Abstract
Topical application of coal tar is one of the oldest therapies for atopic dermatitis (AD), a T helper 2 (Th2) lymphocyte–mediated skin disease associated with loss-of-function mutations in the skin barrier gene, filaggrin (FLG). Despite its longstanding clinical use and efficacy, the molecular mechanism of coal tar therapy is unknown. Using organotypic skin models with primary keratinocytes from AD patients and controls, we found that coal tar activated the aryl hydrocarbon receptor (AHR), resulting in induction of epidermal differentiation. AHR knockdown by siRNA completely abrogated this effect. Coal tar restored filaggrin expression in FLG-haploinsufficient keratinocytes to wild-type levels, and counteracted Th2 cytokine–mediated downregulation of skin barrier proteins. In AD patients, coal tar completely restored expression of major skin barrier proteins, including filaggrin. Using organotypic skin models stimulated with Th2 cytokines IL-4 and IL-13, we found coal tar to diminish spongiosis, apoptosis, and CCL26 expression, all AD hallmarks. Coal tar interfered with Th2 cytokine signaling via dephosphorylation of STAT6, most likely due to AHR-regulated activation of the NRF2 antioxidative stress pathway. The therapeutic effect of AHR activation herein described opens a new avenue to reconsider AHR as a pharmacological target and could lead to the development of mechanism-based drugs for AD.
Introduction
Atopic dermatitis (AD) is a common inflammatory skin disease that has both genetic and environmental factors in its etiology. The pathogenesis is thought to be driven by cells of the adaptive immune system, mainly T helper 2 (Th2) lymphocytes. Cytokines derived thereof, such as IL-4, IL-5, and IL-13 contribute to disease symptoms in a STAT6-dependent fashion. Clinical and histopathological features include erythema, itch, loss of skin barrier function, intercellular epidermal edema (spongiosis), and keratinocyte apoptosis. The disease is considered to be initiated by abnormal exposure to external triggers (e.g., allergens) and could be due to an impaired skin barrier function, as exemplified by loss-of-function mutations in the filaggrin (FLG) gene (1, 2). Recent work has demonstrated that not only is FLG haploinsufficiency (observed in >25% of AD patients) or complete deficiency (as in ichthyosis vulgaris patients) associated with AD, but the quantity of FLG repeats determines the risk for developing the disease as well (3). Therefore, upregulation of filaggrin expression in AD patients might be of clinical significance. Aside from the association of FLG with AD, other important skin barrier proteins, such as involucrin and loricrin, are known to be downregulated in AD by IL-4 and IL-13 via STAT6 signaling pathways (4). Constitutive expression of STAT6 in mice leads to decreased expression of epidermal barrier proteins and subsequent development of allergic skin inflammation (5). Furthermore, variation in a newly discovered terminal differentiation protein called hornerin, and decreased protein expression levels thereof, have been associated with AD (6, 7). A recent replication study, however, did not find a significant association between hornerin variation and AD (8). Collectively, these data underscore the contributions of biological interaction of skin barrier biology and immune mechanisms to the pathophysiology of AD.
Most of the current therapies for AD target the immune system (corticosteroids, calcineurin inhibitors, UVB irradiation), whereas emollients are used as an adjuvant to improve skin barrier function. Topical application of coal tar is an effective AD therapy for reducing inflammation and itch, and has been used to treat skin diseases for more than 2000 years. Coal tar contains over 10,000 different organic compounds, which has precluded identification of its molecular mode of action, and has hampered pharmacological investigation or drug development of its active ingredients.
Coal tar consists of a wide range of polycyclic aromatic hydrocarbons (PAHs) (9). Keratinocytes metabolize and detoxify internalized PAHs via CYP450 enzymes, as indicated by their high levels in the skin of coal tar–treated individuals (10). The CYP450 enzymes are induced by activation of the aryl hydrocarbon receptor (AHR) (11). AHR is a cytoplasmic transcription factor that translocates to the nucleus upon ligand recognition (12), where it binds to specific motifs in the DNA known as xenobiotic response elements (XREs) (13). The recent discovery of endogenous AHR ligands revealed a physiological role of AHR in cell behavior and development. AHR was found to be crucial in regulating the development of lymphoid cells (14) and the induction of regulatory T cells (15, 16). Recently, the environmental pollutant and AHR ligand, tetrachloro-dibenzo-dioxin (TCDD), has been described to induce epidermal differentiation, thereby providing an explanation for the occurrence of chloracne (17). Thus far, no endogenous AHR ligands affecting epidermal gene expression programs have been described.
Using submerged cultured human keratinocytes and human organotypic skin models from AD patients and healthy volunteers, we studied the effects of coal tar exposure on the epidermal aspects of AD. Our results show that coal tar activates the AHR signaling pathway, resulting in enhanced epidermal differentiation, increased levels of filaggrin, and inhibition of the IL-4/STAT6 signaling pathway. These results indicate that coal tar enhances skin barrier function and dampens keratinocyte response to the major cytokines involved in AD, and point to a key role of the AHR signaling pathway in the molecular mechanism by which the oldest known drug in dermatology corrects epidermal abnormalities in a common skin disease.
Results
AHR regulates coal tar–induced epidermal gene and protein expression.
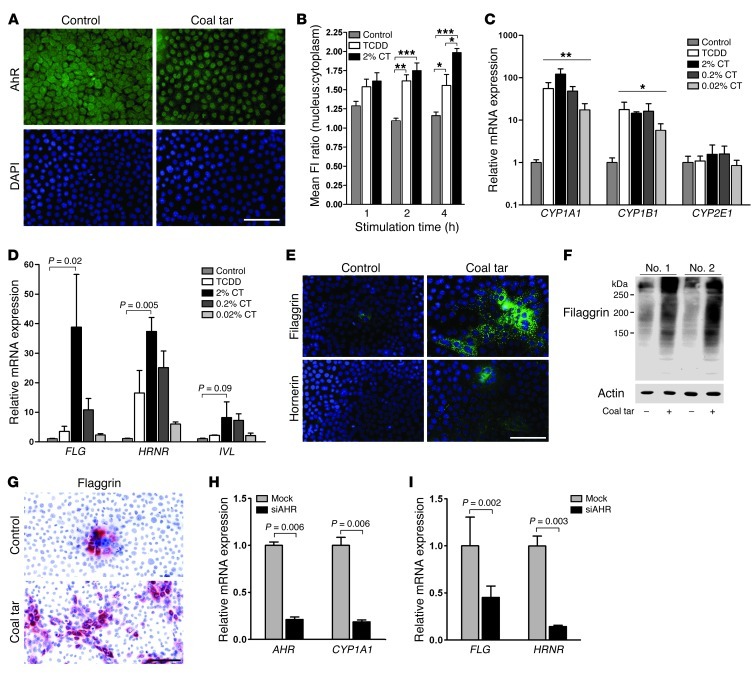
First, we investigated the potential of coal tar to activate AHR using cultured adult primary human keratinocytes. A coal tar formulation was prepared in culture medium and various concentrations were tested for toxicity. After 48 hours of treatment, none of the concentrations tested resulted in enhanced cell death (Supplemental Figure 1A; supplemental material available online with this article; doi: 10.1172/JCI65642DS1). In a variety of cells, it has been shown that upon ligand binding, AHR translocates to the nucleus. We indeed observed that coal tar caused AHR to relocate from the cytoplasm to the nucleus within 4 hours (Figure 1A), and we found a significant increase over time in the AHR nucleus/cytoplasm ratio upon TCDD and coal tar stimulation (Figure 1B). Preincubation of the antibody with recombinant AHR before immunofluorescence analysis completely eliminated nuclear staining of the coal tar–treated cells, which indicates the specificity of the immunofluorescence staining (Supplemental Figure 1B). We found a strong time- and concentration-dependent increase in expression of typical AHR-responsive genes such as CYP1A1 and CYP1B1 in coal tar–treated keratinocytes, while expression of CYP2E1 (not an AHR target) was unaffected (Figure 1C and Supplemental Figure 2). The response of keratinocytes to coal tar was similar in magnitude to their response to TCDD, a dioxin-like compound used as a positive control. Aside from CYP450 target gene expression, we also observed a highly significant and concentration-dependent induction of the epidermal differentiation genes FLG, hornerin (HRNR), and involucrin (IVL) (Figure 1D), all pivotal for barrier function and all functionally related to AD (1, 2, 4, 7). For these genes, the induction rate achieved by high and intermediate coal tar concentrations was even greater than that achieved by TCDD. Other epidermal differentiation genes such as cystatin M/E (CST6), repetin (RPTN), and small proline-rich proteins 2A and 2B (SPRR2A and SPRR2B) were also induced after coal tar treatment, but to a lesser extent (Supplemental Figure 3). The induced mRNA expression was confirmed at the protein level by staining of coal tar–treated and untreated keratinocytes cultured on coverslips (Figure 1E) and by Western blotting (Figure 1F). Filaggrin protein expression in untreated keratinocytes was confined to a few multilayered foci, while in coal tar–treated keratinocytes, its expression was already present in large patches of the keratinocyte monolayer (Figure 1G). These results indicate that coal tar treatment induced filaggrin expression at early stages of keratinocyte differentiation.
Figure 1. Coal tar induces epidermal differentiation via AHR signaling.
(A) AHR staining of coal tar–treated or untreated keratinocytes. Nuclei were counterstained using DAPI. Scale bar: 100 μM. (B) Semiquantitative analysis of nuclear and cytoplasmic fluorescence intensity (FI) measured by ImageJ software. *P < 0.05, **P < 0.01, and ***P < 0.001, relative to untreated keratinocytes. Bars indicate mean ± SEM (n = 3). (C) CYP450 mRNA expression levels after 48 hours of stimulation with TCDD or a coal tar concentration series. Expression levels are relative to untreated (control) keratinocytes. *P < 0.05 and **P < 0.001. Bars indicate mean ± SEM (n = 3). (D) mRNA expression levels of epidermal differentiation genes after 48 hours of stimulation with TCDD or a coal tar concentration series. Expression levels are relative to untreated (control) keratinocytes. Bars indicate mean ± SEM (n = 3). (E) Filaggrin and hornerin staining of 2% coal tar–treated or untreated keratinocytes. Nuclei were counterstained using DAPI. Scale bar: 100 μM. (F) Western blot analysis of filaggrin protein in lysates of coal tar–treated and untreated keratinocytes from 2 keratinocyte donors. (G) Filaggrin staining of 2% coal tar–treated or untreated keratinocytes. Nuclei were counterstained using hematoxylin. Scale bar: 100 μM. (H) AHR and CYP1A1, and (I) FLG and HRNR mRNA expression levels after siRNA-mediated AHR knockdown and subsequent 2% coal tar stimulation. Expression levels are relative to mock-treated, coal tar–stimulated keratinocytes. Bars indicate mean ± SEM (n = 3).
To confirm the regulatory role of the AHR signaling pathway in the coal tar–mediated induction of epidermal differentiation genes, we studied the coal tar response after siRNA-mediated knockdown of AHR in keratinocytes. As shown in Figure 1H, a 79% decrease in AHR expression was achieved, with a concomitant 82% decrease in expression of its classical target gene CYP1A1. AHR knockdown also caused a similar decrease in coal tar–induced FLG and HRNR gene expression (Figure 1I) and other terminal differentiation genes (Supplemental Table 1). The induction rate after coal tar treatment and subsequent inhibition by AHR knockdown was most pronounced in terminal differentiation genes with a reported xenobiotic response element in their promoter region (17), such as FLG, HRNR, and filaggrin family member 2 (FLG2). In contrast, the early differentiation marker keratin 10, or the basal keratinocyte markers keratin 5 and keratin 14, were not affected by coal tar stimulation or AHR knockdown. Altogether, these data show that coal tar induces epidermal differentiation, which is regulated by the AHR signaling pathway.
Accelerated epidermal differentiation by coal tar treatment.
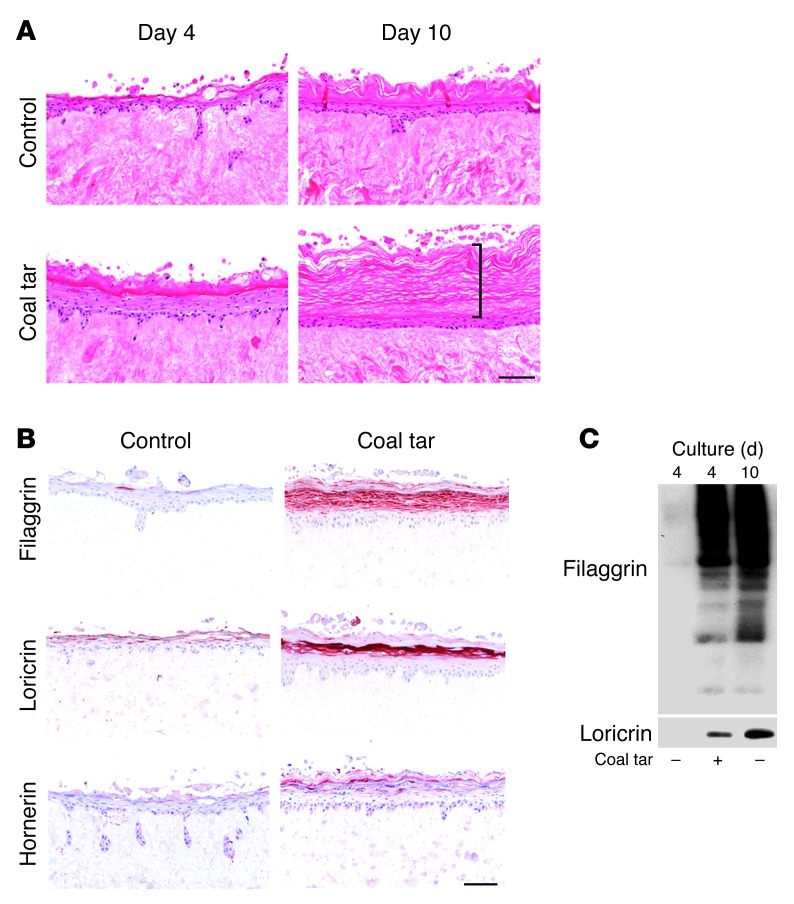
Next, we used an organotypic human skin model (hereinafter referred to as “skin equivalents”) to evaluate the effects of coal tar during epidermal morphogenesis. We have previously shown that skin equivalents can be used successfully to mimic many aspects of normal epidermis or diseased epidermis (e.g., psoriasis and AD) (18, 19). After 3 days of submerged culture, the skin equivalents were lifted at the air-liquid interface and the culture medium was supplemented with coal tar. Already after 4 days of culture at the air-liquid interface, a fully developed epidermis was observed in coal tar–treated skin equivalents. The untreated skin equivalents required 10 days of air exposure to generate a well-developed epidermis (Figure 2A). From day 4 onward, the epidermis of coal tar–treated skin equivalents continuously produced terminally differentiated cells, resulting in a very thick layer of stratum corneum at the air-liquid interface on day 10 of culture (Figure 2A; bracket). To further examine the differentiation process of coal tar–treated skin equivalents, we analyzed protein expression at several time points using immunohistochemistry. Terminal differentiation markers, which are normally expressed from day 6 of air-exposed culture onward (20), were already expressed at high levels at day 4 in coal tar–treated skin equivalents. In particular, filaggrin, loricrin, and hornerin were barely present in the standard (control) cultures at day 4, while they were abundantly expressed in coal tar–treated skin equivalents (Figure 2, B and C). Therefore, we conclude that coal tar treatment in vitro results in accelerated epidermal differentiation, and is accompanied by induced expression of terminal differentiation proteins.
Figure 2. Accelerated epidermal differentiation by coal tar.
(A) H&E staining of human skin equivalents cultured in the presence or absence (control) of coal tar during the entire air-liquid interface culture. Bracket indicates the thickness of the stratum corneum. (B) Immunohistochemical staining of filaggrin, loricrin, and hornerin in skin equivalents at day 4 of the air-liquid interface culture when cultured in the presence or absence (control) of coal tar. Images are representative of 3 keratinocyte donors, and the experiment was replicated twice. Scale bar: 100 μM. (C) Filaggrin and loricrin Western blotting of skin equivalents harvested on day 4 or day 10 of air-liquid interface culture, treated with or without coal tar during the entire culture period.
Coal tar increases filaggrin expression in skin equivalents and in lesional AD skin.
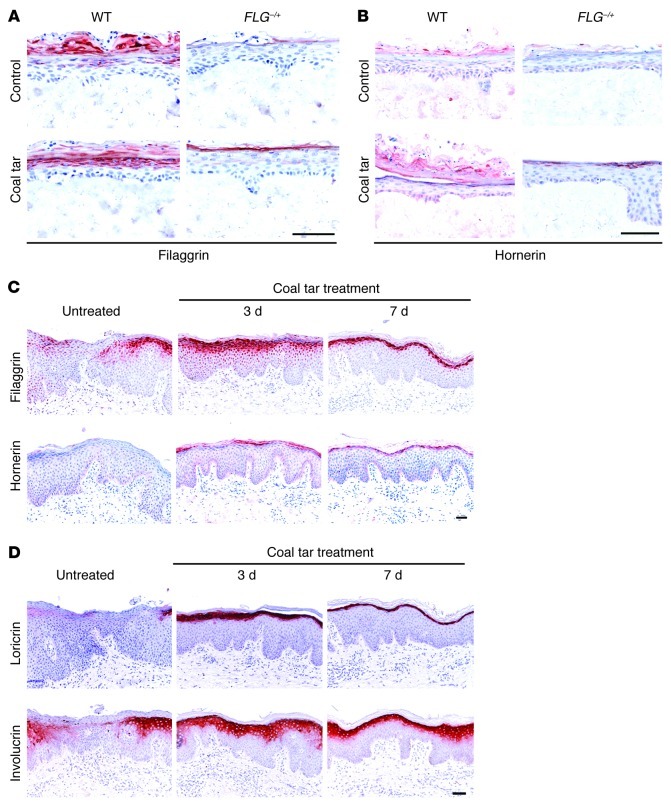
Based on the observed association of AD with FLG haploinsufficiency and the increased risk of lower copy numbers of intragenic FLG repeats, it has been suggested that treatments resulting in increased filaggrin expression may have therapeutic value (3). To address this issue experimentally, we generated skin equivalents with keratinocytes from both healthy volunteers and AD patients, with a wild-type genotype or a heterozygous mutation in the FLG gene, respectively (Supplemental Table 2). During the last 3 days of culture, we supplemented the culture medium with coal tar and evaluated whether coal tar would induce filaggrin in these patient-derived skin equivalents. Filaggrin protein expression in skin equivalents of heterozygously mutated keratinocytes was barely present in control conditions, whereas after coal tar treatment, filaggrin expression was evident in the granular layer of the epidermis (Figure 3A). As expected, wild-type keratinocytes responded more strongly to coal tar treatment, since they have 2 intact FLG alleles. Since downregulated hornerin expression in AD might be important in the pathophysiology of the disease (7), we also evaluated hornerin protein expression and found coal tar–mediated upregulated hornerin expression to be similar to that of filaggrin (Figure 3B). To correlate our in vitro model to the in vivo situation, we obtained skin biopsies from AD patients before and during coal tar therapy. In lesional AD skin, expression of filaggrin and hornerin was markedly lower and discontinuous, or even absent, compared with normal skin, as described previously (7). Already after 3 days of coal tar treatment, expression of these proteins was strongly induced in lesional AD skin, and after 7 days of coal tar therapy, a continuous expression pattern was observed (Figure 3C), resembling that of normal skin. We also analyzed other epidermal differentiation proteins and found strongly upregulated expression of loricrin and involucrin proteins both in vitro and in vivo (Figure 3D), suggesting that AD patients may benefit from enhanced epidermal differentiation in general, as well as improved barrier function.
Figure 3. Restored filaggrin and hornerin protein expression by coal tar.
Skin equivalents generated from keratinocytes from AD patients harboring a heterozygous FLG mutation (FLG–/+; n = 3) and healthy control wild-type (WT; n = 3) keratinocytes were treated with coal tar during the last 3 days of air-liquid interface culture. Skin equivalents were stained for (A) filaggrin, and (B) hornerin protein expression analysis. (C) Immunohistochemical analysis of filaggrin and hornerin protein expression in skin biopsies from AD patients receiving coal tar therapy (n = 3). Scale bar: 100 μM. (D) Immunohistochemical analysis of loricrin and involucrin protein expression in skin biopsies from AD patients receiving coal tar therapy (n = 3). Scale bar: 100 μM.
Coal tar normalizes histopathological and molecular hallmarks of AD in skin equivalents.
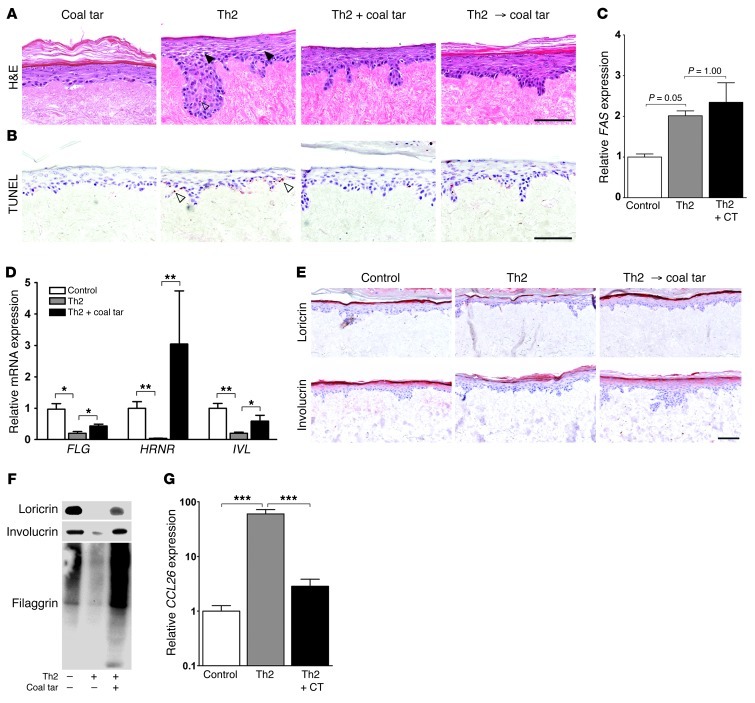
Th2 cytokines like IL-4 and IL-13 induce spongiosis and apoptosis in skin equivalents, probably via STAT6 signaling (18). These cytokines were also shown to downregulate involucrin and loricrin expression via STAT6 (4). Furthermore, the constitutive expression of STAT6 in transgenic mice has been demonstrated to lead to an increased risk of developing AD (5). We therefore stimulated skin equivalents with IL-4 and IL-13 to induce an AD phenotype in vitro as previously described (18), and tested the effect of coal tar. We found that coal tar treatment prevented the occurrence of spongiosis and apoptosis and could “cure” these symptoms in a therapeutic experimental design; both simultaneous addition of Th2 cytokines and coal tar, and coal tar treatment after Th2 stimulation, resulted in improvement of the phenotype (Figure 4, A and B). Although Th2 cytokine–mediated apoptosis was reported to be associated with increased expression of FAS (21), we did not observe downregulated FAS levels after coal tar treatment (Figure 4C). In our skin equivalent model, we confirmed the reported Th2 cytokine–mediated downregulation of epidermal differentiation proteins (4) and found coal tar treatment to restore both mRNA (Figure 4D) and protein expression (Figure 4, E and F). This was also confirmed in coal tar–treated AD patients in vivo (Figure 3D). Eosinophilia has been associated with AD, and the degree of eosinophilia correlates with AD severity (22). CCL26, or eotaxin-3, a chemokine for eosinophils, is upregulated in keratinocytes by IL-4 via the STAT6 signaling pathway (23). Our results have shown coal tar to diminish the downstream effects of IL-4, therefore we analyzed CCL26 levels in our AD skin equivalent model after coal tar treatment. The highly upregulated CCL26 expression levels in skin equivalents after Th2 cytokine stimulation were completely normalized after coal tar treatment (Figure 4G), indicating that coal tar directly targets the innate immune system by interfering with the keratinocyte response following Th2 cytokine stimulation.
Figure 4. Coal tar attenuates Th2 cytokine–induced AD hallmarks.
(A) H&E staining of skin equivalents stimulated with Th2 cytokines for 3 days, or treated with coal tar thereafter (Th2 → coal tar), or simultaneously stimulated with Th2 cytokines and coal tar (Th2 + coal tar). Closed arrowheads indicate spongiosis. Images are representative of 3 keratinocyte donors. The experiment was replicated twice. Scale bar: 100 μM. (B) TUNEL assay on matching skin equivalents as depicted in A, showing apoptotic cells indicated by open arrowheads. Scale bar: 100 μM. (C) FAS mRNA expression levels in skin equivalents after Th2 cytokine stimulation and coal tar treatment. Expression levels are relative to untreated (control) keratinocytes. Bars indicate mean ± SEM (n = 3). (D) mRNA expression levels of epidermal differentiation genes after Th2 cytokine stimulation and coal tar treatment. Expression levels are relative to untreated (control) keratinocytes. Bars indicate mean ± SEM (n = 3). *P < 0.05 and **P < 0.01. (E) Immunohistochemical staining of loricrin and involucrin in skin equivalents stimulated with Th2 cytokines for 3 days, or treated with coal tar thereafter (Th2 → coal tar). Images are representative of 3 keratinocyte donors. The experiment was replicated twice. Scale bar: 100 μM. (F) Loricrin, involucrin, and filaggrin Western blotting of skin equivalents are depicted in E. (G) CCL26 mRNA expression levels after Th2 cytokine stimulation and coal tar treatment by qPCR analysis. Expression levels are relative to untreated (control) keratinocytes. Bars indicate mean ± SEM (n = 3). ***P < 0.001.
Coal tar interferes with the Th2 cytokine–mediated STAT6 signaling cascade.
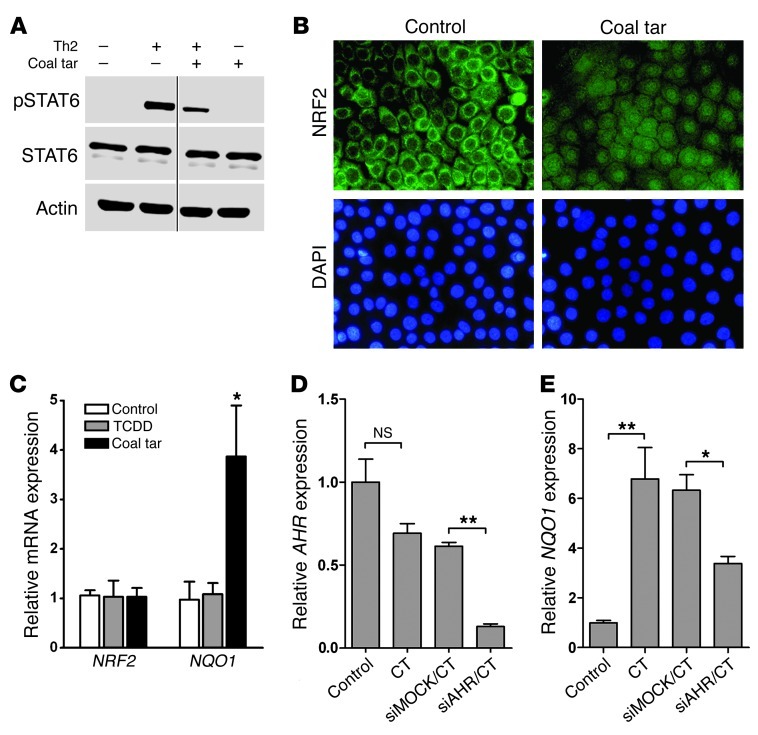
Since most of the aforementioned effects of Th2 cytokine stimulation in keratinocytes act via STAT6 signaling, we hypothesized that coal tar interferes with this signaling cascade. Both phosphorylated STAT6 (pSTAT6) and total STAT6 levels were determined using Western blot analysis, which revealed that stimulation with IL-4 and IL-13 induced STAT6 phosphorylation in keratinocytes (Figure 5A). Thereafter, we supplemented Th2 cytokine–stimulated keratinocytes with coal tar for 6 hours and observed markedly reduced pSTAT6 levels in these cell lysates (Figure 5A). pSTAT6 is a known substrate for protein tyrosine phosphatase 1B (PTPN1) (24). In addition, oxidative stress induced by Th2 cytokine stimulation in keratinocytes results in oxidative inactivation of PTPN1, and thus sustained STAT6 phosphorylation (25). We therefore hypothesized that the molecular mechanism of coal tar–mediated inhibition of STAT6 phosphorylation could, at least in part, act via inhibition of the oxidative inactivation of PTPN1. AHR is known as a sensor of the redox system against oxidative stress and regulates nuclear factor erythroid 2–related factor 2 (NRF2), a master switch of the redox machinery (26). We found coal tar to induce both nuclear translocation of NRF2 (Figure 5B) and subsequent induction of its target gene, NAD(P)H quinone oxidoreductase 1 (NQO1) in keratinocytes (Figure 5C). Remarkably, no induction of NQO1 was observed in keratinocytes following TCDD exposure. Upon AHR knockdown, induction of NQO1 expression was inhibited, indicating the regulatory role of AHR in NRF2-mediated signaling in keratinocytes (Figure 5D). Our data indicate that coal tar activates the antioxidative stress pathway via NRF2 signaling, which might inhibit Th2-induced oxidative stress and result in STAT6 dephosphorylation and abrogation of the inflammatory Th2-mediated STAT6 signaling cascade in keratinocytes (Figure 6).
Figure 5. STAT6 dephosphorylation and NRF2 activation by coal tar.
(A) Western blot analysis of phosphorylated STAT6 (pSTAT6) and total STAT6 levels in lysates of 2 keratinocyte donors. Keratinocytes were stimulated for 12 hours with differentiation medium (–/–), Th2 cytokines (+/–), or 2% coal tar (–/+), and with Th2 cytokines during the first 6 hours, followed by the addition of 2% coal tar and stimulation for another 6 hours (+/+). Thin black line indicates that the lanes were run on the same gel, but were noncontiguous. (B) Immunofluorescence staining of NRF2 in coal tar–treated or untreated keratinocytes. Nuclei were counterstained using DAPI. Images are representative of 3 keratinocyte donors. Original magnification, ×400. (C) qPCR analysis of NRF2 and NQO1 mRNA expression levels in keratinocytes. Expression levels are relative to untreated (control) keratinocytes. *P = 0.002 relative to control condition. Bars indicate mean ± SEM (n = 3). qPCR analysis of mRNA expression levels of AHR (D) and NQO1 (E) after siRNA-mediated AHR knockdown and subsequent coal tar stimulation by qPCR analysis. Expression levels are relative to mock-treated, coal tar–stimulated keratinocytes. *P = 0.006 and **P = 0.032, relative to mock-treated keratinocytes. Bars indicate mean ± SEM (n = 3).
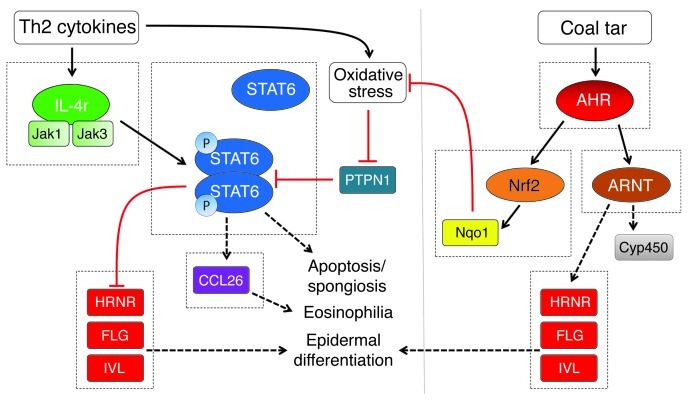
Figure 6. Model of the molecular mechanism of coal tar therapy in AD.
Th2 cytokines IL-4 and IL-13 activate the STAT6 signaling cascade, leading to downregulated expression of epidermal differentiation proteins, induction of the eosinophilic chemoattractant CCL26, and histopathological features such as spongiosis and apoptosis. Under normal conditions, PTPN1 dephosphorylates STAT6, resulting in a regulatory feedback loop. However, oxidative stress caused by Th2 cytokines inactivates PTPN1, leading to sustained STAT6 signaling. Coal tar–mediated activation of the AHR signaling pathway leads to enhanced epidermal differentiation and possible improvement of epidermal barrier function, thereby attenuating allergen exposure and reducing inflammatory cues. Importantly, coal tar activates the AHR/NRF2 signaling pathway, enabling detoxification of reactive oxygen species. This may prevent the oxidative inactivation of PTPN1 and lead to decreased STAT6 signaling, normalization of skin barrier protein expression, and downregulation of CCL26 expression.
Discussion
AD is a highly prevalent inflammatory skin disease caused by skin barrier dysfunction and chronic immune activation (27, 28). No mechanism-based therapies are available for AD patients and treatment is mainly focused on symptom relief, predominantly targeting dry skin, itch, and inflammation with emollients and corticosteroids. Since the seminal work of the McLean Lab has underscored the importance of skin barrier abnormalities as an etiological factor in AD, we focused on the effects of coal tar on the epidermal biology of this disease (1, 3, 8). Our results reveal a key role of the AHR signaling pathway in breaching the vicious circle of chronic inflammation by improving skin barrier function and interfering with Th2 cytokine signaling and downstream pathological processes.
The immunopathogenesis of AD has been studied directly on patients or in animal models based on allergic immune responses (29–31). We have recently developed in vitro organotypic models for psoriasis and AD (18, 19), and have shown that the application of genetic techniques in these models could be used to study epidermal aspects of diseases (32). Others showed that FLG knockdown in human skin equivalents could mimic AD skin by impaired epidermal differentiation and barrier dysfunction (33, 34). We have chosen keratinocyte-only human skin equivalents to evaluate the effect of coal tar on the epidermis, thereby excluding the possible effects of coal tar and subsequent AHR activation on other skin cell types such as fibroblasts and immune cells, as these cells could in turn affect epidermal processes. Genetic approaches to modify keratinocyte gene expression were introduced in this system by using an siRNA knockdown approach to prove the role of AHR, or by using patient-derived keratinocytes harboring one FLG-null allele. These findings illustrate the power of skin equivalents to study human skin diseases and, to a certain extent, to replace mouse models.
In the early seventies, attempts were made to unravel the mechanism of action of coal tar therapy. Suppression of DNA synthesis (35, 36) and induction of a granular layer using the mouse tail test (37, 38) have been reported. The mouse tail test has previously demonstrated the effects of coal tar on epidermal differentiation. Since the discovery of FLG mutations in AD, it is now widely accepted that epidermal barrier dysfunction is key in the pathophysiology of this disease. These new insights are strongly guiding current efforts in AD research and therapy development. The accelerated filaggrin expression and barrier function in fetal mouse skin due to dioxin-mediated AHR activation (17, 39) suggested to us that there is a potential role for AHR in AD treatment. Here, we show that coal tar activates AHR signaling and found that AHR regulates and induces epidermal differentiation and stimulates filaggrin expression in keratinocytes harboring a monoallelic FLG loss-of-function mutation. These findings may have a major impact on the reappraisal of coal tar therapy, which is gradually being abandoned by dermatologists due to its cosmetic side effects, safety concerns, and a hitherto unknown mechanism of action.
The AHR signaling pathway is currently of great interest. Though it was previously only considered to be related to immunotoxicity, recent findings have underscored this pathway’s physiological role. AHR appears to be predominantly involved in the development and function of the immune system (14, 40–43). Genetic approaches in mice, however, have also revealed that AHR signaling plays a role in epidermal pathophysiology. Deficiency or constitutive expression of AHR interaction partners ARNT (44, 45) and NRF2 (46), respectively, has detrimental effects on epidermal differentiation and barrier function, whereas AHR transgenic mice develop inflammatory skin lesions with hyperkeratinization (47). All these studies show that disturbance of normal AHR signaling, either by genetic approaches or TCDD exposure, leads to epidermal abnormalities. Our study is the first to demonstrate the beneficial therapeutic effects of AHR activation on epidermal differentiation and barrier function in a skin disease characterized by low levels of terminal differentiation proteins and the resultant poor barrier function. The therapeutic use of AHR ligands appears controversial in light of the complete ban on all AHR ligands instituted by the pharmaceutical industry because of the widely known dioxin-related toxic effects of AHR activation. However, recent interest in the AHR signaling pathway has led to a variety of reports describing diverse downstream effects by different exogenous and endogenous AHR agonists. Nowadays, therefore, it is recognized that the specific ligand-receptor interaction determines the downstream effects of AHR activation, and that it is not merely a matter of drug potency. Hence, CYP1A1 induction and/or AHR activation are not synonymous with dioxin-like toxicity.
In addition to the improved epidermal differentiation and filaggrin induction by coal tar–mediated AHR activation, we made the exciting observation that coal tar normalizes histopathological and molecular hallmarks of AD, and found STAT6 to be the key player in this process in keratinocytes. Although we were unable to directly measure the putative phosphatase (PTPN1) activity responsible for inactivating STAT6 activity (24) in keratinocytes, we do provide evidence that both upstream (NRF2) and downstream (STAT6) pathways are affected by coal tar. Therefore, it is very likely that coal tar inhibits oxidative inactivation of PTPN1, and thereby attenuates STAT6 activation. The Th2 immune response, as observed in atopic diseases like AD, is also driven by STAT6 via IL-4–induced maturation and development of Th2 cells (48, 49). Based on our data, we believe that AHR has great potential as a pharmacological target in atopic or allergic diseases, especially because it was recently shown that the AHR agonist FICZ (a tryptophan metabolite) suppresses eosinophilia, Th2 cytokine production, and STAT6 activation in allergic asthma in mice (50). Moreover, PTPN1 deficiency results in exacerbated lung inflammation in mice due to early recruitment of leukocytes and elevated levels of lung CCL26 and Th2 cytokine levels (51). Since coal tar metabolites are found in urine of patients receiving coal tar therapy (52), suggesting the systemic entrance of coal tar components following topical application, the therapeutic success of coal tar therapy may, in addition to its epidermal effects, also rely on suppression of the immune response by dampening STAT6 activation in immune cells. The reported role of AHR in Treg development is likely to contribute to the therapeutic efficacy of coal tar in two major skin diseases: psoriasis and AD. The scope of our current investigations, however, was limited to epidermal aspects of AD and the effect of coal tar.
In addition to the recently uncovered role of NRF2 in epidermal barrier function, its main function lies in the defense against oxidative stress, and it is therefore proposed as a potential pharmacological target for chemoprevention (53). NRF2 has been linked to the AHR signaling pathway mostly in mice liver tissue after dioxin exposure (54). Here we show that coal tar, but not TCDD, induces NRF2 activation and subsequent expression of NQO1 in human keratinocytes. Upon knockdown of AHR, significantly less NQO1 was expressed, indicating an interaction between AHR and NRF2 in human keratinocytes. The discrepancy between the previously reported TCDD-induced NQO1 levels and the lack of such induction in keratinocytes is in itself not unprecedented, and may be explained by species and cell type differences and ligand-specific effects (55). Therefore, the induction of a protective mechanism (NQO1 induction) in keratinocytes after coal tar exposure might explain the lack of toxicity and carcinogenicity associated with the medicinal use of coal tar (56), while AHR activation by TCDD leads to severe symptoms such as chloracne and immunotoxicity, as well as an increased risk of cancer.
Although coal tar therapy is the oldest known dermatological treatment, questions still remain regarding its safety and possible carcinogenicity (57). A comprehensive study by Roelofzen et al. in 2010 (56) did not find a relation between an increased risk of skin or non-skin malignancies and coal tar therapy in a large cohort of 13,200 psoriasis and eczema patients. AHR ligands are currently excluded from drug discovery and development pipelines due to the exposure-related toxicity and carcinogenicity of some of its known ligands, such as dioxin and benzo[a]pyrene. The emerging evidence of a more physiological role of AHR, and the therapeutic effect of AHR activation herein described, suggest that it might be the right time to reconsider whether it is justifiable to exclude AHR as a pharmacological target.
Methods
Coal tar formulations.
Pix Licantrix (medicinal coal tar) obtained from BUFA (Spruyt Hillen) was added to keratinocyte differentiation medium supplemented with 0.5 mg/ml delipidized BSA at an initial concentration of 2% (v/v). After overnight vortexing at room temperature, the saturated coal tar suspension was centrifuged and the supernatant was sterilized by 0.2 μm filtration. This solution was diluted 10 times in culture medium to obtain the highest nontoxic concentration (this condition was further designated as the “2%” concentration in all experiments). Further dilutions in culture medium were made from this solution. Coal tar is known to be composed of a large number (>10,000) of organic compounds, many of which are nonpolar and practically insoluble in aqueous solution. The presence of delipidized BSA in the medium is likely to have facilitated the solubility of part of the nonpolar fraction, but no attempt was made to quantify the fraction of the crude coal tar that was actually dissolved.
Toxicity testing.
Cellular toxicity was measured using cell supernatant and lactate dehydrogenase analysis according to the manufacturer’s recommendations (Roche). Percentage of cell death, which was set at 100%, was relative to 1% Triton-X/PBS–treated keratinocytes.
Submerged keratinocyte culture.
Keratinocytes were isolated from human abdominal skin derived from donors who underwent surgery for abdominal wall correction, as previously described (17). For submerged culture, keratinocytes were cultured in keratinocyte growth medium (Lonza) and differentiated by depletion of growth factors, as described previously (58). Coal tar was supplemented during the differentiation phase at indicated concentrations. The study was conducted in accordance with Declaration of Helsinki principles.
Skin equivalent development.
Human skin equivalents were generated using de-epidermized dermis, as described previously, and seeded with 105 human primary keratinocytes (17). Skin equivalents were cultured submerged for 3 days, after which the medium level was decreased and equivalents were cultured at the air-liquid interface at indicated time points in the presence of coal tar.
Skin morphology.
Six-micrometer sections were obtained from paraffin-embedded, formalin-fixed skin equivalents or skin biopsies and were stained with H&E.
Indirect immunostaining of skin equivalents or biopsies and keratinocyte cultures.
Indirect immunoperoxidase technique with avidin-biotin complex enhancement (Vectastain; Vector Laboratories) was used to analyze epidermal differentiation. The following antibodies were used: antibodies against filaggrin (1:6000; Biomedical Technologies Inc.); loricrin (1:500; BabCO); involucrin (1:50; Mon150; generated by our lab, van Duijnhoven [1992], University of Geneva, Switzerland); and hornerin (antibody was raised against full-length recombinant HRNR repeat domains, 1:200; ref. 59). For translocation of AHR and NRF2 (1:200, Santa Cruz), direct immunofluorescence labeling was performed using Alexa Fluor 488 conjugate in conjunction with fluorescence microscopy, and fluorescence intensity of nuclei and cytoplasm was measured by ImageJ software. The average mean FI nucleus/cytoplasm ratio of 10 cells per experimental condition was calculated from 3 keratinocyte donors. Preincubation of 2 μg AHR human recombinant protein (Abnova) with a 1:200 dilution of AHR antibody for 1 hour at room temperature was performed prior to incubation with the cell cultures to determine antibody specificity.
Transcriptional analysis.
Epidermis was separated from the skin equivalents by dispase treatment (Roche) for 2 hours at 4°C. Total RNA from epidermis and cultured keratinocytes were isolated using Trizol reagent (Life Technologies) and a microprep kit (QIAGEN). Dnase I–treated (Invitrogen; Life Technologies) and reverse transcription PCR products were analyzed using the MyiQ Single-Color Real-Time Detection System for quantification with SYBR Green and melting curve analysis (Bio-Rad Laboratories). Expression of target genes was normalized to expression of the housekeeping gene human ribosomal phosphoprotein P0 (RPLP0) in the same sample (60). Relative mRNA expression levels were calculated using the ΔΔCt method (61).
siRNA knockdown.
Keratinocytes were grown to 60% confluency, and 500 nM of smartpool AHR targeting or nontargeting siRNA (Accell Dharmacon; Thermo Scientific) was added for 48 hours. Culture medium was subsequently refreshed and supplemented with siRNA for another 24 hours. Keratinocytes were thereafter allowed to differentiate for 48 hours in the presence of coal tar. Cells were harvested for transcriptional analysis as described above.
Clinical studies.
Three AD patients hospitalized to receive coal tar treatment in our medical center were included in the study. Coal tar treatment consisted of the application of 5% crude coal tar ointment (pix lithantracis in zinc oxide paste) to the affected body area twice daily. Three-millimeter biopsies were taken under local anesthesia from lesional skin prior to treatment and on days 3 and 7 of treatment. Biopsies were taken from the center of active lesions, avoiding blisters or wounds.
FLG mutation analysis.
Genomic DNA was extracted from saliva samples using the Oragene kit (DNA Genotek Inc.) according to the manufacturer’s protocol. For analysis of the p.R501X (c.1501C>T) and c.2282del4 mutations, we used the previously published primers FilH1F3/RPT1P6 and RPT1P7/RPT2P1, respectively (62). DNA (50 ng) was diluted in PCR reaction buffer containing 1.6 mM MgCl2 and Platinum Taq Polymerase (Invitrogen; Life Technologies). PCR conditions were as follows: 94°C for 5 minutes; 35 cycles of 94°C for 30 seconds; 58°C for 45 seconds; 72°C for 75 seconds; and the final extension step at 72°C for 7 minutes. The amplified PCR products were resolved on a 1.5% agarose gel, purified by using the NucleoSpin Gel and PCR Clean-up kit (BIOKω), and sequenced using dye termination chemistry on a 3730 DNA analyzer (Applied Biosystems; Life Technologies).
Western blot analysis.
Keratinocytes or epidermal sheets from skin equivalents separated by dispase treatment were lysed in RIPA lysis buffer. After a single cycle of freeze-thawing, the lysates were centrifuged at maximum speed for 10 minutes at 4°C. The supernatant was used for involucrin immunoblotting. Total protein concentration of the supernatant was determined, and a total of 12.5 μg protein was loaded for each sample. The pellet containing the insoluble fraction was resuspended in sample buffer, sonicated, and boiled for 5 minutes and used for filaggrin and loricrin detection. For submerged cultured keratinocyte lysates, actin antibody (Sigma-Aldrich) was used to control equal protein loading. To control equal loading of the insoluble fraction from skin equivalent epidermis, Coomassie Blue staining of gels and Poncea–u S staining of blots were used (data not shown). Prior to immunoblotting, proteins were separated by SDS page and transferred to PVDF membranes using the NuPAGE system (Life Technologies). LumiGLO (Cell Signaling Technology Inc.) was used for chemiluminescent detection by the Bio-Rad Universal Hood Gel Imager (Bio-Rad Laboratories).
STAT6 phosphorylation was analyzed in primary human keratinocytes after IL-4 and IL-13 stimulation as described previously (18), with coal tar supplemented in the culture medium at indicated time points.
Statistics.
Data are given as mean ± SEM of at least 3 biological replicates. Statistical analysis of quantitative PCR (qPCR) data was performed on ΔCt values using commercially available software (PASW Statistics 18; SPSS Inc.). One-way analysis of variance, followed by Bonferroni post hoc testing and 2-tailed, paired t tests were performed. P < 0.05 was considered statistically significant.
Study approval.
The study was approved by the local medical ethical committee and was conducted according to Declaration of Helsinki principles. Informed consent was obtained from all patients and healthy volunteers.
Supplementary Material
Acknowledgments
The authors thank Daniëlle Hansen, Josan van der Heijden, and Heleen de Koning for performing skin biopsies. Patients and healthy volunteers are acknowledged for their kind cooperation. Diana Rodijk-Olthuis is acknowledged for isolation of the primary human keratinocytes. This work was supported by a grant from the Nijmegen Institute for Infection, Inflammation, and Immunity (N4i), Radboud University Nijmegen Medical Centre (to J. Schalkwijk), and Deutsche Forschungsgemeinschaft (to J.M. Schröder).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2013;123(2):917–927. doi:10.1172/JCI65642.
See the related Commentary beginning on page 551.
References
- 1.Palmer CN, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38(4):441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 2.Jakasa I, et al. Skin barrier function in healthy subjects and patients with atopic dermatitis in relation to filaggrin loss-of-function mutations. J Invest Dermatol. 2011;131(2):540–542. doi: 10.1038/jid.2010.307. [DOI] [PubMed] [Google Scholar]
- 3.Brown SJ, et al. Intragenic copy number variation within filaggrin contributes to the risk of atopic dermatitis with a dose-dependent effect. J Invest Dermatol. 2012;132(1):98–104. doi: 10.1038/jid.2011.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim BE, Leung DY, Boguniewicz M, Howell MD. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin Immunol. 2008;126(3):332–337. doi: 10.1016/j.clim.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sehra S, et al. IL-4 regulates skin homeostasis and the predisposition toward allergic skin inflammation. J Immunol. 2010;184(6):3186–3190. doi: 10.4049/jimmunol.0901860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esparza-Gordillo J, et al. A common variant on chromosome 11q13 is associated with atopic dermatitis. Nat Genet. 2009;41(5):596–601. doi: 10.1038/ng.347. [DOI] [PubMed] [Google Scholar]
- 7.Henry J, et al. Hornerin is a component of the epidermal cornified cell envelopes. FASEB J. 2011;25(5):1567–1576. doi: 10.1096/fj.10-168658. [DOI] [PubMed] [Google Scholar]
- 8.O’Regan GM, Campbell LE, Cordell HJ, Irvine AD, McLean WH, Brown SJ. Chromosome 11q13.5 variant associated with childhood eczema: an effect supplementary to filaggrin mutations. J Allergy Clin Immunol. 2010;125(1):170–174. doi: 10.1016/j.jaci.2009.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright CW, Later DW, Pelroy RA, Mahlum DD, Wilson BW. Comparative chemical and biological analysis of coal tar-based therapeutic agents to other coal-derived materials. J Appl Toxicol. 1985;5(2):80–88. doi: 10.1002/jat.2550050208. [DOI] [PubMed] [Google Scholar]
- 10.Smith G, et al. Regulation of cutaneous drug-metabolizing enzymes and cytoprotective gene expression by topical drugs in human skin in vivo. Br J Dermatol. 2006;155(2):275–281. doi: 10.1111/j.1365-2133.2006.07317.x. [DOI] [PubMed] [Google Scholar]
- 11.Ma Q, Lu AY. CYP1A induction and human risk assessment: an evolving tale of in vitro and in vivo studies. Drug Metab Dispos. 2007;35(7):1009–1016. doi: 10.1124/dmd.107.015826. [DOI] [PubMed] [Google Scholar]
- 12.Burbach KM, Poland A, Bradfield CA. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc Natl Acad Sci U S A. 1992;89(17):8185–8189. doi: 10.1073/pnas.89.17.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denison MS, Fisher JM, Whitlock JP. Inducible, receptor-dependent protein-DNA interactions at a dioxin-responsive transcriptional enhancer. Proc Natl Acad Sci U S A. 1988;85(8):2528–2532. doi: 10.1073/pnas.85.8.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu J, et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36(1):92–104. doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Apetoh L, et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010;11(9):854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gandhi R, et al. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat Immunol. 2010;11(9):846–853. doi: 10.1038/ni.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sutter CH, Bodreddigari S, Campion C, Wible RS, Sutter TR. 2,3,7,8-Tetrachlorodibenzo-p-dioxin increases the expression of genes in the human epidermal differentiation complex and accelerates epidermal barrier formation. Toxicol Sci. 2011;124(1):128–137. doi: 10.1093/toxsci/kfr205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamsteeg M, et al. Type 2 helper T-cell cytokines induce morphologic and molecular characteristics of atopic dermatitis in human skin equivalent. Am J Pathol. 2011;178(5):2091–2099. doi: 10.1016/j.ajpath.2011.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tjabringa G, Bergers M, van Rens D, de BR, Lamme E, Schalkwijk J. Development and validation of human psoriatic skin equivalents. Am J Pathol. 2008;173(3):815–823. doi: 10.2353/ajpath.2008.080173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng T, et al. The cystatin M/E-controlled pathway of skin barrier formation: expression of its key components in psoriasis and atopic dermatitis. Br J Dermatol. 2009;161(2):253–264. doi: 10.1111/j.1365-2133.2009.09156.x. [DOI] [PubMed] [Google Scholar]
- 21.Trautmann A, et al. T cell-mediated Fas-induced keratinocyte apoptosis plays a key pathogenetic role in eczematous dermatitis. J Clin Invest. 2000;106(1):25–35. doi: 10.1172/JCI9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kagami S, et al. Significant elevation of serum levels of eotaxin-3/CCL26, but not of eotaxin-2/CCL24, in patients with atopic dermatitis: serum eotaxin-3/CCL26 levels reflect the disease activity of atopic dermatitis. Clin Exp Immunol. 2003;134(2):309–313. doi: 10.1046/j.1365-2249.2003.02273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bao L, Shi VY, Chan LS. IL-4 regulates chemokine CCL26 in keratinocytes through the Jak1, 2/Stat6 signal transduction pathway: Implication for atopic dermatitis. Mol Immunol. 2012. 50 (1-2): 91 97 10.1016/j.molimm.2011.12.008 [DOI] [PubMed] [Google Scholar]
- 24.Lu X, et al. PTP1B is a negative regulator of interleukin 4-induced STAT6 signaling. Blood. 2008;112(10):4098–4108. doi: 10.1182/blood-2008-03-148726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirakawa S, Saito R, Ohara H, Okuyama R, Aiba S. Dual oxidase 1 induced by Th2 cytokines promotes STAT6 phosphorylation via oxidative inactivation of protein tyrosine phosphatase 1B in human epidermal keratinocytes. J Immunol. 2011;186(8):4762–4770. doi: 10.4049/jimmunol.1000791. [DOI] [PubMed] [Google Scholar]
- 26.Tsuji G, et al. Identification of ketoconazole as an AHR-Nrf2 activator in cultured human keratinocytes: the basis of its anti-inflammatory effect. J Invest Dermatol. 2012;132(1):59–68. doi: 10.1038/jid.2011.194. [DOI] [PubMed] [Google Scholar]
- 27.Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011;365(14):1315–1327. doi: 10.1056/NEJMra1011040. [DOI] [PubMed] [Google Scholar]
- 28.Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242(1):233–246. doi: 10.1111/j.1600-065X.2011.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oyoshi MK, et al. Epicutaneous challenge of orally immunized mice redirects antigen-specific gut-homing T cells to the skin. J Clin Invest. 2011;121(6):2210–2220. doi: 10.1172/JCI43586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masuoka M, et al. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Invest. 2012;122(7):2590–2600. doi: 10.1172/JCI58978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Man MQ, et al. Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. J Invest Dermatol. 2008;128(1):79–86. doi: 10.1038/sj.jid.5701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van den Bogaard EH, et al. Rho kinase inhibitor Y-27632 prolongs the life span of adult human keratinocytes, enhances skin equivalent development, and facilitates lentiviral transduction. Tissue Eng Part A. 2012;18(17–18):1827–1836. doi: 10.1089/ten.tea.2011.0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuchler S, et al. Hallmarks of atopic skin mimicked in vitro by means of a skin disease model based on FLG knock-down. Altern Lab Anim. 2011;39(5):471–480. doi: 10.1177/026119291103900508. [DOI] [PubMed] [Google Scholar]
- 34.Mildner M, et al. Knockdown of filaggrin impairs diffusion barrier function and increases UV sensitivity in a human skin model. J Invest Dermatol. 2010;130(9):2286–2294. doi: 10.1038/jid.2010.115. [DOI] [PubMed] [Google Scholar]
- 35.Lowe NJ, Breeding J, Wortzman MS. The pharmacological variability of crude coal tar. Br J Dermatol. 1982;107(4):475–479. doi: 10.1111/j.1365-2133.1982.tb00391.x. [DOI] [PubMed] [Google Scholar]
- 36.Stoughton RB, DeQuoy P, Walter JF. Crude coal tar plus near ultraviolet light suppresses DNA synthesis in epidermis. Arch Dermatol. 1978;114(1):43–45. doi: 10.1001/archderm.1978.01640130007001. [DOI] [PubMed] [Google Scholar]
- 37.Bladon PT, Taylor M, Wood EJ, Cunliffe WJ. Effect of crude coal tar in the mouse-tail model of psoriasis. Arch Dermatol Res. 1985;277(2):121–125. doi: 10.1007/BF00414109. [DOI] [PubMed] [Google Scholar]
- 38.Wrench R, Britten AZ. Evaluation of coal tar fractions for use in psoriasiform diseases using the mouse tail test. (II) Tar oil acids. Br J Dermatol. 1975;92(5):575–579. doi: 10.1111/j.1365-2133.1975.tb03127.x. [DOI] [PubMed] [Google Scholar]
- 39.Loertscher JA, Lin TM, Peterson RE, len-Hoffmann BL. In utero exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin causes accelerated terminal differentiation in fetal mouse skin. Toxicol Sci. 2002;68(2):465–472. doi: 10.1093/toxsci/68.2.465. [DOI] [PubMed] [Google Scholar]
- 40.Lee JA, et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin modulates functional differentiation of mouse bone marrow-derived dendritic cells Downregulation of RelB by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Lett. 2007;173(1):31–40. doi: 10.1016/j.toxlet.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147(3):629–640. doi: 10.1016/j.cell.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 42.Quintana FJ, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453(7191):65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 43.Veldhoen M, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453(7191):106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 44.Geng S, Mezentsev A, Kalachikov S, Raith K, Roop DR, Panteleyev AA. Targeted ablation of Arnt in mouse epidermis results in profound defects in desquamation and epidermal barrier function. J Cell Sci. 2006;119(pt 23):4901–4912. doi: 10.1242/jcs.03282. [DOI] [PubMed] [Google Scholar]
- 45.Robertson ED, Weir L, Romanowska M, Leigh IM, Panteleyev AA. ARNT controls the expression of epidermal differentiation genes through. J Cell Sci. 2012;125(pt 14):3320–3332. doi: 10.1242/jcs.095125. [DOI] [PubMed] [Google Scholar]
- 46.Wakabayashi N, et al. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet. 2003;35(3):238–245. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- 47.Tauchi M, et al. Constitutive expression of aryl hydrocarbon receptor in keratinocytes causes inflammatory skin lesions. Mol Cell Biol. 2005;25(21):9360–9368. doi: 10.1128/MCB.25.21.9360-9368.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4(3):313–319. doi: 10.1016/S1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 49.Shimoda K, et al. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380(6575):630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 50.Jeong KT, Hwang SJ, Oh GS, Park JH. FICZ, a tryptophan photoproduct, suppresses pulmonary eosinophilia and Th2-type cytokine production in a mouse model of ovalbumin-induced allergic asthma. Int Immunopharmacol. 2012;13(4):377–385. doi: 10.1016/j.intimp.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 51.Berdnikovs S, et al. PTP1B deficiency exacerbates inflammation and accelerates leukocyte trafficking in vivo. J Immunol. 2012;188(2):874–884. doi: 10.4049/jimmunol.1004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roelofzen JH, et al. DNA adducts in skin biopsies and 1-hydroxypyrene in urine of psoriasis patients and healthy volunteers following treatment with coal tar. Toxicol Lett. 2012;213(1):39–44. doi: 10.1016/j.toxlet.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 53.Haarmann-Stemmann T, Abel J, Fritsche E, Krutmann J. The AHR-Nrf2 pathway in keratinocytes: on the road to chemoprevention? J Invest Dermatol. 2012;132(1):7–9. doi: 10.1038/jid.2011.359. [DOI] [PubMed] [Google Scholar]
- 54.Yeager RL, Reisman SA, Aleksunes LM, Klaassen CD. Introducing the “TCDD-inducible AHR-Nrf2 gene battery”. Toxicol Sci. 2009;111(2):238–246. doi: 10.1093/toxsci/kfp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Denison MS, Soshilov AA, He G, DeGroot DE, Zhao B. Exactly the same but different: promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol Sci. 2011;124(1):1–22. doi: 10.1093/toxsci/kfr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roelofzen JH, et al. No increased risk of cancer after coal tar treatment in patients with psoriasis or eczema. J Invest Dermatol. 2010;130(4):953–961. doi: 10.1038/jid.2009.389. [DOI] [PubMed] [Google Scholar]
- 57.Cosmetic Ingredient Review Expert Panel. Final safety assessment of coal tar as used in cosmetics. Int J Toxicol. 2008;27(suppl 2):1–24. doi: 10.1080/10915810802244405. [DOI] [PubMed] [Google Scholar]
- 58.van Ruissen F, de Jongh GJ, Zeeuwen PL, van Erp PE, Madsen P, Schalkwijk J. Induction of normal and psoriatic phenotypes in submerged keratinocyte cultures. J Cell Physiol. 1996;168(2):442–452. doi: 10.1002/(SICI)1097-4652(199608)168:2<442::AID-JCP23>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 59.Wu Z, et al. Highly complex peptide aggregates of the S100 fused-type protein hornerin are present in human skin. J Invest Dermatol. 2009;129(6):1446–1458. doi: 10.1038/jid.2008.370. [DOI] [PubMed] [Google Scholar]
- 60.Minner F, Poumay Y. Candidate housekeeping genes require evaluation before their selection for studies of human epidermal keratinocytes. J Invest Dermatol. 2009;129(3):770–773. doi: 10.1038/jid.2008.247. [DOI] [PubMed] [Google Scholar]
- 61.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 62.Smith FJ, et al. Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris. Nat Genet. 2006;38(3):337–342. doi: 10.1038/ng1743. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.