD cells in vitro is unknown, however, and could need further investigation.
Results of this study also showed that Gal-1 increased both NP and AF cell attachment to laminins (LM-111 and LM-511) in vitro and exhibited a similar binding capacity to both cell types as determined by flow cytometry analysis and MACS. Our findings that Gal-1 promotes NP and AF cell adhesion on both LM-111 and LM-511 substrates are consistent with the previously reported function of Gal-1 as a substrate adhesion molecule that cross-links cells to extracellular matrix proteins 17, 29 in human melanoma cells 29, 30, hamster CHO cells 31, mouse F9 cells 31 and porcine articular chondrocytes 32. In this current study, we chose to use exogenous Gal-1 for studying cell-matrix and cell-Gal-1 interactions. Exogenous Gal-1 may act in a manner similar to that of a growth factor, and drive enhanced expression of Gal-1 binding molecules that mediate IVD cell adhesion to laminin substrates. Another possibility is that exogenous Gal-1 may promote the expression and secretion of endogenous Gal-1 proteins that may additionally contribute to the maintenance of the NP and AF phenotype over longer durations of culture. Overall, our results indicate that the pre-incubation step before cell attachment to laminin substrates is critical for promoting sufficient Gal-1 molecules to bind to the cell surface.
In general, all galectins exert their biological effects by binding to specific carbohydrate structures, although the mechanism by which Gal-1 exerts its biological functions remains unknown. It has been shown that Gal-1 interacts with many cell-surface receptors such as integrin β1 (CD29), CD107a, CD90 and CD45 16. Also, recent studies have shown that Gal-1 stimulates rat hepatic stellate cell proliferation through a MEK1/2-ERK1/2 signaling pathway 33 and that altering protein phosphorylation levels affects Gal-1-mediated 3T3 cell attachment and proliferation on chitosan membranes 34. In our current studies, the function of Gal-1 was explored to select for IVD cells from other cell populations that may be present in pathological IVD tissues. Pre-incubation with Gal-1 and selection via MACS was a novel tool that successfully selected IVD cells from a mixed population including macrophages and lymphocytes. This approach may have some utility for a cell-baesd therapy that makes use of MACS with Gal-1 sorting to enrich disc cell populations from degenerate and herniated tissues; these tissues often contain a mixture of NP, AF and other inflammatory cell types (i.e. macrophages, lymphocytes) 8,9 that could interfere with a cell-mediated regenerative process.
A limitation of this current study was that only laminins were used as extracellular matrix substrates for cell adhesion. Future work is needed to investigate if Gal-1 promotes cell adhesion to other matrix proteins such as fibronectin, and type I and type II collagens. Another limitation of this study was that human IVD cells were studied only after culture in monolayer to evaluate the ability of Gal-1 to interact with IVD cells. Future studies are needed to assess if Gal-1 can modify adhesion of freshly isolated IVD cells from different sources (young healthy vs. old diseased).
In summary, NP cells of immature IVDs expressed higher levels of Gal-1 in situ as compared to AF cells. These Gal-1 levels in NP region of IVD tissue appeared to decrease with age. Similar staining patterns for Gal-1 and LM-511 in IVD tissue suggest that Gal-1 may serve as an adhesion molecule that may interact with both cells and laminin matrix. Exogenous Gal-1 increased both NP and AF cell attachment to laminins and was bound to both cell types at equivalent levels in vitro. Incubation with B-Gal-1 was also able to promote the retention of more than 50% of NP and AF cells by MACS. Thus, a MACS protocol based on IVD cell binding to B-Gal-1 may be useful for selecting and enriching pure IVD cells from mixed cells of pathological tissue for future studies.
Acknowledgments
We gratefully acknowledge Steve Johnson and Tish Griffin for assistance with tissue harvesting, Melissa Tsuboyama for assistance with immunostaining, Bob Nielsen for assistance with confocal microscopy, and Dr. Mike Cook for assistance with flow cytometry analysis. This study was supported by NIH R01AR057410, R01EB002263, R01AR047442 and P01AR050245 and the Pratt Undergraduate Research Fellowship (SWL).
References
- 1.Andersson GB, An HS, Oegema TR, Jr, et al. Intervertebral disc degeneration. Summary of an AAOS/NIH/ORS workshop, September 2005. J Bone Joint Surg Am. 2006;88:895–899. doi: 10.2106/JBJS.F.00028. [DOI] [PubMed] [Google Scholar]
- 2.Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(2):21–24. doi: 10.2106/JBJS.E.01273. [DOI] [PubMed] [Google Scholar]
- 3.Biyani A, Andersson GB. Low back pain: pathophysiology and management. J Am Acad Orthop Surg. 2004;12:106–115. doi: 10.5435/00124635-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Boos N, Weissbach S, Rohrbach H, et al. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine. 2002;27:2631–44. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- 5.Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 1995;20:1307–1314. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5:120–130. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meisel HJ, Ganey T, Hutton WC, et al. Clinical experience in cell-based therapeutics: intervention and outcome. Eur Spine J. 2006;15(3):S397–405. doi: 10.1007/s00586-006-0169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arai Y, Yasuma T, Shitoto K, et al. Immunohistological study of intervertebral disc herniation of lumbar spine. J Orthop Sci. 2000;5:229–231. doi: 10.1007/s007760050156. [DOI] [PubMed] [Google Scholar]
- 9.Kanerva A, Kommonen B, Gronblad M, et al. Inflammatory cells in experimental intervertebral disc injury. Spine. 1997;22:2711–2715. doi: 10.1097/00007632-199712010-00002. [DOI] [PubMed] [Google Scholar]
- 10.Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–284. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- 11.Nettles DL, Richardson WJ, Setton LA. Integrin expression in cells of the intervertebral disc. J Anat. 2004;204:515–520. doi: 10.1111/j.0021-8782.2004.00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilchrist CL, Chen J, Richardson WJ, et al. Functional integrin subunits regulating cell-matrix interactions in the intervertebral disc. J Orthop Res. 2007;25:829–840. doi: 10.1002/jor.20343. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Yan W, Setton LA. Molecular phenotypes of notochordal cells purified from immature nucleus pulposus. Eur Spine J. 2006;15(15):303–311. doi: 10.1007/s00586-006-0088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J, Jing L, Gilchrist CL, et al. Expression of laminin isoforms, receptors, and binding proteins unique to nucleus pulposus cells of immature intervertebral disc. Connect Tissue Res. 2009;50:294–306. [PMC free article] [PubMed] [Google Scholar]
- 15.Gilchrist CL, Francisco AT, Plopper GE, et al. Nucleus pulposus cell interactions with laminins. Eur Cell Mater. 2011;21:523–532. doi: 10.22203/ecm.v021a39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elola MT, Chiesa ME, Alberti AF, et al. Galectin-1 receptors in different cell types. J Biomed Sci. 2005;12:13–29. doi: 10.1007/s11373-004-8169-5. [DOI] [PubMed] [Google Scholar]
- 17.Moiseeva EP, Williams B, Goodall AH, et al. Galectin-1 interacts with beta-1 subunit of integrin. Biochem Biophys Res Commun. 2003;310:1010–1016. doi: 10.1016/j.bbrc.2003.09.112. [DOI] [PubMed] [Google Scholar]
- 18.Camby I, Le Mercier M, Lefranc F, et al. Galectin-1: a small protein with major functions. Glycobiology. 2006;16:137R–157R. doi: 10.1093/glycob/cwl025. [DOI] [PubMed] [Google Scholar]
- 19.Poirier F, Timmons PM, Chan CT, et al. Expression of the L14 lectin during mouse embryogenesis suggests multiple roles during pre- and post-implantation development. Development 1992. 1992;115:143–155. doi: 10.1242/dev.115.1.143. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Jing L, Richardson WJ, et al. Gene expression profiling reveals age and zonal-specific differences in intervertebral disc tissue during aging. Transactions of the Orthopaedic Research Society. 2007;32:1104. [Google Scholar]
- 21.Ahmed H, Du SJ, O'Leary N, et al. Biochemical and molecular characterization of galectins from zebrafish (Danio rerio): notochord-specific expression of a prototype galectin during early embryogenesis. Glycobiology. 2004;14:219–232. doi: 10.1093/glycob/cwh032. [DOI] [PubMed] [Google Scholar]
- 22.Parsons MJ, Pollard SM, Saude L, et al. Zebrafish mutants identify an essential role for laminins in notochord formation. Development. 2002;129:3137–3146. doi: 10.1242/dev.129.13.3137. [DOI] [PubMed] [Google Scholar]
- 23.Choi KS, Cohn MJ, Harfe BD. Identification of nucleus pulposus precursor cells and notochordal remnants in the mouse: implications for disk degeneration and chordoma formation. Dev Dyn. 2008;237:3953–3958. doi: 10.1002/dvdy.21805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gotz W, Kasper M, Miosge N, et al. Detection and distribution of the carbohydrate binding protein galectin-3 in human notochord, intervertebral disc and chordoma. Differentiation. 1997;62:149–157. doi: 10.1046/j.1432-0436.1997.6230149.x. [DOI] [PubMed] [Google Scholar]
- 25.Oguz E, Tsai TT, Di Martino A, et al. Galectin-3 expression in the intervertebral disc: a useful marker of the notochord phenotype? Spine. 2007;32:9–16. doi: 10.1097/01.brs.0000250302.74574.98. [DOI] [PubMed] [Google Scholar]
- 26.Thompson JP, Pearce RH, Schechter MT, et al. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine. 1990;15:411–415. doi: 10.1097/00007632-199005000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Chen J, Baer AE, Paik PY, et al. Matrix protein gene expression in intervertebral disc cells subjected to altered osmolarity. Biochem Biophys Res Commun. 2002;293:932–938. doi: 10.1016/S0006-291X(02)00314-5. [DOI] [PubMed] [Google Scholar]
- 28.Seyrek K, Ozcan A, Erbas H. Histochemical study of expression of galectin-1 and its reactive carbohydrate epitopes in normal bovine embryonal and adult pancreas. Israel J Vet Med. 2000;56:25–30. [Google Scholar]
- 29.Hughes RC. Galectins as modulators of cell adhesion. Biochimie. 2001;83:667–676. doi: 10.1016/s0300-9084(01)01289-5. [DOI] [PubMed] [Google Scholar]
- 30.van den Brule FA, Buicu C, Baldet M, et al. Galectin-1 modulates human melanoma cell adhesion to laminin. Biochem Biophys Res Commun. 1995;209:760–767. doi: 10.1006/bbrc.1995.1564. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Q, Cummings RD. L-14 lectin recognition of laminin and its promotion of in vitro cell adhesion. Arch Biochem Biophys. 1993;300:6–17. doi: 10.1006/abbi.1993.1002. [DOI] [PubMed] [Google Scholar]
- 32.Marsich E, Mozetic P, Ortolani F, et al. Galectin-1 in cartilage: expression, influence on chondrocyte growth and interaction with ECM components. Matrix Biol. 2008;27:513–525. doi: 10.1016/j.matbio.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Maeda N, Kawada N, Seki S, et al. Stimulation of proliferation of rat hepatic stellate cells by galectin-1 and galectin-3 through different intracellular signaling pathways. J Biol Chem. 2003;278:18938–18944. doi: 10.1074/jbc.M209673200. [DOI] [PubMed] [Google Scholar]
- 34.Chang YY, Chen SJ, Liang HC, et al. The effect of galectin 1 on 3T3 cell proliferation on chitosan membranes. Biomaterials. 2004;25:3603–3611. doi: 10.1016/j.biomaterials.2003.10.039. [DOI] [PubMed] [Google Scholar]


