Abstract
Study Objective
To quantify the effect of acetaminophen at therapeutic doses on serum ALT in subjects who use ethanol, using data from all randomized placebo-controlled trials (RCT).
Design
A systematic review and meta-analysis of RCTs identified in a comprehensive literature search of PubMed EMBASE, International Pharmaceutical Abstracts and the Cochrane Registry of Clinical Trials. The search included multiple terms for “acetaminophen” combined with multiple terms for “ethanol”.
Patients We included RCT that enrolled subjects who had consumed ethanol either during or just prior to study entry, and who were administered up to 4 g daily of acetaminophen.
Measurements
The primary outcome was the change in serum ALT activity from baseline compared to placebo.
Main Results
184 unique articles were identified; six articles met all criteria. Five of the six articles reported ALT values at study day 4 for 551 acetaminophen-treated and 350 placebo-treated subjects; thus the changes in ALT from baseline to study day 4 were used for the meta-analysis. The difference in mean change from baseline ALT values between the acetaminophen and placebo groups on study day 4 was 0.0 IU/L (95%CI: −0.2 to 0.1 IU/L). There were no reports of liver dysfunction, failure or death in any of the prospective trials.
Conclusion
In published randomized, placebo controlled trials of subjects who consume ethanol, there was no elevation of ALT on study day four when subjects ingest 4g/day of acetaminophen.
Introduction
Hepatotoxicity following acetaminophen (paracetamol) use in patients who consume ethanol is an important issue for the medical community. Specific concern has been raised about the potential for the maximal recommended doses of acetaminophen (4g/day) to cause liver injury in alcoholics. The current label for non-prescription acetaminophen products in the United States reads “severe liver damage may occur if you take more than the maximum number of daily dosage units in 24 hours, if you take with other drugs containing acetaminophen, and if you take three or more alcoholic drinks every day while using this product.”
A previous systematic review of the pre-1999 literature identified only two placebo-controlled, randomized and blinded clinical trials that measured serum transaminase activity in heavy ethanol users given acetaminophen.1 The authors concluded that the available controlled trials did not show evidence that therapeutic doses of acetaminophen caused liver injury in alcoholics. The only reports of therapeutic doses of acetaminophen producing hepatic toxicity are uncontrolled, retrospective cohort studies and single case reports of variable quality.
While case reports are useful for identifying rare events 2 they cannot establish causality and do not provide an estimate incidence of disease. Therefore, we performed a meta-analysis of published randomized controlled trials to quantify the effect of therapeutic doses of acetaminophen on serum alanine amniotransferase (ALT) activity in subjects who consume ethanol. A secondary outcome was to identify any cases of acute liver dysfunction, failure or death reported in these trials.
Methods
The study protocol including analysis methods and study inclusion criteria were pre-specified. We sought to identify every randomized prospective, placebo-controlled trial in which subjects reporting ethanol consumption immediately prior to enrollment were administered therapeutic doses of paracetamol. The study protocol was not registered and, as this was a review of published results of studies that had undergone human subjects review, we did not seek additional human subjects approval.
A systematic review of the medical literature was conducted on November 17, 2010 using MEDLINE (1950–2010), EMBASE (1980–2010) and International Pharmaceutical Abstracts (1967–2009) databases using the terms “acetaminophen”, “paracetamol”, “APAP”, and the CAS Registry number (103-90-2). The resulting citations were stored in an internal bibliographic database. Abstracts of these citations were reviewed, and full reports of studies where subjects were treated with acetaminophen were obtained. The internal database was searched using the keywords “alcohol”, “ETOH”, “ethanol”, or “drinker”. Additionally, a search of the Cochrane Central Register of Controlled Trials (1800-11/22/2010) was performed with use of the terms “acetaminophen”, “paracetamol”, “APAP”, or the CAS Registry number #103-90-2 and “ethanol”, “alcohol”, “ETOH”, or “drinker”. No restriction was placed on language of publication.
Abstracts of articles were screened to determine if it reported a randomized clinical trial. Full articles of randomized trials were then reviewed to determine if the study met the complete criteria of 1) subjects consumed ethanol either just prior to (usually based on self-report) or during the trial, and 2) subjects were administered multiple doses of acetaminophen (up to 4 g/day) and 3) subjects had measurement of serum ALT.
Two independent abstractors assessed all studies for eligibility using a structured abstraction form. Disagreements were resolved by previously published consensus methods.3 The variables abstracted included: study inclusion and exclusion criteria, and study design factors including timing and frequency of follow-up visits, the number and allocation of subjects, demographic characteristics, acetaminophen dosage and duration, self-reported ethanol use, serum ALT, aspartate aminotransferase (AST), gamma glyutamyltransferase (GGT), and international normalized ratio (INR). We also identified any report of subjects experiencing liver dysfunction (defined as a serum total bilirubin > 2 mg/dl or an increase in the INR above 2.0), liver failure or death. We performed no specific assessment for bias in the studies.
For the meta-analysis, we retained only studies that measured the serum ALT because it was the most commonly reported and because it is more specific than AST. If published data were not sufficient for the meta-analysis, the authors were contacted to obtain additional data. While raw ALT values were not normally distributed, the change in ALT from baseline was. Therefore, we selected the difference in mean change in ALT from baseline between treatment groups (i.e. the mean change from baseline for the acetaminophen groups minus the mean change from baseline for the placebo groups at each time point) as our mean effect size for the meta-analysis. For this outcome, a greater increase in ALT from baseline in acetaminophen-treated subjects versus placebo would be expressed as a positive difference. A test for heterogeneity across studies was assessed using the Q statistic and determination of the I2. Funnel plots were generated to explore the potential for publication bias. All analyses are conducted using Comprehensive Meta-Analysis® software (Biostat™, Englewood, New Jersey).
Results
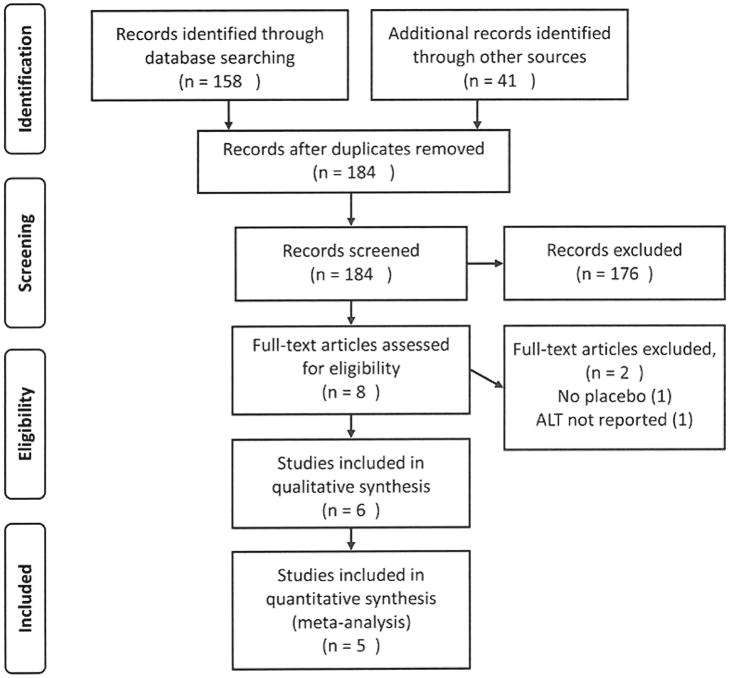
The initial search based on the keyword selection criteria retrieved 199 articles, of which 15 were deemed to be duplicates. Figure 1 shows a flow chart of the article selection process. After abstract review, the reviewers identified eight unique randomized controlled trials. Two of these were excluded from the meta-analysis because ALT was not measured4 or because the serum ALT was not compared between active treatment and placebo.5 Neither trial reported any cases of acute liver dysfunction, liver failure or death.
Figure 1.
PRISMA diagram showing article selection for review and analysis. Study subjects were ethanol users who were administered therapeutic doses of acetaminophen or placebo and had serum ALT measured.
The remaining six trials were randomized control trials published in English, comparing acetaminophen to placebo.6–11 Acetaminophen dosing ranged from 2 to 4g daily for 2 to 28 days. Four studies enrolled heavy ethanol users who were admitted to a detoxification center.6, 7, 9, 10 Subjects in these studies initiated acetaminophen within 24 hours of their last drink, and were not allowed access to ethanol during the trial. One study enrolled moderate drinkers (history of 1–3 drinks/day) who continued their normal drinking pattern during the study.8 The final study excluded heavy drinkers (>2 oz ethanol/day) but a majority of the enrolled subjects (58%) reported consuming less than 2 oz ethanol/day. 11 All studies were approved by the appropriate IRB and all subjects provided informed consent.
Because the effect of acetaminophen on serum transaminase activity depends on duration of treatment in non-ethanol consuming subjects,12–14 we planned to quantify the effect of acetaminophen on ALT at several time points. However, the studies identified did not consistently report ALT measurements at the same time points, so the meta-analysis was restricted to the most commonly used time point (study day 4) that was reported in five of the six studies (Table 1).
Table 1.
Characteristics of studies included in the meta-analysis. Study subjects were ethanol users who were administered therapeutic doses of acetaminophen or placebo.
| Study | Dose | Duration | APAP | Placebo | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean change ALT day 4 | SD | n | Mean change ALT day 4 | SD | |||
| Kuffner9 | 4 g/day | 2 days | 98 | 5.70 | 23.9 | 95 | 5.40 | 28.1 |
| Heard8 | 4 g/day | 10 days | 102 | −0.2 | 5.1 | 51 | −0.6 | 4.1 |
| Kuffner10 | 4 g/day | 3 days | 262 | −0.3 | 29.6 | 120 | 3.6 | 23.5 |
| Bartels6 | 3.9 g/day | 4 days | 21 | 3.4 | 10.7 | 19 | 4.1 | 10.6 |
| Dart7 | 4 g/day | 5 days | 68 | −2.3 | 26.2 | 65 | −3.0 | 21.1 |
The overall mean change in ALT between acetaminophen- and placebo-treated subjects at day 4 was 0.0 IU/L (97% CI: −0.2 to 0.1 IU/L) (Table 1, Figure 2). Testing for heterogeneity among studies produced a Q=1.7 (p = 0.8) and an I2 = 0.0. These results support the assumption of a uniform response among studies, and the use of a fixed-effect meta-analysis.
Figure 2.
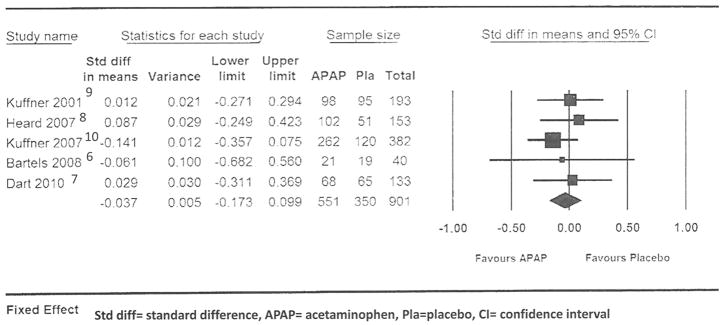
Forest plot of standardized differences in means for acetaminophen and placebo groups at day 4.
Changes in other liver related laboratory tests were measured in several studies. The change in AST6, 8–10, INR9, 10, total bilirubin6, 8, 10, or glutathione-s-transferase6 was similar for the placebo and acetaminophen treated subjects in the studies that reported these outcomes. One study noted elevation of the INR in acetaminophen treated subjects who were taking warfarin.11
A funnel plot of the standard error versus the estimated standardized difference was examined for an indication of publication bias and heterogeneity. Within the limits of the small number of studies in the meta-analysis, these findings suggest that the overall point estimate is not subject to publication bias, and that the published results would appear to describe a single population.
The serum ALT activities at each study day as reported in the original trials are shown in Table 2. These results consistently show that ALT did not change substantially during the first four days of administration. However, in one study, the ALT values observed in the acetaminophen group at days 6 and 7 (one and two days after treatment was stopped) were 5.5 and 12.6 IU/L (respectively) higher than the placebo group values.7 In another study, the day 11 ALT (one day after treatment was stopped) in the acetaminophen treated group was 8.4 IU/L higher than the placebo group and this difference was statistically significant.8 In a third study (the study that was not included in the meta-analysis as there were no day 4 values) the day 14 ALT values were 18.7 IU/L higher than placebo in the 4g/day group and 8.1 IU/L higher than placebo in the 2g/day group.11 Importantly, these differences had disappeared by day 28 of treatment. None of the randomized placebo controlled trials reported any cases of liver dysfunction, failure or death.
Table 2.
Mean ALT activity for treatment groups in identified randomized controlled trials. Study subjects were ethanol users who were administered acetaminophen or placebo. Shaded areas show measurements obtained during administration of acetaminophen, unshaded those either at baseline, or after acetaminophen administration had ended.
| Study | Day | N | Placebo | Acetaminophen | |
|---|---|---|---|---|---|
| Mean (SD) | N | Mean (SD) | |||
| Kuffner9 | 0 | 98 | 36.5 (20.3) | 101 | 34.5 (19.3) |
| 2 | 95 | 37.3 (23.9) | 101 | 32.9 (22.0) | |
| 4 | 96 | 41.9 (33.9) | 98 | 40.2 (31.1) | |
| Heard8 | 0 | 52 | 22.9 (8.4) | 104 | 21.4 (7.5) |
| 4 | 51 | 22.1 (8.8) | 102 | 21.1 (7.5) | |
| 11 | 50 | 21.6 (7.9) | 100 | 30.0 (18.6) | |
| Kuffner10 | 0 | 137 | 46.7 (36.6) | 306 | 49.4 (38.2) |
| 2 | 129 | 45.3 (33.6) | 282 | 45.7 (38.6) | |
| 3 | 123 | 46.7 (37.1) | 269 | 45.9 (36.6) | |
| 4 | 120 | 50.5 (42.1) | 262 | 49.0 (39.2) | |
| 5 | 119 | 54.9 (47.5) | 256 | 57.1 (45.2) | |
| Parra11 (2g) | 0 | 12 | 37.3 (10.2) | 12 | 32.3 (12.8) |
| 14 | 12 | 36.9 (11.3) | 12 | 45 (9) | |
| 28 | 12 | 34.5 (11) | 12 | 36.5 (10.9) | |
| Parra11 (4g) | 0 | * | 12 | 37.3 (10.2) | |
| 14 | * | 12 | 55.2 (30) | ||
| 28 | * | 12 | 36.5 (9.8) | ||
| Bartels6 | 0 | 22 | 38.7 (24.8) | 23 | 32.9 (20.8) |
| 1 | 21 | 38.8 (24.3) | 23 | 34.3 (23.2) | |
| 2 | 20 | 40.6 (21.0) | 22 | 34.4 (25.6) | |
| 3 | 19 | 42.1 (22.8) | 21 | 37.3 (25.6) | |
| 4 | 17 | 47.2 (27.8) | 17 | 44.8 (34.7) | |
| Dart7 | 0 | 68 | 47.2 (32.6) | 74 | 48.2 (36.9) |
| 2 | 68 | 41.9 (27.3) | 71 | 41.9 (33.9) | |
| 4 | 65 | 44.1 (37.5) | 68 | 47.2 (41.9) | |
| 6 | 64 | 49.3 (42.1) | 66 | 55.9 (44.8) | |
| 7 | 64 | 49.2 (42.4) | 66 | 61.8 (50.4) | |
Parra11 compared placebo to 2g/day and 4/g day. Shaded areas show measurements obtained during administration of acetaminophen, unshaded those either at baseline, or after acetaminophen administration had ended.
Discussion
Based on available published trials involving 551 moderate to heavy users of alcohol, daily administration of acetaminophen at or near the maximum recommended dose does not affect the serum at study day 4. Two studies that measured serum ALT activity 6 to 14 days after start of therapy reported a transient increase of less than 20 IU/L average after acetaminophen dosing had ended. No prospective, randomized trial reported any cases of liver dysfunction, liver failure, or death.
The potential for ethanol consumption to increase the risk of hepatic injury from acetaminophen has been suggested for many years.15 N-acetyl-p-benzo-quinoneimine (NAPQI), the acetaminophen metabolite responsible for acetaminophen toxicity, is formed when acetaminophen is oxidized by CYP2E1. When acetaminophen is consumed by patients with ethanol in their system, the ethanol acts a competitive inhibitor of CYP2E1 so the production of NAPQI is decreased and the toxic effects of acetaminophen are reduced.16–19 However ethanol consumption increases CYP2E1 activity via transcriptional, post-transcriptional and post-translational mechanisms, including the stabilization of the enzyme.20 Induction of CYP2E1 by a 6 hour ethanol infusion (titrated to maintain a serum ethanol concentration of 100 mg/dL) prior to acetaminophen administration has been shown to increase NAPQI formation by 22% compared to controls.21 Therefore subjects who ingest ethanol but who are recently abstinent at the time of acetaminophen of administration should have increased NAPQI formation and be at maximal risk of hepatic injury from therapeutic doses of acetaminophen. The risk of toxicity decreases as the CYP2E1 activity returns to baseline.22 The risk should also be lower in patients who have ethanol in their system when they ingest acetaminophen as CYP2E1 will be inhibited at clinically relevant serum ethanol concentrations.17 Four of the six studies included in our meta-analysis included ethanol users who should have been near maximal CYP2E1 induction (the “vulnerable period”).
The second condition that may predispose alcoholics to liver injury from acetaminophen is glutathione depletion. Under normal circumstances, the NAPQI that is formed by CYP2E1 is detoxified by glutathione conjugation. This represents about 4% of acetaminophen metabolism. Glutathione depletion increases acetaminophen toxicity in animal models.23 Malnutrition can reduce glutathione and its regeneration, and in many cases chronic alcoholics are malnourished.24 However, CYP2E1 activity is also reduced by malnutrition, and the net effect on susceptibility to toxicity from these competing changes on acetaminophen metabolism has not been systematically studied.25 The results of the trials included in our analysis suggest that metabolic effects of alcohol consumption do not uniformly increase the risk of liver injury from therapeutic doses of acetaminophen.
Our systematic review and meta-analysis of the available randomized controlled clinical trials suggests that concerns about increased risk for hepatic injury from therapeutic doses of acetaminophen in alcoholics are overstated. 26 Basing risk estimates on case reports and retrospective cohort studies would be expected to overstate the likelihood of hepatic injury from therapeutic doses of acetaminophen in any vulnerable population.27 In a previously published systematic review of the use of acetaminophen in alcoholics, the only reports of hepatic injury from therapeutic doses were single case reports and case series.1 By their nature, such reports have substantial selection bias, rely on patient recollection, and in some cases report contradictory data such as serum acetaminophen concentrations inconsistent with patient self-report. The authors of this systematic review concluded that all rigorous prospective studies available indicate that therapeutic dosing of acetaminophen in alcoholics is not associated with hepatic injury.1 A similar dichotomy was noted in studies of non-alcoholic patients; no cases of liver failure or death were identified in prospective studies of more than 30,000 subjects while approximately 1% of cases in retrospective reports had evidence of liver injury, liver failure or death from therapeutic does.28 This dichotomy highlights the limitations of reports based on retrospective data collection. Case reports and case series are useful for describing phenomenon, but they cannot be used to determine causality or estimate rates of events. Findings must be validated in prospective trials before being accepted as valid.
While our findings demonstrate that four days of acetaminophen has no appreciable effect on serum ALT in subjects who consume ethanol, we also noted a small elevation in ALT when measured after 4 days.7, 8, 11 This effect has also been reported in non-drinkers, although the effect size was slightly larger (approximately 20 IU/L) than the effect observed in any of the reports included in our review.12–14 The course of ALT changes in subjects who have mild ALT elevations and continue acetaminophen should be investigated further.
Limitations
Meta-analyses have several potential limitations to the internal and external validity of the results. Internal validity may be limited by the quality of the included trials and the analytic technique. Our meta-analysis only included studies that were randomized-placebo controlled and used blinded outcome assessment. These methods make selection or misclassification bias unlikely. We used a fixed effects model which assumes that the effects are similar among the included trials. This assumption was supported by the low Cochrane Q score and I2.
The external validity of a meta-analysis is limited by the identification of studies, the design of the included studies and the characteristics of the populations included in the original studies. Our search was comprehensive, but we only included six published trials. We contacted the authors of all included studies and these authors were not aware of any unpublished work relevant to our review. We attempted to evaluate publication bias using a funnel plot, but the small number of studies limited our evaluation. While it seems unlikely that trials with a large effect would remain unpublished, we cannot exclude publication bias as a threat to the validity of our findings. Additionally, the small number of trials may have prevented us from identifying relevant subgroups that would differ in their response from the included population.
The major design limitation was that the studies measured different markers of liver injury and on different study days. We were obliged to use the most common marker (ALT) at the most common time point (study day 4). While we could not provide a summary estimate for the effect size on other study days, we have reported each trial’s point estimate for the other study days as measured.
A second limitation specific to this study is that subjects for three of the five studies were recruited at an alcohol detoxification center. While these subjects do not represent the general population of drinkers, alcoholics are generally considered to be at the highest risk for acetaminophen toxicity. The lack of measurable effect in recently abstinent alcoholics under the carefully controlled circumstances of a randomized, placebo-controlled trial with near maximal dosing provides some reassurance regarding the risks of up to 4 g/day of acetaminophen for up to 4 days in the general population including those who use ethanol. We also recognize that one study used 3.9g/day of a modified release preparation; however as toxicity from repeated doses of acetaminophen is based on an cumulative exposure leading to glutathione depletion, we don’t believe that the effects should differ between 1 g/every 6 hours and 1.3 g every 8 hours.
Conclusions
Based on data from all available randomized, placebo-controlled trials, 4g/day of acetaminophen minimal effects on serum ALT in drinkers, including recently abstinent alcoholics, when measured on study day 4. No cases of hepatic failure or death were observed in any published prospective trials of moderate to heavy drinkers, even when administered acetaminophen during the theoretically most vulnerable period from the perspective of hepatic CYP2E1 activity and glutathione regeneration. The significance of small, transient rises in serum ALT activity during the second week of continuous therapy including shortly after acetaminophen is discontinued remains unclear, but appears to be similar to the changes observed in non-drinkers.
Footnotes
This work was presented at the North American Congress of Clinical Toxicology Sept 23–27 2011 Washington DC
Financial disclosure: This study was supported by an investigator initiated grant from McNeil Consumer Healthcare to Denver Health. KH, JG, BB, DA and RD are employees of Denver Health and Hospitals. Denver Health has clinical, consulting and research contracts with Cumberland Pharmaceuticals (a manufacturer of IV acetylcysteine), Cadence Pharmaceuticals (a manufacturer of acetaminophen products) and McNeil Consumer Healthcare (a manufacturer of acetaminophen products). The investigators received only their salaries for work performed on this project. The investigators were responsible for the design, performance and analysis of the study and for the drafting and revisions of the manuscript. The sponsors had no role in the study design, performance, analysis or authorship of the article. The sponsor reviewed the final manuscript, but the authors are responsible for the final content. KH was supported by Award Number K08DA020573 from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health. BHR has consulting services to McNeil Consumer Healthcare on behalf of the Denver Health and Hospital Authority. In addition BHR has testified on behalf of McNeil Consumer Healthcare in litigation in 2010.
References
- 1.Dart RC, Kuffner EK, Rumack BH. Treatment of pain or fever with paracetamol (acetaminophen) in the alcoholic patient: a systematic review. Am J Ther. 2000;7:123–34. doi: 10.1097/00045391-200007020-00009. [DOI] [PubMed] [Google Scholar]
- 2.Aronson JK. Anecdotes as evidence. BMJ. 2003;326:1346. doi: 10.1136/bmj.326.7403.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vermeulen LC, Jr, Ratko TA, Erstad BL, Brecher ME, Matuszewski KA. A paradigm for consensus. The University Hospital Consortium guidelines for the use of albumin, nonprotein colloid, and crystalloid solutions. Arch Intern Med. 1995;155:373–9. doi: 10.1001/archinte.155.4.373. [DOI] [PubMed] [Google Scholar]
- 4.Edwards C, Gard PR, Handley SL, Hunter M, Whittington RM. Distalgesic and ethanol-impaired function. Lancet. 1982;2:384. doi: 10.1016/s0140-6736(82)90571-2. [DOI] [PubMed] [Google Scholar]
- 5.Critchley JA, Dyson EH, Scott AW, Jarvie DR, Prescott LF. Is there a place for cimetidine or ethanol in the treatment of paracetamol poisoning? Lancet. 1983;1:1375–6. doi: 10.1016/s0140-6736(83)92150-5. [DOI] [PubMed] [Google Scholar]
- 6.Bartels S, Sivilotti M, Crosby D, Richard J. Are recommended doses of acetaminophen hepatotoxic for recently abstinent alcoholics? A randomized trial. Clin Toxicol (Phila) 2008;46:243–9. doi: 10.1080/15563650701447020. [DOI] [PubMed] [Google Scholar]
- 7.Dart RC, Green JL, Kuffner EK, et al. The effects of paracetamol (acetaminophen) on hepatic tests in patients who chronically abuse alcohol - a randomized study. Aliment Pharmacol Ther. 2010;32:478–86. doi: 10.1111/j.1365-2036.2010.04364.x. [DOI] [PubMed] [Google Scholar]
- 8.Heard K, Green JL, Bailey JE, Bogdan GM, Dart RC. A randomized trial to determine the change in alanine aminotransferase during 10 days of paracetamol (acetaminophen) administration in subjects who consume moderate amounts of alcohol. Aliment Pharmacol Ther. 2007;26:283–90. doi: 10.1111/j.1365-2036.2007.03368.x. [DOI] [PubMed] [Google Scholar]
- 9.Kuffner EK, Dart RC, Bogdan GM, et al. Effect of maximal daily doses of acetaminophen on the liver of alcoholic patients: a randomized, double-blind, placebo-controlled trial. Arch Intern Med. 2001;161:2247–52. doi: 10.1001/archinte.161.18.2247. [DOI] [PubMed] [Google Scholar]
- 10.Kuffner EK, Green JL, Bogdan GM, et al. The effect of acetaminophen (four grams a day for three consecutive days) on hepatic tests in alcoholic patients--a multicenter randomized study. BMC Med. 2007;5:13. doi: 10.1186/1741-7015-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parra D, Beckey NP, Stevens GR. The effect of acetaminophen on the international normalized ratio in patients stabilized on warfarin therapy. Pharmacotherapy. 2007;27:675–83. doi: 10.1592/phco.27.5.675. [DOI] [PubMed] [Google Scholar]
- 12.Harrill AH, Watkins PB, Su S, et al. Mouse population-guided resequencing reveals that variants in CD44 contribute to acetaminophen-induced liver injury in humans. Genome Res. 2009;19:1507–15. doi: 10.1101/gr.090241.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heard KJ, Green JL, Dart RC. Serum alanine aminotransferase elevation during 10 days of acetaminophen use in nondrinkers. Pharmacotherapy. 2010;30:818–22. doi: 10.1592/phco.30.8.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watkins PB, Kaplowitz N, Slattery JT, et al. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily: a randomized controlled trial. JAMA. 2006;296:87–93. doi: 10.1001/jama.296.1.87. [DOI] [PubMed] [Google Scholar]
- 15.Whitcomb DC, Block GD. Association of acetaminophen hepatotoxicity with fasting and ethanol use. JAMA. 1994;272:1845–50. doi: 10.1001/jama.1994.03520230055038. [DOI] [PubMed] [Google Scholar]
- 16.Lee SM, Cho TS, Kim DJ, Cha YN. Protective effect of ethanol against acetaminophen-induced hepatotoxicity in mice: role of NADH:quinone reductase. Biochem Pharmacol. 1999;58:1547–55. doi: 10.1016/s0006-2952(99)00248-8. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt LE, Dalhoff K, Poulsen HE. Acute versus chronic alcohol consumption in acetaminophen-induced hepatotoxicity. Hepatology. 2002;35:876–82. doi: 10.1053/jhep.2002.32148. [DOI] [PubMed] [Google Scholar]
- 18.Thummel KE, Slattery JT, Nelson SD. Mechanism by which ethanol diminishes the hepatotoxicity of acetaminophen. J Pharmacol Exp Ther. 1988;245:129–36. [PubMed] [Google Scholar]
- 19.Waring WS, Stephen AF, Malkowska AM, Robinson OD. Acute ethanol coingestion confers a lower risk of hepatotoxicity after deliberate acetaminophen overdose. Acad Emerg Med. 2008;15:54–8. doi: 10.1111/j.1553-2712.2007.00019.x. [DOI] [PubMed] [Google Scholar]
- 20.Oneta CM, Lieber CS, Li J, et al. Dynamics of cytochrome P4502E1 activity in man: induction by ethanol and disappearance during withdrawal phase. J Hepatol. 2002;36:47–52. doi: 10.1016/s0168-8278(01)00223-9. [DOI] [PubMed] [Google Scholar]
- 21.Thummel KE, Slattery JT, Ro H, et al. Ethanol and production of the hepatotoxic metabolite of acetaminophen in healthy adults. Clin Pharmacol Ther. 2000;67:591–9. doi: 10.1067/mcp.2000.106574. [DOI] [PubMed] [Google Scholar]
- 22.Prescott LF. Paracetamol, alcohol and the liver. Br J Clin Pharmacol. 2000;49:291–301. doi: 10.1046/j.1365-2125.2000.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther. 1973;187:211–7. [PubMed] [Google Scholar]
- 24.Teixeira J, Mota T, Fernandes JC. Nutritional evaluation of alcoholic inpatients admitted for alcohol detoxification. Alcohol Alcohol. 2011;46:558–60. doi: 10.1093/alcalc/agr062. [DOI] [PubMed] [Google Scholar]
- 25.Rumack BH. Acetaminophen hepatotoxicity: the first 35 years. J Toxicol Clin Toxicol. 2002;40:3–20. doi: 10.1081/clt-120002882. [DOI] [PubMed] [Google Scholar]
- 26.Rumack BH. Acetaminophen misconceptions. Hepatology. 2004;40:10–5. doi: 10.1002/hep.20300. [DOI] [PubMed] [Google Scholar]
- 27.Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–72. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 28.Dart RC, Bailey E. Does therapeutic use of acetaminophen cause acute liver failure? Pharmacotherapy. 2007;27:1219–30. doi: 10.1592/phco.27.9.1219. [DOI] [PubMed] [Google Scholar]