Abstract
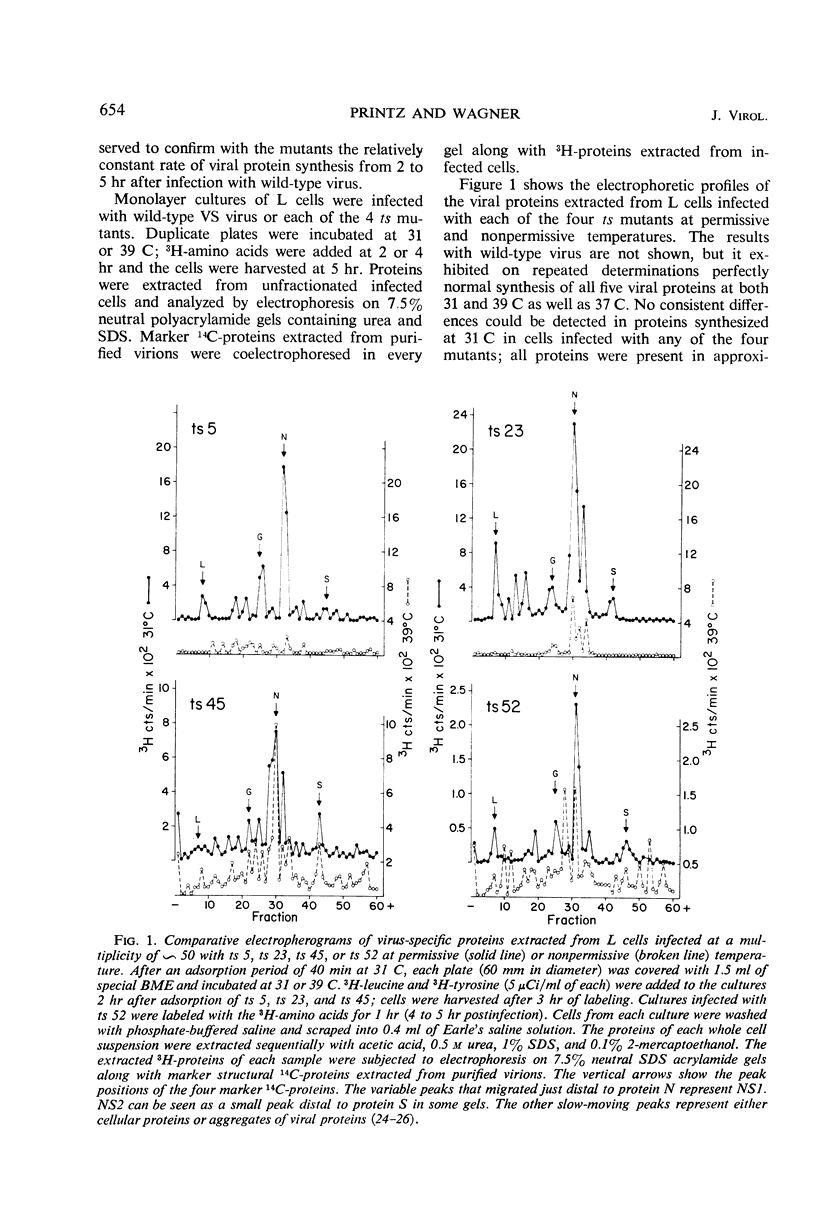
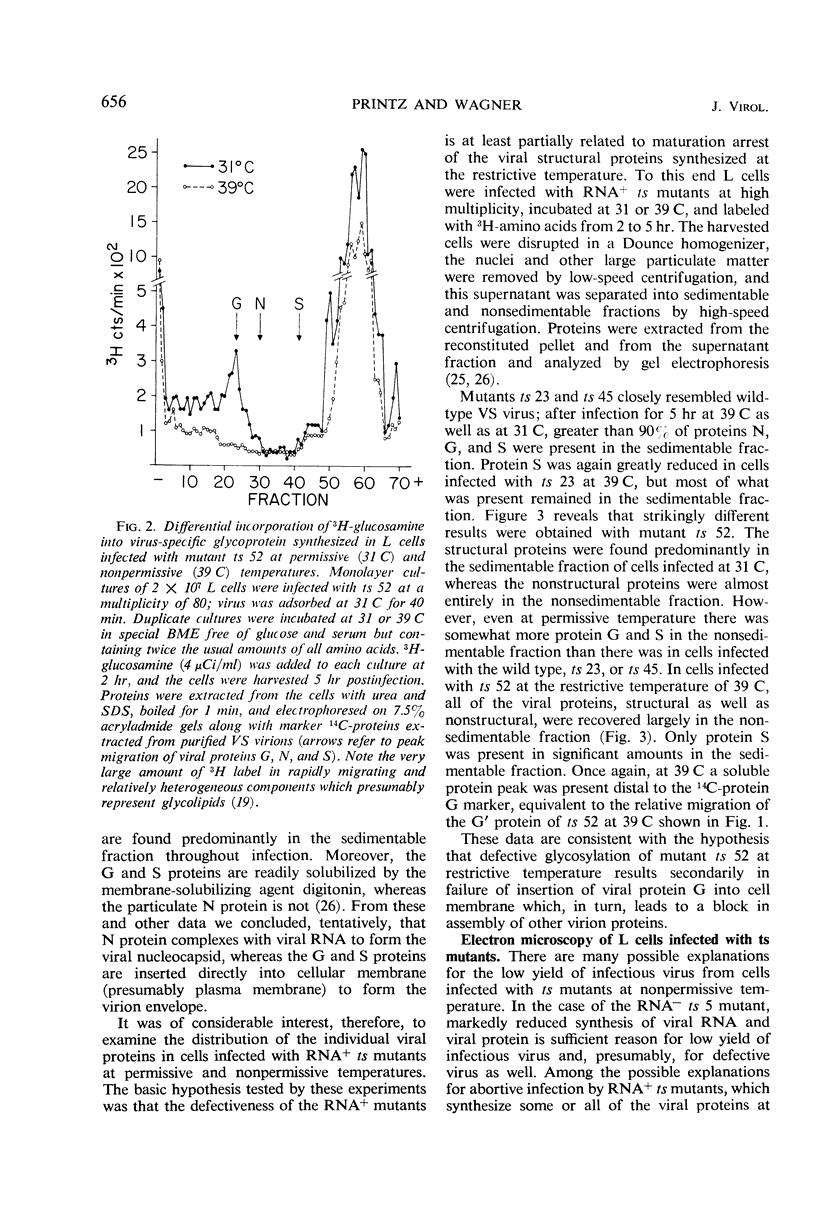
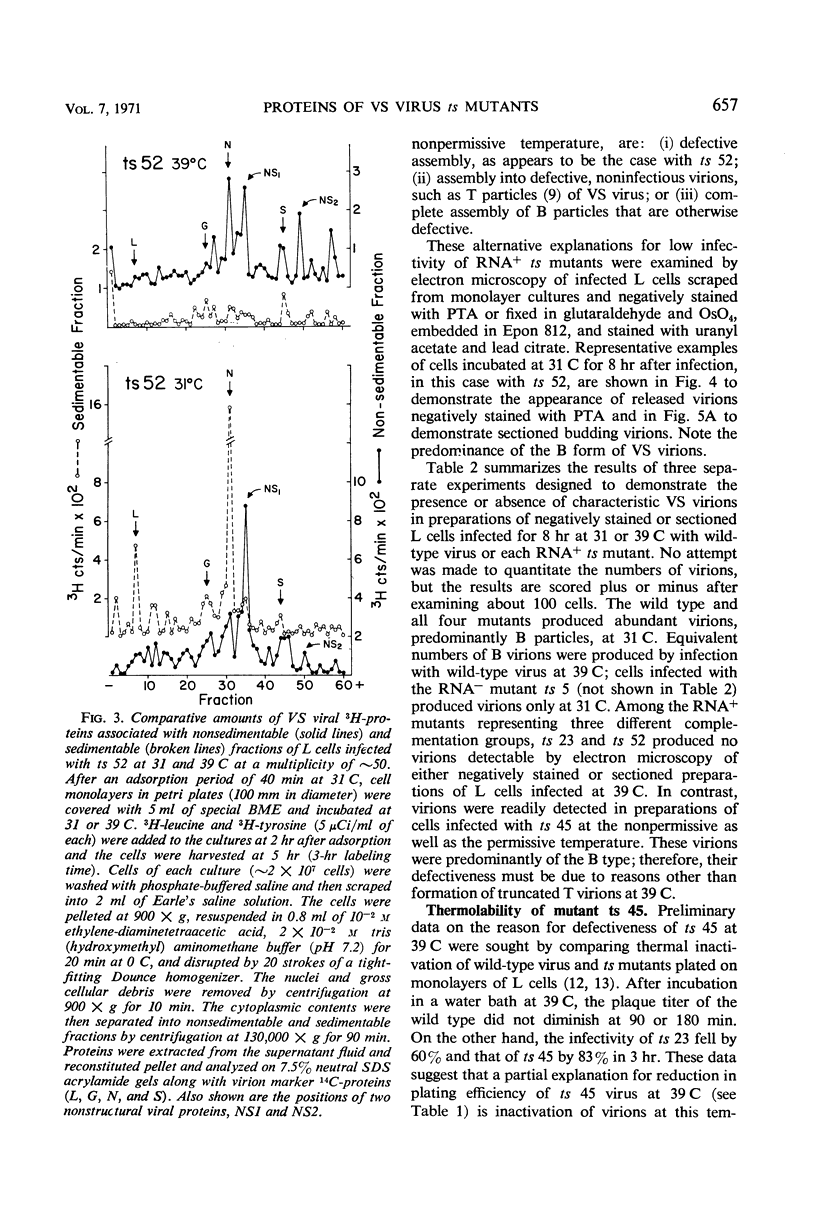
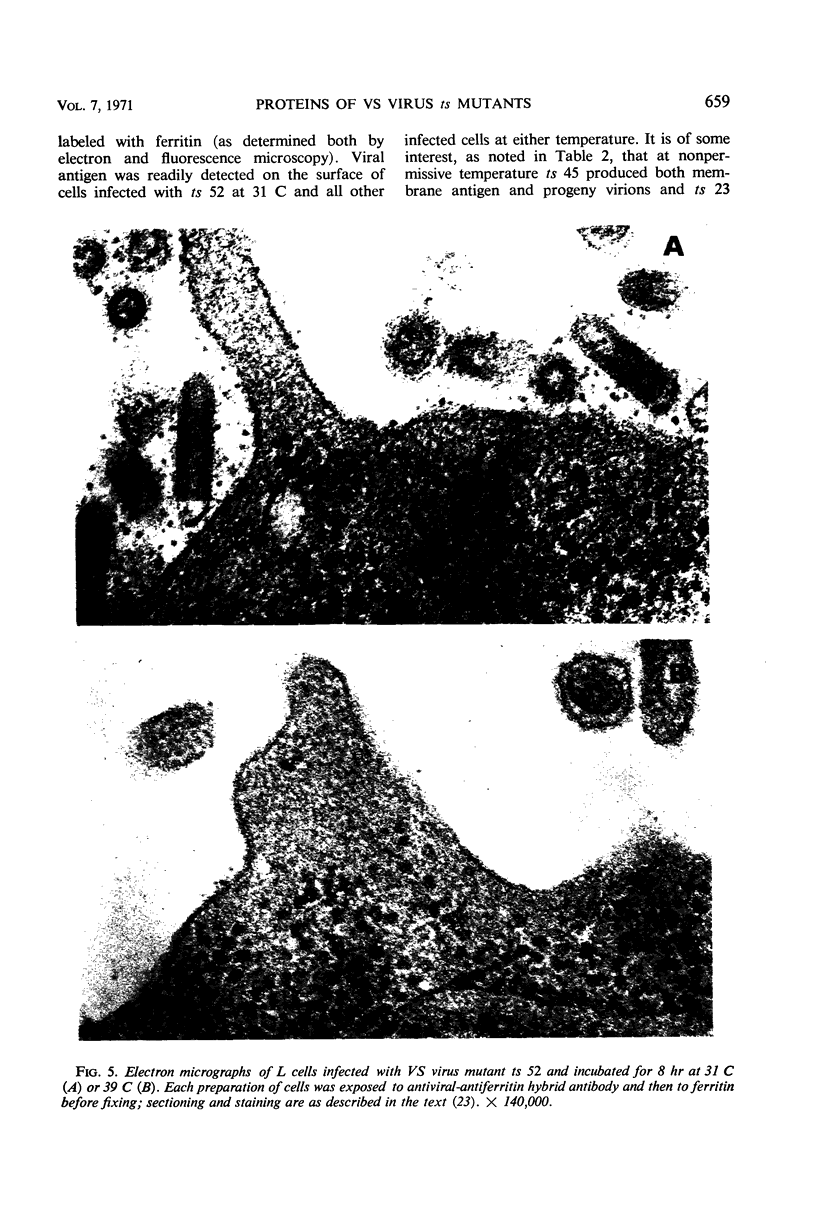
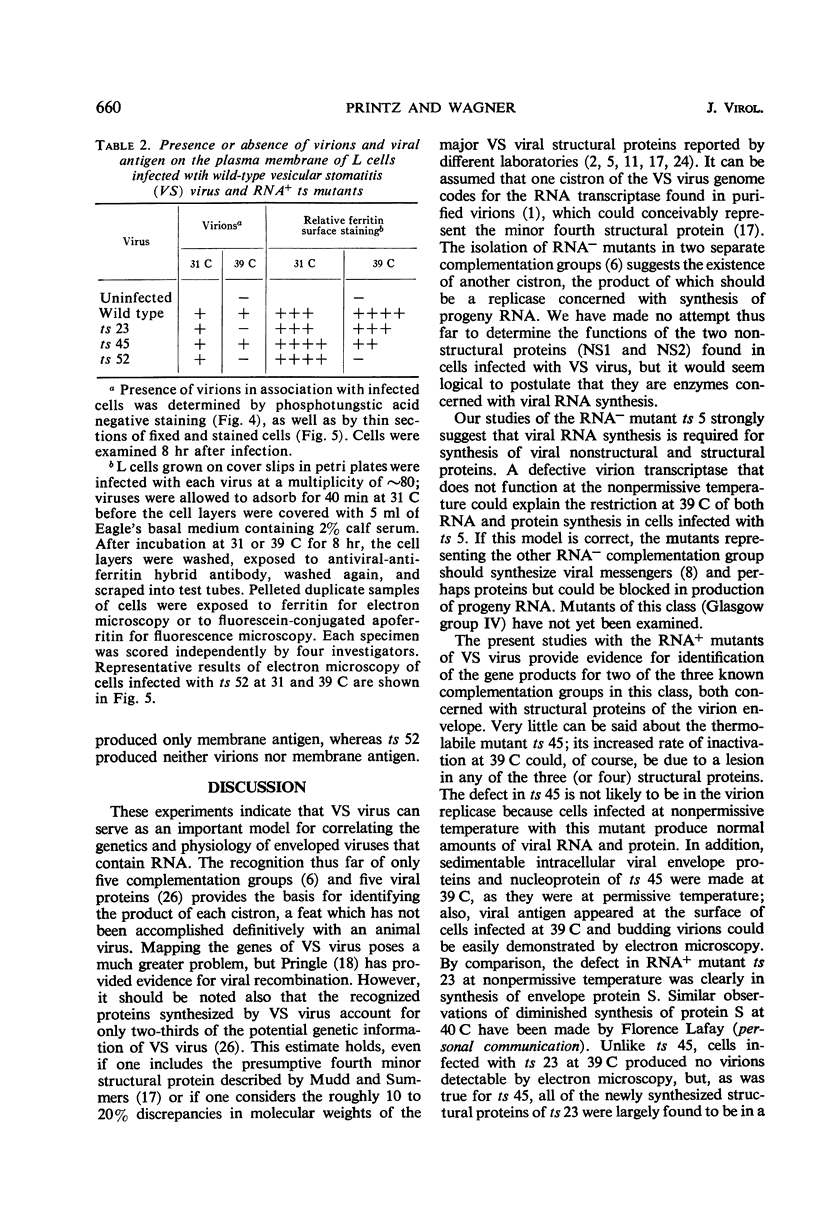
Viral proteins synthesized in L cells infected with temperature-sensitive (ts) mutants of vesicular stomatitis (VS) virus at permissive (31 C) and nonpermissive (39 C) temperatures were compared by polyacrylamide gel electrophoresis. Mutant ts 5, deficient in synthesis of viral ribonucleic acid (RNA−), failed to synthesize any of the five identifiable viral proteins at 39 C. Each of three RNA+ mutants, representing three separate complementation groups, showed distinctive patterns of viral protein synthesis at nonpermissive temperature. Equivalent amounts of 3H-amino acids were incorporated into the five viral proteins made in cells infected with RNA+ mutant ts 45 at 31 and 39 C. Complete virions of ts 45 could be identified by electron microscopy of infected cells incubated at the nonpermissive temperature; the defect in ts 45 appeared to be due in part to greater thermolability of virions as compared with the wild-type. RNA+ mutant ts 23 was deficient in synthesis of viral envelope protein S and failed to make detectable virions at the nonpermissive temperature. Infection of cells at 39 C with the third RNA+ mutant, ts 52, resulted in synthesis of all five viral proteins, but the peak of radioactivity representing the viral membrane glycoprotein migrated more rapidly on gels than coelectrophoresed authentic virion 14C-glycoprotein or viral 3H-glycoprotein extracted from cells infected at 31 C. These data and results of experiments on incorporation of radioactive glucosamine suggest that the primary defect in mutant ts 52 at nonpermissive temperature is failure of glycosylation of the viral glycoprotein. The viral structural proteins made in cells infected with ts 52 at the nonpermissive temperature did not assemble into sedimentable components as they did at permissive temperature; this observation indicates failure of insertion of the nonglycosylated protein (G′) into cell membrane. In support of this hypothesis was the finding that antiviral-antiferritin hybrid antibody did not detect VS viral antigen on the plasma membrane of L cells infected at 39 C with ts 52. In contrast, VS viral antigen localized in plasma membrane of L cells infected at 39 C with mutants ts 23 and ts 45 was readily detected by electron microscopy and fluorescence microscopy.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D., Huang A. S., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus, II. An RNA polymerase in the virion. Proc Natl Acad Sci U S A. 1970 Jun;66(2):572–576. doi: 10.1073/pnas.66.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge B. W., Huang A. S. Comparison of membrane protein glycopeptides of Sindbis virus and vesicular stomatitis virus. J Virol. 1970 Aug;6(2):176–182. doi: 10.1128/jvi.6.2.176-182.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Complementation between temperature-sensitive mutants of Sindbis virus. Virology. 1966 Oct;30(2):214–223. doi: 10.1016/0042-6822(66)90097-3. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Functional defects of temperature-sensitive mutants of Sindbis virus. J Mol Biol. 1968 Jul 14;35(1):193–205. doi: 10.1016/s0022-2836(68)80047-6. [DOI] [PubMed] [Google Scholar]
- Cartwright B., Talbot P., Brown F. The proteins of biologically active sub-units of vesicular stomatitis virus. J Gen Virol. 1970 Jun;7(3):267–272. doi: 10.1099/0022-1317-7-3-267. [DOI] [PubMed] [Google Scholar]
- Flamand A. Etude génétique du virus de la stomatite vésiculaire: classement de mutants thermosensibles spontanés en groupes de complémentation. J Gen Virol. 1970 Sep;8(3):187–195. doi: 10.1099/0022-1317-8-3-187. [DOI] [PubMed] [Google Scholar]
- Heine J. W., Schnaitman C. A. Fusion of vesicular stomatitis virus with the cytoplasmic membrane of L cells. J Virol. 1969 Jun;3(6):619–622. doi: 10.1128/jvi.3.6.619-622.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus. 3. Multiple complementary messenger RNA molecules. Virology. 1970 Dec;42(4):946–957. doi: 10.1016/0042-6822(70)90343-0. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Greenawalt J. W., Wagner R. R. Defective T particles of vesicular stomatitis virus. I. Preparation, morphology, and some biologic properties. Virology. 1966 Oct;30(2):161–172. doi: 10.1016/0042-6822(66)90092-4. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Prevec L. Proteins of vesicular stomatitis virus. I. Polyacrylamide gel analysis of viral antigens. J Virol. 1969 Apr;3(4):404–413. doi: 10.1128/jvi.3.4.404-413.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafay F., Berkaloff A. Etude des mutants thermosensibles du virus de la stomatite vésiculaire (VSV). Mutants de maturation. C R Acad Sci Hebd Seances Acad Sci D. 1969 Sep 15;269(11):1031–1034. [PubMed] [Google Scholar]
- Lafay F. Etude des mutants thermosensibles du Virus de la Stomatite Vésiculaire (VSV). Classification de quelques mutants d'après des critères de fonctionnement. C R Acad Sci Hebd Seances Acad Sci D. 1969 May 12;268(19):2385–2388. [PubMed] [Google Scholar]
- Lomniczi B., Burke D. C. Interferon production by temperature-sensitive mutants of Semliki Forest virus. J Gen Virol. 1970 Jul;8(1):55–68. doi: 10.1099/0022-1317-8-1-55. [DOI] [PubMed] [Google Scholar]
- Martinet C., Printz Ane C. Analyse de la synthèse de l'ARN viral du virus de la stomatite vésiculaire (VSV). Utilisation de mutants thermosensibles. Ann Inst Pasteur (Paris) 1970 Oct;119(4):411–419. [PubMed] [Google Scholar]
- McSharry J. J., Wagner R. R. Carbohydrate composition of vesicular stomatitis virus. J Virol. 1971 Mar;7(3):412–415. doi: 10.1128/jvi.7.3.412-415.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd J. A., Summers D. F. Protein synthesis in vesicular stomatitis virus-infected HeLa cells. Virology. 1970 Oct;42(2):328–340. doi: 10.1016/0042-6822(70)90277-1. [DOI] [PubMed] [Google Scholar]
- Pringle C. R. Genetic characteristics of conditional lethal mutants of vesicular stomatitis virus induced by 5-fluorouracil, 5-azacytidine, and ethyl methane sulfonate. J Virol. 1970 May;5(5):559–567. doi: 10.1128/jvi.5.5.559-567.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothfield L., Pearlman-Kothencz M. Synthesis and assembly of bacterial membrane components. A lipopolysaccharide-phospholipid-protein complex excreted by living bacteria. J Mol Biol. 1969 Sep 28;44(3):477–492. doi: 10.1016/0022-2836(69)90374-x. [DOI] [PubMed] [Google Scholar]
- Scheele C. M., Pfefferkorn E. R. Virus-specific proteins synthesized in cells infected with RNA+ temperature-sensitive mutants of Sindbis virus. J Virol. 1970 Mar;5(3):329–337. doi: 10.1128/jvi.5.3.329-337.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K. B. Electron microscopy of cells infected with Semliki forest virus temperature-sensitive mutants: correlation of ultrastructural and physiological observations. J Virol. 1970 May;5(5):632–638. doi: 10.1128/jvi.5.5.632-638.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K. B., Sambrook J. F., Bellett A. J. Semliki forest virus temperature-sensitive mutants: isolation and characterization. Virology. 1969 Jul;38(3):427–439. doi: 10.1016/0042-6822(69)90155-x. [DOI] [PubMed] [Google Scholar]
- Wagner R. R., Heine J. W., Goldstein G., Schnaitman C. A. Use of antiviral-antiferritin hybrid antibody for localization of viral antigen in plasma membrane. J Virol. 1971 Feb;7(2):274–277. doi: 10.1128/jvi.7.2.274-277.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Schnaitman T. A., Snyder R. M. Structural proteins of vesicular stomatitis viruses. J Virol. 1969 Apr;3(4):395–403. doi: 10.1128/jvi.3.4.395-403.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Schnaitman T. C., Snyder R. M., Schnaitman C. A. Protein composition of the structural components of vesicular stomatitis virus. J Virol. 1969 Jun;3(6):611–618. doi: 10.1128/jvi.3.6.611-618.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Snyder R. M., Yamazaki S. Proteins of vesicular stomatitis virus: kinetics and cellular sites of synthesis. J Virol. 1970 May;5(5):548–558. doi: 10.1128/jvi.5.5.548-558.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S., Wagner R. R. Action of interferon: kinetics and differential effects on viral functions. J Virol. 1970 Oct;6(4):421–429. doi: 10.1128/jvi.6.4.421-429.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F. H., Lockart R. Z., Jr Maturation defects in temperature-sensitive mutants of Sindbis virus. J Virol. 1968 Jul;2(7):728–737. doi: 10.1128/jvi.2.7.728-737.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F. H. Temperature-sensitive behavior of hemagglutinin in a temperature-sensitive mutant virion of Sindbis. J Virol. 1969 Oct;4(4):547–548. doi: 10.1128/jvi.4.4.547-548.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]