Abstract
To better understand the energetics of accurate DNA replication, we directly measured ΔGO for the incorporation of a nucleotide into elongating dsDNA in solution (ΔGOincorporation). Direct measurements of the energetic difference between synthesis of correct and incorrect base pairs found it to be much larger than previously believed (average ΔΔGOincorporation = 5.2±1.34 kcal mol−). Importantly, these direct measurements indicate that ΔΔGOincorporation alone can account for the energy required for highly accurate DNA replication. Evolutionarily, these results indicate that the earliest polymerases did not have to evolve sophisticated mechanisms to replicate nucleic acids, they may have only had to take advantage of the inherently more favorable ΔGO for polymerization of correct nucleotides. These results also provide a basis for understanding how polymerases replicate DNA (or RNA) with high fidelity.
A hallmark of DNA replication is its low error frequency. Replicative DNA polymerases accurately copy the cell’s genome, discriminating between four chemically similar substrates (dATP, dCTP, dTTP, and dGTP) during each polymerization event. In the absence of proofreading exonucleases, these enzymes typically make a mistake only once every 1,000 to 1,000,000 incorporation events1. While it has been well documented that different polymerases use different mechanisms to achieve their accuracy2–6, how they obtain the energy to so effectively differentiate between right and wrong nucleotides has remained unclear5,7–9. The prevailing hypothesis posits that the energy difference between correct and incorrect base pair formation is small and the polymerase must, therefore, greatly amplify this difference to attain high levels of fidelity1,7–12. However, this idea derives from studies that approximated the ΔΔGO (ca. 0.2–3 kcal mol−1) between right and wrong base pairs using the melting profiles of duplex DNA10,13. We have now directly measured ΔGO for the incorporation of a nucleotide (ΔGOincorporation). These studies showed that the ΔΔGO for forming correct versus incorrect base pairs is large (ΔΔGOincorporation ranges from 3.52±0.80 to 6.98±0.17 kcal mol−1 (mean = 5.2±1.34 kcal mol−1)). Thus, the energetics of base pairing can account for an average misincorporation frequency of <10−3 per nucleotide polymerized without any amplification of ΔΔGOincorporation, discrimination comparable to the level achieved by high-fidelity polymerases.
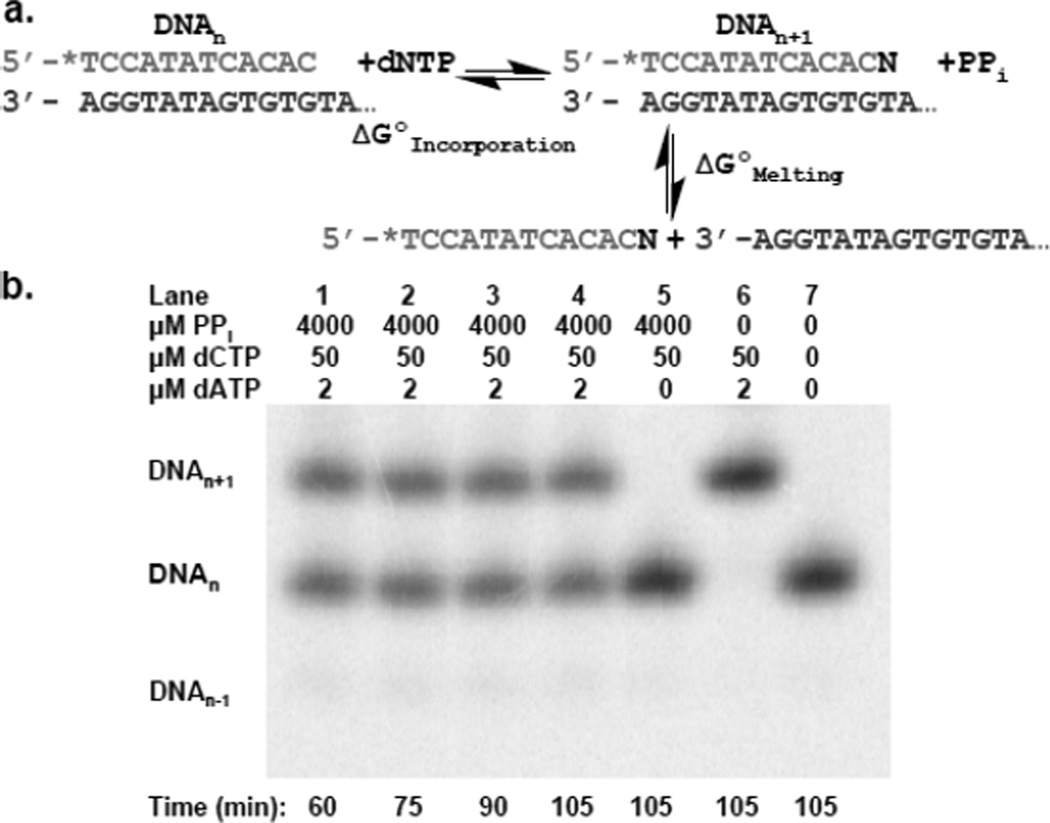
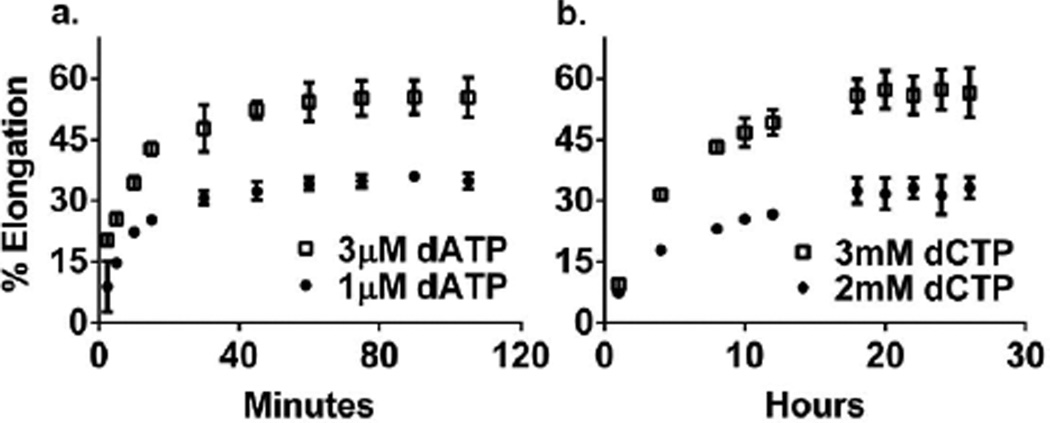
We measured ΔGO for polymerization of a correct dNTP (ΔGOincorporation) for each correct incorporation event (Figure 1a, Table 1). Reactions containing 5’-[32P]-DNAn, the next correct dNTP needed for elongation of DNAn into DNAn+1, pyrophosphate and a trace amount of an exonuclease-deficient DNA polymerase were allowed to reach equilibrium (~60 min (Figure 2a)). ΔGO values were always obtained at three different dNTP concentrations to ensure their accuracy and reproducibility. In contrast to previous studies that measured ΔGO when the DNA was bound to the polymerase4,14,15, we used a large excess of DNA such that the polymerase acted only as a catalyst – i.e., measured ΔGOincorporation for the reaction in solution. To avoid shortening of DNAn via pyrophosphorolysis, the reactions always contained ~50 µM of the dNTP present at the 3’ terminus (the nth position) of DNAn. This concentration sufficed to prevent shortening of the DNAn but did not result in the misincorporation of this dNTP into the n+1 position (See below and Figure 1b, lane 5). The ΔGOincorporation for correct dNTP polymerization ranged from −4.3±0.06 to −6.2±0.10 kcal mol−1 and the average ΔGOincorporation was −5.2 ± 0.4 kcal mol−1. The ΔGO for the polymerization of an incorrect dNTP (ΔGOmisincorporation) was determined for all 12 possible misincorporation events (Table 1), and ranged from 1.52±0.27 to −1.57±0.79 kcal mol−1 with an average ΔGOmisincorporation of 0.13±1.28 kcal mol−1. These reactions differed from those for correct incorporation in that they required ~18 hours to attain equilibrium (Figure 2b) due to the slower rate of misincorporation, they contained higher concentrations of the incorrect dNTP and the template sequences were constructed so as not to require a second dNTP to prevent shortening of the DNAn via pyrophosphorolysis. In the absence of pyrophosphate, both correct and incorrect incorporation reactions were able to proceed to completion (full extension of DNAn to DNAn+1) over the time course of the experiment (Figure 1b, lane 6 and Figure S1, lane 2). With each misincorporation reaction, we observed that the percentage of DNAn that was elongated to DNAn+1 did not change significantly after 18 hours (example shown in Figure 2b) indicating that the DNAn ↔ DNAn+1 reaction had reached equilibrium. Additionally, after 18 hours addition of the correct dNTP (1mM) for conversion of any remaining DNAn to DNAn+1 followed by a 1 hour incubation period resulted in complete extension of any remaining DNAn into DNAn+1, indicating that the enzyme was still active (data not shown).
Figure 1.
DNAn ↔ DNAn+1 reaction. a) Pictorial depiction of ΔGOincorporation versus ΔGOmelting. * indicates [32P]-phosphate. b) Gel of correct incorporation of dATP into Primer C/DNAt. 50 µM dTTP prevents pyrophosphorolysis of DNAn to DNAn−1.
Table1.
Primer-Template Sequences
| Primer-Template | Incorporation Event |
ΔGO kcal/mol |
ΔΔGO kcal/mol |
|---|---|---|---|
| Primer T/DNAt | |||
| TCCATATCACAT | A → T | −4.68±0.10 | |
| AGGTATAGTGTATGTCTTATCATCT | T → T | +0.52±0.15 | 5.20±0.18 |
| Primer T/DNAt (BF) | |||
| TCCATATCACAT | A → T | −4.88±0.15 | |
| AGGTATAGTGTATGTCTTATCATCT | T → T | +0.96±0.04 | 5.84±0.16 |
| Primer T/DNAt (KF) | |||
| TCCATATCACAT | A → T | −4.97±0.17 | |
| AGGTATAGTGTATGTCTTATCATCT | T → T | N/A | N/A |
| Primer C/DNAt | |||
| TCCATATCACAC | A → T | −4.64±0.10 | |
| AGGTATAGTGTGTATCTTATCATCT | C → T | −0.11±0.17 | 4.54±0.20 |
| Primer G/DNAt | |||
| TCCATATCACCG | A → T | −5.12±0.16 | |
| AGGTATAGTGGCTATCTTATCATCT | G → T | −0.32±0.59 | 4.81±0.62 |
| Primer T/DNAc | |||
| TCCATATCACAT | G → C | −5.08±0.14 | |
| AGGTATAGTGTACTTCTTATCATCT | T → C | −0.55±0.13 | 4.52±0.19 |
| Primer C/DNAc | |||
| TCCATATCACAC | G → C | −5.73±0.11 | |
| AGGTATAGTGTGCTTCTTATCATCT | C → C | +0.81±0.12 | 6.54±0.16 |
| Primer A/DNAc | |||
| TCCATATCACGA | G → C | −6.20±0.10 | |
| AGGTATAGTGCTCAACTTATCATCT | A → C | −0.61±0.12 | 5.58±0.16 |
| Primer T/DNAg | |||
| TCCATATCACAT | C → G | −5.09±0.08 | |
| AGGTATAGTGTAGTTCTTATCATCT | T → G | −1.57±0.79 | 3.52±0.80 |
| Primer A/DNAg | |||
| TCCATATCACGA | C → G | −6.04±0.04 | |
| AGGTATAGTGCTGAACTTATCATCT | A → G | +0.95±0.17 | 6.98±0.17 |
| Primer G/DNAg | |||
| TCCATATCACCG | C → G | −5.78±0.20 | |
| AGGTATAGTGGCGAACTTATCATCT | G → G | +0.18±0.55 | 5.96±0.58 |
| Primer C/DNAa | |||
| TCCATATCACAC | T → A | −4.30±0.06 | |
| AGGTATAGTGTGATTCTTATCATCT | C → A | +0.19±0.29 | 4.49±0.29 |
| Primer A/DNAa | |||
| TCCATATCACGA | T → A | −4.86±0.09 | |
| AGGTATAGTGCTAGGCTTATCATCT | A → A | −1.14±0.29 | 3.73±0.31 |
| Primer G/DNAa | |||
| TCCATATCACCG | T → A | −4.63±0.08 | |
| AGGTATAGTGGCATTCTTATCATCT | G → A | +1.52±0.27 | 6.15±0.28 |
Average results of two independent experiments are displayed with the estimated error (±standard deviation). Within each experiment, ΔG° was determined at three different dNTP concentrations in quadruplicate. The underlined base is the templating position. BF denotes that Bacillus stearothermophilus Large Fragment was used. KF denotes that Klenow Fragment (3’→5’ exo−) was used. VentR (exo−) DNA Polymerase was used in all other cases.
Figure 2.
Time course of Primer C/DNAt elongation. a) Correct incorporation of 1 µM and 3 µM dATP. b) Misincorporation of 2 mM and 3 mM dCTP. All assays contained 4 mM pyrophosphate. Average results of two independent experiments are displayed with the estimated error (±standard deviation).
The sequences of the primer-templates used to measure misincorporation were designed to prevent net pyrophosphorolysis of the primer strand (DNAn) during the long incubations required to allow the reactions to reach equilibrium. Both the misincorporated nucleotide (i.e., at the n+1 position) and the nth nucleotide of primer strand were identical1. Thus, if pyrophosphorolysis of the nucleotide at the primer terminus occurred, a relatively high concentration of this just removed dNTP was present, thereby allowing the polymerase to immediately replace the terminal nucleotide. Ultimately, this approach succeeds because ΔGO for a correct incorporation reaction is much more negative than ΔGO for a misincorporation reaction.
We used three different exonuclease deficient polymerases from two different evolutionary families to demonstrate that the enzyme acts only as a catalyst and does not affect ΔGOincorporation. Bacillus stearothermophilus Large Fragment (BF, an A family enzyme), VentR (exo−) DNA Polymerase (a B family enzyme), and Klenow Fragment (KF (exo−), an A family enzyme) were compared using Primer T/DNAt. All three enzymes gave similar ΔGOincorporation values for correct incorporation of dATP opposite a templating T (Table 1). Only the thermostable enzymes, BF and VentR, could be compared for misincorporation of dTTP opposite the templating T due to the 18 hour incubation required to achieve equilibrium at 37°C. Again, similar ΔGOmisincorporation values were measured with both polymerases (Table 1). Together, these data indicate that ΔGOincorporation is polymerase independent, as one would predict for the polymerase acting as a catalyst.
The ΔΔGOincorporation between right and wrong dNTPs varied from 3.52±0.80 to 6.98±0.17 kcal mol−1 with an average ΔΔGOincorporation of 5.2±1.34 kcal mol−1, enough energy on average to account for misincorporation frequencies <10−3 per nucleotide polymerized and close to those observed with high fidelity polymerases10. Thus, DNA polymerases could achieve high fidelity with little, if any, amplification of ΔΔGOincorporation.
To determine if the large ΔΔGOincorporation is independent of primer-template length, we compared polymerization of a correct (dTTP) and incorrect (dCTP) nucleotide using two DNAs with different duplex lengths but identical sequences around the polymerization site, Primer Clong/DNAa (a 27 base pair duplex) and Primer C/DNAa, (a 12 base pair duplex). The incorporation of dTTP and dCTP opposite a template A yielded a ΔΔGOincorporation of 4.37±0.13 kcal mol−1 on Primer Clong/DNAa, very similar to the ΔΔGOincorporation of 4.49±0.29 kcal mol−1 on Primer C/DNAa (Table 1 and Table S1). Thus, the large ΔΔGOincorporation is independent of template length for identical sequence contexts.
We measured the correct incorporation events within the context of three different sequences to ask if sequence could affect ΔGOincorporation. Comparing these values showed that while sequence affected ΔGOincorporation by up to 1.1 kcal mol−1 the values were always highly negative and a large ΔΔGOincorporation between right and wrong dNTPs was always observed (Table 1). Elucidating the cause of this sequence dependence of ΔGOincorporation will, however, require a much more extensive investigation.
To provide insights into the importance of Watson-Crick hydrogen bonding during dNTP polymerization, we examined Primer C/DNAabasic1. This DNA is identical to Primer C/DNAt except the T in the templating position has been replaced by an abasic site (Table S1). Unlike the generation of a correct base pair, only phosphodiester bond formation and stacking of the base from the incoming dNTP can drive incorporation. Polymerization of purine dNTPs was significantly more favorable than polymerization of pyrimidine dNTPs (by ~1.8 kcal mol−1) consistent with stacking of purines being more favorable than stacking of pyrimidines (Table S1) and as predicted by the differing stacking potentials of the bases16. Similar results were obtained with a DNA that contained 4 consecutive abasic sites, indicating that the identity of the templating nucleotide at the n+2 position does not affect dNTP incorporation opposite an abasic site at the n+1 position (Table S1, Primer C/DNAabasic4). The lack of a templating base resulted in a much less favorable ΔGOincorporation than when the correct templating base was present. Potentially, this could result either from the lack of Watson-Crick hydrogen bonds and/or altered stacking interactions of the template base at the n+1 and/or n+2 position upon dNTP incorporation. We suspect that hydrogen bonding and base stacking are intrinsically linked; if a base pair can form Watson-Crick hydrogen bonds it will help position the bases for optimum base stacking, and the stacking of bases will likewise favorably align the base pair for hydrogen bonding.
These data show that the ΔΔGO between right and wrong base pair formation in DNA is much larger than previously believed and is sufficient to account for most, but not quite all, of the discrimination exhibited by high fidelity polymerases. This contrasts with current dogma, which postulates that polymerases must greatly amplify ΔΔGOincorporation to achieve high fidelity1,7–12. However, this model is based upon melting profiles of dsDNA containing matched or mismatched base-pairs at the 3’-terminus of a primer-template10,13 (i.e., ΔGOmelting (Figure 1a)) rather than from direct measurements of ΔGOincorporation. Why, however, should these melting studies give such different results than direct measurement of ΔΔGOincorporation? DNA melting is a highly cooperative process, and previous studies have shown that the effect of a mismatch is very position dependent17,18. If the mismatch is placed in the middle of a DNA duplex as opposed to near one end, ΔGOmelting is much more greatly altered, raising the question of whether melting profiles are the best way to determine the energetics for the generation of new base pairs, as occurs during DNA synthesis (i.e., ΔGOincorporation (Figure 1a))10,19. The smaller effect of a mismatch at the primer terminus likely results from the mismatch at the primer terminus only disrupting one neighboring stacking interaction, whereas an internal mismatch disrupts two stacking interactions (one on either side of the mismatch). Previous studies have shown that stacking interactions, even in the absence of Watson-Crick hydrogen bonding, have a significant impact on DNA melting thermodynamics20.
Evolutionarily, this large ΔΔGO may have simplified the fidelity problem for the first nucleotide polymerases. Rather than having to develop sophisticated mechanisms to accurately replicate nucleic acids, they could have taken advantage of the much greater stability of correct base pairs. The more favorable binding of a correct dNTP to a templating base would favor the synthesis of correct base pairs opposite a nucleic acid template.
However, in terms of today’s enzymes and thinking about how polymerases obtain fidelity, several issues must be considered. First, DNA synthesis inside of a cell operates under non-equilibrium conditions since one of the products, PPi, is rapidly destroyed by pyrophosphatase21. Second, polymerases generally synthesize DNA quite rapidly (> 1000 nucleotides s−1 in some cases9), and it is unlikely that allowing a reaction to reach equilibrium on an enzyme could accommodate rapid DNA synthesis. Assuming the enzyme can “harvest” this ΔΔGO, it could be expressed at any stage of the reaction cycle (dNTP binding, chemistry, etc.) and this could vary for different enzymes, as one observes when comparing how different polymerases discriminate against wrong dNTPs2–6,12,22–24 Recent simulations of ΔΔGO of transition state binding between correct and incorrect bases within the DNA polymerase β active site are within the range of our ΔΔGOincorporation observations (~5 kcal/mol)25. In light of these constraints, polymerases may well have developed catalytic strategies to amplify the ΔΔGO between right and wrong base pairs. Finally, it remains to be seen if the different structures of DNA/RNA and RNA/RNA duplexes provide different base pairing energetics, thus requiring polymerases that generate these duplexes to adopt different catalytic strategies.
Supplementary Material
AKNOWLEGEMENTS
This work was supported by NIH grant AI59764
Footnotes
The conditions required to measure ΔGOmisincorporation (high PPi concentrations and long incubation times) could result in substantial pyrophosphorolysis of DNAn. Avoiding this problem required that the nucleotide at the primer 3’ terminus (the nth position) of DNAn be the same as the nucleotide for which we measured misincorporation at the n+1 position For example, misincorporation of only dCTP could be measured with Primer C/DNAt (Table 1). If the nth nucleotide were removed via pyrophosphorolysis, the high level of dCTP in combination with the favorable ΔGO of correct dNTP polymerization ensured it was rapidly replaced. If the nth nucleotide were different (Ex., A) than the misincorporated nucleotide (dCTP), the high levels of dATP needed to replace an A removed via pyrophosphorolysis would have competed with dCTP during misincorporation.
ASSOCIATED CONTENT
Supporting Information Available
Supporting information including the abbreviations used, the experimental details and the data for polymerization of dNTPs opposite an abasic site and on longer templates is available. This information is available free of charge via theinternet at http://pubs.acs.org.
REFERENCES
- 1.Kunkel TA, Bebenek K. Annual review of biochemistry. 2000;69:497. doi: 10.1146/annurev.biochem.69.1.497. [DOI] [PubMed] [Google Scholar]
- 2.Capson TL, Peliska JA, Kaboord BF, Frey MW, Lively C, Dahlberg M, Benkovic SJ. Biochemistry. 1992;31:10984. doi: 10.1021/bi00160a007. [DOI] [PubMed] [Google Scholar]
- 3.Kuchta RD, Benkovic P, Benkovic SJ. Biochemistry. 1988;27:6716. doi: 10.1021/bi00418a012. [DOI] [PubMed] [Google Scholar]
- 4.Kuchta RD, Mizrahi V, Benkovic PA, Johnson KA, Benkovic SJ. Biochemistry. 1987;26:8410. doi: 10.1021/bi00399a057. [DOI] [PubMed] [Google Scholar]
- 5.McCulloch SD, Kunkel TA. Cell research. 2008;18:148. doi: 10.1038/cr.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong I, Patel SS, Johnson KA. Biochemistry. 1991;30:526. doi: 10.1021/bi00216a030. [DOI] [PubMed] [Google Scholar]
- 7.Echols H, Goodman MF. Annual review of biochemistry. 1991;60:477. doi: 10.1146/annurev.bi.60.070191.002401. [DOI] [PubMed] [Google Scholar]
- 8.Goodman MF, Creighton S, Bloom LB, Petruska J. Critical reviews in biochemistry and molecular biology. 1993;28:83. doi: 10.3109/10409239309086792. [DOI] [PubMed] [Google Scholar]
- 9.Berdis AJ. Chemical reviews. 2009;109:2862. doi: 10.1021/cr800530b. [DOI] [PubMed] [Google Scholar]
- 10.Petruska J, Goodman MF, Boosalis MS, Sowers LC, Cheong C, Tinoco I., Jr Proceedings of the National Academy of Sciences of the United States of America. 1988;85:6252. doi: 10.1073/pnas.85.17.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodman MF. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:10493. doi: 10.1073/pnas.94.20.10493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joyce CM, Benkovic SJ. Biochemistry. 2004;43:14317. doi: 10.1021/bi048422z. [DOI] [PubMed] [Google Scholar]
- 13.Loeb LA, Kunkel TA. Annual review of biochemistry. 1982;51:429. doi: 10.1146/annurev.bi.51.070182.002241. [DOI] [PubMed] [Google Scholar]
- 14.Dahlberg ME, Benkovic SJ. Biochemistry. 1991;30:4835. doi: 10.1021/bi00234a002. [DOI] [PubMed] [Google Scholar]
- 15.Eger BT, Benkovic SJ. Biochemistry. 1992;31:9227. doi: 10.1021/bi00153a016. [DOI] [PubMed] [Google Scholar]
- 16.Guckian KM, Schweitzer BA, Ren RX, Sheils CJ, Tahmassebi DC, Kool ET. Journal of the American Chemical Society. 2000;122:2213. doi: 10.1021/ja9934854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo Z, Liu Q, Smith LM. Nature biotechnology. 1997;15:331. doi: 10.1038/nbt0497-331. [DOI] [PubMed] [Google Scholar]
- 18.Piao X, Sun L, Zhang T, Gan Y, Guan Y. Acta biochimica Polonica. 2008;55:713. [PubMed] [Google Scholar]
- 19.Aboul-ela F, Koh D, Tinoco I, Jr, Martin FH. Nucleic acids research. 1985;13:4811. doi: 10.1093/nar/13.13.4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bommarito S, Peyret N, SantaLucia J., Jr Nucleic acids research. 2000;28:1929. doi: 10.1093/nar/28.9.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kornberg A, Rao NN, Ault-Riche D. Annual review of biochemistry. 1999;68:89. doi: 10.1146/annurev.biochem.68.1.89. [DOI] [PubMed] [Google Scholar]
- 22.Frey MW, Sowers LC, Millar DP, Benkovic SJ. Biochemistry. 1995;34:9185. doi: 10.1021/bi00028a031. [DOI] [PubMed] [Google Scholar]
- 23.Johnson KA. Annual review of biochemistry. 1993;62:685. doi: 10.1146/annurev.bi.62.070193.003345. [DOI] [PubMed] [Google Scholar]
- 24.Purohit V, Grindley ND, Joyce CM. Biochemistry. 2003;42:10200. doi: 10.1021/bi0341206. [DOI] [PubMed] [Google Scholar]
- 25.Rucker R, Oelschlaeger P, Warshel A. Proteins. 2010;78:671. doi: 10.1002/prot.22596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.