Abstract
Background
Controversy surrounds the cardiac effects of competitive sports and the athlete’s heart. In this review, we present and discuss the main cardiological findings in competitive athletes.
Method
Selective review of pertinent literature retrieved by a search with the keywords “athlete’s heart,” “ECG,” “echocardiography,” “endurance exercise,” “longevity,” and others.
Results
Regular exercise leads to functional and structural adaptations that improve cardiac function. Athlete’s heart, which develops rarely, is a typical finding in endurance athletes. This condition is characterized by physiological, harmonically eccentric hypertrophy of all cardiac chambers. The athlete’s ECG can be used to distinguish physiological, training-related changes from pathological training-unrelated changes. The athlete’s heart function is normal at rest and increases appropriately during exercise. The cardiac markers troponin and B-type natriuretic peptide are within the normal range in healthy athletes at rest, but can temporarily be mildly elevated after exhausting endurance-exercise, without evidence of myocardial damage. The epidemiological data suggest that participation in competitive sports increases life expectancy.
Conclusion
Competitive exercise does not induce cardiac damage in individuals with healthy hearts, but does induce physiological functional and structural cardiac adaptations which have positive effects on life expectancy.
Physicians of the ancient and early modern world often recommended physical exercise as a means of staying healthy. Today, various medical specialty societies recommend exercise and athletic activity to maintain and preserve health, on the basis of strong evidence from numerous single studies and meta-analyses (1). On the other hand, ever since the “athlete’s heart” was described more than 100 years ago (e1), there has been concern that prolonged and intense athletic activity might confer certain risks, while exercise-induced cardiac changes have been interpreted as a potential sign of damage (e2, e3). The question whether sport has a net positive or negative effect on health was already debated in 1912 at the first sports-medicine conference in Germany (e4). Among physicians, those with no special training or experience in sports medicine tend to view athlete’s heart as a potentially dangerous condition and to think that the associated cardiac enlargement and ECG changes indicate an increased cardiac risk. Reindell et al. (2), and later Kindermann (3), interpreted the radiological, electrocardiographic, and hemodynamic changes seen in healthy athletes as physiological adaptations of the heart; yet the recurring reports of cardiac events, and even sudden cardiac death, among athletes (4, e5) continue to sustain the debate over the potential pathological effects of sports on the heart, rare as they may be (e6).
Strong evidence.
Various medical specialty societies recommend exercise and athletic activity to maintain and preserve health, on the basis of strong evidence from numerous single studies and meta-analyses.
Learning objectives
We critically review the current evidence about the risks and benefits of athletic activity and describe the proper classification of cardiac findings in competitive athletes from the point of view of the sports cardiologist. Readers should become well informed about the following matters:
physiological cardiovascular adaptations,
ECG changes,
structural and functional findings of imaging studies,
stress-induced elevation of the concentrations of the cardiac markers troponin and BNP,
and the life span of competitive athletes.
Methods
We selectively searched the PubMed database for articles published up to June 2012 that contained the keywords “athletes,” “athlete’s heart,” “BNP,” “ECG,” “echocardiography,” “electrocardiography,” “endurance exercise,” “magnetic resonance imaging,” “MRI,” “NT-proBNP,” “troponin,” “longevity,” and others. We present their most important findings in this article. The recommendations given here regarding ECG interpretation in patients who participate in sports correspond to the current criteria of the European Society of Cardiology (5).
Physiological cardiovascular changes
Oxygen uptake rises by a factor of 10 to 12 during exercise in healthy, untrained individuals and by a factor of 20 or more in highly trained endurance athletes (e7). This rise is accounted for by increases in cardiac output and in the arteriovenous oxygen concentration gradient. The increased cardiac output is mainly due to a faster heart rate; as a rule of thumb, the maximum attainable number of beats per minute can be estimated as 220 minus the age in years. The increased heart rate is caused initially by a drop in parasympathetic activity, and then, during intermediate or intense exercise, by an increase in sympathetic activity. The stroke volume increases by only 30% to 50%.
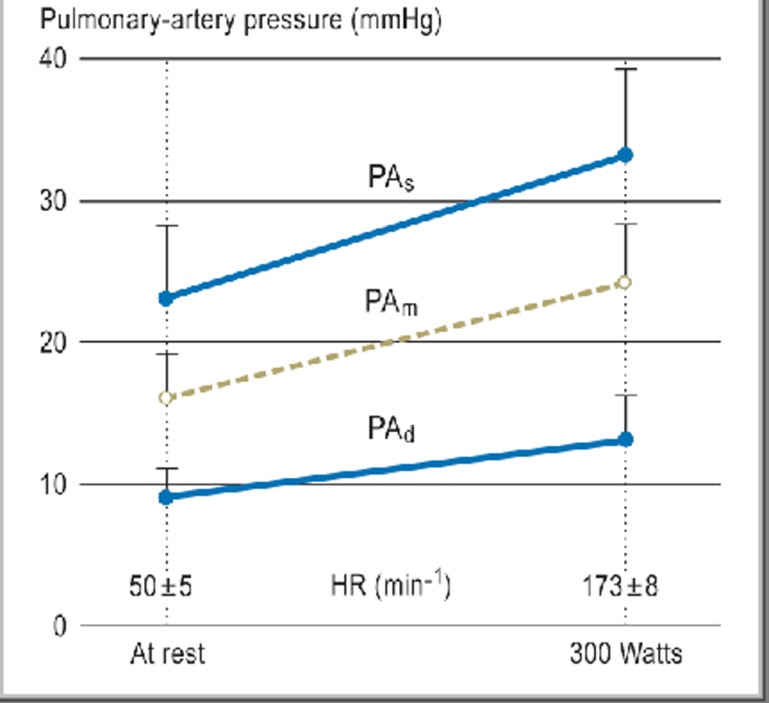
Dynamic exercise causes a rise in systolic blood pressure that is linearly related to exercise intensity, while the diastolic pressure is only marginally changed. On the other hand, static exercise—in particular, maximal exertion—or high-intensity dynamic exercise or Valsalva maneuvers causes a much greater rise in both systolic and diastolic blood pressure (e8). The pressures in the pulmonary artery and in the heart itself rise only slightly during exercise and remain in the normal range even under exercise stress (Figure 1) (3, e9). In general, dynamic exercise mainly increases the heart’s volume load, while static stress mainly increases its pressure work (e10).
Figure 1.
Pulmonary-arterial pressure in athlete’s heart
Measurements, by Swan-Ganz catheterization, of pulmonary-arterial pressures (PAs = systolic; PAd = diastolic; PAm = mean) and heart rate (HR) at rest and during maximal bicycle ergometry in nine endurance athletes (age. 21 ± 3 years) with athlete’s heart (heart volume 1148 ± 133 mL, 15.1 ± 1.5 mL/kg). From Reference (3).
Oxygen uptake in untrained persons and in highly trained endurance athletes.
Oxygen uptake rises by a factor of 10 to 12 during exercise in healthy, untrained persons and by a factor of 20 or more in highly trained endurance athletes.
Cardiovascular adaptation differs depending on the type of physical exercise and on the extent and intensity of training. Functional adaptations appear within a few weeks, requiring an additional energy consumption of at least 500 to 1000 kcal per week (6, e11, e12), which corresponds, for example, to brisk walking for one hour two to three times a week. Aerobic training lowers the heart rate and increases stroke volume without changing cardiac output at rest or for a given exercise intensity representing an economization of cardiac function (e13). Maximal cardiac output rises because the heart-rate reserve capacity increases while the maximal heart rate remains constant. The stroke volume is greater because of improved filling dynamics of the left ventricle, including greater compliance (e9, e14) and reduced peripheral vascular resistance (e15), so that the end-diastolic volume rises and the end-systolic volume falls. Increased shear stress during exercise improves endothelial function, with secretion of vasodilatory substances (e16).
The response of blood pressure to dynamic stress.
Dynamic stress causes a rise in systolic blood pressure that is linearly related to the intensity of stress, while the diastolic pressure is only marginally changed.
Training beyond a certain level (which differs from one individual to another) leads to structural adaptations as well. These dimensional changes of the heart are known as athlete’s heart; they generally arise only after endurance training in an amount that is generally only undertaken by competitive athletes. All chambers of the heart become dilated and hypertrophic. This eccentric hypertrophy is a harmonic enlargement of the heart (7) in which the heart mass does not exceed the critical value of 7.5 g/kg, corresponding on average to 500 g. In some cases, an athlete’s heart can be nearly twice as big as that of an untrained, healthy person (e17, e18).
Blood pressure during static exercise.
Static exercise causes a much greater rise in both systolic and diastolic blood pressure.
Athlete’s heart.
Athlete’s heart is rarer than generally thought. It typically affects endurance athletes and is characterized by harmonic, biventricular, eccentric hypertrophy. Strength and speed athletes generally do not develop athlete’s heart.
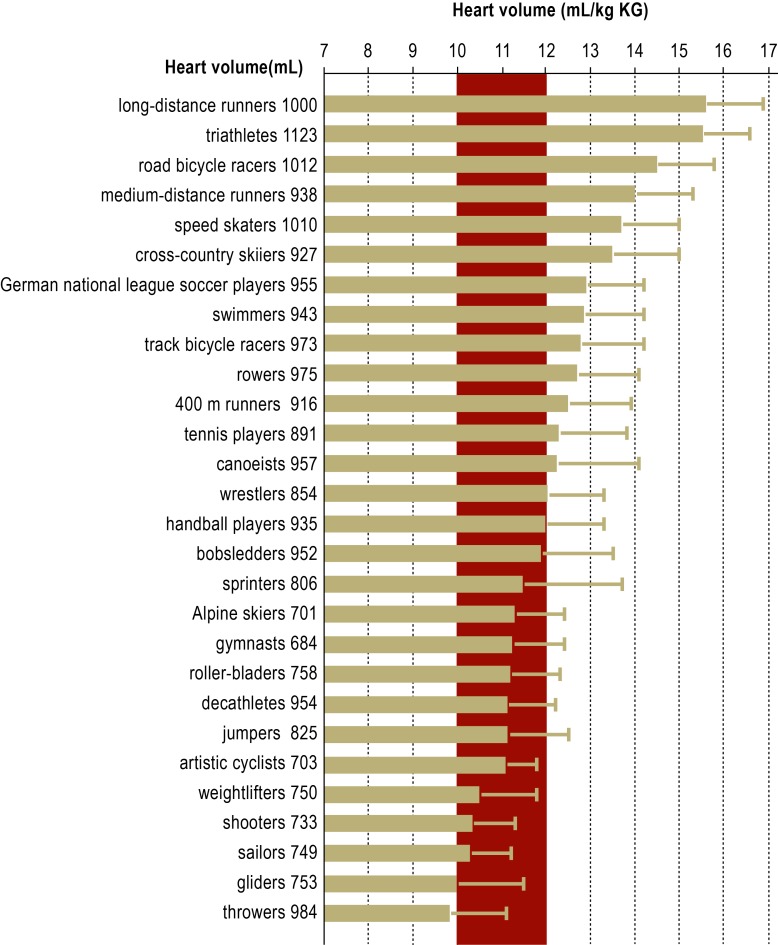
Athlete’s heart is rarer than generally thought and is not a prerequisite for a beneficial effect of training on health. At least five hours of endurance training per week, and more in many cases, are necessary for the volume load to result in dimensional changes (e19). The amount is highly variable: Running 60–70 km per week leads to the development of athlete’s heart in some persons, while others do not develop it even if they run 100 km per week. Athlete’s heart appears most prominent in long-distance runners, road bicycle racers, cross-country skiers, and triathletes. Athlete’s heart may also develop in older endurance athletes. On the other hand, strength and speed athletes, such as weightlifters, gymnasts, sprinters, high-jumpers, discus- and javelin-throwers, or Alpine skiers, generally do not develop athlete’s heart (Figure 2). The so-called strength athlete’s heart, which—in contrast with the eccentric hypertrophy characteristic of endurance athlete’s heart—shows concentric hypertrophy (8, e20), is discussed in the literature, not primarily in connection with exercise-related cardiac load, but more frequently in connection with the abuse of anabolic steroids and other performance-enhancing drugs (9, 10, e21). Pulmonary-arterial pressure and intracardiac pressure is not elevated in athlete’s heart (Figure 1) (3).
Figure 2.
Heart volumes in different types of competitive athletes
Means and standard deviations of relative heart volumes in male athletes in different sports. The mean absolute heart volumes are given at the left edge of the figure. Red: normal range. Athlete’s heart is defined, in men, as a heart volume ≥13 mL/kg body weight. Gray zone: 12–13 mL/kg body weight
When training ceases, an athlete’s heart size reduces again (e22, e23), with individually variable rates of regression. Immobilization (e.g., bed rest) leads to rapid regression of heart size (2). Athlete’s heart often regresses incompletely, however, with persistent left ventricular enlargement despite normalization of chamber thickness (e13, e23). Incomplete regression is thought to be due to genetic factors combined with continued athletic activity at a lower level than before. An important point for clinical practice is that, as long as there is still some degree of cardiac enlargement from athlete’s heart, the individual will still have a higher than average ergometric performance capacity for age.
The evaluation of an athlete’s ECG.
A distinction must be drawn between physiological training-related ECG changes and training-unrelated changes which may be pathological.
ECG changes in athletes
Various authors have reported ECG changes in athletes and have attempted to distinguish them from pathological findings (2, 5, 11, 12, e25– e27). There is, however, a gray zone between physiological and pathological changes. Depending on the particular study and method of classification used, 5% to 40% of athletes are found to have an abnormal or moderately to distinctly abnormal ECG (e25, e28, e29). Even higher percentages of abnormal findings have been found in selected groups of highly trained athletes, and only a small fraction of these persons (ca. 5%) have structural heart disease unrelated to their athletic activity (e25). The necessary distinction between common, training-related ECG changes and uncommon, training-unrelated ones that may be pathological should be drawn according to the current criteria and recommendations (Box) (5, e27). Hence, false-positive findings can be markedly reduced, and specificity increased without loss of sensitivity (11, e28), compared to earlier recommendations (12). Uncommon ECG changes are more frequent in men than in women, and they are also more frequent in black athletes, and require further evaluation (13, e29, e30).
Box. ECG changes.
-
Common, training-related ECG changes
sinus bradycardia
1st-degree AV block, 2nd-degree AV block of Wenckebach type
incomplete right bundle branch block
early repolarization
isolated QRS voltage criteria for left heart hypertrophy
-
Uncommon, training-unrelated ECG changes
T wave inversion in at least two adjacent leads
epsilon wave*1
ST segment depression
pathological Q waves
left atrial enlargement
left anterior hemiblock, left axis deviation
left posterior hemiblock, right axis deviation
right ventricular hypertrophy
ventricular preexcitation syndrome (Wolff-Parkinson-White syndrome)
complete left or right bundle branch block
long or short QT intervall (long or short QT syndrome)
Brugada-like early repolarization*2
Common ECG changes that require no further evaluation in healthy, asymptomatic athletes, versus uncommon ECG changes unrelated to athletic training that require evaluation in athletes; modified from Corrado et al. (5).
*1Post-excitation with a small wave in the ST segment in leads V1 to V3 as evidence of an arrhythmogenic right ventricular cardiomyopathy (ARVC)
*2Brugada-syndrome: an ion-channel disease characterized by ST elevation in leads V1 through V3, with an elevated risk of sudden cardiac death
Atrial fibrillation.
Atrial fibrillation is more common among middle-aged and older endurance athletes who have been training for many years than among athletically inactive persons of the same age.
ECG changes are most commonly seen in endurance athletes (e25). Sinus arrhythmia and sinus bradycardia are very common : on Holter monitoring, the heart rate may be found to dip as low as 30 beats per minute, or rarely even lower, usually at night. First-degree AV block (asymptomatic; resolves under stress) and second-degree AV block of Mobitz type I (Wenckebach block) are often seen in athletes, but second-degree AV block of Mobitz type II and third-degree AV block are atypical and require further evaluation.
Occasional ventricular and supraventricular extrasystoles are seen in athletes as well and are of no significance as long as they are asymptomatic. Frequent extrasystoles (more than 2000 in 24 hours on Holter monitoring [e31]) and paroxysmal supraventricular tachycardia require evaluation. Any arrhythmia that worsens during exercise must be evaluated; in athletes with a very low basal heart rate, extrasystoles usually disappear during exercise as sympathetic activity increases. In unclear cases, ambulatory ECG monitoring—ideally including the performance of usual athletic activities—should be performed to rule out significant arrhythmias.
Repolarization changes in an athlete’s ECG.
50% to 80% of all highly-trained athletes have early repolarization, which is reflected in the ECG by elevation of the beginning of the ST segment.
Atrial fibrillation is more common among middle-aged and older endurance athletes who have been training for many years than it is among athletically inactive persons of the same age (for elderly persons, 23% versus 12.5%) (14). The proposed pathophysiological mechanisms involve altered autonomic regulation due to athletic training, leading to a more intense vagal reaction, often at night; a lesser degree of sympathetic stimulation; and atrial remodeling. Endurance athletes aged 20 to 30 do not have atrial fibrillation any more commonly than non-athletes (e32). On the other hand, it has been found that moderate endurance training, as performed in preventive-fitness programs, may actually lower the risk of atrial fibrillation in old age (e33).
Alterations in the QRS complex and during repolarization are also more commonly seen in athletes and are usually physiological. 35% to 50% of athletes have an incomplete right bundle branch block; endurance athletes are the most likely to exhibit this finding (5). In contrast, complete right or left bundle branch block is not due to athletic activity and requires further evaluation. Isolated QRS voltage elevations should not be taken as an indicator of hypertrophy among athletes.
50% to 80% of all highly-trained athletes present with early repolarization, which is reflected in the ECG by elevation of the beginning of the ST segment (by at least 0.1 mV at the J point), usually seen in leads V2 through V4. The ST segment is then either concave (the typical finding in white athletes) or convex (the typical finding in black athletes). Moreover, as many as 25% of healthy black athletes with ST-segment elevation may also have negative T waves (5). The latter repolarization abnormality, which is more common in both male and female black athletes than in white athletes (13, e29, e30), may represent an ethnic variant of athlete’s heart (e31). Negative T waves that are at least 2 mm deep and are seen in two or more adjacent leads (frequency among athletes, ca. 3%) and ST-segment depressions require further evaluation (5, e25).
The QT interval is generally somewhat longer in athletes because of their lower heart rate. When correcting the QT interval for the lower rate, one must bear in mind that the correction is imprecise for rates under 40 beats per minute or over 80–100 beats per minute. A QTc of 500 ms or above is considered unequivocally pathological, while the range from 440 to 500 ms (in men) or from 460 to 500 ms (in women) is considered a gray zone (5).
ECG changes in black athletes.
Repolarization changes with negative T waves are more common in black athletes than in white athletes.
Findings of imaging studies
Echocardiography
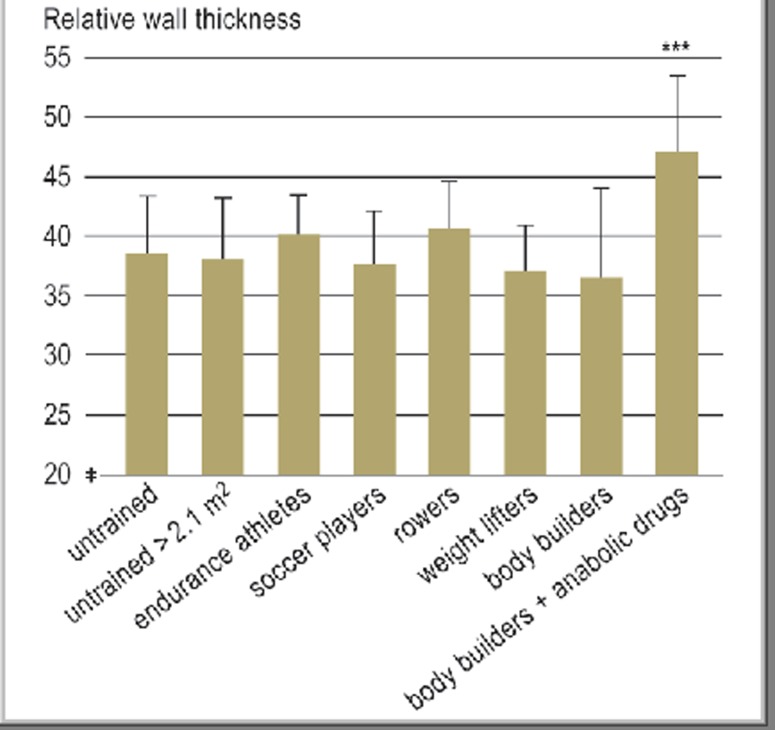
The most important routine method for differentiating physiological from pathological cardiac hypertrophy is echocardiography, which can also be used to determine cardiac volume (e34, e35). The normal cardiac volume depends on body weight and lies in the range of 10–12 mL/kg in men and 9–11 mL/kg in women (Figures 1 and 2) (e36). Athlete’s heart is defined as a heart volume <12 mL/kg body weight in women and 13 mL/kg body weight in men, up to a maximum of 19 and 20 mL/kg, respectively. The end-diastolic diameter of the left ventricle is enlarged in an athlete’s heart (60 mm or more in 15% of athletes), while the left ventricular wall thicknesses are normal or in the upper portion of the normal range (13–15 mm in 2% to 4% of athletes) (Table 1) (15, 16). The left ventricular wall may be 1 or 2 mm thicker in black athletes (15). The clinical relevance of these findings remains unclear. The relative wall thickness, defined as the ratio of left ventricular thickness to end-diastolic inner diameter, normally does not exceed 42–43% in athletes (Figure 3) (10, e37). The left artrium is enlarged in 20% of athletes, with maximum values of 50 mm (men) and 45 mm (women) (e32) (Table 1). Physiological atrial remodeling is closely related to left ventricular dilatation; thus, left atrial enlargement is mainly seen in endurance athletes and strength-endurance athletes (e.g., rowers, canoeists) (e32).
Table 1. Echocardiographic upper limits for athlete’s heart.
| Men | Women | |
| Heart volume (mL/kg) | 20 | 19 |
| Heart mass (g/kg) | 7.5 | 7 |
| LV myocardial mass (g/m2) | ||
| - Devereux (e76) | 165−170 | 130 |
| - Teichholz (e77) | 135 | |
| – Dickhuth (e34) | 137 | |
| LV EDD (mm) | 63 (–67*1) | 60 (–63*1) |
| LV EDD (mm/m2 BSA) | 32 | 33 |
| LV wall thickness (mm) | 13 (–15*2) | 12 |
| Left atrium (mm) | 45 (– 50) | 45 |
| RV EDD (mm) | 32 | |
| RV EDD (mm/m2 BSA) | 17 |
LV, left ventricle; RV, right ventricle; EDD, end-diastolic diameter; BSA, body surface area
*1upper limit for individuals with large body dimensions
*2gray zone: 13–15 mm
Figure 3.
Relative wall thickness (mean and standard deviation) in healthy, athletically untrained persons of varying body size, endurance athletes, soccer players, strength-endurance athletes (rowers), and strength athletes with and without anabolic drug abuse (total, 230 subjects). Only body-builders taking anabolic drugs had concentric cardiac hypertrophy with a significantly elevated relative wall thickness (p < 0.001). m2, body surface area in square meters. Reprinted from (10) with the kind permission of Springer publishers.
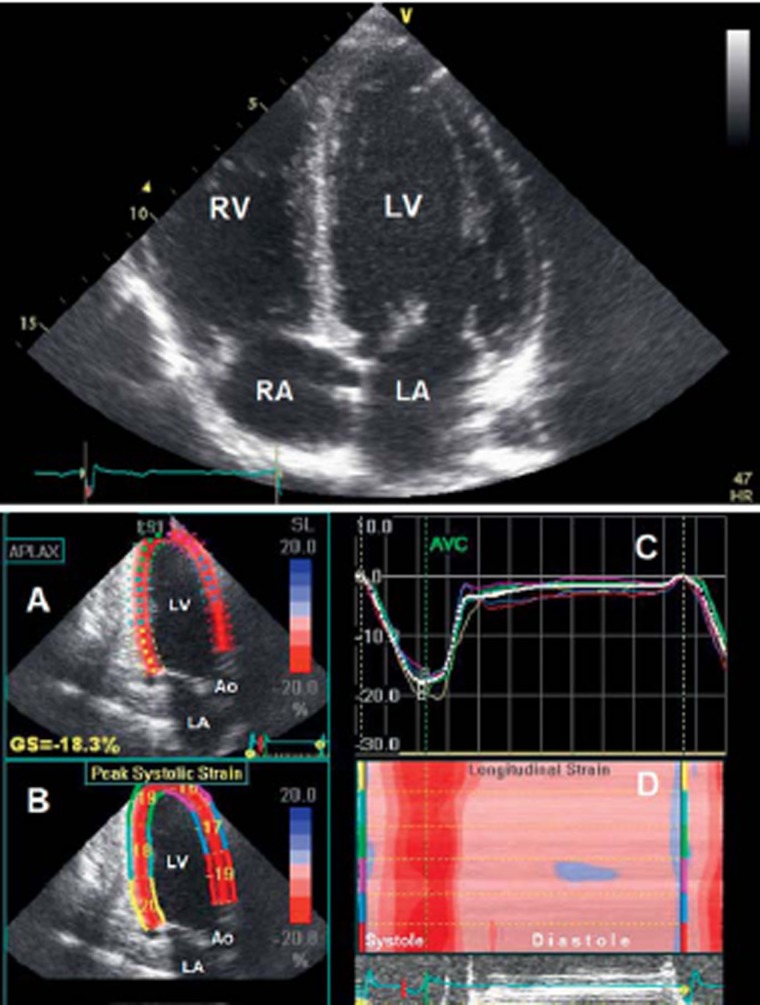
In healthy athletes, systolic function is normal at rest. For those with athlete’s heart, it may be in the low-normal range or even slightly below it, while the stroke volume remains normal (7, 10, e24, e36). Stress echo-cardiography reveals a normal increase in systolic function (10). Diastolic function is normal or in the high-normal range (10, e38). Depending on the type of study and the particular variables measured, regional systolic and diastolic left and right ventricular myocardial function, which can be visualized on the basis of myocardial deformation with tissue Doppler and with two- and three-dimensional speckle tracking (Figure 4), are found to lie in the low- to high-normal range; the same is true of left and right atrial function (e38– e49).
Figure 4.
Above: Echocardiography (four-chamber view) of an athlete’s heart in a male world-class endurance athlete with a heart volume of 19.0 mL/kg body weight. There is a harmonic relationship between the left and right ventricle (LV and RV) and between the left and right atrium (LA and RA).
Below: Echocardiography of an athlete’s heart in a healthy, female, nationally top-ranked endurance athlete with a heart rate of 29 beats per minute at rest.
A: Three-chamber view with wall-motion analysis by 2D speckle tracking.
B: Display of the maximal relative deformation (strain) and shortening of the myocardial segments in systole.
C: The course of relative deformation of the myocardial segments over the cardiac cycle.
D: Display of all analyzed myocardial segments in a three-chamber view (y-axis with color labeling of the segments from B) with synchronous short contraction (red) of all myocardial segments, followed by a long period of relaxation and filling in diastole (pink). LV, left ventricle; LA, left atrium; Ao, aorta
Relative wall thickness in athlete’s heart.
The echocardiographically measured relative wall thickness, defined as the ratio of left ventricular thickness to end-diastolic inner diameter, normally does not exceed 42–43% in athletes.
Echocardiography has often been performed before and immediately after exhausting endurance exercise, or endurance competitions, to determine whether there has been any acute exercise-induced damage to the heart (e50– e52). Such studies generally reveal a mild reduction of diastolic function, and sometimes of systolic function as well, which some authors have called “cardiac fatigue” (e50– e53). However, the validity of such comparisons is limited by the different physiological conditions that prevail before and after exercise (e.g., differences in plasma volume, heart rate, or blood pressure). Prolonged endurance exercise may acutely impair right ventricular function more than left ventricular function (17, 18, e54). In general, the echocardiographically demonstrable acute exercise-induced functional changes in healthy athletes are only transient; unlike those seen in persons with heart disease, they are mild and apparently clinically insignificant (e51, e52, e55).
Function of the athlete’s heart.
In healthy athletes, systolic function is normal at rest. For those with athlete’s heart, it may be in the low-normal range or even slightly below it, while the stroke volume remains normal. Diastolic function is normal or in the high-normal range.
Cardiac magnetic resonance imaging (MRI)
The biventricular, eccentric cardiac hypertrophy characteristic of athlete’s heart, which Reindell originally described on the basis of conventional diagnostic x-rays (2), has been confirmed by cardiac magnetic imaging (MRI) and shown to consist of balanced hypertrophy of the left and right ventricle (7). In strength athletes who do not take anabolic drugs, MRI (like echocardiography) does not reveal concentric hypertrophy of either ventricle (e56, e57). The reported figures for left and right ventricular volume and myocardial mass in athletes cover a wide range because of the widely differing groups of individuals studied and measuring techniques used. These volumes and masses are, however, well correlated with maximal oxygen uptake (VO2max) (7, e58); spiroergometry is, therefore, recommended in unclear cases for an objective assessment of endurance capacity. In this context, it has to be remembered that MRI generally yields higher values than echocardiography for the atrial and ventricular dimensions and lower values for wall thicknesses and muscle masses (19).
45% is considered the lower reference limit of normal for the left and right ventricular systolic ejection fraction in athletes, as measured by MRI (e59). Lower values may be physiological in some cases, particularly in endurance athletes with athlete’s heart.
Right and left ventricular function on MRI.
45% is considered the lower reference limit for the left and right ventricular systolic ejection fraction in athletes, as measured by MRI.
Cardiac biomarkers
Exercise-induced increases in the concentrations of the cardiac biomarkers troponin I and T (TnI, TnT) and B-type natriuretic peptide (BNP and N-terminal proBNP [NT-proBNP]) have been extensively studied in the last decade and a half (20, 21). In non-athletes, these biomarkers are found at pathologically high levels only after myocardial infarction or in congestive heart failure.
Troponin
A meta-analysis of studies involving a total of more than 1000 endurance athletes showed that 47% had an elevation of third-generation TnT concentration beyond the upper reference limit of normal after exhausting endurance exercise (e.g., a marathon or triathlon) (e60). In studies that showed a percentage of TnT-positive athletes in this range, the percentage of TnI-positive athletes was even higher, ca. 75% to 80% (e61– e63). More recent studies employing high-sensitivity troponin tests have revealed comparable or even higher percentages of troponin-positive athletes after exhausting endurance exercise (e62, e63). Presumably, nearly all athletes engaging in such activities have a transient, reversible increase in troponin concentration.
Exercise-induced rises in troponin concentration.
In healthy athletes, elevated troponin levels induced by endurance exercise usually decline markedly within 24 hours and are back in the normal range within 24 to 48 hours (at most, 72 hours).
In myocardial infarction, the troponin concentration increases when bound troponin is released from the tropomyosin complex of necrotic cardiomyocytes. In contrast, the elevation of troponin concentration in athletes is attributed to the release of unbound troponin from the cytoplasmic pool by membrane vesicles, without cell necrosis (20, 21, e64). How this comes about is currently unknown. The putative mechanisms include changes of intracellular metabolism, changes of intracellular calcium concentration that activate intracellular proteases, free-radical effects, and exercise-induced ischemia (20, 21, e51, e55). Further experimental studies are needed to clarify the matter.
BNP and NT-proBNP
BNP and NT-proBNP also rise, particularly after exhausting endurance activities. The percentage of athletes with elevated concentrations of these biomarkers after such activity is in the same range as that mentioned above for troponin. Well-trained marathon runners or those with high training volumes seem to have lower increases of BNP and NT-proBNP concentrations, as well as lower increases of troponin concentration, than less well-trained marathon runners do (17). The elevation of BNP-/NT-proBNP, unlike that of troponin, is affected by the duration of exercise, not just by its intensity (e37).
BNP/NT-proBNP.
Compared to healthy, but athletically untrained individuals, athletes with athlete’s heart do not have elevated BNP-/NT-proBNP concentrations at rest.
Compared to healthy, but athletically untrained persons of like age, athletes with or without athlete’s heart do not have elevated BNP-/NT-proBNP concentrations at rest (20, e66– e68), nor do these concentrations rise to any greater degree than in untrained persons in response to standardized exercise, despite greater ergometric and cardiac performance (e69). The recently reported moderate association between exercise-induced elevation of the BNP or TnI concentration amd transient right ventricular dysfunction after prolonged endurance stress may indicate that endurance exercise has a stronger effect on the right ventricle (17, 18).
The important point for clinical practice with respect to exercise-induced elevation of troponin and BNP-/NT-proBNP concentrations is that a majority of athletes may have a transient elevation after exhausting endurance exercise in the absence of any pathological abnormality. In healthy athletes, the troponin values usually decline markedly within 24 hours and reach normal limits within 24 to 48 hours (at most, 72 hours). Doubtful cases with unclear findings require further cardiological evaluation.
Life expectancy.
The epidemiological data imply that endurance sports prolong life even when they are pursued competitively.
The life expectancy of competitive athletes
The benefits and risks of competitive sports can also be judged in terms of life expectancy. Although there is good evidence for a higher life expectancy among persons who regularly participate in recreational sports (1), there has always been concern that the much more intense, exhausting physical activity demanded of competitive and high-performance athletes might actually shorten their lives (22, e70). A recent review article (23) and editorial (e70) addressed this question by summarizing the findings of 14 (resp. 15) published studies: it was concluded that endurance athletes who achieve national and international success in sports such as long-distance running, cross-country skiing, and bicycle racing, as well as athletes in mixed types of sport, such as soccer, basketball, and ice hockey, have a longer life span than the general population. This, in turn, is largely due to lower cardiovascular mortality, particularly among athletes who continue endurance training even after they stop competing. Strength-based sports, on the other hand, seem not to prolong life (23).
Because of the recent debate over potential danger to the heart from extreme endurance sports, studies on elite endurance athletes whose extensive training and high performance make them likely to have an athlete’s heart (i.e., physiological cardiac remodeling) are presented separately in Table 2 (24, 25, e71, e72). One such study, recently published, is a retrospective assessment of 834 cyclists who took part in the Tour de France bicycle race from 1930 to 1964: 50% of them were still alive at age 82, while 50% of the general population born from 1892 to 1942 was already dead by age 74 (24). It remains an open question, however, whether endurance and high-performance exercise can still be as beneficial as this in the modern era, in which athletes’ life expectancy may be shortened by the cardiovascular and other side effects of doping drugs such as anabolic steroids, growth hormone, and erythropoietin (9, e73).
With respect to amateur endurance sports performed on a competitive level, a study of more than 73 000 participants in the Swedish Vasa cross-country ski race (over 30 and 90 km) from 1989 to 1998 revealed that only 410 of them had died by 1999, a much lower number than the 851 deaths that would have been expected in the general population, after adjustment for age (standardized mortality ratio [SMR] 0.48) (e74). The relative reduction of cardiovascular mortality among participants was 57% (SMR 0.43). In a Dutch study of 2129 men who skated in the single-day Eleven Cities Ice-Skating Tour (with a course of more than 200 km), the observed number of deaths in athletes from any cause over 32 years of observation was 24% lower than would have been expected for the general population (SMR 0.76) (e75).
Conclusion.
Regular athletic activity brings about functional adaptations that improve cardiac function. Athlete’s heart develops only after intensive training with a high endurance component and is not a pathological condition.
Taken together, the foregoing epidemiological data imply that endurance sports prolong life even when they are pursued competitively. The athletes’ healthier lifestyle, with regular physical activity even when their sporting careers are over, is presumably decisive.
Conclusion
Regular athletic activity brings about functional adaptations that improve cardiac function. Athlete’s heart, which develops only after intensive training with a high endurance component, is not a pathological condition and is much rarer than commonly thought. An athlete’s ECG may well reveal certain common, training-related changes which must be distinguished from uncommon, training-unrelated ECG-changes that require further evaluation. The variables measured in cardiac imaging studies exceed the normal range for untrained individuals only in persons who have undergone competitive endurance training. Cardiac function in athlete’s heart is normal at rest and increases appropriately during exercise. The concentrations of the cardiac biomarkers troponin and BNP/NT-proBNP may rise transiently in healthy athletes in response to endurance exercise; this is not currently thought to represent exercise-induced damage to the heart. Epidemiological data show that endurance sports prolong life even when they are pursued at the competitive level.
Table 2. Illustrative findings from studies on the longevity of elite endurance athletes.
| Author / type of sporting activity | Number of athletes | Control group | Athletes | Effect |
| Grimsmo (e71) Cross-country skiing | 122 | 40% dead | 31% dead | 9% lower mortality at the time of the study |
| Karvonen (e72) Cross-country skiing | 396 | Finnish general population, no data about age | 73 years mean life span | 95% CI: life prolonged by 2.8 to 4.3 years |
| Sarna (25) Cross-country skiing,long-distance running | 303 (2613 athletes total) | age attained: 69.9 years 95% CI: 69.0–70.9 (1712 persons) | age attained: 75.6 years 95% CI: 73.6–77.5 (303 persons) | life prolonged by a mean of 5.7 years |
| Sanchis-Gomar (24) Bicycle racing (Tour de France) | 834 | 50% dead by the age of 73.5 years | 50% dead by the age of 81.5 years | 17% prolongation of mean life span |
CI: confidence interval
Please answer the following questions to participate in our certified Continuing Medical Educationprogram. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
Which of the following is a typical feature of athlete’s heart in a male athlete?
heart volume between 10 and 12 mL/kg body weight
septum thickness 17 mm
end-diastolic left ventricular diameter 61 mm
right ventricle not enlarged
diastolic function in low-normal range
Question 2
What ECG change is frequently due to training?
first-degree AV block
left atrial hemiblock
complete right bundle branch block
prolonged QT interval
pre-excitation syndrome
Question 3
What type of arrhythmia is more common among middle-aged and older endurance athletes than in athletically inactive persons of the same age?
supraventricular extrasystoles
ventricular extrasystoles
atrial fibrillation
ventricular tachycardia
third-degree AV block
Question 4
A leisure-time jogger (45 minutes, three times a week) presents for cardiovascular assessment. Which of the following findings requires further evaluation?
incomplete right bundle branch block
end-diastolic left ventricular diameter 52 mm
septum thickness 16 mm
ejection fraction 60%
Sinus bradycardia with a resting heart rate of 55 beats per minute
Question 5
How does the blood pressure change during dynamic exercise?
systolic and diastolic pressures rise as an exponential function of stress intensity
systolic pressure rises exponentially, diastolic pressure rises linearly
diastolic pressure rises exponentially, systolic pressure rises linearly
diastolic pressure rises linearly, systolic pressure barely changes
systolic pressure rises linearly, diastolic pressure barely changes
Question 6
What cardiovascular adaptation is physiological in endurance athletes?
concentric hypertrophy of the left ventricle
increased end-systolic volume of the left ventricle
an up to 20-fold increase of oxygen uptake during excercise
elevated pulmonary-arterial pressure at rest
elevated peripheral vascular resistance at rest
Question 7
Which of the following is true of the blood levels of cardiac biomarkers in healthy endurance athletes with athlete’s heart?
Cardiac troponin levels are elevated.
BNP/NT-proBNP levels are elevated at rest.
Increases in cardiac troponin levels are unlikely in a competitive endurance event.
Well trained endurance athletes have higher exercise-induced elevations of BNP and NT-proBNP.
Exercise-induced elevation of troponin usually reverts to the normal range within 24 to 48 hours.
Question 8
Which of the following findings on echocardiography or MRI would be unusual for an endurance athlete?
low-normal systolic left ventricular function at rest
high-normal diastolic left ventricular function at rest
only slight rise of systolic function in stress echocardiography
harmonic biventricular eccentric hypertrophy on MRI
atrial diameter of 45 mm with athlete’s heart
Question 9
Which of the following ECG findings is attributable to training in an asymptomatic competitive endurance athlete?
evidence of left atrial enlargement
incomplete right bundle branch block
Wolff-Parkinson-White syndrome
long QT syndrome
short QT syndrome
Question 10
There is epidemiological evidence that competitive and high-performance athletes live longer than the population at large. What is the most probable reason for this?
persistently prominent athlete’s heart
regular strength training
regular medical check-ups
regular endurance training even after retirement from competition
moderate nicotine consumption
Further information on CME.
This article has been certified by the North Rhine Academy for Postgraduate and Continuing Medical Education. Deutsches Ärzteblatt provides certified continuing medical education (CME) in accordance with the requirements of the Medical Associations of the German federal states (Länder). CME points of the Medical Associations can be acquired only through the Internet, not by mail or fax, by the use of the German version of the CME questionnaire within 6 weeks of publication of the article. See the following website: cme.aerzteblatt.de
Participants in the CME program can manage their CME points with their 15-digit “uniform CME number” (einheitliche Fortbildungsnummer, EFN). The EFN must be entered in the appropriate field in the cme.aerzteblatt.de website under “meine Daten” (“my data”), or upon registration. The EFN appears on each participant’s CME certificate.
The solutions to the following questions will be published in issue 9/2013. The CME unit “The Treatment of Hallux Valgus” (issue 49/2012) can be accessed until 18 January 2013.
For issue 5/2013, we plan to offer the topic “Postoperative Care and Follow up After Coronary Stenting.”
Solutions to the CME questionnaire in issue 45/2012:5
Kaufmann L, Aster v M: The Diagnosis and Management of Dyscalculia.
Answers: 1c, 2a, 3d, 4d, 5b, 6a, 7e, 8b, 9e, 10c
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
The authors state that no conflict of interest exists.
References
- 1.Löllgen H, Löllgen D. Risikoreduktion kardiovaskulärer Erkrankungen durch körperliche Aktivität. Internist. 2012;53:20–29. doi: 10.1007/s00108-011-2889-1. [DOI] [PubMed] [Google Scholar]
- 2.Reindell H, Klepzig H, Steim H, Musshoff K, Roskamm H, Schildge E. Herz, Kreislaufkrankheiten und Sport. Barth, München. 1960 [Google Scholar]
- 3.Kindermann W, Keul J, Reindell H. Grundlagen zur Bewertung leistungsphysiologischer Anpassungsprozesse. Dtsch Med Wochenschr. 1974;99:1372–1379. doi: 10.1055/s-0028-1107950. [DOI] [PubMed] [Google Scholar]
- 4.Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980-2006. Circulation. 2009;119:1085–1092. doi: 10.1161/CIRCULATIONAHA.108.804617. [DOI] [PubMed] [Google Scholar]
- 5.Corrado D, Pelliccia A, Heidbuchel H, et al. Recommendations for interpretation of 12-lead electrocardiogram in the athlete. Eur Heart J. 2010;31:243–259. doi: 10.1093/eurheartj/ehp473. [DOI] [PubMed] [Google Scholar]
- 6.Paffenbarger RS, Hyde RT, Wing AL, Hsieh CC. Physical activity, all cause mortality, and longevity of college alumni. N Engl J Med. 1986;314:605–613. doi: 10.1056/NEJM198603063141003. [DOI] [PubMed] [Google Scholar]
- 7.Scharhag J, Schneider G, Urhausen A, Rochette V, Kramann B, Kindermann W. Athlete’s heart: right and left ventricular mass and function in male endurance athletes and untrained individuals determined by magnetic resonance imaging. J Am Coll Cardiol. 2002;40:1856–1863. doi: 10.1016/s0735-1097(02)02478-6. [DOI] [PubMed] [Google Scholar]
- 8.Pluim BM, Zwinderman AH, van der Laarse A, van der Wall EE. The athlete’s heart. A meta-analysis of cardiac structure and function. Circulation. 2000;101:336–344. doi: 10.1161/01.cir.101.3.336. [DOI] [PubMed] [Google Scholar]
- 9.Kindermann W. Kardiovaskuläre Nebenwirkungen von anabol-androgenen Steroiden. Herz. 2006;31:566–573. doi: 10.1007/s00059-006-2856-0. [DOI] [PubMed] [Google Scholar]
- 10.Urhausen A, Kindermann W. Sports-specific adaptions and differentiation of the athlete`s heart. Sports Med. 1999;28:237–244. doi: 10.2165/00007256-199928040-00002. [DOI] [PubMed] [Google Scholar]
- 11.Baggish AL, Hutter AM, Wang F, et al. Cardiovascular screening in college athletes with and without electrocardiography: A cross-sectional study. Ann Intern Med. 2010;152:269–275. doi: 10.7326/0003-4819-152-5-201003020-00004. [DOI] [PubMed] [Google Scholar]
- 12.Pelliccia A, Fagard R, Bjørnstad HH, et al. Recommendations for competitive sports participation in athletes with cardiovascular disease: a consensus document from the Study Group of Sports Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005;26:1422–1445. doi: 10.1093/eurheartj/ehi325. [DOI] [PubMed] [Google Scholar]
- 13.Papadakis M, Carre F, Kervio G, et al. The prevalence, distribution, and clinical outcomes of electrocardiographic repolarization patterns in male athletes of African/Afro-Caribbean origin. Eur Heart J. 2011;32:2304–2313. doi: 10.1093/eurheartj/ehr140. [DOI] [PubMed] [Google Scholar]
- 14.Abdulla J, Nielsen JR. Is the risk of atrial fibrillation higher in athletes than in the general population? A systematic review and meta-analysis. Europace. 2009;11:1156–1159. doi: 10.1093/europace/eup197. [DOI] [PubMed] [Google Scholar]
- 15.Basavarajaiah S, Boraita A, Whyte G, et al. Ethnic differences in left ventricular remodeling in highly-trained athletes relevance to differentiating physiologic left ventricular hypertrophy from hypertrophic cardiomyopathy. J Am Coll Cardiol. 2008;51:2256–2262. doi: 10.1016/j.jacc.2007.12.061. [DOI] [PubMed] [Google Scholar]
- 16.Pelliccia A, Maron BJ, Spataro A, Proschan MA, Spirito P. The upper limit of physiologic cardiac hypertrophy in highly trained elite athletes. N Eng J Med. 1991;324:295–301. doi: 10.1056/NEJM199101313240504. [DOI] [PubMed] [Google Scholar]
- 17.Neilan TG, Januzzi JL, Lee-Lewandrowski E, et al. Myocardial injury and ventricular dysfunction related to training levels among nonelite participants in the Boston marathon. Circulation. 2006;114:2325–2333. doi: 10.1161/CIRCULATIONAHA.106.647461. [DOI] [PubMed] [Google Scholar]
- 18.La Gerche A, Burns AT, Mooney DJ, et al. Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur Heart J. 2012;33:998–1006. doi: 10.1093/eurheartj/ehr397. [DOI] [PubMed] [Google Scholar]
- 19.Prakken NHJ, Teske AJ, Cramer MJ, et al. Head-to-head comparison between echocardiography and cardiac MRI in the evaluation of the athlete’s heart. Br J Sports Med. 2012;46:348–354. doi: 10.1136/bjsm.2010.077669. [DOI] [PubMed] [Google Scholar]
- 20.Scharhag J, George K, Shave R, Urhausen A, Kindermann W. Exercise-associated increases in cardiac biomarkers. Med Sci Sports Exerc. 2008;40:1408–1415. doi: 10.1249/MSS.0b013e318172cf22. [DOI] [PubMed] [Google Scholar]
- 21.Shave R, Baggish A, George K, et al. Exercise-Induced Cardiac Troponin Elevation: Evidence, Mechanisms, and Implications. J Am Coll Cardiol. 2010;56:169–176. doi: 10.1016/j.jacc.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 22.Vina J, Sanchis-Gomar F, Martinez-Bello V, Gomez-Cabrera M. Exercise acts as a drug. Pharmacological benefits of exercise. Br J Pharmacol. 2012;167:1–12. doi: 10.1111/j.1476-5381.2012.01970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teramoto M, Bungum TJ. Mortality and longevity of elite athletes. J Sci Med Sport. 2010;13:410–416. doi: 10.1016/j.jsams.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Sanchis-Gomar F, Olaso-Gonzalez G, Corella D, Gomez-Cabrera MC, Vina J. Increased average longevity among the “Tour de France” cyclists. Int J Sports Med. 2011;32:644–647. doi: 10.1055/s-0031-1271711. [DOI] [PubMed] [Google Scholar]
- 25.Sarna S, Sahi T, Koskenvuo M, Kaprio J. Increased life expectancy of world class male athletes. Med Sci Sports Exerc. 1993;25:237–244. [PubMed] [Google Scholar]
- e1.Henschen S. Skilanglauf und Skiwettkampf: Eine medizinische Sportstudie. Fischer, Jena. 1899 [Google Scholar]
- e2.Löllgen H. Herz und Sport. Herz. 2012 doi: 10.1007/s00059-012-3633-x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- e3.Rost R. The athlete’s heart. Historical perspectives. Cardiol Clin. 1992;10:197–207. [PubMed] [Google Scholar]
- e4.Greiner E, Arndt K. Der erste deutsche Sportärztekongress 1912 - Programm für ein Jahrhundert. Dtsch Z Sportmed. 2004;55:310–314. [Google Scholar]
- e5.Kim JH, Malhotra R, Chiampas G, et al. Cardiac arrest during long-distance running races. N Engl J Med. 2012;366:130–140. doi: 10.1056/NEJMoa1106468. [DOI] [PubMed] [Google Scholar]
- e6.Myerson M, Sanchez-Ross M, Sherrid MV. Preparticipation athletic screening for genetic heart disease. Prog Cardiovasc Dis. 2012;54:543–552. doi: 10.1016/j.pcad.2012.03.003. [DOI] [PubMed] [Google Scholar]
- e7.Hoppeler H, Weibel ER. Limits for oxygen and substrate transport in mammals. J Exp Biol. 1998;201:1051–1064. doi: 10.1242/jeb.201.8.1051. [DOI] [PubMed] [Google Scholar]
- e8.MacDougall JD, Tuxen D, Sale DG, Moroz JR, Sutton JR. Arterial blood pressure response to heavy resistance exercise. J Appl Physiol. 1985;58:785–790. doi: 10.1152/jappl.1985.58.3.785. [DOI] [PubMed] [Google Scholar]
- e9.Stickland MK, Welsh RC, Petersen SR, et al. Does fitness level modulate the cardiovascular hemodynamic response to exercise? J Appl Physiol. 2006;100:1895–1901. doi: 10.1152/japplphysiol.01485.2005. [DOI] [PubMed] [Google Scholar]
- e10.Friedman DB, Peel C, Mitchell JH. Cardiovascular responses to voluntary and nonvoluntary static exercise in humans. J Appl Physiol. 1992;73:1982–1985. doi: 10.1152/jappl.1992.73.5.1982. [DOI] [PubMed] [Google Scholar]
- e11.Lee IM, Paffenbarger RS., Jr. Associations of light, moderate, and vigorous intensity physical activity with longevity. The Harvard Alumni Health Study. Am J Epidemiol. 2000;151:293–299. doi: 10.1093/oxfordjournals.aje.a010205. [DOI] [PubMed] [Google Scholar]
- e12.Paffenbarger RS, Jr., Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med. 1993;328:538–545. doi: 10.1056/NEJM199302253280804. [DOI] [PubMed] [Google Scholar]
- e13.Keul J, Dickhuth H, Lehmann M, Staiger J. The athlete’s heart - haemodynamics and structure. Int J Sports Med. 1982;3:33–43. doi: 10.1055/s-2008-1026103. [DOI] [PubMed] [Google Scholar]
- e14.Levy WC, Cerqueira MD, Abrass IB, Schwartz RS, Stratton JR. Endurance exercise training augments diastolic filling at rest and during exercise in healthy young and older men. Circulation. 1993;88:116–126. doi: 10.1161/01.cir.88.1.116. [DOI] [PubMed] [Google Scholar]
- e15.Snell PG, Martin WH, Buckey JC, Blomqvist CG. Maximal vascular leg conductance in trained and untrained men. J Appl Physiol. 1987;62:606–610. doi: 10.1152/jappl.1987.62.2.606. [DOI] [PubMed] [Google Scholar]
- e16.Di Francescomarino S, Sciartilli A, Di Valerio V, Di Baldassarre A, Gallina S. The effect of physical exercise on endothelial function. Sports Med. 2009;39:797–812. doi: 10.2165/11317750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- e17.Rost R, Hollmann W. Athlete`s heart - a review of its historical assessment and new aspects. Int J Sports Med. 1983;4:147–165. doi: 10.1055/s-2008-1026028. [DOI] [PubMed] [Google Scholar]
- e18.Urhausen A, Kindermann W. Echocardiographic findings in strength- and endurance-trained athletes. Sports Med. 1992;13:270–284. doi: 10.2165/00007256-199213040-00004. [DOI] [PubMed] [Google Scholar]
- e19.Urhausen A, Kindermann W. Nichtinvasive Differentialdiagnostik vergrößerter Herzen bei Sporttreibenden. Dtsch Z Sportmed. 1987;38:290–296. [Google Scholar]
- e20.Morganroth J, Maron BJ, Henry WL, Epstein SE. Comparative left ventricular dimensions in trained athletes. Ann Intern Med. 1975;82:521–524. doi: 10.7326/0003-4819-82-4-521. [DOI] [PubMed] [Google Scholar]
- e21.Achar S, Rostamian A, Narayan SM. Cardiac and Metabolic Effects of Anabolic-Androgenic Steroid Abuse on Lipids, Blood Pressure, Left Ventricular Dimensions, and Rhythm. Am J Cardiol. 2010;106:893–901. doi: 10.1016/j.amjcard.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e22.Dickhuth HH, Horstmann T, Staiger J, Reindell H, Keul J. The long-term evolution of physiological cardiomegaly and cardiac hypertrophy. Med Sci Sports Exerc. 1989;21:244–249. [PubMed] [Google Scholar]
- e23.Pelliccia A, Maron B, De Luca R, Di Paolo F, Spataro A, Culasso F. Remodeling of left ventricular hypertrophy in elite athletes after long-term deconditioning. Circulation. 2002;105:944–949. doi: 10.1161/hc0802.104534. [DOI] [PubMed] [Google Scholar]
- e24.Kindermann W. Physiologische Anpassungen des Herz-Kreislauf-Systems an körperliche Belastung. In: Kindermann W, Dickhuth HH, Niess A, Röcker K, Urhausen A, editors. Sportkardiologie. 2nd Edition. Steinkopff Verlag Darmstadt; 2007. pp. 1–20. [Google Scholar]
- e25.Pelliccia A, Maron BJ, Culasso F, et al. Clinical significance of abnormal electrocardiographic patterns in trained athletes. Circulation. 2000;102:278–284. doi: 10.1161/01.cir.102.3.278. [DOI] [PubMed] [Google Scholar]
- e26.Scharhag J. Das Sportler-EKG. Dtsch Z Sportmed. 2007;58:184–185. [Google Scholar]
- e27.Uberoi A, Stein R, Perez MV, et al. Interpretation of the Electrocardiogram of Young Athletes. Circulation. 2011;124:746–757. doi: 10.1161/CIRCULATIONAHA.110.013078. [DOI] [PubMed] [Google Scholar]
- e28.Weiner RB, Hutter AM, Wang F, et al. Performance of the 2010 European Society of Cardiology criteria for ECG interpretation in athletes. Heart. 2011;97:1573–1577. doi: 10.1136/hrt.2011.227330. [DOI] [PubMed] [Google Scholar]
- e29.Wilson MG, Chatard JC, Carre F, et al. Prevalence of electrocardiographic abnormalities in West-Asian and African male athletes. Br J Sports Med. 2012;46:341–347. doi: 10.1136/bjsm.2010.082743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e30.Rawlins J, Carre F, Kervio G, et al. Ethnic differences in physiological cardiac adaptation to intense physical exercise in highly trained female athletes. Circulation. 2010;121:1078–1085. doi: 10.1161/CIRCULATIONAHA.109.917211. [DOI] [PubMed] [Google Scholar]
- e31.Biffi A, Pelliccia A, Verdile L, et al. Long-term clinical significance of frequent and complex ventricular tachyarrhythmias in trained athletes. J Am Coll Cardiol. 2002;40:446–452. doi: 10.1016/s0735-1097(02)01977-0. [DOI] [PubMed] [Google Scholar]
- e32.Pelliccia A, Maron BJ, Di Paolo FM, et al. Prevalence and clinical significance of left atrial remodeling in competitive athletes. J Am Coll Cardiol. 2005;46:690–696. doi: 10.1016/j.jacc.2005.04.052. [DOI] [PubMed] [Google Scholar]
- e33.Mozaffarian D, Furberg CD, Psaty BM, Siscovick D. Physical activity and incidence of atrial fibrillation in older adults: the cardiovascular health study. Circulation. 2008;118:800–807. doi: 10.1161/CIRCULATIONAHA.108.785626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e34.Dickhuth H, Roecker K, Niess A, Hipp A, Heitkamp H. The echocardiographic determination of volume and muscle mass of the heart. Int J Sports Med. 1996;17:132–139. doi: 10.1055/s-2007-972914. [DOI] [PubMed] [Google Scholar]
- e35.Urhausen A, Kindermann W. Ein- und zweidimensionale echokardiographische Herzvolumenbestimmung bei Herzgesunden im Vergleich zur röntgenologischen Methode und zu spiroergometrischen Parametern. Herz/Kreisl. 1987;19:525–528. [Google Scholar]
- e36.Kindermann W. Das Sportherz. Dtsch Z Sportmed. 2000;51:307–308. [Google Scholar]
- e37.Scharhag J. Akute und chronische Effekte von Ausdauerbelastungen auf das Herz bei Sportlern, gesunden Normalpersonen und Patienten. Habilitationsschrift, Universität des Saarlandes. 2009 [Google Scholar]
- e38.George K. Left ventricular diastolic function in athletes. Dtsch Z Sportmed. 2012;63:63–68. [Google Scholar]
- e39.D’Andrea A, Riegler L, Golia E, et al. Range of right heart measurements in top-level athletes: The training impact. Int J Cardiol. 2011 doi: 10.1016/j.ijcard.2011.06.058. E Pub ahead of print. [DOI] [PubMed] [Google Scholar]
- e40.D’Andrea A, Caso P, Bossone E, et al. Right ventricular myocardial involvement in either physiological or pathological left ventricular hypertrophy: an ultrasound speckle-tracking two-dimensional strain analysis. Eur J Echocardiogr. 2010;11:492–500. doi: 10.1093/ejechocard/jeq007. [DOI] [PubMed] [Google Scholar]
- e41.D’Ascenzi F, Cameli M, Zaca V, et al. Supernormal diastolic function and role of left atrial myocardial deformation analysis by 2D speckle tracking echocardiography in elite soccer players. Echocardiography. 2011;28:320–326. doi: 10.1111/j.1540-8175.2010.01338.x. [DOI] [PubMed] [Google Scholar]
- e42.D’Ascenzi F, Cameli M, Padeletti M, et al. Characterization of right atrial function and dimension in top-level athletes: a speckle tracking study. Int J Cardiovasc Imaging. 2012 doi: 10.1007/s10554-012-0063-z. Epud ahead of print. [DOI] [PubMed] [Google Scholar]
- e43.D’Ascenzi F, Cameli M, Lisi M, et al. Left Atrial Remodelling in Competitive Adolescent Soccer Players. Int J Sports Med. 2012 doi: 10.1055/s-0032-1304660. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- e44.De Luca A, Stefani L, Pedrizzetti G, Pedri S, Galanti G. The effect of exercise training on left ventricular function in young elite athletes. Cardiovasc Ultrasound. 2011;9 doi: 10.1186/1476-7120-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e45.Florescu M, Stoicescu C, Magda S, et al. “Supranormal” Cardiac Function in Athletes Related to Better Arterial and Endothelial Function. Echocardiography. 2010;27:659–667. doi: 10.1111/j.1540-8175.2009.01121.x. [DOI] [PubMed] [Google Scholar]
- e46.Krieg A, Scharhag J, Kindermann W, Urhausen A. Cardiac tissue Doppler imaging in sports medicine. Sports Med. 2007;37:15–30. doi: 10.2165/00007256-200737010-00002. [DOI] [PubMed] [Google Scholar]
- e47.Krol W, Braksator W, Kasprzak JD, et al. The influence of extreme mixed exertion load on the right ventricular dimensions and Function in Elite Athletes: A Tissue Doppler Study. Echocardiography. 2011;28:753–760. doi: 10.1111/j.1540-8175.2011.01437.x. [DOI] [PubMed] [Google Scholar]
- e48.Knebel F, Schimke I, Schroeckh S, et al. Myocardial Function in Older Male Amateur Marathon Runners: Assessment by Tissue Doppler Echocardiography, Speckle Tracking, and Cardiac Biomarkers. J Am Soc Echocardiogr. 2009;22:803–809. doi: 10.1016/j.echo.2009.04.009. [DOI] [PubMed] [Google Scholar]
- e49.Scharhag J, Mayer F. BISp-Jahrbuch Forschungsförderung 2010/11 Bundesinstitut für Sportwissenschaft. Bonn: 2011. Kardiale Funktion im Saisonverlauf bei hochtrainierten Ausdauerathleten; pp. 41–44. [Google Scholar]
- e50.George K, Spence A, Naylor LH, Whyte GP, Green DJ. Cardiac adaptation to acute and chronic participation in endurance sports. Heart. 2011;97:1999–2004. doi: 10.1136/heartjnl-2011-300536. [DOI] [PubMed] [Google Scholar]
- e51.Shave R, Oxborough D. Exercise-Induced Cardiac Injury: Evidence From Novel Imaging Techniques and Highly Sensitive Cardiac Troponin Assays. Prog Cardiovasc Dis. 2012;54:407–415. doi: 10.1016/j.pcad.2012.01.007. [DOI] [PubMed] [Google Scholar]
- e52.Scharhag J, Knebel F, Mayer F, Kindermann W. Schadet Marathonlaufen dem Herz? Ein Update. Dtsch Z Sportmed. 2011;62:293–298. [Google Scholar]
- e53.Shave R, George K, Whyte G, Hart E, Middleton N. Postexercise changes in left ventricular function: the evidence so far. Med Sci Sports Exerc. 2008;40:1393–1399. doi: 10.1249/MSS.0b013e318172cf36. [DOI] [PubMed] [Google Scholar]
- e54.Heidbuchel H, La Gerche A. The right heart in athletes. Evidence for exercise-induced arrhythmogenic right ventricular cardiomyopathy. Herzschrittmacherther Elektrophysiol. 2012;23:82–86. doi: 10.1007/s00399-012-0180-3. [DOI] [PubMed] [Google Scholar]
- e55.Kindermann W, Corrado D, Scharhag J. The right heart in athletes. Do we really have sufficient evidence for exercise-induced arrhythmogenic right ventricular cardiomyopathy? Herzschrittmacherther Elektrophysiol. 2012;23:144–145. doi: 10.1007/s00399-012-0207-9. author reply 145-6. [DOI] [PubMed] [Google Scholar]
- e56.Scharhag J, Schneider G, Kürschner R, Urhausen A, Mayer F, Kindermann W. Right and left ventricular mass and function in strength athletes determined by MRI. Med Sci Sports Exerc. 2009;41(Suppl 5) [Google Scholar]
- e57.Spence AL, Naylor LH, Carter HH, et al. A prospective randomised longitudinal MRI study of left ventricular adaptation to endurance and resistance exercise training in humans. J Physiol. 2011;589:5443–5452. doi: 10.1113/jphysiol.2011.217125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e58.Fagard R. Athlete’s heart. Heart. 2003;89:1455–1461. doi: 10.1136/heart.89.12.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e59.Prior DL, La Gerche A. The athlete’s heart. Heart. 2012;98:947–955. doi: 10.1136/heartjnl-2011-301329. [DOI] [PubMed] [Google Scholar]
- e60.Shave R, George K, Atkinson G, et al. Exercise-Induced Cardiac Troponin T release: A Meta-Analysis. Med Sci Sports Exerc. 2007;39:2099–2106. doi: 10.1249/mss.0b013e318153ff78. [DOI] [PubMed] [Google Scholar]
- e61.Mingels A, Jacobs L, Michielsen E, Swaanenburg J, Wodzig W, van Dieijen-Visser M. Reference population and marathon runner sera assessed by highly sensitive cardiac troponin T and commercial cardiac troponin T and I assays. Clin Chem. 2009;55:101–108. doi: 10.1373/clinchem.2008.106427. [DOI] [PubMed] [Google Scholar]
- e62.Saravia SG, Knebel F, Schroeckh S, et al. Cardiac troponin T release and inflammation demonstrated in marathon runners. Clin Lab. 2010;56:51–58. [PubMed] [Google Scholar]
- e63.Scharhag J, Herrmann M, Urhausen A, Haschke M, Herrmann W, Kindermann W. Independent elevations of N-terminal pro-brain natriuretic peptide and cardiac troponins in endurance athletes after prolonged strenuous exercise. Am Heart J. 2005;150:1128–1134. doi: 10.1016/j.ahj.2005.01.051. [DOI] [PubMed] [Google Scholar]
- e64.Hickman PE, Potter JM, Aroney C, et al. Cardiac troponin may be released by ischemia alone, without necrosis. Clin Chim Acta. 2010;411:318–323. doi: 10.1016/j.cca.2009.12.009. [DOI] [PubMed] [Google Scholar]
- e65.Koller A. Exercise-induced increases in cardiac troponins and prothrombotic markers. Med Sci Sports Exerc. 2003;35:444–448. doi: 10.1249/01.MSS.0000053736.51903.0E. [DOI] [PubMed] [Google Scholar]
- e66.Almeida SS, Azevedo A, Castro A, et al. B-type natriuretic peptide is related to left ventricular mass in hypertensive patients but not in athletes. Cardiology. 2002;98:113–115. doi: 10.1159/000066319. [DOI] [PubMed] [Google Scholar]
- e67.Scharhag J, Urhausen A, Herrmann M, et al. No difference in N-terminal pro-brain natriuretic peptide (NT-proBNP) concentrations between endurance athletes with athlete’s heart and healthy untrained controls. Heart. 2004;90:1055–1056. doi: 10.1136/hrt.2003.020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e68.Pagourelias ED, Giannoglou G, Kouidi E, et al. Brain natriuretic peptide and the athlete’s heart: a pilot study. Int J Clin Pract. 2010;64:511–517. doi: 10.1111/j.1742-1241.2009.02184.x. [DOI] [PubMed] [Google Scholar]
- e69.Scharhag J, Kindermann W. Bundesinstitut für Sportwissenschaft. Bonn: 2007. Verhalten der kardialen Marker BNP und Troponin nach standardisierten Belastungen bei Leistungssportlern mit und ohne Sportherz BISp-Jahrbuch Forschungsförderung 2006/2007; pp. 73–78. [Google Scholar]
- e70.Ruiz JR, Moran M, Arenas J, Lucia A. Strenuous endurance exercise improves life expectancy: it’s in our genes. Br J Sports Med. 2011;45:159–161. doi: 10.1136/bjsm.2010.075085. [DOI] [PubMed] [Google Scholar]
- e71.Grimsmo J, Maehlum S, Moelstad P, Arnesen H. Mortality and cardiovascular morbidity among long-term endurance male cross country skiers followed for 28-30 years. Scand J Med Sci Sports. 2011;21:e351–e358. doi: 10.1111/j.1600-0838.2011.01307.x. [DOI] [PubMed] [Google Scholar]
- e72.Karvonen MJ, Klemola H, Virkajarvi J, Kekkonen A. Longevity of endurance skiers. Med Sci Sports. 1974;6:49–51. [PubMed] [Google Scholar]
- e73.Baggish AL, Weiner RB, Kanayama G, et al. Long-term anabolic-androgenic steroid use is associated with left ventricular dysfunction. Circ Heart Fail. 2010;3:472–476. doi: 10.1161/CIRCHEARTFAILURE.109.931063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e74.Farahmand BY, Ahlbom A, Ekblom O, et al. Mortality amongst participants in Vasaloppet: a classical long-distance ski race in Sweden. J Intern Med. 2003;253:276–283. doi: 10.1046/j.1365-2796.2003.01122.x. [DOI] [PubMed] [Google Scholar]
- e75.van Saase JL, Noteboom WM, Vandenbroucke JP. Longevity of men capable of prolonged vigorous physical exercise: a 32 year follow up of 2259 participants in the Dutch eleven cities ice skating tour. BMJ. 1990;301:1409–1411. doi: 10.1136/bmj.301.6766.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e76.Devereux R, Alonso D, Lutas E, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- e77.Teichholz L, Kreulen T, Herman M, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence or absence of asynergy. Am J Cardiol. 1976;37:7–11. doi: 10.1016/0002-9149(76)90491-4. [DOI] [PubMed] [Google Scholar]