Abstract
Recombinant vaccinia virus has been widely employed as a cancer vaccine in several clinical trials. In this study we explored, employing BALB/c mice transgenic for the rat neu oncogene, the ability of the recombinant vaccinia virus neu (rV-neuT) vaccine to inhibit growth of neu+ mammary carcinomas and whether the efficacy of vaccination was dependent on: (a) carcinogenesis stage at which the vaccination was initiated; (b) number of vaccinations and (c) route of delivery (systemic vs. local). BALB-neuT mice were vaccinated one, two and three times by subcutaneous (s.c.) and intramammary gland (im.g.) injection with rV-neuT or V-wt (wild-type vaccinia virus) starting at the stage in which mouse mammary gland displays atypical hyperplasia, carcinoma in situ or invasive carcinoma. We demonstrated that vaccination using rV-neuT was more effective when started at an earlier stage of mammary carcinogenesis and after three vaccinations. The im.g. vaccination was more effective than the s.c. vaccination in inhibiting mammary carcinogenesis, eliciting anti-Neu antibodies, increasing anti-Neu IgG2a/G3 isotypes and inducing antibodies able to trigger mammary tumor cells apoptosis and antibody-dependent cellular cytotoxicity. The better protective ability of rV-neuT im.g. vaccination was associated with its capacity to induce a superior degree of in vivo mammary cancer cells apoptosis. Our research suggests that intratumoral vaccination using recombinant vaccinia virus could be employed to increase the activity of a genetic cancer vaccine. This study may have important implications for the design of cancer vaccine protocols for the treatment of breast cancer and of accessible tumors using recombinant vaccinia virus.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-010-0850-0) contains supplementary material, which is available to authorized users.
Keywords: Cancer vaccine, Intratumoral vaccination, Recombinant vaccinia virus, Serum antibodies
Introduction
Attenuated vaccinia virus delivery produces a mild infection in humans, which protects against smallpox caused by the variola virus, due to its ability to provide long-term immunity against the natural form of the virus [1, 2]. The ability of vaccinia virus to insert and express foreign genes encoding for weak immunogenic proteins has led to its use as a delivery vehicle for cancer and infectious disease vaccines in experimental models [3–5]. Engineered attenuated recombinant vaccinia virus has now been widely employed as a cancer vaccine in a large number of clinical trials. The results of these trials demonstrated that vaccinia virus infection upon vaccination was safe and that a specific humoral or T cell response against the foreign inserted tumor-associated antigen could be induced in several cancer patients [3, 5–14]. Vaccination with recombinant vaccinia virus can be achieved by systemic or local intratumoral injection [3, 6–19]. Systemic vaccination employs subcutaneous (s.c.), intradermal, or intramuscular delivery [20, 21]. Although the majority of anticancer vaccine strategies employ systemic vaccination, recent data support the effectiveness of the intratumoral vaccination both in human and experimental models [11, 13, 15–25]. Recently, it was demonstrated that the antitumor activity induced by intratumoral vaccination with an avipox virus expressing carcinoembryonic antigen (CEA) and multiple co-stimulatory molecules was superior to that induced by systemic (subcutaneous) vaccination in CEA-transgenic mice [21]. A prerequisite to employing intratumoral vaccine therapy is the simple access for antigen delivery to the tumor site. Among others, mammary cancer is a typical accessible primary tumor. BALB-neuT mice that are transgenic for the neu oncogene are suitable animal models to study the efficiency of Her-2/neu cancer vaccine in counteracting autochthonous mammary carcinogenesis [26, 27]. Tumor vaccine studies have used the BALB-neuT model in the past, which included different delivery strategies employing DNA [28], synthetic peptides [29], adenoviral vector [30, 31] or a cell-based vaccine [32, 33]. These vaccines have been shown to protect BALB-neuT mice to a variable degree from neu oncogene-induced tumorigenesis. The stepwise progression of mammary carcinogenesis in BALB-neuT allows one to begin the vaccination based on the progressive stage of the disease. BALB-neuT mice exhibit reproducible transition from normal epithelium to atypical hyperplasia (week 6) and to multifocal breast carcinoma that becomes palpable around week 16 [28].
To our knowledge there are no studies that employed recombinant vaccinia virus as a vaccination vehicle to deliver high recombinant protein levels of Her-2/neu in BALB-neuT mice. In this report we explored the mammary tumor inhibitory ability of the recombinant vaccinia virus neu (rV-neuT) vaccine in BALB-neuT mice. In addition, we investigated whether the efficacy of vaccination was dependent on the carcinogenesis stage at which the vaccination was initiated as well as the usefulness of multiple rV-neuT injections. This study compares the antitumor effect induced by systemic versus local route of rV-neuT administration, by employing subcutaneous (s.c.) versus intramammary gland (im.g.), respectively. This study may have important implications for the design of cancer vaccine protocols for the treatment of breast cancer and of accessible tumors using recombinant vaccinia virus.
Materials and methods
Antibodies, peptides and cells
Synthetic peptides located in the extracellular (Neu 15.3, aa 66–74; Neu 42, aa 169–183; Neu 98, aa 393–407; Neu 141, aa 566–580; Neu 156, aa 626–640), transmembrane (Neu 166, aa 666–680) domains of rat Neu sequence [34, NCI, PubMed Accession 1202344A] were previously described [33]. Neu-overexpressing BALB-neuT mammary cancer cells (H-2d) (TUBO) were previously described [27]. NIH3T3 cells encoding normal rat Neu (LTR-Neu) have been previously characterized and kindly provided by Dr. Eddi Di Marco (Istituto Tumori di Genova) [35]. Polyclonal rabbit anti-neu antibody Ab1 (PC04) was purchased from Oncogene Science (Cambridge, MA, USA). Rabbit polyclonal antibody recognizing the activated cleaved caspase-3 was purchased from Cell Signaling Technology (Beverly, MA, USA; catalog #9661).
Poxviruses
The recombinant vaccinia virus encoding the neu oncogene was designated rV-neuT (vT67RR-1-1, original lot from Therion Biologics Corp: #SC012197). It encodes the full length activated rat neu oncogene [34, NCI, PubMed Accession 1202344A]. The wild-type control vaccinia virus was designated V-wt (original lot from Therion Biologics Corp: #062797-NYCBH). Therion Biologics Corp. (Cambridge, MA, USA) kindly provided the poxviruses. Expression of recombinant NeuT encoded by rV-neuT was detected by Western blotting after infection of BSC-1 cells with V-wt or rV-neuT. Cells were infected with 10 pfu (plaque forming unit)/cell of poxviruses and cultured at 37°C for 18 h. Cell lysates, protein concentrations and immunoblotting were conducted as previously described [33, 36]. Polyclonal anti-ErbB2/neu antiserum was used to detect recombinant NeuT.
Transgenic BALB-neuT mouse colony
Transgenic BALB-neuT male mice were routinely mated with BALB/c females (H-2d; Charles River, Calco, Italy) in the animal facilities of Tor Vergata University. Progenies were confirmed for presence of the transgene by PCR [27]. Individually tagged virgin females were used in this study. Mice were bred under pathogen-free conditions and handled in compliance with European Union and institutional standards for animal research.
Recombinant vaccinia neu vaccination
The protocol of vaccination was approved by the Ethical Committee of the University of Rome “Tor Vergata” and submitted to the Italian Health Department. Groups of BALB-neuT mice were vaccinated by s.c. or im.g. injection. Subcutaneous injection was performed at the base of the tail. Viruses were diluted in PBS (phosphate-buffered saline) such that the entire dose was delivered in 100 μl. Mice received for each vaccination a total of 108 pfu (plaque forming unit) of either rV-neuT or V-wt. For intramammary gland delivery, mice received 107 pfu in each mammary gland for a total of 108 pfu each dose. BALB-neuT mice were vaccinated starting at the age at which they displayed atypical breast hyperplasia (6 weeks). One group of mice was vaccinated only one time (1×), while other groups were vaccinated once and then boosted one (2×) or two times (3×) at 4-week intervals (complete schedules 6, 10 or 6, 10, 14 weeks). Other groups of BALB-neuT mice were vaccinated one time with rV-neuT or V-wt starting at the age coinciding with in situ (11 weeks) or invasive breast carcinoma (16 weeks). Groups of mice were boosted one or two more times every 4 weeks (complete schedules 11, 15, and 11, 15, 19 or 16, 20, and 16, 20, 24 weeks, respectively). Depending on the immunogen, groups of 5–17 mice were vaccinated. The number of BALB-neuT mice receiving one (1×) or two (2×) doses of rV-neuT or V-wt were five in each group, independently of the time of initial vaccination and type of delivery. The numbers of mice receiving three doses (3×) of s.c. rV-neuT or V-wt were 10, 17, 12 and 8, 13, 10 when the vaccination was started at 6, 11 and 16 weeks of age, respectively. The numbers of mice receiving 3× of im.g. rV-neuT or V-wt were 9, 13, 16 and 7, 7, 13 when the vaccination was started at 6, 11 and 16 weeks of age, respectively.
Analysis of antitumor activity in vivo
Mammary glands were checked weekly and tumors recorded at 3 mm in diameter. Tumor growth was monitored until all mammary glands displayed a palpable tumor or tumor mass exceeded 15 mm in diameter. At this point mice were killed. The time of initial tumor appearance as well as tumor multiplicity was averaged as the mean ± standard deviation of incidental tumors [33].
Antibody immunity following vaccination with rV-neuT
Sera from vaccinated BALB-neuT mice were collected prior to vaccination and 7 days after the final boost. Serum from animals vaccinated one time was collected 4 weeks after the vaccination. The presence of antibodies reactive to Neu was assayed using NIH3T3, LTR-Neu and TUBO cells by immunoblotting, immunofluorescence or enzyme-linked immunosorbent assay (ELISA) as previously described [33, 36]. For immunofluorescence, mouse serum was used at 1:2,000 [33]. For ELISA, individual rV-neuT mouse serum at different dilutions (1:500, 1:4,000, 1:16,000) was assayed against LTR-Neu and NIH3T3 control (5 × 104 cells/well). The specific absorbance of each sample was calculated by subtracting LTR-Neu absorbance from that of NIH3T3 cells. Antibody titer was estimated as the highest immune serum dilution generating a specific absorbance of 2.2 at 492 nm. Sera titer is the mean value of individual serum titer [37]. Individual serum samples from mice receiving 3× of rV-neuT at 11 (n = 12) and 16 (n = 10) weeks were randomly chosen. Individual V-wt mouse serum was assayed at 1:250 dilution. Immunoglobulin subclasses were determined by ELISA using a Mouse Typer Isotyping Kit (Bio-Rad, Richmond, CA, USA) using individual serum of rV-neuT vaccinated mice as previously described [37, 38].
Biologic activity of vaccinated mouse immune sera in vitro
Antibody-dependent cellular cytotoxicity (ADCC) was conducted as previously described [32, 33]. BALB-neuT mammary tumor cells (5 × 103 cells/well) were used as targets (T), while spleen cells from normal BALB/c mice were used as effectors at 50:1. Dilutions of sera pooled from four mice vaccinated 3× with rV-neuT or V-wt starting at the age of 6 and 16 weeks were assayed. Dilutions of sera from s.c. (1:10, 1:20, 1:40) or im.g. (1:12, 1:24, 1:48 and 1:11, 1:22, 1:44 for mice vaccinated at 6 and 16 weeks, respectively) rV-neuT vaccinated mice were normalized according to their magnitude of reactivity to Neu as determined by ELISA. Percentage of specific lysis was calculated as described [32, 33]. The results represent average percentage of cytotoxicity of three independent experiments. Four randomly chosen serum samples were pooled and used for two independent experiments. Four other randomly chosen serum samples were pooled and used for the third experiment.
For in situ detection of programmed cell death of BALB-neuT cancer cells, immunoglobulins (Ig) from BALB-neuT mice pooled sera were purified by protein G and dialyzed against PBS. BALB-neuT cancer cells (2.5 × 103 cells/well) were incubated in serum-free DMEM containing 0.2% BSA containing Ig (10 μg/ml) from rV-neuT or V-wt vaccinated mice starting at the age of 6 weeks. Ig’s were replenished every 24 h. Cells were fixed in 4% formaldehyde for 15 min and after washing they were incubated with the polyclonal anti-activated caspase-3 antibody for 1 h. After another washing the cells were labeled with goat anti-rabbit IgG Alexa fluor-594-conjugated antibody (Invitrogen) for 30 min [36]. After a third washing the cells were incubated with 0.1 μg/ml Hoechst 33342 and mounted under a coverslip in glycerol. Staurosporine at 1 μM for 24 h was used as positive control. The percentage of apoptotic cells was calculated by determining the activated caspase-3 positive cells/total cells evaluating five randomly chosen microscopic fields. Count of apoptotic cells was done in a blinded fashion.
Detection of apoptotic cells in vivo
Mammary tissue from 2 to 3 BALB-neuT mice vaccinated with rV-neuT or V-wt starting at the age (6 weeks) coinciding with atypical hyperplasia was processed for immunohistochemical analyses as previously described [33, 36]. Three tumors were used for each group of vaccinated mice. Deparaffinized tissue sections were incubated with the anti-activated caspase-3 antibody [39]. Apoptotic cells were counted at 200× in five microscopic randomly chosen fields. This result represents the mean ± standard deviation of positive cells/field evaluated by immunohistochemistry. The count of apoptotic cells was done in a blinded fashion. Electron microscopy analysis was performed as previously described [40].
IL-2 and IFN-γ release assay
Spleen cells from BALB-neuT vaccinated mice at 16 weeks of age were harvested 7 days after the final vaccination as previously described [33]. Spleen mononuclear cells (2 × 106/well in 24-well plates) were incubated with Concanavalin A (ConA, 2 μg/ml), various Neu peptides (10 μg/ml) or control gag peptide. Neu peptides were selected based on immunoreactivity in vitro with lymphocytes from BALB-neuT mice vaccinated with recombinant adenovirus or NIH3T3 fibroblasts (LTR-Neu) expressing Neu [30, 33]. IL-2 and IFN-γ release into the supernatant was measured using an enzymatic immunocapture assay (Quantikine, R&D Systems, Minneapolis, MN, USA). Results represent the mean of two independent experiments.
Statistical analysis
Mean and standard deviation describes continuous variables. Survival curves were estimated using the Kaplan–Meier method and compared by the log-rank test. The effects of vaccine, route of administration, number of injections and starting point of the vaccination on time to initial tumor appearance were estimated using the Cox proportional hazards model. Ties in the failure times were handled by computing the exact conditional probability, under the proportional hazards assumption, that all tied event times occur before censored times of the same value or before larger values. Diagnostics based on the weighted Schoenfeld residuals did not show any significant departure from the proportional hazards assumption. The same factors were considered as potential predictors of reduction of tumor multiplicity at 25 weeks; their effects were estimated by the Poisson model, introducing an additional parameter to adjust for over-dispersion. Differences in number of apoptotic cells, titer of the serum, isotype of immunoglobulins and percentage of antibody-dependent cellular cytotoxicity were evaluated by a two-tailed t test. A preliminary test was performed to compare variances between groups; if a significant difference was detected, the classical t test statistics were modified and the Satterthwaite’s approximation utilized.
Results
Expression of recombinant NeuT encoded by rV-neuT
Expression of recombinant NeuT encoded by rV-neuT was detected by Western blotting after infection of BSC-1 with rV-neuT. Polyclonal anti-HER-2/neu antibody detected a 185 kDa protein product on BSC-1 cells infected with rV-neuT but not in those infected with the wild-type virus, V-wt (data not shown).
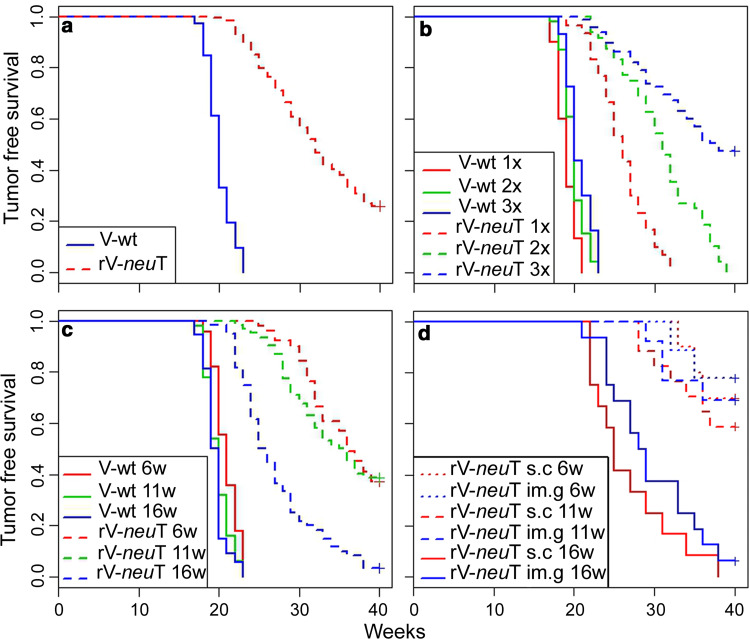
Inhibition of mammary carcinogenesis by recombinant vaccinia neu (rV-neuT) vaccine: effect of stage of initial vaccination, multiple injections and route of delivery
To compare the effectiveness of systemic versus local route of rV-neuT administration, we employed s.c. or im.g. vaccination, respectively. To determine whether efficacy of vaccination was dependent on the carcinogenesis stage at which the vaccination was initiated, BALB-neuT mice were initially vaccinated at the stage of atypical hyperplasia (6 weeks), carcinoma in situ (11 weeks) and invasive carcinoma (16 weeks). In addition, to determine the usefulness of multiple rV-neuT injections, a dose response study (1×, 2× and 3×) was carried out. Control groups of mice received wild-type vaccinia virus (V-wt). Results by Cox’s model show that all of the four considered variables (i.e., V-wt vs. rV-neuT vaccination, multiple injections, route of delivery and disease stage of initial vaccination) significantly affect the tumor-free survival (Table 1). Overall, our results indicated specific interference of the rV-neuT vaccine with tumor growth and dependency of the effect on the number of injections, starting point and modality of the vaccination (Table 1). When considering the effectiveness of the rV-neuT vaccine independently of the number of injections, route and beginning of vaccinations, the estimated tumor-free survival of mice vaccinated with rV-neuT versus those receiving the V-wt was 0.24 (SD = 0.037) versus 0 at 40 weeks, respectively, and the estimated median tumor-free survival time was 31 versus 20 weeks (Fig. 1a). Overall, at 30 weeks of age, the estimated tumor-free survival of mice vaccinated with one, two or three rV-neuT doses was 0.1 (SD = 0.055), 0.5 (SD = 0.091) and 0.7 (SD = 0.299), respectively, with an estimated median tumor-free survival time of 26, 30.5 and 36 weeks (Fig. 1b). The dose dependent response was observed independently of the time in which the rV-neuT vaccination was initiated and route of injection (Table 1). Thus, our results indicate that the regimen protocol using three rV-neuT injections is the most effective in inducing antitumor activity (Table 1). In addition, our results indicate that early vaccination (6 and 11 weeks of age) improves the antitumor effectiveness of the rV-neuT vaccine (Table 1) (Fig. 1c). Reduction of tumor multiplicity at 25 weeks was also dependent on the starting point and number of vaccinations (Supplementary Table S1).
Table 1.
Multivariate analysis of tumor-free survival of BALB-neuT mice after rV-neuT vaccination according to the Cox model
| Variable | Contrast | Hazard ratio | 95% hazard ratio confidence limits | p value | |
|---|---|---|---|---|---|
| Vaccine | rV-neuT vs. V-wt | 0.007 | 0.003 | 0.014 | <0.0001 |
| Route of administration | im.g. vs. s.c. | 0.529 | 0.396 | 0.706 | <0.0001 |
| Number of injections | 2 vs. 1 | 0.363 | 0.245 | 0.539 | <0.0001 |
| 3 vs. 2 | 0.333 | 0.214 | 0.452 | <0.0001 | |
| Starting point of the vaccination (weeks) | 11 vs. 6 | 1.857 | 1.290 | 2.671 | 0.0009 |
| 16 vs. 11 | 3.256 | 2.131 | 4.380 | <0.0001 | |
Fig. 1.
Inhibition of neu oncogene-mediated mammary carcinogenesis in vivo by rV-neuT vaccination. a Differences in tumor-free survival between V-wt and rV-neuT vaccinated BALB-neuT mice independently of dose, starting point and route of vaccination, b differences in tumor-free survival between V-wt and rV-neuT vaccinated BALB-neuT mice after multiple injections, c differences in tumor-free survival between V-wt and rV-neuT vaccinated BALB-neuT mice when the vaccination was started at 6, 11 and 16 weeks of age, d differences in tumor-free survival between s.c. or im.g. rV-neuT 3× vaccinated mice when the vaccination was started at 6, 11 and16 weeks of age. Numbers of vaccinated mice are reported in “Materials and methods”
Overall, the risk of developing tumors in the im.g. rV-neuT vaccinated group was 0.529 in comparison to the s.c. vaccinated group (Table 1). For example, the Kaplan–Meier method showed that when rV-neuT vaccination was started at 16 weeks, three vaccinations provided an estimated median tumor-free survival time of 28.5 versus 25 weeks, respectively, for im.g. versus the s.c. rV-neuT vaccination (Fig. 1d). At this stage of vaccination the estimated tumor-free survival at 30 weeks was 0.375 (SD = 0.121) versus 0.25 (SD = 0.125) when three vaccinations were performed by im.g. versus s.c. rV-neuT injection, respectively (Fig. 1d). Differences in tumor-free survival between s.c. or im.g. rV-neuT 3× vaccinated mice when the vaccination was started at 6 and 11 weeks of age are also shown in Fig. 1d. In addition, at 25 weeks of age, tumor multiplicity was significantly different between s.c. and im.g. vaccination (Supplementary Table S1).
Our findings indicate that im.g. rV-neuT vaccination is superior to s.c. vaccination in inhibiting the neu oncogene-mediated mammary carcinogenesis.
Anti-Neu humoral response following rV-neuT vaccination
Previous studies demonstrated that a potent anti-Neu humoral response is necessary to prevent mammary tumor growth in BALB-neuT vaccinated mice [32]. To determine whether differences in humoral response exist between multiple rV-neuT injections or the carcinogenesis stage at which the rV-neuT vaccination was initiated and between rV-neuT s.c. and im.g. route of administration, specific antibody response to Neu was quantitatively and qualitatively evaluated by ELISA, immunoprecipitation following Western blotting and immunofluorescence. Specific anti-Neu reactivity of rV-neuT vaccinated mouse serum was visualized by immunoblotting of immunoprecipitates using the anti-HER-2/neu-specific antibody and corroborated by immunofluorescence (Supplementary Figure S1, panel A and B). The magnitude of the immune response elicited by varying doses of rV-neuT and the most effective route of rV-neuT vaccination were quantitated by ELISA. As shown in Table 2, mice vaccinated 3× with rV-neuT by the s.c. route developed a significantly higher titer of anti-Neu antibodies than those vaccinated 1× or 2×, independently of the starting point of vaccination. Similar results were obtained when mice received one, two or three im.g. rV-neuT doses. A comparison between the two routes of administration clearly showed that the im.g. vaccination was more effective in eliciting anti-Neu antibodies than the s.c. vaccination when two or three vaccinations were performed independently of the starting point of the vaccination (Table 2). Furthermore, when the BALB-neuT vaccination started at the stage in which the mice displayed atypical breast hyperplasia, even the administration of 1× im.g. rV-neuT resulted in antibody titers higher than those observed with 1× s.c. rV-neuT. The administration of V-wt did not result in the induction of anti-Neu antibodies.
Table 2.
Immunoreactivity of rV-neuT vaccinated Balb-neuT mouse sera with Neu
| Starting point and dose (number of mice with immune response/total)a | Type of delivery of rV-neuT | Number of pooled mouse sera | Serum titer mean (SD)b |
im.g. vs. s.c. p value (t test) | 2× vs. 1×* 3× vs. 2×♦ p value (t test) |
|---|---|---|---|---|---|
| 6 weeks | |||||
| 1× (5/5) | s.c. | 5 | 1,295c (72) | <0.0001 | |
| 1× (5/5) | im.g. | 5 | 2,328 (213) | ||
| 2× (5/5) | s.c. | 5 | 2,270 (441) | 0.0001 | 0.0002* |
| 2× (5/5) | im.g. | 5 | 3,720 (164) | 0.0335* | |
| 3× (10/10) | s.c. | 10 | 8,660 (207) | 0.0371 | <0.0001♦ |
| 3× (9/9) | im.g. | 9 | 10,800 (1,565) | <0.0001♦ | |
| 11 weeks | |||||
| 1× (5/5) | s.c. | 5 | 1,780 (76) | 0.0813 | |
| 1× (5/5) | im.g. | 5 | 2,460 (657) | ||
| 2× (5/5) | s.c. | 5 | 2,705 (273) | 0.0067 | 0.0351* |
| 2× (5/5) | im.g. | 5 | 3,320 (45) | 0.0391* | |
| 3× (17/17) | s.c. | 12 | 11,083 (970) | 0.0004 | <0.0001♦ |
| 3× (13/13) | im.g. | 12 | 13,667 (753) | <0.0001♦ | |
| 16 weeks | |||||
| 1× (5/5) | s.c. | 5 | 1,065 (251) | 0.1249 | |
| 1× (5/5) | im.g. | 5 | 1,390 (342) | ||
| 2× (5/5) | s.c. | 5 | 2,710 (286) | 0.0099 | <0.0001* |
| 2× (5/5) | im.g. | 5 | 3,620 (533) | <0.0001* | |
| 3× (12/12) | s.c. | 10 | 12,880 (164) | 0.0104 | <0.0001♦ |
| 3× (16/16) | im.g. | 10 | 14,006 (583) | <0.0001♦ | |
SD standard deviation
aImmune response was determined by ELISA against LTR-Neu and NIH3T3 at 1:500 serum dilution. Specific absorbance for all rV-neuT sera was >1.0. These values were considered positive as compared to that obtained with V-wt sera. Optical density of V-wt sera (1×, 2× and 3×, 5–13 mice in each group) at 1:250 to LTR-Neu was <0.3. These values were considered negative
bImmune sera titers of BALB-neuT vaccinated mice were determined by ELISA against LTR-Neu and NIH3T3 using individual serum at different dilutions. Sera titer represents the mean value of individual serum titer
cTiter was estimated as the highest immune serum dilution generating a specific absorbance of 2.2 at 492 nm
Experiments were then carried out to evaluate the isotype of the immunoglobulins elicited by rV-neuT vaccination. For comparison, sera of BALB-neuT mice vaccinated 1× or 3× with rV-neuT by the s.c. or im.g. routes were analyzed (Table 3). As shown in Table 3, after three vaccinations the population of IgM significantly decreased in mice vaccinated three times compared to those receiving only one dose of rV-neuT independently of the route of vaccination. However, when one or three doses were given beginning at 6 or 16 weeks of age, the im.g. route of vaccination resulted in significant enhancement of the IgG2a population compared with the s.c. vaccination. Furthermore, after three vaccinations, a significant increase of the IgG3 population was observed in those animals receiving rV-neuT by the im.g. route as compared to those vaccinated by the s.c. route. The increase of the IgG2a/G3 populations was paralleled in the im.g. vaccinated mice by the decrease of the IgG1 population. These results indicated that the rV-neuT route of vaccination affects the anti-Neu immunoglobulin specific isotype.
Table 3.
Effect of rV-neuT vaccination on Balb-neuT immunoglobulin isotype sera
| Starting point of vaccination | Dose | Type of delivery | Immunoglobulin isotype against Neu | |||||
|---|---|---|---|---|---|---|---|---|
| IgG1 | IgG2a | IgG2b | IgG3 | IgM | IgA | |||
| 6 weeks | ||||||||
| rV-neuT | 1× | s.c. | 19.5 ± 1.6a | 20.8 ± 2.4 | 22 ± 1.6 | 8 ± 1.2 | 22.3 ± 5.9 | 7.8 ± 1.6 |
| 1× | im.g. | 16.2 ± 2.4 | 25.8 ± 2 | 20.2 ± 2.2 | 10.5 ± 0.8 | 19 ± 1.1 | 8 ± 2.5 | |
| p | 0.001b | 0.03b | ||||||
| 16 weeks | ||||||||
| rV-neuT | 1× | s.c. | 18.6 ± 1.1 | 19.6 ± 1 | 23.7 ± 3.4 | 9.7 ± 1.9 | 20.9 ± 1.1 | 7.2 ± 3.3 |
| 1× | im.g. | 19.7 ± 1.3 | 24.2 ± 1.1 | 19.9 ± 1.1 | 12.3 ± 2.9 | 16.2 ± 2.5 | 7.5 ± 0.4 | |
| p | 0.01b | |||||||
| 6 weeks | ||||||||
| rV-neuT | 3× | s.c. | 25.9 ± 2.1 | 38 ± 2.4 | 11.9 ± 1.4 | 8.8 ± 1.6 | 8.8 ± 1.1 | 6.9 ± 0.6 |
| 3× | im.g. | 18.7 ± 2 | 45.1 ± 2 | 11 ± 0.9 | 12.9 ± 0.6 | 7.7 ± 0.5 | 4.4 ± 0.9 | |
| p | 0.02 | 0.005b | 0.016b | |||||
| 16 weeks | ||||||||
| rV-neuT | 3× | s.c. | 28.1 ± 0.7 | 36.5 ± 1.3 | 12.9 ± 0.5 | 8.6 ± 0.5 | 8 ± 1.1 | 5.7 ± 1.4 |
| 3× | im.g. | 19.8 ± 1.7 | 44.1 ± 0.7 | 12.5 ± 1.5 | 12.4 ± 1.2 | 6.8 ± 1.3 | 4.1 ± 0.6 | |
| p | 0.005b | 0.002b | 0.024b | |||||
aResults are the mean of the percent (±standard deviation) of each immunoglobulin isotype relative to the total sera immunoglobulin content (at 1:1,500)
bim.g. versus s.c
Biological activity in vitro of immune sera of rV-neuT vaccinated mice
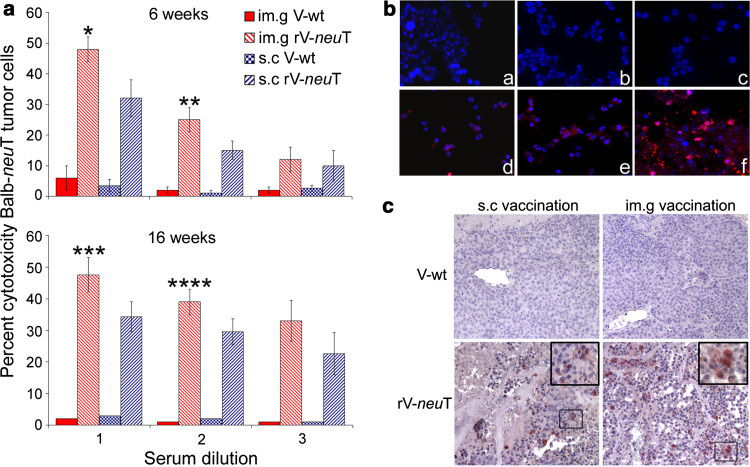
Differences in IgG populations induced by the s.c. and im.g. routes of vaccination might possibly mirror dissimilarities in biological activity of immune sera of rV-neuT vaccinated mice. To test this hypothesis, antibody-dependent cellular cytotoxicity (ADCC) of BALB-neuT mammary tumor cells (TUBO) was analyzed using sera of mice vaccinated at 6 and 16 weeks of age (Fig. 2a). Spleen cells produced no cytotoxicty in the presence of the sera of V-wt vaccinated mice. Conversely, spleen cells in the presence of sera of s.c. or im.g. rV-neuT vaccinated mice showed a high degree of cytotoxicity. However, spleen cells stimulated with the im.g. rV-neuT serum at 1:10 and 1:20 dilutions were more effective in their ability to elicit ADCC than those in the presence of the s.c. rV-neuT serum both for sera of mice vaccinated at 6 and 16 weeks of age (Fig. 2a).
Fig. 2.
Biological activity in vitro of immune sera or purified immunoglobulins of rV-neuT vaccinated mice and in vivo detection of apoptosis induced by rV-neuT vaccination. a Biological activity in vitro of sera from rV-neuT vaccinated mice. Specific antibody-dependent cell-mediated cytotoxicity was elicited by rV-neuT sera from mice vaccinated at 6 and 16 weeks of age. BALB-neuT mammary cancer cells were exposed for 2 h to sera pooled from s.c. or im.g. rV-neuT or V-wt vaccinated mice at different dilutions (for s.c., 1 1:10, 2 1:20, 3 1:40 and for im.g., 1 1:12, 2 1:24, 3 1:48 (mice vaccinated at 6 weeks) and 1 1:11, 2 1:22, 3 1:44 (mice vaccinated at 16 weeks). Results represent average percent cytotoxicity of three independent experiments. *0.0287, **0.0362, ***0.033, ****0.046: p values for im.g. versus s.c. rV-neuT 3× vaccination, b Induction of apoptosis by rV-neuT immunoglobulins in BALB-neuT mammary tumor cells. Apoptotic cells were identified when positively stained by the anti-activated caspase-3 polyclonal antibody. Immunoglobulins from vaccinated mice starting at the age of 6 weeks: a s.c. V-wt, b im.g. V-wt, c negative control, d s.c. rV-neuT, e im.g. rV-neuT, and f staurosporine treated cells. Nuclei were counterstained with Hoechst. Original magnification ×200, c Immunohistochemical analysis detecting apoptotic cells by anti-activated caspase-3 polyclonal antibody in mammary tumors developed in s.c. and im.g. rV-neuT or V-wt vaccinated BALB-neuT mice starting at the age coinciding with atypical hyperplasia (6 weeks). Immunoperoxidase counterstained with hematoxylin. Original magnification ×200
To determine whether specific immunoglobulins were able to trigger apoptosis, BALB-neuT tumor cells were labeled with anti-activated caspase-3 polyclonal antibody upon chronic treatment with Ig (10 μg/ml) from BALB-neuT mice vaccinated with rV-neuT or V-wt starting at the age of 6 weeks. Figure 2b shows detection of cleaved caspase-3 in BALB-neuT cells. The fraction of apoptotic cells was determined relative to cleaved caspase-3 positive cells. Purified Ig from s.c. or im.g. rV-neuT-vaccinated mice induced apoptosis of 41.7 and 55.5%, respectively (p = 0.0007). In comparison, treatment with Ig from V-wt vaccinated mice triggered irrelevant BALB-neuT cells apoptosis (0.6 and 1.5% for the s.c. or im.g. route, respectively). Treatment of cells with 1 μg/ml staurosporine resulted in 90% apoptotic cells.
Our results demonstrate that in vitro biologic activity including ADCC and induction of apoptosis by sera from mice vaccinated by s.c. or im.g. route corresponded to their differential ability of interfering with tumor growth in vivo.
T cell immune response by rV-neuT vaccination
Studies were then undertaken to determine whether the different routes of rV-neuT administration elicit dissimilar Neu T cell immunity. Splenocytes isolated from mice vaccinated at 16 weeks of age after the third vaccination were examined for their ability to proliferate under various Neu peptides. Release of IL-2 and IFN-γ was measured in the supernatant to assess T cell immunoreactivity with specific Neu epitopes. Results are reported in Table 4. T cell proliferative response to ConA was similar for all the vaccinated groups. All Neu peptides analyzed, but not an unrelated gag peptide, were able to specifically activate splenocytes from BALB-neuT mice vaccinated with rV-neuT. However, the magnitude of IL-2 and IFN-γ secretion was conditioned by the stimulating Neu peptide. The strongest T cell response was observed for both groups of rV-neuT vaccinated mice upon stimulation with the 166, 156, 141 and 15.3 peptides, the first located in the transmembrane domain while the remaining were in the extracellular domains of rat Neu sequence. A weaker IL-2 and IFN-γ release was detected upon stimulation with other Neu peptides located in the extracellular domain (r41 and r98). No significant divergence for specific recognition of Neu peptides was observed between splenocytes from s.c. or im.g. rV-neuT vaccinated mice. T cells from im.g. rV-neuT vaccinated mice release, in general, higher IL-2 and IFN-γ than those from s.c. rV-neuT mice upon stimulation with the 166, 156 and 141 Neu peptides. However, these differences were not significant (Table 4).
Table 4.
T cell immune response of BALB-neuT mice following vaccination with rV-neuT
| T cell in vitro stimulus | Neu peptide sequence | IL-2 releasea | IFN-γ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| rV-neuT s.c. | V-wt s.c. | rV-neuT im.g. | V-wt im.g. | rV-neuT s.c. | V-wt s.c. | rV-neuT im.g. | V-wt im.g. | ||
| r15.3 | TYVPANASL | 200 | 4 | 198 | 11 | 205 | 4 | 209 | 4 |
| r41 | DMVLWKDVFRKNNQL | 177 | 3 | 145 | 7 | 30 | 1 | 46 | 3 |
| r98 | IAPLRPEQLQVFETL | 179 | 2 | 161 | 4 | 53 | 2 | 35 | 4 |
| r141 | LPCHPECQPQNSSET | 209 | 9 | 267 | 7 | 191 | 2 | 232 | 6 |
| r156 | GICQPCPINCTHSCV | 325 | 13 | 417 | 15 | 196 | 2 | 219 | 6 |
| r166 | VLLFLILVVVVGILI | 436 | 13 | 536 | 8 | 143 | 3 | 183 | 3 |
| GAG | 19 | 4 | 17 | 1 | 3 | 2 | 2 | 4 | |
| ConA | 1,784 | 1,869 | 1,621 | 1,696 | 1,071 | 1,021 | 1,161 | 956 | |
aSpleen cells from vaccinated mice were stimulated in vitro with Neu-specific peptides. IL-2 and IFN-γ were quantitated in the supernatant (pg/ml) as a measure of T cell immunoreactivity with specific Neu epitopes. Concanavalin A (ConA) for global T cell activation and an unrelated gag peptide served as positive and negative control, respectively
In vivo induction of apoptosis in mammary tumors following rV-neuT vaccination
To determine whether rV-neuT vaccination of BALB-neuT mice induces in vivo mammary cancer cells apoptosis, tumor breast tissues from rV-neuT or V-wt vaccinated mice by s.c. or im.g. route, starting at the age coinciding with atypical hyperplasia, were analyzed by immunohistochemistry for expression of activated caspase-3 on cancer cells. Figure 2c shows detection of apoptotic cancer cells in mammary tissue. Activated caspase-3 positive cancer cells were counted in microscopic randomly chosen fields. Mammary tumors from V-wt vaccinated mice showed a very small number of apoptotic cancer cells (0.2 ± 0.5 and 0.3 ± 0.5 for the s.c. and im.g. vaccination, respectively). Conversely, vaccination with rV-neuT was associated with a noticeable number of apoptotic cancer cells detected among areas of ischemic and hemorrhagic necrosis in mammary tumors. Mammary tissue from rV-neuT vaccinated mice displayed 7.1 ± 2.2 and 12 ± 3.5 apoptotic cancer cells per field when the s.c. and im.g. routes of vaccination were employed, respectively. Differences in the number of cancer apoptotic cells were significant between rV-neuT and V-wt vaccination (1 × 10−7 and 1 × 10−8 for s.c. and im.g. vaccination, respectively) as well as between rV-neuT s.c. and im.g. vaccination (p = 0.005). This latter evidence further confirms differences in antitumor activity elicited by im.g. versus s.c. route of vaccination. The presence of apoptotic cancer cells within necrotic areas and tumor infiltrating lymphocytes has also been demonstrated by ultrastructural analysis in mammary tissue from rV-neuT im.g. vaccinated mice (supplementary figure S2).
Discussion
Clinical studies have demonstrated the ability of trastuzumab, a recombinant humanized monoclonal antibody, which recognizes the extracellular domain of the ErbB2 protein to induce an objective response in breast cancer patients [41, 42]. These studies, however, have also revealed that the objective response to trastuzumab monotherapy had a median duration of 9 months, and that the majority of responsive patients displayed resistance within 1 year [42]. Conversely, it has been also demonstrated that combination therapy with trastuzumab and a HER2/neu vaccine was associated with minimal toxicity and results in prolonged, robust, antigen-specific immune responses in treated patients [43]. In light of these findings it is realistic to explore ErbB2 cancer vaccine approaches with the aim to improve the objective tumor inhibitory response [44]. Active vaccination using ErbB2 as immunogen might maintain tumor inhibition more effectively than passive immunotherapy based on the induction of a persistent memory immune response and induction of T and B cell immunity to multiple immunodominant epitopes. However, there are safety concerns in vaccination involving a potent oncogene like ErbB2. Since oncogenic ErbB2 function relies on its intrinsic tyrosine kinase activity, elimination by mutation of its kinase domain represents a feasible alternative. Mice transgenic for the rat neu oncogene (Balb-neuT) are used to evaluate the capacity of ErbB2/neu vaccines to inhibit the progression of neu-driven carcinogenesis [28]. Recombinant vaccinia virus encoding for tumor-associated antigens has been widely employed in phase I clinical trials for the treatment of advanced stage cancer patients [6–11, 13, 14, 17, 19].
Although these trials proved the safety of vaccinia virus vaccination, as well as T and B cell responses to the encoded tumor-associated antigen in some cases, they showed only a small degree of clinical benefit for cancer patients [6–11, 13, 14, 17, 19]. Poxvirus represents an attractive delivery vehicle of tumor antigens due to the normal post-translational modification of the inserted antigen and strong immunogenicity [3–5]. In this study we explored for the first time the mammary tumor inhibitory ability of the recombinant vaccinia virus neu (rV-neuT) vaccine when administered in BALB-neuT mice. We also set out to determine whether the vaccination efficiency of rV-neuT vaccine was dependent on the carcinogenesis stage at which the vaccination was initiated and on the use of multiple rV-neuT injections.
Our observations indicated that tumor suppression was more effective when started at an earlier stage of the disease. The degree of tumor growth interference in vivo reflected the titers of anti-Neu serum antibodies elicited upon rV-neuT vaccination. Regression of established tumors following vaccination was less effective, most likely due to insufficient antibody accessibility or higher antibody requirement in vivo. In addition, we showed that immune response and antitumor activity were increased by repeated rV-neuT vaccinations. However, one of the potential drawbacks in the use of multiple recombinant vaccinia administrations is that pre-existing and/or induced antibody and T cell response to vaccinia virus will prevent the spread of the inoculated vaccinia virus and thus diminish the expression of inserted antigen. On the other hand, it should be noted that smallpox was eradicated worldwide more than 25 years ago; thus, young women are no longer vaccinated. In addition, recombinant avipox virus, which has a limited viral replication, can be used to boost immune response after recombinant vaccinia priming [4]. It was previously demonstrated that the mechanism of tumor protection in BALB-neuT depends on the antibody-mediated blockade of Her-2/neu function [31, 32]. In the current study we provided evidence that immune sera from rV-neuT-vaccinated mice induced apoptosis of BALB-neuT tumor cells in vitro, which corresponded to the relative extent of tumor inhibitory effect in vivo. We also demonstrated that immune rV-neuT sera were able to mediate ADCC. Furthermore, we demonstrated in vivo induction of cancer cells apoptosis in BALB-neuT mammary tumor sections following rV-neuT vaccination, by detecting activated caspase-3 positive cancer cells. This immunohistochemical finding was corroborated by the presence of apoptotic bodies within necrotic areas and tumor infiltrating lymphocytes in the tumor mass, as demonstrated by ultrastructural analysis.
Vaccination with recombinant vaccinia virus can be achieved by systemic or local intratumoral injection [3, 6–19]. Although the majority of anticancer vaccine strategies employ systemic vaccination, recent data support the effectiveness of the intratumoral vaccination both in human and experimental models [11, 13, 15–25]. A prerequisite to employing intratumoral vaccine therapy is the access to the tumor site. The accessibility of breast tumors and the standard surgical removal of the tumor allow one to envision intratumoral immunotherapy in a neoadjuvant setting [22].
In the current study we compared the antitumor effect induced by systemic versus local route of rV-neuT administration, by employing subcutaneous (s.c.) versus intramammary gland (im.g.), respectively. Our findings indicate that rV-neuT im.g. vaccination is superior to s.c. vaccination in inhibiting the neu oncogene-mediated mammary carcinogenesis. Furthermore, we demonstrated that rV-neuT im.g. vaccination was more effective in eliciting anti-Neu antibodies and in increasing anti-Neu IgG2a/G3 isotypes than s.c. vaccination. Immunoglobulins of the IgG2a isotype have been shown to mediate in mice a more potent ADCC than other Ig isotypes [45]. The in vitro biologic activity including ADCC and induction of cancer cells apoptosis by sera from im.g. vaccinated mice was superior to that induced by sera from s.c. vaccinated mice. It has been demonstrated that cytokines release and antibody production are the immune mechanisms mostly responsible for tumor protection in BALB-neuT mice, whereas cytotoxic T lymphocytes appear to play a marginal role [32, 46]. In addition, cytotoxic T cells reacting with high affinity with rat Neu are deleted in BALB-neuT mice by central tolerance [47]. Here, we showed that T cells from s.c. and im.g. rV-neuT vaccinated mice release IL-2 and IFN-γ upon stimulation with the 166, 156, 141 and 15.3 peptides, the first located in the transmembrane domain, while the remaining are in the extracellular domains of rat neu sequence. However, we could not detect significant differences in response to Neu peptides between splenocytes from s.c. or im.g. rV-neuT vaccinated mice.
The differential level of humoral immune response between the s.c. and im.g. routes of vaccination paralleled their differential ability of interfering with tumor growth in vivo. A superior degree of in vivo induction of mammary cancer cells apoptosis in rV-neuT im.g. vaccinated mice further supports this finding. Poxvirus infection leads to the production of immunomodulatory proteins that activate the innate immune system, a crucial event to induce a strong adaptive immune response. Such immunomodulatory proteins include interferons, chemokines, inflammatory cytokines, and the toll-like receptor family of pattern recognition receptors [2]. According to the “danger” model proposed by Matzinger, the immune system is activated by danger signals from injured tissues so that any molecule independently, whether foreign or self, can induce a specific immune response if it is able to alert and activate a specialized APC which in turn expresses costimulatory molecules and promotes T and B cell activation [48, 49]. Local vaccination with recombinant vaccinia virus might provide danger signals more proficiently than systemic vaccination. As a matter of fact, BALB-neuT V-wt im.g. vaccinated mice had a detectable superior tumor-free survival than those vaccinated by s.c. vaccination. Furthermore, the combination of a neu genetic vaccine and novel agonist of TLR9 had potent antitumor activity associated with antibody isotype switch and antibody-dependent cellular cytotoxicity activities. Mice treated with the combination produced greater antibody titers with IgG2a isotype switch and antibody-dependent cellular cytotoxicity activity than did mice treated with the vaccine alone [50]. It was also reported that intratumoral delivery of CpG has advantages in the treatment of tumors [51]. Rituximab, a chimeric monoclonal antibody against the protein CD20, plus intratumoral CpG could eradicate B cell lymphoma from 42% of mice, whereas systemically administered CpG, with or without rituximab, did not achieve tumor eradication [52].
Our findings may have important implications for the design of cancer vaccine protocols for the treatment of breast cancer and other accessible tumors using recombinant vaccinia virus.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study was supported by grants from PRIN and AIRC. We wish to thank Therion Biologics (Cambridge, MA) and Dr. G. Mazzara, which kindly provided vaccinia viruses, IRBM P. Angeletti (Pomezia, Rome) for peptides, and Dr. Eddi Di Marco (Istituto Tumori di Genova) for providing LTR-Neu cells. The authors thank Debra Weingarten for her editorial assistance in the preparation of the manuscript.
References
- 1.Jacobs BL, Langland JO, Kibler KV, et al. Vaccinia virus vaccines: past, present and future. Antiviral Res. 2009;84:1–13. doi: 10.1016/j.antiviral.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kennedy RB, Ovsyannikova IG, Jacobson RM, Poland GA. The immunology of smallpox vaccines. Curr Opin Immunol. 2009;21:314–320. doi: 10.1016/j.coi.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Essajee S, Kaufman HL. Poxvirus vaccines for cancer and HIV therapy. Expert Opin Biol Ther. 2004;4:575–588. doi: 10.1517/14712598.4.4.575. [DOI] [PubMed] [Google Scholar]
- 4.Kaufman HL. The role of poxviruses in tumor immunotherapy. Surgery. 2003;134:731–737. doi: 10.1016/S0039-6060(03)00294-0. [DOI] [PubMed] [Google Scholar]
- 5.Moss B. Genetically engineered poxviruses for recombinant gene expression, vaccination, and safety. Proc Natl Acad Sci U S A. 1996;93:11341–11348. doi: 10.1073/pnas.93.21.11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lechleider RJ, Arlen PM, Tsang KY, et al. Safety and immunologic response of a viral vaccine to prostate-specific antigen in combination with radiation therapy when metronomic-dose interleukin 2 is used as an adjuvant. Clin Cancer Res. 2008;14:5284–5291. doi: 10.1158/1078-0432.CCR-07-5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaufman HL, Kim-Schulze S, Manson K, et al. Poxvirus-based vaccine therapy for patients with advanced pancreatic cancer. J Transl Med. 2007;26(5):60. doi: 10.1186/1479-5876-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gulley J, Chen AP, Dahut W, et al. Phase I study of a vaccine using recombinant vaccinia virus expressing PSA (rV-PSA) in patients with metastatic androgen-independent prostate cancer. Prostate. 2002;53:109–117. doi: 10.1002/pros.10130. [DOI] [PubMed] [Google Scholar]
- 9.Marshall JL, Hoyer RJ, Toomey MA, et al. Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. J Clin Oncol. 2000;18:3964–3973. doi: 10.1200/JCO.2000.18.23.3964. [DOI] [PubMed] [Google Scholar]
- 10.Scholl SM, Balloul JM, Le Goc G, et al. Recombinant vaccinia virus encoding human MUC1 and IL2 as immunotherapy in patients with breast cancer. J Immunother. 2000;23:570–580. doi: 10.1097/00002371-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Mastrangelo MJ, Maguire HC, Lattime EC. Intralesional vaccinia/GM-CSF recombinant virus in the treatment of metastatic melanoma. Adv Exp Med Biol. 2000;465:391–400. doi: 10.1007/0-306-46817-4_34. [DOI] [PubMed] [Google Scholar]
- 12.Conry RM, Allen KO, Lee S, et al. Human autoantibodies to carcinoembryonic antigen (CEA) induced by a vaccinia-CEA vaccine. Clin Cancer Res. 2000;6:34–41. [PubMed] [Google Scholar]
- 13.Conry RM, Khazaeli MB, Saleh MN, et al. Phase I trial of a recombinant vaccinia virus encoding carcinoembryonic antigen in metastatic adenocarcinoma: comparison of intradermal versus subcutaneous administration. Clin Cancer Res. 1999;5:2330–2337. [PubMed] [Google Scholar]
- 14.Wallack MK, Sivanandham M, Balch CM, et al. Surgical adjuvant active specific immunotherapy for patients with stage III melanoma: the final analysis of data from a phase III, randomized, double-blind, multicenter vaccinia melanoma oncolysate trial. J Am Coll Surg. 1998;187:69–77. doi: 10.1016/S1072-7515(98)00097-0. [DOI] [PubMed] [Google Scholar]
- 15.Kim-Schulze S, Kim HS, Wainstein A, et al. Intrarectal vaccination with recombinant vaccinia virus expressing carcinoembronic antigen induces mucosal and systemic immunity and prevents progression of colorectal cancer. J Immunol. 2008;181:8112–8119. doi: 10.4049/jimmunol.181.11.8112. [DOI] [PubMed] [Google Scholar]
- 16.Kaufman HL, Cohen S, Cheung K, et al. Local delivery of vaccinia virus expressing multiple costimulatory molecules for the treatment of established tumors. Hum Gene Ther. 2006;17:239–244. doi: 10.1089/hum.2006.17.239. [DOI] [PubMed] [Google Scholar]
- 17.Hörig H, Kaufman HL. Local delivery of poxvirus vaccines for melanoma. Semin Cancer Biol. 2003;13:417–422. doi: 10.1016/j.semcancer.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Kaufman HL, DeRaffele G, Divito J, et al. A phase I trial of intralesional rV-Tricom vaccine in the treatment of malignant melanoma. Hum Gene Ther. 2001;12:1459–1480. doi: 10.1089/104303401750298616. [DOI] [PubMed] [Google Scholar]
- 19.Gomella LG, Mastrangelo MJ, McCue PA, Maguire HC, Jr, Mulholland SG, Lattime EC. Phase I study of intravescical vaccinia virus as a vector for gene therapy of bladder cancer. J Urol. 2001;166:1291–1295. doi: 10.1016/S0022-5347(05)65755-2. [DOI] [PubMed] [Google Scholar]
- 20.Kudo-Saito C, Schlom J, Hodge JW. Induction of an antigen cascade by diversified subcutaneous/intratumoral vaccination is associated with antitumor responses. Clin Cancer Res. 2005;11:2416–2426. doi: 10.1158/1078-0432.CCR-04-1380. [DOI] [PubMed] [Google Scholar]
- 21.Kudo-Saito C, Schlom J, Hodge JW. Intratumoral vaccination and diversified subcutaneous/intratumoral vaccination with recombinant poxviruses encoding a tumor antigen and multiple costimulatory molecules. Clin Cancer Res. 2004;10:1090–1099. doi: 10.1158/1078-0432.CCR-03-0145. [DOI] [PubMed] [Google Scholar]
- 22.Crittenden MR, Thanarajasingam U, Vile RG, Gough MJ. Intratumoral immunotherapy: using the tumour against itself. Immunology. 2005;114:11–22. doi: 10.1111/j.1365-2567.2004.02001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufman HL, Kim DW, Deraffele G, Mitcham J, Coffin RS, Kim-Schulze S. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann Surg Oncol. 2010;17:718–730. doi: 10.1245/s10434-009-0809-6. [DOI] [PubMed] [Google Scholar]
- 24.Yang AS, Monken CE, Lattime EC. Intratumoral vaccination with vaccinia-expressed tumor antigen and granulocyte macrophage colony-stimulating factor overcomes immunological ignorance to tumor antigen. Cancer Res. 2003;63:6956–6961. [PubMed] [Google Scholar]
- 25.Wright P, Zheng C, Moyana T, Xiang J. Intratumoral vaccination of adenoviruses expressing fusion protein RM4/tumor necrosis factor (TNF)-alpha induces significant tumor regression. Cancer Gene Ther. 1998;5:371–379. [PubMed] [Google Scholar]
- 26.Boggio K, Nicoletti G, Di Carlo E, et al. Interleukin 12-mediated prevention of spontaneous mammary adenocarcinomas in two lines of Her-2/neu transgenic mice. J Exp Med. 1998;188:589–596. doi: 10.1084/jem.188.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rovero S, Amici A, Di Carlo E, et al. DNA vaccination against rat her-2/Neu p185 more effectively inhibits carcinogenesis than transplantable carcinomas in transgenic BALB/c mice. J Immunol. 2000;165:5133–5142. doi: 10.4049/jimmunol.165.9.5133. [DOI] [PubMed] [Google Scholar]
- 28.Cavallo F, Offringa R, van der Burg SH, Forni G, Melief CJ. Vaccination for treatment and prevention of cancer in animal models. Adv Immunol. 2006;90:175–213. doi: 10.1016/S0065-2776(06)90005-4. [DOI] [PubMed] [Google Scholar]
- 29.Allen SD, Garrett JT, Rawale SV, et al. Peptide vaccines of the HER-2/neu dimerization loop are effective in inhibiting mammary tumor growth in vivo. J Immunol. 2007;179:472–482. doi: 10.4049/jimmunol.179.1.472. [DOI] [PubMed] [Google Scholar]
- 30.Gallo P, Dharmapuri S, Nuzzo M, et al. Adenovirus vaccination against neu oncogene exerts long-term protection from tumorigenesis in BALB/neuT transgenic mice. Int J Cancer. 2007;120:574–584. doi: 10.1002/ijc.22274. [DOI] [PubMed] [Google Scholar]
- 31.Park JM, Terabe M, Steel JC, et al. Therapy of advanced established murine breast cancer with a recombinant adenoviral ErbB-2/neu vaccine. Cancer Res. 2008;68:1979–1987. doi: 10.1158/0008-5472.CAN-07-5688. [DOI] [PubMed] [Google Scholar]
- 32.Nanni P, Landuzzi L, Nicoletti G, et al. Immunoprevention of mammary carcinoma in HER-2/neu transgenic mice is IFN-gamma and B cell dependent. J Immunol. 2004;173:2288–2296. doi: 10.4049/jimmunol.173.4.2288. [DOI] [PubMed] [Google Scholar]
- 33.Masuelli L, Focaccetti C, Cereda V, et al. Gene-specific inhibition of breast carcinoma in BALB-neuT mice by active immunization with rat Neu or human ErbB receptors. Int J Oncol. 2007;30:381–392. [PubMed] [Google Scholar]
- 34.Bargmann CI, Hung MC, Weinberg RA. The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature. 1986;319:226–230. doi: 10.1038/319226a0. [DOI] [PubMed] [Google Scholar]
- 35.Di Marco E, Pierce JH, Knicley CL, Di Fiore PP. Transformation of NIH 3T3 cells by overexpression of the normal coding sequence of the rat neu gene. Mol Cell Biol. 1990;10:3247–3252. doi: 10.1128/mcb.10.6.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bei R, Budillon A, Masuelli L, et al. Frequent overexpression of multiple ErbB receptors by head and neck squamous cell carcinoma contrasts with rare antibody immunity in patients. J Pathol. 2004;204:317–325. doi: 10.1002/path.1642. [DOI] [PubMed] [Google Scholar]
- 37.Bei R, Kantor J, Kashmiri SV, Abrams S, Schlom J. Enhanced immune responses and anti-tumor activity by baculovirus recombinant carcinoembryonic antigen (CEA) in mice primed with the recombinant vaccinia CEA. J Immunother Emphasis Tumor Immunol. 1994;16:275–282. doi: 10.1097/00002371-199411000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Bei R, Guptill V, Masuelli L, et al. The use of a cationic liposome formulation (DOTAP) mixed with a recombinant tumor-associated antigen to induce immune responses and protective immunity in mice. J Immunother. 1998;21:159–169. doi: 10.1097/00002371-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Gown AM, Willingham MC. Improved detection of apoptotic cells in archival paraffin sections: immunohistochemistry using antibodies to cleaved caspase 3. J Histochem Cytochem. 2002;50:449–454. doi: 10.1177/002215540205000401. [DOI] [PubMed] [Google Scholar]
- 40.Masuelli L, Trono P, Marzocchella L, et al. Intercalated disk remodeling in delta-sarcoglycan-deficient hamsters fed with an alpha-linolenic acid-enriched diet. Int J Mol Med. 2008;21:41–48. [PubMed] [Google Scholar]
- 41.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.20.3.719. [DOI] [PubMed] [Google Scholar]
- 42.Nahta R, Esteva FJ. Herceptin: mechanisms of action and resistance. Cancer Lett. 2006;232:123–138. doi: 10.1016/j.canlet.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 43.Disis ML, Wallace DR, Gooley TA, et al. Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer. J Clin Oncol. 2009;27:4685–4692. doi: 10.1200/JCO.2008.20.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Disis ML, Schiffman K. Cancer vaccines targeting the HER2/neu oncogenic protein. Semin Oncol. 2001;28(6 Suppl 18):12–20. doi: 10.1053/sonc.2001.29721. [DOI] [PubMed] [Google Scholar]
- 45.Denkers EY, Badger CC, Ledbetter JA, Bernstein ID. Influence of antibody isotype on passive serotherapy of lymphoma. J Immunol. 1985;135:2183–2186. [PubMed] [Google Scholar]
- 46.Nanni P, Nicoletti G, De Giovanni C, et al. Combined allogeneic tumor cell vaccination and systemic interleukin 12 prevents mammary carcinogenesis in HER-2/neu transgenic mice. J Exp Med. 2001;194:1195–1205. doi: 10.1084/jem.194.9.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rolla S, Nicolo C, Malinarich S, Orsini M, et al. Distinct and non-overlapping T cell receptor repertoires expanded by DNA vaccination in wild-type and HER-2 transgenic BALB/c mice. J Immunol. 2006;177:7626–7633. doi: 10.4049/jimmunol.177.11.7626. [DOI] [PubMed] [Google Scholar]
- 48.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 49.Bei R, Masuelli L, Palumbo C, et al. A common repertoire of autoantibodies is shared by cancer and autoimmune disease patients: inflammation in their induction and impact on tumor growth. Cancer Lett. 2009;281:8–23. doi: 10.1016/j.canlet.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 50.Aurisicchio L, Peruzzi D, Conforti A, et al. Treatment of mammary carcinomas in HER-2 transgenic mice through combination of genetic vaccine and an agonist of Toll-like receptor 9. Clin Cancer Res. 2009;15:1575–1584. doi: 10.1158/1078-0432.CCR-08-2628. [DOI] [PubMed] [Google Scholar]
- 51.Mastini C, Becker PD, Iezzi M, et al. Intramammary application of non-methylated-CpG oligodeoxynucleotides (CpG) inhibits both local and systemic mammary carcinogenesis in female BALB/c Her-2/neu transgenic mice. Curr Cancer Drug Targets. 2008;8:230–242. doi: 10.2174/156800908784293604. [DOI] [PubMed] [Google Scholar]
- 52.Betting DJ, Yamada RE, Kafi K, et al. Intratumoral but not systemic delivery of CpG oligodeoxynucleotide augments the efficacy of anti-CD20 monoclonal antibody therapy against B cell lymphoma. J Immunother. 2009;32:622–631. doi: 10.1097/CJI.0b013e3181ab23f1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.