Abstract
Xerogel films were synthesized via sol-gel chemistry to fabricate optical nitroxyl (HNO) sensors. Selective detection of HNO in solution was achieved by monitoring the rates of manganese(III) meso-tetrakis(4-sulfonatophenyl) porphyrinate (MnIIITPPS) reductive nitrosylation in the anaerobic interior of aminoalkoxysilane-derived xerogel films. Nitroxyl permeability in sensor films deposited in round-bottom 96-well plates was enhanced via incorporation of trimethoxysilyl-terminated poly(amidoamine-organosilicon) (PAMAMOS) dendrimers in the xerogel network. The selectivity of MnIIITPPS for HNO, the overall sensitivity, and the working dynamic range of the resulting sensors were characterized. The HNO-sensing microtitre plates were used to quantify pH-dependent HNO generation by the recently described HNO-donor sodium-1-(isopropylamino)diazene-1-ium-1,2-diolate (IPA/NO), and compare HNO-production efficiency between IPA/NO and Angeli’s salt, a traditional HNO-donor.
INTRODUCTION
Nitrogen oxide compounds have become a topic of considerable interest in medicinal and biological chemistry.1 Nitric oxide (NO) is the most widely studied and well-known of these molecules, and multiple reports have catalogued the role of NO in numerous physiological processes, including vasodilation, neurotransmission, wound-healing, and thrombosis.2 As the effects of NO on physiology become better understood, more attention has focused on structurally similar nitrogen oxide compounds that may also have direct biochemical effects and/or function as intermediates in the NO-signaling cascade. Nitroxyl (HNO), the one electron reduced congener of NO, is one such molecule that has displayed a number of interesting physiological effects.3 Nitroxyl’s high reactivity with thiols and metalloproteins has led to potential pharmaceutical application in protective preconditioning of ischemic injury,4 treatment for alcohol abuse through targeted inhibition of aldehyde dehydrogenase,5, 6 and as an efficient means of enhancing left ventricular contractility in victims of congestive heart failure.7 These discoveries have led to efforts focusing on the design and development of new HNO-donor molecules and prodrugs.8–1 Research in this area has been hindered by an inability to easily monitor HNO. Indeed, no simple method currently exists for the quantitative determination of HNO in solution. Herein, we seek to address this issue through the development of optical sensors designed for the rapid quantitative screening of HNO released by nitroxyl-donor molecules.
Despite their similarities, HNO and NO have distinct chemical properties and reactivity. The relatively high standard reduction potentials for the formation of HNO from NO (< −0.4 V vs. NHE)12 preclude the creation of HNO from NO under most physiological conditions. Conversely, while the oxidation of nitroxyl to NO would be expected to occur readily in vivo, this has not been observed. Oxidation to NO occurs most readily from nitroxyl’s triplet-state anion form (NO−) which is not present in significant concentration at physiological pH due to a pKa > 11.13 The ground spin states of HNO and NO− are singlet and triplet, respectively, making proton transfer between the two species spin-forbidden and kinetically slow.13 The recently described biological activities of HNO-donor compounds thus reflect reactivity of the HNO molecule itself and not a transformation to NO.
Knowledge of HNO chemistry lags behind that of other nitrogen oxides such as NO, which can in large part be explained by HNO’s greater reactivity and difficulties in its handling and quantitation. Nitroxyl in solution undergoes dimerization to hyponitrous acid followed by rapid dehydration and the formation of nitrous oxide (N2O):
| (1) |
The study and application of HNO must therefore be accomplished by in situ generation of nitroxyl by HNO-donor molecules such as Angeli’s salt (Na2N2O3) which decomposes upon protonation to form HNO and nitrite:
| (2) |
The situation is further complicated by the lack of a simple means to detect and quantify HNO. While specialized techniques such as infrared chemiluminescence may directly assay HNO,14 such procedures are impractical in most laboratories. More commonly, indirect screening methods for HNO are employed. Gas chromatographic detection of the N2O dimerization product is often viewed as a marker of HNO formation, but may be misleading as alternate NO reaction pathways not involving nitroxyl also result in the production of N2O.15, 16 A more selective strategy reported by Donzelli et al. employed the rapid reaction of HNO with thiols as a marker of HNO generation in biological systems.17 Treatment of HNO producing systems with glutathione resulted in the formation of a sulfinamide reaction product that was subsequently detected via HPLC. Another commonly employed strategy involves reaction of HNO with ferric heme-containing proteins such as metmyoglobin.18 In the presence of HNO, ferric hemes undergo reductive nitrosylation, resulting in detectable shifts in the absorbance spectrum of the enzyme. This reaction provides a highly selective means of screening for HNO generation, but the resulting FeII(NO) complex is unstable in aerobic conditions and readily undergoes reoxidation. Ferric porphyrin analogues have been reported that display similar absorbance shifts upon reaction with HNO.19 While easier to handle than the bulky metmyoglobin protein, ferric porphyrins suffered from the same oxygen instability and also react with NO, a common interferent in HNO-generating systems. Recently, Marti et al. reported on the kinetics of reductive nitrosylation of manganese porphyrins via HNO.20 The reaction of manganese(III) meso-tetrakis(4-sulfonatophenyl) porphyrinate (MnIIITPPS; Figure 1A) with nitroxyl:
| (3) |
resulted in strong shifts in the absorbance spectrum and displayed no cross-reactivity with NO or catalytic decomposition of the Angeli’s salt HNO donor compound. This molecule may thus provide a simple and robust alternative to metmyoglobin for the selective detection of HNO. Unfortunately, the resultant MnII(NO)TPPS complex was found to be unstable in the presence of O2 and rapidly oxidized back to MnIII. The instability of the MnII(NO) complex in aerated solution makes it difficult to quantitatively determine HNO concentrations. Complex formation/oxidation is slower than the HNO dimerization rate (8 × 106 M−1 s−1),13 and thus a measured absorbance is not reflective of an instantaneous HNO concentration. As such, HNO complexation at metal centers has been limited to nonquantitative screening procedures and qualitative tests for the presence of HNO.
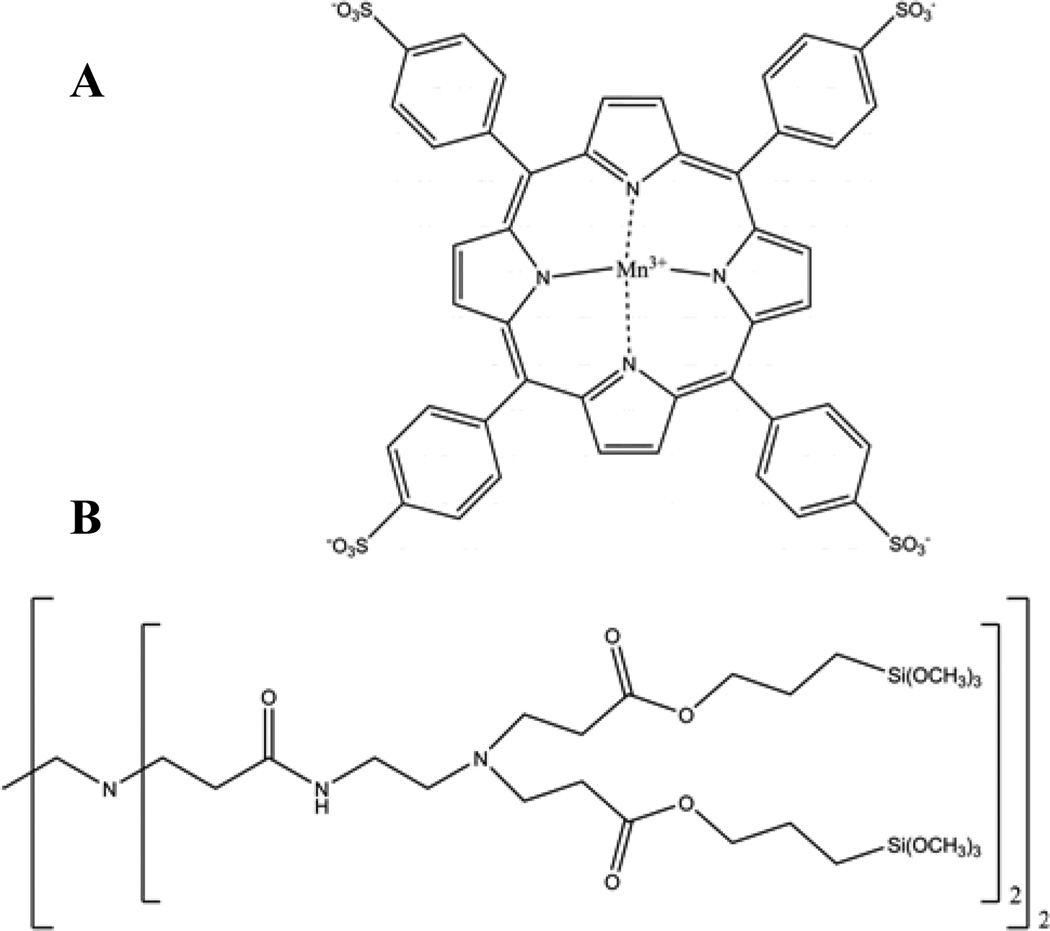
Figure 1.
Chemical structures of (A) MnIIITPPS and (B) generation-zero trimethoxysilyl-terminated PAMAMOS dendrimers.
Herein, we seek to develop optical sensor coatings suitable for quantitative determination of HNO in solution by increasing the stability of the MnII(NO)TPPS complex through encapsulation in an aminoalkoxysilane-based xerogel matrix. Xerogels are polymeric materials formed through the catalytic hydrolysis and condensation of alkoxy-terminated silane precursors.21 While NO diffusion through aminoalkoxysilane-derived xerogels has been shown to occur readily, oxygen permeability in these materials is quite low.22 Encapsulation of MnIIITPPS inside aminoalkoxysilane-xerogel films is thus expected to permit HNO diffusion while creating a localized anaerobic environment for the porphyrin, slowing oxidation of the MnII(NO)TPPS complex. Over short periods, HNO complexation by MnIIITPPS in these films may be considered largely irreversible, resulting in the creation of a cumulative HNO trap. By utilizing the detected rate of intra-film MnII(NO)TPPS formation, a kinetic strategy may be employed to estimate HNO concentrations. We have optimized aminoalkoxysilane-xerogel film properties for this application through the inclusion of trimethoxysilyl-terminated poly(amidoamine-organosilicon) (PAMAMOS) dendrimers (Figure 1B). Such dendrimers create hydrophilic nanodomains in the material and speed HNO diffusion through the membrane, increasing sensitivity and allowing application of the sensor films in a convenient 96-well plate format. The resulting HNO-sensing microtitre plates were used to quantify HNO and confirm the pH-dependent nature of nitroxyl generation from a recently described HNO/NO donor compound, sodium-1-(isopropylamino)diazene-1-ium-1,2-diolate (IPA/NO).23–25
EXPERIMENTAL SECTION
Materials
Manganese(III) meso-tetrakis(4-sulfonatophenyl) porphine chloride (MnIIITPPS) was purchased from Frontier Scientific (Logan, UT). (Aminoethylaminomethyl)phenethyltrimethoxysilane (AEMP3) was purchased from Gelest (Tullytown, PA). Methyltrimethoxysilane (MTMOS) and diethylenetriamine-pentaacetic acid (DTPA) were purchased from Fluka. Generation zero (G-0) trimethoxysilyl-terminated PAMAMOS dendrimer (10 wt% in isopropanol) was purchased from Aldrich (Milwaukee, WI). Angeli’s Salt (disodium diazen-1-ium-1,2,2-triolate; Na2N2O3) was purchased from Cayman Chemical (Ann Arbor, MI). Sodium-1-(isopropylamino)diazene-1-ium-1,2-diolate (IPA/NO) was provided as a generous gift from Dr. Katrina Miranda at the University of Arizona. Nitric oxide (99.5%) was purchased from National Welders Supply (Durham, NC). Untreated polystyrene round-bottom 96-well microtitre plates were purchased from Fisher Scientific. Other solvents and chemicals were analytical-reagent grade and used as received. Distilled water was purified to 18.2 MΩ·cm with a Millipore Milli-Q Gradient A-10 water purification system (Bedford, MA).
Response of MnIIITPPS to NO and HNO
The selectivity of HNO binding over NO by MnIIITPPS in aerobic conditions was examined in a PerkinElmer Lamba 40 UV/Vis spectrophotometer (Norwalk, CT). A ~0.01 M stock solution of the Angeli’s salt HNO donor was prepared in 0.01 M NaOH and stored on ice until use. A deoxygenated, saturated NO stock solution (~1.9 mM) was created by sparging 30 mL pH 7.4 10 mM phosphate-buffered saline (PBS) with Ar for 40 min, and then bubbling with NO for 2 h. Aliquots (20–100µL) of each solution were mixed with 2 mL 5.5 µM MnIIITPPS in 10 mM PBS at room temperature, and the visible absorbance spectrum of the resulting solution was monitored for ~2 h.
Xerogel Film Preparation
Nitroxyl-sensing films were first evaluated as thin films on glass slides using a previously described 20/80% (v:v total silane) AEMP3/MTMOS aminoalkoxysilane-xerogel formulation.22, 26 Briefly, MTMOS (160 µL) and ethanol (200 µL) were combined with 0.25 – 2 mM (aq) MnIIITPPS solution (11 µL). After 5 min sonication, AEMP3 (40 µl) was added and sonicated for an additional 5 min. The resulting sol was cast in 10 – 30 µL aliquots onto 9 × 25 mm2 glass substrates that had been previously sonicated 20 min in ethanol, dried under N2, and UV/O3 cleaned for 20 min in a BioForce TipCleaner (Ames, IA). The resulting films were allowed to dry a minimum of 3 d in ambient conditions prior to subsequent characterization.
Film Characterization
Film depths and the corresponding volume of cured xerogel on glass slides were determined using a Tencor Alpha-Step 200 Profilometer (San Jose, CA). Spectral characterization of MnIIITPPS encapsulated in AEMP3/MTMOS films was performed by affixing the slides inside PBS-containing (2 mL) cuvettes normal to the light path of the spectrophotometer. The extinction coefficient of immobilized MnIIITPPS was determined by plotting the measured 467 nm absorbance of xerogel films (depth ~29 µm) containing 0.32 – 2.55 × 10−7 moles/cm3 MnIIITPPS versus porphyrin concentration. Aliquots of 10–100 µL Angeli’s salt stock solution (~0.01 M in 10 mM NaOH) were added to pH 7.4 PBS to a final volume of 2.5 mL. Nitroxyl-sensing xerogel films that had been previously hydrated in PBS for a minimum of 20 min were placed in cuvettes with 2 mL of the Angeli’s salt/PBS solution. The rates of MnII(NO)TPPS formation and oxidation in AEMP3/MTMOS films were determined by monitoring changes in the absorbance maxima of MnIIITPPS and MnII(NO)TPPS at 467 and 432 nm, respectively, for ~2 h. The concentrations of Angeli’s salt stock solutions were determined via Beer’s law prior to each experiment by monitoring the absorbance at 250 nm (ε = 8000 M−1 cm−1).27
Kinetic HNO Quantification
Stabilization of the MnII(NO)TPPS complex in AEMP3/MTMOS sensor films provides a means for kinetic quantification of HNO in solution. By eliminating the uncertain rate of competitive complex oxidation, steady-state reaction kinetics for HNO-donor systems are greatly simplified. Calibration with a well-characterized HNO-donor such as Angeli’s salt under controlled reaction conditions allows determinations of an experimental kinetic coefficient (kon) for MnII(NO)TPPS formation in xerogel films. The resulting rate constant may then be used to extrapolate solution HNO concentrations generated by alternate HNO-donors. In our system, three kinetic expressions (Eq. 4 – 6) were considered during calibration, corresponding to Angeli’s salt decomposition (Eq. 2), MnII(NO)TPPS formation (Eq. 3), and HNO scavenging through dimerization (Eq. 1):
| (4) |
| (5) |
| (6) |
Application of steady-state kinetics to Eq. 4 – 6 yields:
| (7) |
where k1[AS] is the rate of Angeli’s salt decomposition, and k2 is the kinetic coefficient of the HNO dimerization reaction (8 × 106 M−1 s−1).13 Angeli’s salt decomposition rates were determined in PBS by monitoring the decrease in 250 nm absorbance over 60 min and fitting to an exponential decay model (absorbance = Ae−kt + c).28 For the experimental conditions employed herein (25 °C, pH 7.4, 10−4 DTPA, aerobic), k1 was calculated at 8.1 × 10−4 s−1. After determination of kon via Angeli’s salt calibration, the observed rates of MnII(NO)TPPS formation were used to calculate HNO concentrations according to:
| (8) |
where [MnII(NO)TPPS] and [MnIIITPPS] concentrations in the film were monitored spectroscopically at 432 and 467 nm, respectively. Considering xerogel film thicknesses (~29 µm) as the optical path length, the extinction coefficient of MnIIITPPS encapsulated in AEMP/MTMOS was calculated to be 90000 M−1 cm−1 at 467 nm via Beer’s law. Although complete conversion of MnIIITPPS to MnII(NO)TPPS was not attainable even with large excesses of Angeli’s salt, the differential rates of absorbance decrease at 467 nm and increase at 432 nm were used to estimate ε ~ 160000 M−1 cm−1 at 432 nm for MnII(NO)TPPS.
Xerogel-coating of 96-well Plates
Nitroxyl-sensing xerogel films were optimized for deposition in 96-well plates. Sols were created using the general reaction scheme described above and varying the following: the volume and identity of solvent employed, including methanol, ethanol, isopropanol, and n-butanol; the concentration of aqueous MnIIITPPS solution included in the sol (2–8 mM); and the volume percentage of AEMP3 to MTMOS employed (10–50%). Aliquots (4–10 µL) of the resulting sols were pipetted into the bottom of round-bottom 96-well polystyrene microtitre plates and allowed to cure under ambient conditions. Xerogel films were evaluated for their transparency, smoothness, stability, and detectable absorbance at 467 nm. Film thickness was monitored via optical microscopy using a Zeiss Axiovert 200 inverted microscope.
Microtitre Plate Sensor Optimization
Sols containing 40/60% (v:v) AEMP/MTMOS and ~0–2% PAMAMOS dendrimer (v:v total silane) were created by shaking MTMOS (120 µL), 10% (w:w) PAMAMOS dendrimer in isopropanol (0 – 40 µL), methanol (420-380 µL; total methanol and PAMAMOS volume = 420 µL), and 4mM (aq) MnIIITPPS (11 µl) for 10 min, followed by the addition of AEMP3 (80 µL) and a subsequent 5 min additional shaking. Aliquots (4–10 µL) of the resulting sol were pipetted into wells and allowed to cure for a minimum of 3 d under ambient conditions. Initial screening procedures monitoring the rate of MnII(NO)TPPS complex formation in the gel were performed using a Labsystems Multiskan RC plate reader outfitted with 430 and 467 nm narrow bandpass interference filters (10 nm full-width at half max) purchased from Edmund Optics (Barrington, NJ). All wells were soaked with 50–100 µL PBS for a minimum of 3 h prior to use to achieve a steady 430 nm baseline absorbance. To initiate HNO generation, 40 µL of the initial Angeli’s salt stock solution was mixed and shaken with 1960 µL pH 7.4 PBS containing 100 µM DTPA for 30 s. The pre-soaked wells were emptied and 10–100 µL of the Angeli’s salt-PBS solution and a balance PBS/DTPA was added to wells for a total volume of 100 µL. To ensure accurate kinetics, care was taken so that timing remained consistent. The HNO-sensing wells were loaded and the recording of kinetic absorbance data began at exactly 40 and 60 s, respectively, after initial mixing of Angeli’s salt and PBS. The absorbance at 430 nm, corresponding to formation of MnII(NO)TPPS in the films, was monitored every 10 s for 15 min. Initial rates were obtained from the slope of linear fits of the first 5 min of collected data. Only fits with R2 ≥ 0.99 were retained for subsequent calculations. After optimization, characterization of the most favorable sensor formulation’s dynamic range and sensitivity was performed following the same procedures with a monochromator-equipped Molecular Devices Spectramax 340PC plate reader (Sunnyvale, CA) capable of monitoring both 432 and 467 nm absorbance maxima simultaneously. Baseline absorbance from control xerogel films containing no MnIIITPPS was subtracted from all measurements.
Oxygen Permeability Testing
The permeability of oxygen (O2) through PAMAMOS-modified HNO-sensing xerogels was examined by measuring O2 reduction at xerogel-coated electrodes. Sols containing 40/60% (v:v total silane) AEMP3/MTMOS with ~0, 0.25, 0.5, 1.0, and 2.0 % (v:v total silane) trimethoxysilyl-terminated PAMAMOS dendrimer were prepared using the formula described above. Control MTMOS sols containing no AEMP3 were synthesized by shaking MTMOS (750 µL) with 0.1 M HCl (140 µL) for 10 min. Platinum electrodes (2 mm dia.) were mechanically polished with a 0.05 µM alumina slurry, rinsed, sonicated in water for 15 min, coated with 2 µL of the corresponding sol and allowed to dry for three days. The diffusion of O2 through the xerogel films as a function of PAMAMOS inclusion was examined with a CH Instruments 660A potentiostat (Austin, TX). A three-electrode system consisting of the xerogel-modified Pt electrode, Pt wire auxiliary electrode (0.5 mm), and a Ag/AgCl (3.0 M KCl) reference electrode was used to monitor O2 reduction. Electrodes placed in 40 mL of PBS (100 µM DTPA, pH 7.4) with constant stirring were exposed to atmospheric levels of O2. Current was recorded at an applied potential of −0.65 V vs. Ag/AgCl with a sampling frequency of 1 Hz. Xerogel permeability to oxygen was determined by taking the ratio of the peak current for O2 for the xerogel-coated electrode to that of the corresponding uncoated electrode. Coated electrodes were soaked in PBS (100 µM DTPA, pH 7.4) for 3 h prior to the electrochemical experiments to mimic hydration conditions of the HNO sensing films at time of use.
HNO-Generation from IPA/NO
Generation of HNO from an IPA/NO nitroxyl donor compound was quantified using the developed 96-well microtitre plate sensing scheme. Sols containing 40/60% (v:v total silane) AEMP3/MTMOS and ~0.25% PAMAMOS dendrimer (v:v total silane) were prepared and pre-hydrated as described above. An IPA/NO stock solution was prepared by dissolving 29.3 mg IPA/NO in 1 mL 0.01 M NaOH. The solution was stored at −20 °C until use. Immediately prior to measurement, 80 µL of the IPA/NO stock was added to 1.92 mL 10 mM PBS/100µM DTPA, and shaken for 30 s. Using the strategy described above, 25–100 µL of the IPA/NO-PBS solution was added to wells containing a balance of PBS/DTPA buffer for a total volume of 100 µL. The formation of MnII(NO)TPPS from MnIIITPPS was monitored by measuring the absorbance at 432 and 467 nm, respectively. The effect of pH on HNO production was examined by adjusting the starting pH of the PBS solution employed prior to mixing (pH 3–10). To compensate for the poor buffering capacity of PBS outside the neutral pH region, final solution pH was determined after IPA/NO stock addition. The concentrations of IPA/NO stock solutions were determined prior to each experiment by monitoring absorbance at 250 nm (ε = 10000 M−1 cm−1).25
RESULTS AND DISCUSSION
MnIIITPPS HNO Selectivity
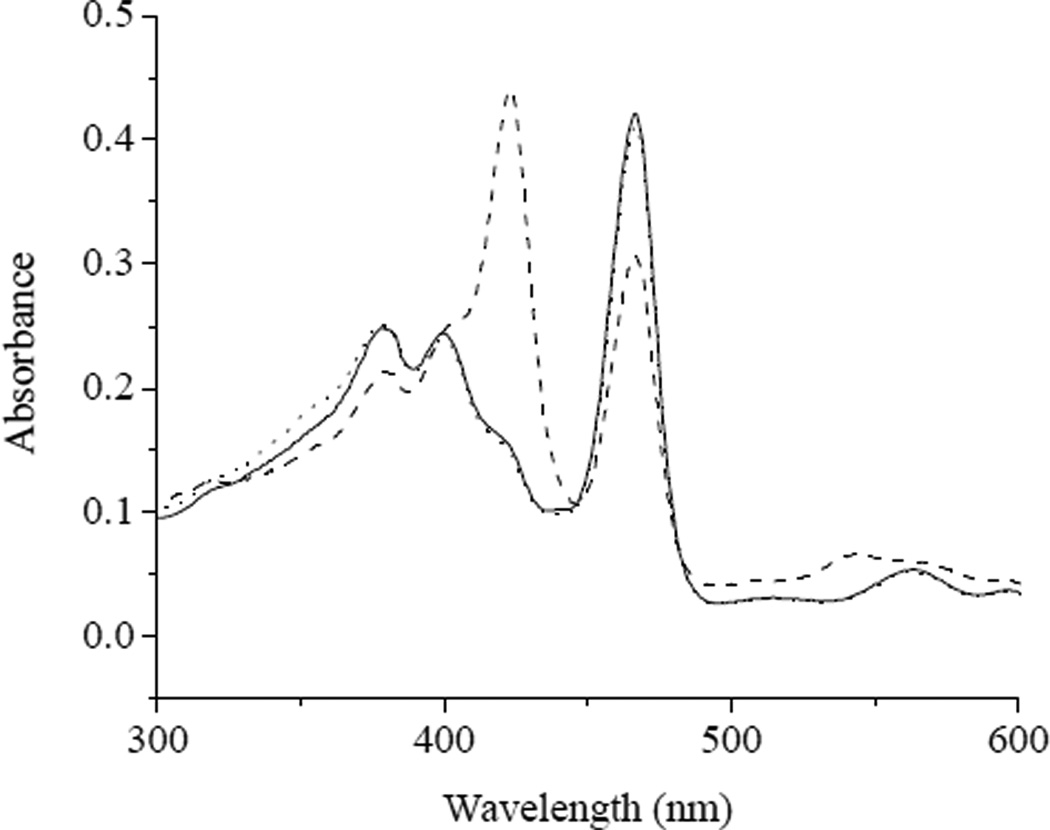
The selectivity of MnIIITPPS for complexation of HNO over NO in oxygenated media was examined directly using a UV/Vis spectrophotometer. As expected, mixing MnIIITPPS with HNO generated via Angeli’s salt resulted in a rapid increase in the absorbance of the solution at 423 nm and a concurrent decrease in absorbance at 467 nm, corresponding to the formation of MnII(NO)TPPS from MnIIITPPS (Figure 2). Maximum 423 nm absorbance occurred within 5 min of HNO addition and then slowly decreased to baseline absorbance within 90 min. Negligible absorbance change was observed upon addition of saturated NO solution, indicating that MnIIITPPS retains its selectivity for HNO in aerobic solution conditions.
Figure 2.
The UV/vis spectral response of (——) MnIIITPPS in pH 7.4 PBS upon exposure to 100 µM (---) Angeli’s salt and (···) NO. Spectra were recorded 8 min after the addition of Angeli’s salt or NO.
MnII(NO)TPPS Formation in Xerogel Films
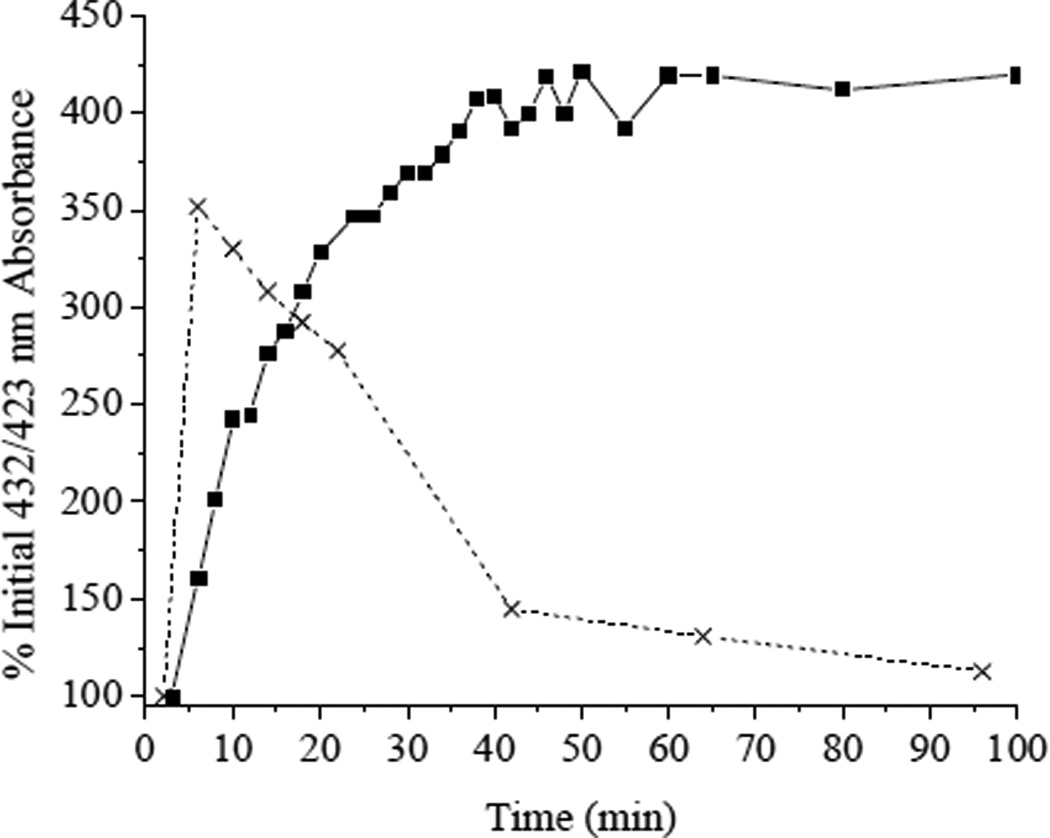
Amine-terminated xerogels have previously been used in the production of multiple optical (e.g., pH),29 amperometric (e.g., CO2, NO)30, 31 and enzyme-based (e.g. glucose)32, 33 sensors and biosensors. While the diffusion of NO through these materials was rapid, the intrinsic permeability of O2, a highly nonpolar molecule, was quite low.34 Robbins and co-workers measured oxygen permeability at xerogel-coated electrodes, and reported that the oxygen permeability at a Pt electrode coated with 20/80% (v:v total silane) AEMP3/MTMOS was diminished by ~97% compared to a bare electrode.22 By encapsulating MnIIITPPS in this material we thus sought to provide an anaerobic localized environment for the porphyrin, slowing reoxidation of the MnII(NO)TPPS complex formed through reaction with HNO. Spectroscopic observation of MnIIITPPS encapsulated in an AEMP3/MTMOS xerogel revealed the presence of both the expected absorbance maxima at 467 nm and a second unidentified peak centered at 445 nm. Upon immersion of the film in PBS, the 445 nm peak disappeared rapidly concomitant with a rise in 467 nm absorbance. This behavior may be attributed to temporary association of the Mn center with surface silanols or amines present in the dry xerogel film. All films were hydrated a minimum of 20 min prior to HNO exposure to overcome this effect. Exposure of xerogel-encapsulated MnIIITPPS to saturated NO solution elicited no detectable absorbance change. Conversely, upon introduction of HNO, a rise and fall of absorbance maxima at 432 and 467 nm, respectively, were observed. While red-shifted compared to the 423 nm absorbance maximium observed in solution, the correlation between the 432 and 467 nm maxima is a strong indication that 432 nm absorbance may be assigned to MnII(NO)TPPS formation via reductive nitrosylation of MnIIITPPS. As shown in Figure 3, encapsulation of the porphyrin in AEMP3/MTMOS xerogel resulted in a dramatic decrease in MnII(NO)TPPS oxidation. While MnII(NO)TPPS formation was slowed relative to in solution, the resulting complex remained stable over the time course of the experiment (2 h), indicating that MnIIITPPS in AEMP3/MTMOS functions largely as a cumulative HNO trap over short periods.
Figure 3.
MnII(NO)TPPS formation in (■) AEMP3/MTMOS or (x) free in aerobic PBS as measured by absorbance at 432 or 423 nm, respectively, after the addition of 100 µM Angeli’s salt.
Microtitre Plate Sensor Optimization via PAMAMOS
Long-term exposure (>24 h) of HNO-sensing films to media containing ambient O2 concentrations was found to regenerate MnIIITPPS and restore initial absorbance maxima. However, the largely irreversible short-term nature of HNO complexation in AEMP3/MTMOS films makes HNO quantification with a single film impractical for analytical measurements. To maximize the utility of HNO-sensing films, xerogel coatings were optimized for use in a 96-well microtitre plate format. Such a configuration allows a large number of HNO assays to be run in parallel while permitting calibration to be performed at the time of analysis to account for variations in individual films and changes that may occur in the xerogel with time. Curing of sols deposited in flat-bottom polystyrene microtitre plates resulted in clustering of xerogel around the edges of the well and the formation of concave films. More uniform coatings were achieved using round-bottom plates. In general, the reduced sensitivity of the microtitre plate reader format versus the UV/Vis spectrophotometer required thicker xerogel layers and larger MnIIITPPS concentrations to bring measured absorbance values above baseline noise. Several solvents and reaction conditions were evaluated to form reproducibly thicker, crack-free xerogel films in the round-bottom wells. Ultimately, a mixture of 40/60% (v:v total silane) AEMP3/MTMOS, 4mM MnIIITPPS (aq) and methanol (co-solvent) provided the proper balance between sol-gel condensation rates and solvent evaporation, resulting in smooth, uniform films of adequate thickness (~90–140 µm, for 6–10 µL sol cast).
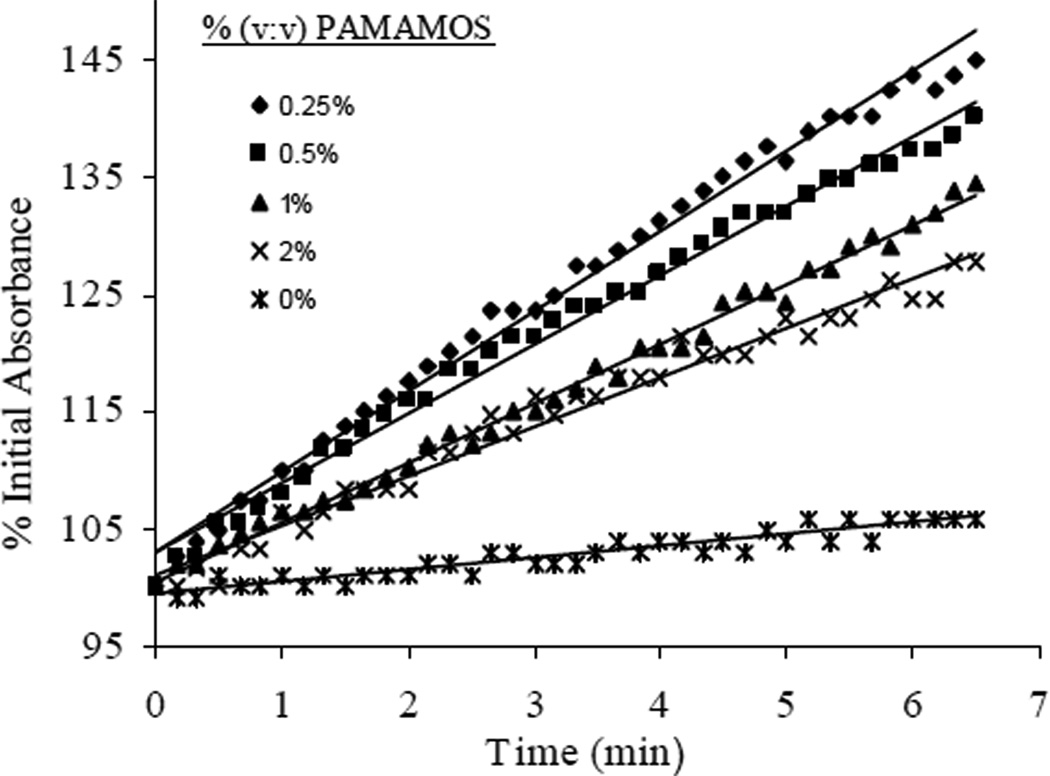
Unfortunately, nitroxyl diffusion through pure AEMP3/MTMOS films proved inadequate for the sensitivity constraints imposed by the 96-well plate format. To increase the rate of MnII(NO)TPPS formation, trimethoxysilyl-terminated PAMAMOS dendrimers was evaluated as a means of selectively enhancing HNO permeability through the xerogel. Dvornic and co-workers previously reported on the utility of such macromolecules to form honeycomb-like hydrophilic-nanodomained networks via sol-gel hydrolysis and condensation.35, 36 The generation-zero PAMAMOS dendrimers employed are characterized by a branched hydrophilic polyamidoamine interior surrounded by 8 terminal trimethoxysilyl groups that enable their incorporation into the surrounding xerogel structure via condensation. By incorporating PAMAMOS dendrimers as bridging molecules in the surrounding AEMP3/MTMOS network, hydrophilic pockets were created in the final xerogel, providing preferential enhancement of HNO diffusion over the more lipophilic O2 molecule. The percentage change in 430 nm absorbance observed from xerogel films containing ~0.25–2.0% (v:v total silane) PAMAMOS dendrimer upon introduction of Angeli’s salt is shown in Figure 4. In films containing ~0.25% (v:v total silane) PAMAMOS, the initial rates of MnII(NO)TPPS formation increased by nearly an order of magnitude versus rates measured in pure AEMP3/MTMOS films. Surprisingly, increasing the percentages of PAMAMOS >0.25% resulted in an overall decrease in the rate of MnII(NO)TPPS formation measured in the films. This effect may be attributed to increased competition with oxygen as larger amounts of the PAMAMOS bridge would lead to less dense crosslinking of the alkoxysilane precursors and thus a more open xerogel network. Indeed, macroscopic holes were observed in multiple wells at percentages of PAMAMOS inclusion above 2%, likely due to termination of growing silane networks by the inclusion of multiple dendrimers.
Figure 4.
Initial rates of MnII(NO)TPPS formation detected as 430 nm absorbance in AEMP3/MTMOS films modified with 0–2% (v:v total silane) trimethoxysilyl-terminated PAMAMOS dendrimers upon the addition of 320 µM Angeli’s salt/PBS.
Oxygen Permeability in PAMAMOS-Modified Films
To evaluate the effect of PAMAMOS dendrimer incorporation on O2 diffusion in the HNO-sensing xerogel films, the amperometric response of xerogel-coated O2-reducing electrodes was examined. Films comprised of 40/60% (v:v total silane) AEMP3/MTMOS with ~0, 0.25, 0.5, 1.0 and 2.0% (v:v total silane) trimethoxysilyl-terminated PAMAMOS dendrimers were tested and compared to control films consisting of 100% MTMOS. Initially, the amperometric response of bare Pt electrodes to ambient concentrations of dissolved O2 was evaluated. The electrodes were then coated with MTMOS, AEMP3/MTMOS, and PAMAMOS-modified xerogels. The change in oxygen peak current was determined and correlated to percent O2 permeability relative to the bare electrode (Table 1). Whereas MTMOS-only control films retained nearly 70% of the oxygen signal of a bare electrode, the oxygen permeability of pure AEMP3/MTMOS films was reduced to ~8% of the value observed for bare electrodes. Of note, ANOVA statistical tests showed no significant oxygen permeability difference (P > 0.05) in films modified with 0.25% PAMAMOS compared to AEMP3/MTMOS alone. While O2 permeability in all PAMAMOS-modified AEMP3/MTMOS films remained well below the permeability of MTMOS controls, all films with >0.25% PAMAMOS incorporation were found to yield small, but statistically significant increases in oxygen permeability compared to both 0 and 0.25% PAMAMOS-modified xerogels. Thus, enhanced oxygen permeation may account for the downward trend in the MnII(NO)TPPS formation rates observed with increasing %PAMAMOS inclusion. Nevertheless, the inclusion of small percentages of PAMAMOS dendrimer in the AEMP3/MTMOS network provided a clear enhancement in the sensitivity of HNO-sensing films with minimal detrimental effects on oxygen permeability.
Table 1.
Oxygen permeability of 0–2% (v:v) PAMAMOS-modified AEMP3/MTMOS xerogels and controls.
| % PAMAMOS (v:v total silane) |
O2 Permeability (%) a |
ANOVA P value (vs. 0.0 % PAMAMOS) |
ANOVA P value (vs. MTMOS Control) |
|---|---|---|---|
| 0.0 | 8.19 ± 1.90 | 1 | 3.23E-03 |
| 0.25 | 9.61 ± 0.23 | 2.56E-01 | 5.44E-04 |
| 0.5 | 16.92 ± 1.08 | 1.64E-03 | 1.90E-04 |
| 1.0 | 14.69 ± 0.72 | 4.57E-02 | 4.84E-03 |
| 2.0 | 15.98 ± 0.58 | 5.68E-03 | 9.24E-04 |
| MTMOS Control |
68.90 ± 12.79 | 3.23E-03 | 1 |
relative to uncoated electrode
Nitroxyl Sensing
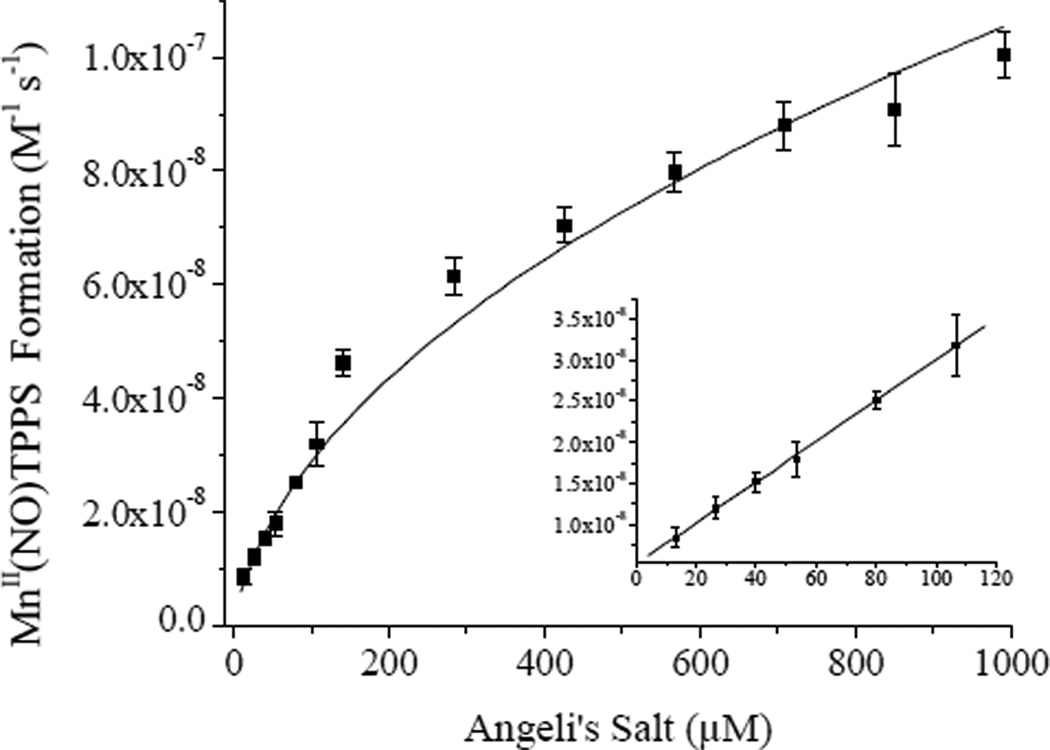
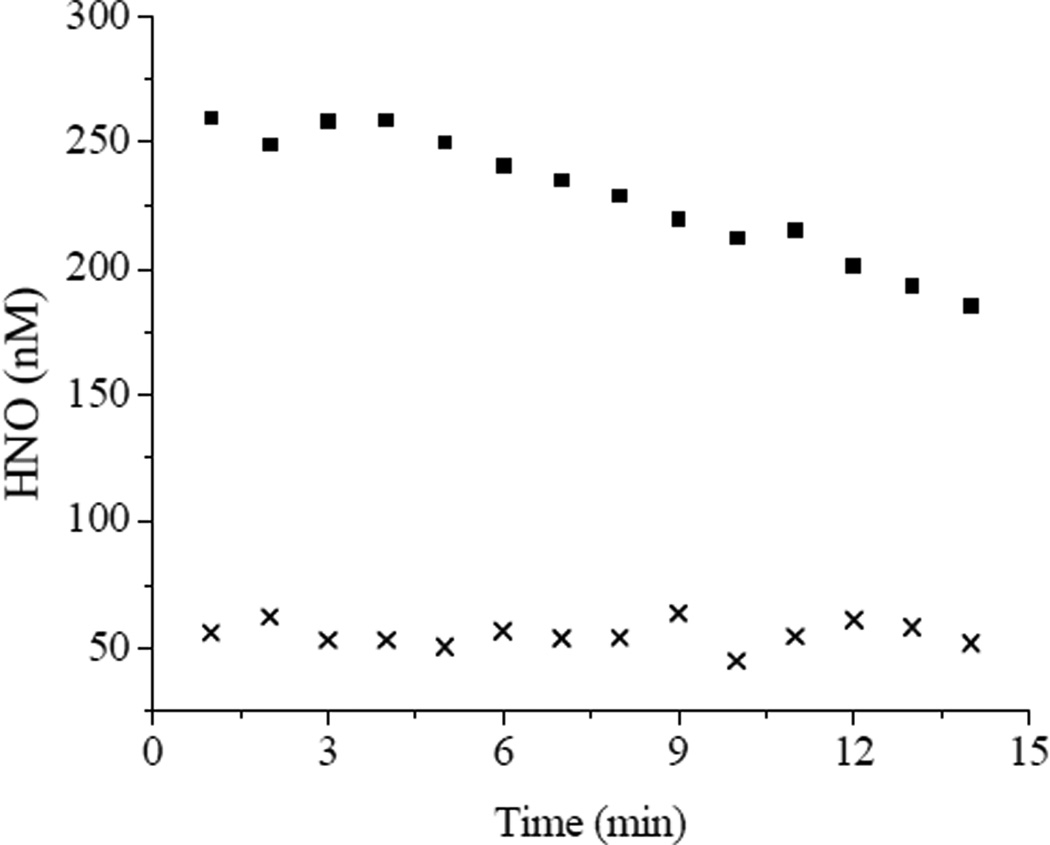
Nitroxyl sensor films comprised of the optimized xerogel formulation were fabricated by depositing 8 µl of 40/60% (v:v total silane) AEMP3/MTMOS with 0.25% (v:v total silane) PAMAMOS dendrimer in each well of a 96-well microtitre plate and cured up to 7 d before use. Sol-gel condensation reactions continue to occur after xerogel solidification, and thus long term storage may have detrimental effects on sensor sensitivity as the siloxane network of the gel becomes more densely crosslinked.37 As such, sensor calibration at the time of nitoxyl detection is important. Calibration of the sensor plate was performed by monitoring the initial rates of MnII(NO)TPPS formation in the films upon exposure to Angeli’s salt and fitting the response to Eq. 7. A representative response curve is shown in Figure 5. Obtaining a strong coefficient of determination (R2 ≥ 0.99) at rates < 8 × 10−9 M−1 s−1 (corresponding to ~13 µM Angeli’s salt) became difficult due to intrinsic noise. Rates > ~1 × 10−7 M−1 s−1 (~1 mM Angeli’s salt) began to deviate from the theoretical model of Eq. 7. Fitting the initial rates over this concentration range using Eq. 7 resulted in an experimental kon ~1 × 103 M−1 s−1 for MnII(NO)TPPS formation in the xerogel films. As expected, this value was significantly lower than that established by Marti for MnIIITPPS in anaerobic solution (4 × 104 M−1 s−1)20 due to diffusion constraints imposed by encapsulation of the porphyrin in the film. Substitution of these values into Eq. 8 resulted in an estimated dynamic range of 24–290 nM HNO, a relatively small range reflective of the rapid scavenging of HNO via dimerization at higher nitroxyl concentrations which limits free HNO in solution. Example real-time HNO release data from ~700 µM and 50 µM Angeli’s salt solutions derived via Eq. 8 by spectroscopically monitoring intra-film MnIIITPPS concentrations and MnII(NO)TPPS formation rates over 60 s time intervals are shown in Figure 6. At the larger HNO-donor concentration, a steady decrease in solution HNO was observed due to the prevalence of the second order HNO dimerization reaction and N2O formation. Dimerization was slowed considerably at the lower HNO concentration. Further improvement of the working dynamic range of HNO-sensing films would therefore best be achieved by increasing kon, expanding the range of measurable initial rates and improving sensitivity at lower donor concentrations. The rates of MnII(NO)TPPS formation scale with the square root of the nitroxyl donor employed (Eq. 7). However, sensor response can be approximated as linear at low donor concentrations (Figure 5 inset). In this region, a sensitivity of ~ 2.5 × 10−10 M−1 s−1 (change in initial rates) per µmol Angeli’s salt was obtained, correlating to an estimated minimum resolvable shift of approximately 10 nM HNO as derived from twice the average standard deviation of the measured initial rates. Although sensors regenerated by long-term (>24 h) exposure to PBS containing ambient O2 concentrations showed consistent response to HNO, the lengthy soaking periods required often had detrimental effects on xerogel stability, leading to cracking and peeling of the xerogel films. Because of this issue and the relative ease of film fabrication, films were typically utilized only once.
Figure 5.
Initial rates of MnII(NO)TPPS formation in 40/60% (v:v total silane) AEMP3/MTMOS films modified with 0.25% (v:v total silane) trimethoxysilyl-terminated PAMAMOS dendrimers. Data corresponds to the average of 7 individual films.
Figure 6.
Time-resolved HNO concentrations obtained from (■) 700 µM and (x) 50 µM Angeli’s salt in pH 7.4 PBS. Rates were acquired over 60 s intervals.
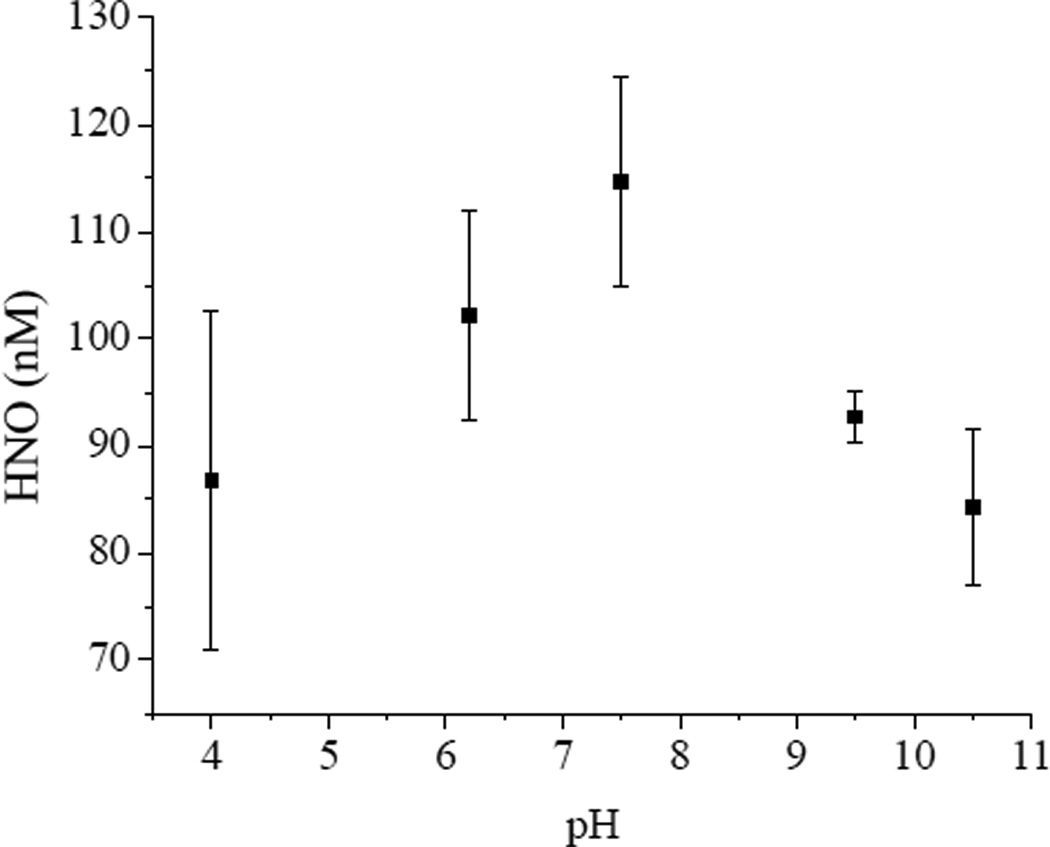
To illustrate the usefulness of the developed HNO-sensing films for the screening of HNO-donor compounds, nitroxyl production from the N-bound diazeniumdiolate (NONOate) compound IPA/NO was measured using the microtitre plate format. Formed upon exposure of amines to high pressures of NO, NONOate compounds have been widely employed as NO-storage and delivery agents. Recent studies indicate that primary amine NONOates such as IPA/NO have the capacity to produce both NO and HNO under certain conditions.25, 38 While overall decomposition of N-bound NONOates is proton initiated, HNO production is predicted to be thermodynamically favored over NO at high pH values.26 The transition of IPA/NO from a HNO to NO-donor is proposed to occur gradually, with NO production increasing at pH < 7 at the expense of HNO generation. We confirmed this behavior experimentally by examining the rates of MnII(NO)TPPS formation using the HNO-sensing plates. After Angeli’s salt calibration, nitroxyl concentrations generated from 6.1 mM IPA/NO in pH 4–10.5 PBS were determined at 15 min time intervals (Figure 7). The nitroxyl concentrations at elevated pH trended upwards with decreasing pH to near neutral conditions, after which HNO concentrations began to decline. Gas evolution did not follow this trend, but rather was observed to consistently increase at decreasing pH values. Enhanced gas evolution is consistent with increased proton-initiated decomposition rates of the NONOate donor, and a strong indication that the reduced HNO concentrations detected were the result of an increase in IPA/NO decomposition to NO. Optical noise due to bubble formation in the sensor light path likely contributed to the high variability in recovered HNO concentrations observed at low pH in Figure 7. To compare the HNO production efficiencies of IPA/NO versus Angeli’s salt, HNO concentrations were calculated from 1–6 mM IPA/NO in pH 7.4 PBS over 15 min intervals and compared to equivalent HNO concentrations derived from Angeli’s salt (Figure 8). Despite similar rates of donor decomposition,27 generation of equivalent HNO concentrations required significantly larger concentrations of IPA/NO. Such a trend is consistent with previous reports of decreased metmyoglobin reductive nitrosylation efficiency by equimolar amounts of IPA/NO and Angeli’s salt,25 and may be attributed to the concurrent formation of NO that is both formed competitively with HNO and scavenges existing HNO from solution.13
Figure 7.
Effect of pH on HNO production from 6.1 mM IPA/NO in PBS. Data corresponds to the average of 7 individual films.
Figure 8.
Concentrations of HNO versus required donor concentrations of (■) IPA/NO or (x) Angeli’s salt in ph 7.4 PBS. Data corresponds to the average of 7 individual films. Note the scale break along the x-axis.
CONCLUSIONS
Despite a growing number of potential pharmaceutical applications, difficulties associated with nitroxyl detection and quantification have complicated the study of HNO and the characterization of new HNO-donor compounds. Through encapsulation of HNO-selective MnIIITPPS within the anaerobic local environment of an aminoalkoxysilane xerogel membrane we have developed the first reported optical sensor films suitable for the quantitative determination of HNO in a convenient 96-well microtitre plate format. At present, the rapid dimerization of HNO at high concentrations and relatively slow rate of HNO complexation in the xerogel film limit optimal sensor performance to environments with restricted HNO scavenging conditions and a narrow dynamic range. Future work should focus on increasing both the sensor range and sensitivity by increasing the rate of HNO diffusion into the films and the corresponding experimental kinetic coefficient (kon) for intra-film complex formation. Nevertheless, we believe that the described HNO-sensing plates provide a simple, rapid means for determining HNO concentrations in aerobic solution and thus will prove useful as tools for characterizing novel HNO-releasing compounds.
ACKNOWLEDGMENTS
This research was supported by the National Institutes of Health (NIH EB000708). The authors thank Dr. Katrina Miranda of the University of Arizona for providing IPA/NO nitroxyl donors. K.P.D. gratefully acknowledges a National Science Foundation Graduate Research Fellowship.
REFERENCES
- 1.Keefer LK. Curr. Top. Med. Chem. 2005;5:625–634. doi: 10.2174/1568026054679380. [DOI] [PubMed] [Google Scholar]
- 2.Moncada S, Higgs A. N. Engl. J. Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 3.Paolocci N, Jackson MI, Lopez BE, Miranda K, Tocchetti CG, Wink DA, Hobbs AJ, Fukuto JM. Pharmacol. Ther. 2007;113:442–458. doi: 10.1016/j.pharmthera.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pagliaro P, Mancardi D, Rastaldo R, Penna C, Gattullo D, Miranda KM, Feelisch M, Wink DA, Kass DA, Paolocci N. Free Radic. Biol. Med. 2003;34:33–43. doi: 10.1016/s0891-5849(02)01179-6. [DOI] [PubMed] [Google Scholar]
- 5.Nagasawa HT, Kawle SP, Elberling JA, Demaster EG, Fukuto JM. J. Med. Chem. 1995;38:1865–1871. doi: 10.1021/jm00011a005. [DOI] [PubMed] [Google Scholar]
- 6.Lee MJC, Nagasawa HT, Elberling JA, Demaster EG. J. Med. Chem. 1992;35:3648–3652. doi: 10.1021/jm00098a008. [DOI] [PubMed] [Google Scholar]
- 7.Paolocci N, Saavedra FW, Miranda KM, Espey MG, Isoda T, Wink DA, Kass DA. Circulation. 2000;102:161–161. [Google Scholar]
- 8.Miranda KM, Nagasawa HT, Toscano JP. Curr. Top. Med. Chem. 2005;5:647–664. doi: 10.2174/1568026054679290. [DOI] [PubMed] [Google Scholar]
- 9.Sha X, Isbell TS, Patel RP, Day CS, King SB. J. Am. Chem. Soc. 2006;128:9687–9692. doi: 10.1021/ja062365a. [DOI] [PubMed] [Google Scholar]
- 10.King SB. Curr. Top. Med. Chem. 2005;5:665–673. doi: 10.2174/1568026054679362. [DOI] [PubMed] [Google Scholar]
- 11.Pennington RL, Sha X, King SB. Bioorg. Med. Chem. Lett. 2005;15:2331–2334. doi: 10.1016/j.bmcl.2005.02.082. [DOI] [PubMed] [Google Scholar]
- 12.Bartberger MD, Liu W, Ford E, Miranda KM, Switzer C, Fukuto JM, Farmer PJ, Wink DA, Houk KN. Proc. Natl. Acad. Sci. U. S. A. 2002;99:10958–10963. doi: 10.1073/pnas.162095599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shafirovich V, Lymar SV. Proc. Natl. Acad. Sci. U. S. A. 2002;99:7340–7345. doi: 10.1073/pnas.112202099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butkovskaya NI, Muravyov AA, Setser DW. Chem. Phys. Lett. 1997;266:223–226. [Google Scholar]
- 15.Cho JY, Dutton A, Miller T, Houk KN, Fukuto JM. Arch. Biochem. Biophys. 2003;417:65–76. doi: 10.1016/s0003-9861(03)00335-7. [DOI] [PubMed] [Google Scholar]
- 16.Yoo J, Fukuto JM. Biochem. Pharmacol. 1995;50:1995–2000. doi: 10.1016/0006-2952(95)02098-5. [DOI] [PubMed] [Google Scholar]
- 17.Donzelli S, Espey MG, Thomas DD, Mancardi D, Tocchetti CG, Ridnour LA, Paolocci N, King SB, Miranda KM, Lazzarino G, Fukuto JM, Wink DA. Free Radic. Biol. Med. 2006;40:1056–1066. doi: 10.1016/j.freeradbiomed.2005.10.058. [DOI] [PubMed] [Google Scholar]
- 18.Bazylinski DA, Hollocher TC. J. Am. Chem. Soc. 1985;107:7982–7986. [Google Scholar]
- 19.Bari SE, Marti MA, Amorebieta VT, Estrin DA, Doctorovich F. J. Am. Chem. Soc. 2003;125:15272–15273. doi: 10.1021/ja036370f. [DOI] [PubMed] [Google Scholar]
- 20.Marti MA, Bari SE, Estrin DA, Doctorovich F. J. Am. Chem. Soc. 2005;127:4680–4684. doi: 10.1021/ja044632n. [DOI] [PubMed] [Google Scholar]
- 21.Brinker J, Scherer G. Sol-Gel Science. New York: Academic Press Inc.; 1990. [Google Scholar]
- 22.Robbins ME, Hopper ED, Schoenfisch MH. Langmuir. 2004;20:10296–10302. doi: 10.1021/la048368n. [DOI] [PubMed] [Google Scholar]
- 23.Katori T, Tocchetti CG, Miranda KM, Champion HC, Fukuto JM, Wink DA, Kass DA, Paolocci N. J. Am. Coll. Cardiol. 2004;43:218A–218A. [Google Scholar]
- 24.Mancardi D, Ridnour L, Donzelli S, Miranda KM, Thomas D, Katori T, Espey M, Paolocci N, Wink D. Free Radic. Biol. Med. 2004;37:S86–S87. [Google Scholar]
- 25.Miranda KM, Katori T, de Holding CLT, Thomas L, Ridnour LA, MeLendon WJ, Cologna SM, Dutton AS, Champion HC, Mancardi D, Tocchetti CG, Saavedra JE, Keefer LK, Houk KN, Fukuto JM, Kass DA, Paolocci N, Wink DA. J. Med. Chem. 2005;48:8220–8228. doi: 10.1021/jm050151i. [DOI] [PubMed] [Google Scholar]
- 26.Dobmeier KP, Schoenfisch MH. Biomacromolecules. 2004;5:2493–2495. doi: 10.1021/bm049632u. [DOI] [PubMed] [Google Scholar]
- 27.Maragos CM, Morley D, Wink DA, Dunams TM, Saavedra JE, Hoffman A, Bove AA, Isaac L, Hrabie JA, Keefer LK. J. Med. Chem. 1991;34:3242–3247. doi: 10.1021/jm00115a013. [DOI] [PubMed] [Google Scholar]
- 28.Miranda KM, Dutton AS, Ridnour LA, Foreman CA, Ford E, Paolocci N, Katori T, Tocchetti CG, Mancardi D, Thomas DD, Espey MG, Houk KN, Fukuto JM, Wink DA. J. Am. Chem. Soc. 2005;127:722–731. doi: 10.1021/ja045480z. [DOI] [PubMed] [Google Scholar]
- 29.Dobmeier KP, Charville GW, Schoenfisch MH. Anal. Chem. 2006;78:7461–7466. doi: 10.1021/ac060995p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marxer SM, Schoenfisch MH. Anal. Chem. 2005;77:848–853. doi: 10.1021/ac0486995. [DOI] [PubMed] [Google Scholar]
- 31.Shin JH, Weinman SW, Schoenfisch MH. Anal. Chem. 2005;77:3494–3501. doi: 10.1021/ac048153i. [DOI] [PubMed] [Google Scholar]
- 32.Schoenfisch MH, Rothrock AR, Shin JH, Polizzi MA, Brinkley MF, Dobmeier KP. Biosens. Bioelectron. 2006;22:306–312. doi: 10.1016/j.bios.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Shin JH, Marxer SM, Schoenfisch MH. Anal. Chem. 2004;76:4543–4549. doi: 10.1021/ac049776z. [DOI] [PubMed] [Google Scholar]
- 34.Marxer SM, Robbins ME, Schoenfisch MH. Analyst. 2005;130:206–212. doi: 10.1039/b412079e. [DOI] [PubMed] [Google Scholar]
- 35.Dvornic PR. J. Polym. Sci. Pol. Chem. 2006;44:2755–2773. [Google Scholar]
- 36.Dvornic PR, Li JM, de Leuze-Jallouli AM, Reeves SD, Owen MJ. Macromolecules. 2002;35:9323–9333. [Google Scholar]
- 37.Tang Y, Tehan EC, Tao ZY, Bright FV. Anal. Chem. 2003;75:2407–2413. doi: 10.1021/ac030087h. [DOI] [PubMed] [Google Scholar]
- 38.Dutton AS, Suhrada CP, Miranda KM, Wink DA, Fukuto JM, Houk KN. Inorg. Chem. 2006;45:2448–2456. doi: 10.1021/ic051505z. [DOI] [PMC free article] [PubMed] [Google Scholar]