Abstract
iRHOM2, encoded by the gene Rhbdf2, regulates the maturation of the TNF-α convertase (TACE), which controls shedding of TNF-α and its biological activity in vivo. TACE is a potential target to treat TNF-α–dependent diseases, such as rheumatoid arthritis, but there are concerns about potential side effects, because TACE also protects the skin and intestinal barrier by activating EGFR signaling. Here we report that inactivation of Rhbdf2 allows tissue-specific regulation of TACE by selectively preventing its maturation in immune cells, without affecting its homeostatic functions in other tissues. The related iRHOM1, which is widely expressed, except in hematopoietic cells, supported TACE maturation and shedding of the EGFR ligand TGF-α in Rhbdf2-deficient cells. Remarkably, mice lacking Rhbdf2 were protected from K/BxN inflammatory arthritis to the same extent as mice lacking TACE in myeloid cells or Tnfa-deficient mice. In probing the underlying mechanism, we found that two main drivers of K/BxN arthritis, complement C5a and immune complexes, stimulated iRHOM2/TACE-dependent shedding of TNF-α in mouse and human cells. These data demonstrate that iRHOM2 and myeloid-expressed TACE play a critical role in inflammatory arthritis and indicate that iRHOM2 is a potential therapeutic target for selective inactivation of TACE in myeloid cells.
Introduction
iRHOM2, an inactive member of the Rhomboid intramembrane proteinase family, was recently identified as regulator of the TNF-α convertase (TACE) (1–3). TACE is essential for activating the EGFR and for releasing TNF-α (4–8). Since biologic TNF-α blockers are widely used to treat rheumatoid arthritis (RA), a destructive, inflammatory joint disease affecting 0.5%–1% of the population, TACE is a potential alternative target for treatment of RA. However, a recently identified TACE-deficient patient revealed key roles for TACE in protecting the skin and intestinal barrier in humans (9). Notably, similar skin defects are recapitulated in mice lacking either TACE or EGFR in keratinocytes (10), and mice with strongly reduced TACE are susceptible to DSS colitis, a model for inflammatory bowel disease, probably because of a lack of EGFR signaling (11, 12). The protective role of TACE in the skin and intestine raises concerns about the potential side effects of targeting of TACE. It would be preferable to selectively inactivate TACE without compromising its protective functions, but, to date, there is no suitable approach to accomplish this. Here, we report that iRHOM2 is a good target to inactivate TACE in myeloid cells without affecting its protective functions and that iRHOM2 might therefore be an attractive new target for treatment of RA.
Results and Discussion
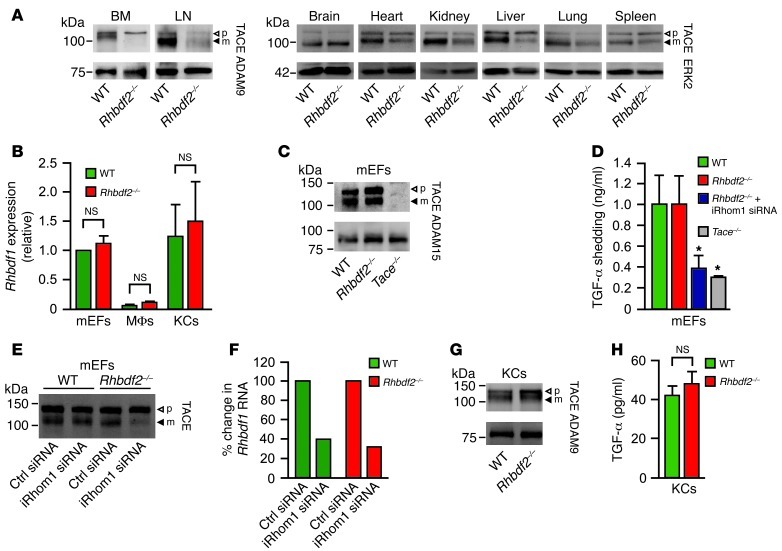
iRHOM2 controls the maturation of TACE, yet Rhbdf2–/– mice are healthy (1–3), whereas Tace–/– mice die perinatally (5, 7). To address this apparent paradox, we assessed whether iRHOM2 affects TACE maturation in tissues other than macrophages. In Western blots of Rhbdf2–/– tissues, mature TACE was not detected in bone marrow and was strongly reduced in lymph nodes but was clearly present in the brain, heart, kidney, liver, lung, and spleen (Figure 1A), in approximate concordance with the expression of the related iRHOM1 (BioGPS atlas, mu-iRHOM1). We therefore tested whether it is iRHOM1 that supports TACE maturation in Rhbdf2–/– mouse embryonic fibroblasts (mEFs), which expressed higher iRHOM1 levels than macrophages (Figure 1B and Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI66168DS1) and had normal levels of mature TACE in Western blots (Figure 1C; control: Tace–/– mEFs). Rhbdf2–/– mEFs shed the TACE substrate and EGFR ligand, TGF-α, at comparable levels to those of WT controls (Figure 1D). However, TGF-α shedding was strongly reduced in Rhbdf2–/– mEFs treated with iRhom1 siRNA (Figure 1D; control: Tace–/– mEFs). Western blots showed normal mature TACE levels in iRhom1 siRNA-treated WT mEFs but strongly reduced mature TACE levels in iRhom1 siRNA-treated Rhbdf2–/– mEFs; iRhom1 siRNA was effective in both WT and mutant cells (Figure 1, E and F). Since iRHOM1 was not upregulated in Rhbdf2–/– mEFs (Figure 1B and Supplemental Figure 1), iRHOM1 was sufficient for TACE maturation and function. In Rhbdf2–/– primary keratinocytes, which expressed similar iRHOM1 levels as mEFs (Figure 1B and Supplemental Figure 1), mature TACE levels and the release of endogenous TGF-α were comparable to those in controls (Figure 1, G and H). Since TACE and TGF are crucial for skin barrier maintenance in mice (10) and TACE/EGFR signaling protects from DSS colitis (11, 12), these findings provide a compelling explanation for the lack of skin and intestinal defects in Rhbdf2–/– mice (3).
Figure 1. iRHOM2 controls TACE maturation in immune cells but not somatic tissues.
(A) Western blots of TACE in tissues and cells from Rhbdf2–/– mice and littermate controls (WT). In Rhbdf2–/– mice, mature TACE is absent in bone marrow and strongly reduced in lymph nodes but present in brain, heart, kidney, liver, lung, and spleen (differences in mature TACE migration are caused by N-linked carbohydrate modifications; blots are representative of 5 experiments). p, pro-TACE; m, mature TACE. (B) qPCR of Rhbdf1 in mEFs, primary macrophages (MΦs), and primary keratinocytes (KCs; Rhbdf2–/– vs. controls; n = 2; mean + SD). (C) Representative TACE Western blot of mEFs from WT, Rhbdf2–/–, or Tace–/– animals (n = 3). (D) Shedding of TGF-α from WT, Rhbdf2–/–, iRhom1 siRNA-treated Rhbdf2–/–, or Tace–/– mEFs (n = 4; mean + SD; *P < 0.05, WT vs. iRhom1 siRNA-treated Rhbdf2–/– mEFs or WT vs. Tace–/– mEFs, 1-way ANOVA followed by Dunnett’s test). (E) TACE Western blot shows reduction of mature TACE only in iRhom1 siRNA-treated Rhbdf2–/– mEFs but not in iRhom1 siRNA-treated WT controls (Ctrl). (F) qPCR confirmed reduction of Rhbdf1 in iRhom1 siRNA-treated WT or Rhbdf2–/– mEFs (representative of 3 experiments). (G) Western blot of TACE and (H) release of endogenous TGF-α from primary keratinocytes from Rhbdf2–/– or WT mice (n = 2; mean + SD). ADAM9, ADAM15, or ERK2 were used as loading controls, as indicated.
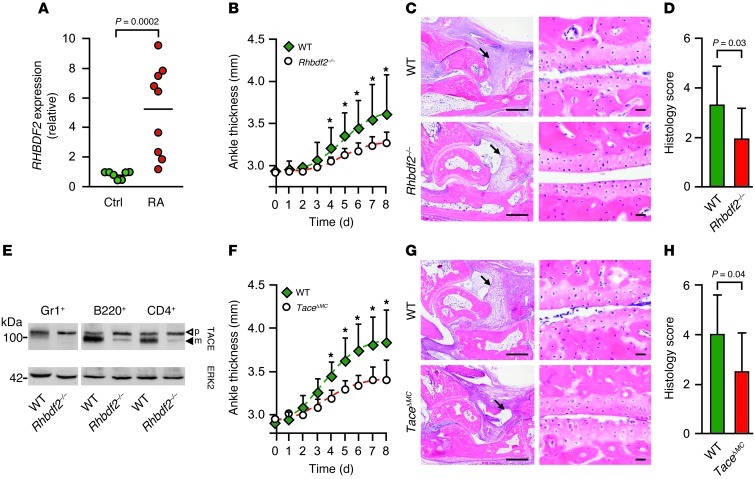
To explore the role of iRHOM2 in RA, we isolated synovial macrophages from patients with RA and found a significant upregulation of iRHOM2 expression compared with that in healthy controls (Figure 2A). We then assessed the role of iRHOM2 in the K/BxN mouse RA model, which has a substantial TNF-α–dependent component (although some Tnfa–/– mice are not protected from disease; refs. 13, 14). K/BxN inflammatory arthritis was significantly attenuated in Rhbdf2–/– mice compared with that in controls, showing less joint swelling (Figure 2B) and lower clinical scores (Supplemental Figure 2A), reflecting less synovial inflammation and cartilage erosion (Figure 2, C and D). These results show for what we believe to be the first time that iRHOM2 promotes inflammatory arthritis in the K/BxN model. Since this model depends on innate immune cells, such as macrophages and neutrophils (15), and since Rhbdf2–/– mice have little, if any, active TACE in these cells (Figure 2E and refs. 1, 2), we tested whether myeloid-specific inactivation of TACE would protect from K/BxN arthritis. As in the case of Rhbdf2–/– mice (Figure 2, F–H, and Supplemental Figure 2B) or Tnfa–/– mice (13, 14), TaceΔMC mice (mice with Tace deleted [Δ] in myeloid cells) (5) were similarly protected from serum transfer arthritis. Thus, the protection of Rhbdf2–/– mice from K/BxN inflammatory arthritis most likely involves inactivation of TACE-dependent TNF-α release from myeloid cells.
Figure 2. iRHOM2 and TACE in myeloid cells are key players in RA.
(A) Relative RHBDF2 expression in RA synovial macrophages (n = 9) compared with that in macrophages from normal control patients (n = 7). The horizontal line indicates the mean. (B) Ankle thickness of Rhbdf2–/– (n = 11) and control (n = 14) mice injected with K/BxN serum (mean + SD). (C) Histology of ankle joints (left) and cartilage erosion (right) in Rhbdf2–/– and control mice (arrows indicate synovitis). Scale bars: 500 μm (left); 20 μm (right). (D) Blinded scoring of ankle joints from Rhbdf2–/– mice (n = 11) or controls (n = 14) for inflammation and cartilage erosion (scale of 0 to 4) (mean + SD). (E) Western blot of TACE on Gr1+ myeloid cells, B220+ B cells, and CD4+ T cells (ERK2 was used as a loading control) (n = 2). (F) Ankle swelling in TaceΔMC and control mice subjected to K/BxN arthritis (n = 14 each; mean + SD). (G) Histology (as in C) and (H) blinded scoring of ankle sections from TaceΔMC mice and controls for inflammation and cartilage erosion (n = 14 each; mean +SD). *P < 0.05, Mann-Whitney U test.
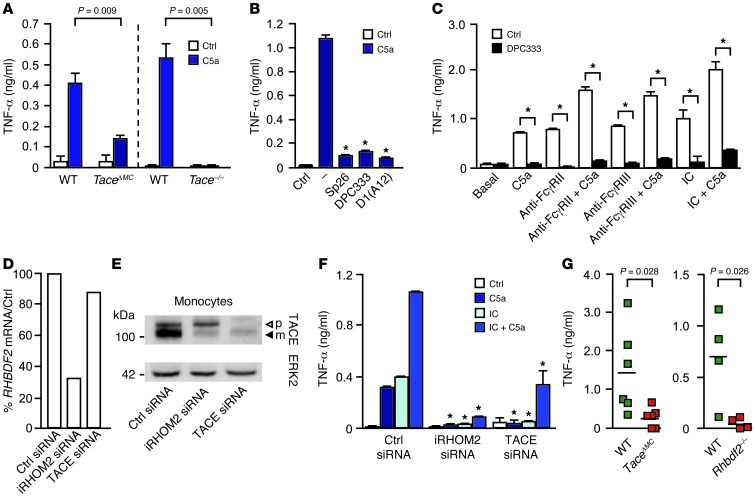
To understand why Rhbdf2–/– and TaceΔMC mice are protected from K/BxN arthritis, we tested whether two crucial drivers of this model, C5a receptor and FcγR (13, 15), activate iRHOM2/TACE-dependent TNF-α shedding. We found that stimulation of TaceΔMC macrophages or Tace–/– fetal liver macrophages with C5a triggered markedly reduced TNF-α release compared with that in controls (Figure 3A). Human monocytes behaved similarly, in that C5a also activated TACE to shed TNF-α, as evidenced by the inhibitory effect of TACE-selective hydroxamates (SP26 and DPC333; ref. 16) and the TACE-specific inhibitory antibody D1(A12) (ref. 17 and Figure 3B). Moreover, crosslinking FcγRIIa or FcγRIII on human monocytes with mAbs or immune complexes (ICs) (18) stimulated TNF-α shedding, which was enhanced by C5a, and inhibited by DPC333 (Figure 3C). Treatment of human macrophages with C5a or IC stimulated the production of RHBDF2 mRNA and TACE (Supplemental Figure 3). Addition of the TACE inhibitor DPC333 or etanercept reduced TNFA mRNA, suggesting that iRHOM2/TACE inhibitors could have unanticipated antiinflammatory benefits by reducing not only TNF-α shedding, but also its biosynthesis (Supplemental Figure 4). Of note, TACE was not required for other C5a receptor–mediated and Fcγ receptor–mediated (C5aR-mediated and FcγR-mediated) effector functions that are essential for microbial host defense, such as phagocytosis and generation of reactive oxidants (Supplemental Figure 5).
Figure 3. Complement C5a and ICs activate TACE to shed TNF-α.
(A) C5a-induced TNF-α shedding from TaceΔMC BMDMs or fetal liver-derived macrophages from Tace–/– mice or controls, assessed by ELISA after 3 to 4 hours of stimulation (n = 4; mean + SD). (B) TNF-α shedding by human monocytes during 2-hour stimulation with C5a (1 μg/ml) plus TACE inhibitors (SP26, DPC333) and anti-TACE antibody D1(A12) (representative of 4 experiments; mean + SD; *P < 0.05). (C) TNF-α shedding by human monocytes induced by FcγR crosslinking (mAbs or model IC [concentration, 0.25 mg/ml IVIg]) and/or C5a (1 μg/ml), assessed after 2 hours (representative of 3 experiments; mean + SD, *P < 0.05, DPC333 vs. control). (D) Human monocytes have reduced RHBDF2 mRNA when treated with iRHOM2 siRNA, but not with TACE siRNA, and (E) iRHOM2 siRNA treatment of human monocytes reduced mature TACE, but not pro-TACE, in a Western blot, whereas TACE siRNA reduced pro- and mature TACE (n = 3). (F) TNF-α release from C5a-, IC-, and IC/C5a-stimulated cells treated with or without iRHOM2 siRNA or TACE siRNA (n = 3; mean + SD; *P < 0.05, stimulated control siRNA vs. iRHOM2 siRNA or TACE siRNA, 1-way ANOVA, followed by Dunnett’s test). (G) Soluble TNF-α in the peritoneal lavage of TaceΔMC (n = 6) or Rhbdf2–/– mice (n = 4) and controls injected with ova plus anti-ova for 3 to 4 hours (n = 6).
To determine whether iRHOM2 regulates TACE in human monocytes, these were treated with iRHOM2 siRNA, which reduced mature TACE, but not pro-TACE, as in Rhbdf2–/– BM-derived macrophages (BMDMs) (1–3), whereas TACE siRNA reduced both forms (Figure 3, D and E). iRHOM2 siRNA or TACE siRNA significantly decreased the C5a-, IC-, or C5a/IC-stimulated release of TNF-α, establishing iRHOM2 as a key molecule in complement- and IC-stimulated TNF-α release from human monocytes (Figure 3F). C5a- and IC-stimulated TNF-α shedding were attenuated, in some cases differentially, by inhibitors of Src family kinases, p38MAPK, PKC, MEK, and Syk, some of which prevent arthritis in rodent models (Supplemental Figure 6). Finally, the in vivo relevance of TACE and iRHOM2 in C5a- and IC-stimulated TNF-α release was confirmed in reverse passive Arthus reaction peritonitis (19), where soluble TNF-α was markedly reduced in the peritoneal lavage in TaceΔMC and Rhbdf2–/– mice (Figure 3G). Taken together, our results demonstrate for the first time to our knowledge that C5a-C5aR and IC-FcγR both activate iRHOM2/TACE to release TNF-α, providing mechanistic insights into how these 2 pathways promote inflammatory RA. The pathway by which C5a and ICs activate TACE is presumably sequential, as both stimuli can activate TACE strongly, but blocking either prevents serum transfer RA (13, 15).
In summary, our results explain why Rhbdf2–/– mice display no obvious spontaneous pathologies: mature TACE is produced in most somatic tissues of Rhbdf2–/– mice. The related iRHOM1, which is expressed in somatic tissues but not in most hematopoietic cells, appears to support TACE maturation and function in the absence of iRHOM2, as shown in fibroblasts. Importantly, the lack of mature TACE in immune cells of Rhbdf2–/– mice offers significant protection from inflammatory arthritis, comparable to what is observed in TaceΔMC mice or Tnfa–/– mice. Because iRHOM2 and TACE are essential for the release of TNF-α from myeloid cells, and because TNF-α is a successful target for the treatment of RA, iRHOM2 may offer the unique and exciting prospect of cell type–specific blockade of TACE and TNF-α shedding. The skin and intestinal inflammation in a patient lacking TACE (9) emphasize the important opportunity provided by targeting iRHOM2 to limit immune cell-specific TACE activation in RA and possibly other TNF-α–dependent pathologies, yet avoid the potential consequences of systemic TACE inactivation.
Methods
Mice.
TaceΔMC, Tace–/–, and Rhbdf2–/– mice (2, 5) were of 129Sv/C57BL/6 mixed background. C5aR–/– mice (C57BL/6) were from C. Gerard (20). KRN-TCR mice (from D. Mathis and C. Benoist, Harvard Medical School, Boston, Massachusetts, USA) were mated with NOD/SHiLtJ mice (The Jackson Laboratory) to generate K/BxN mice.
Reagents.
Recombinant human C5a and C3a (EMD4Biosciences) and recombinant human TNF-α (R&D Systems) were endotoxin free (Limulus Amebocyte Lysate Pyrochrome Kit, Cape Cod Inc.). E. coli LPS (Chemicon); SP26 (16) (Schering Plough); anti-TACE D1(A12) (17) (from G. Murphy and C. Tape, Cambridge University, Cambridge, United Kingdom); DPC333 (16) (from R. Waltermire, Brystol-Myers Squibb); FACS antibodies (BD Pharmingen); TGF-α ELISA (R&D Systems); anti-iRHOM1 (Sigma-Aldrich); anti-TACE, anti-ADAM9, or anti-ADAM15 (Blobel lab); and signaling inhibitors (Milipore) were also used.
Cell culture.
CD14+ monocytes were purified as described previously (21). Macrophages were derived by culturing monocytes for 16 to 24 hours in 10% FBS α-MEM (Invitrogen) and 10 ng/ml hM-CSF (Peprotech). Mouse BMDMs (21) were maintained in DMEM, 20% FBS, and 10 ng/ml mM-CSF. Keratinocytes and mEFs were isolated as described previously (2, 22).
qPCR.
Total RNA (RNeasy) was reverse transcribed (Oligo-dT/SuperScript RT III, Qiagen). qPCR (SYBR Green, ABI PRISM 7900HT; Applied Biosystems) was normalized to actin.
Western blots.
Western blots were performed as described previously (2). Please see Supplemental Figure 7 for details and quantification.
RNA interference.
TACE, iRhom2, or control siRNAs were used for transfection (Lonza Nucleofector). iRhom1 siRNA duplex (Rhbdf1-MSS203813, Rhbdf1-MSS203814, or Rhbdf1-MSS203815; Invitrogen) was also used for transfection (Lipofectamine 2000, Invitrogen). Data shown are representative for Rhbdf1-MSS203814 (see Figure 1, D–F). iRhom2 siRNA duplex (HSS128595, HSS188104, HSS128594; Invitrogen) was used for transfection. Data shown are representative for HSS188104 (see Figure 2, D–F).
Shedding assay.
Shedding assays were for 3 to 4 hours (16).
Fc crosslinking.
CD14+ mononuclear cells were pretreated with or without DPC333 and added to 96-well plates coated with anti-FcγRIII (3G8, Medarex), anti-FcγRII (IV.3, Medarex), or IVIG (Talecris Biotherapeutics).
Reverse passive peritoneal Arthus reaction.
Following i.v. injection of 20 mg/kg chicken egg albumin (Sigma-Aldrich), 800 μg rabbit anti–chicken egg albumin IgG (MP Biomedicals) was i.p. injected. i.p. anti-OVA and i.v. PBS were used as controls. Mice were sacrificed after 3 hours for peritoneal lavage (3 ml PBS, 1% heparin).
K/BxN serum transfer arthritis.
100 μl of serum from 6- to 8-week-old K/BxN mice was injected i.p. at days 0 and 2. Clinical index and ankle thickness were measured (13). Ankle joint sections were scored (blinded) on a scale of 0 to 4 for inflammation and erosion (23).
Synovial macrophages.
Gene expression analysis was performed in RA synovial macrophages (deidentified; use of macrophages was approved by Hospital for Special Surgery IRB), which were isolated as described previously (24).
Statistics.
An unpaired 2-tailed Student’s t test was used unless otherwise stated. SigmaStat3.1 software or Prism (GraphPad Software) was used for all statistical tests.
Study approval.
All animal studies were approved by the Hospital for Special Surgery IACUC. All human studies were approved by the Hospital for Special Surgery IRB.
Supplementary Material
Acknowledgments
We thank G. Murphy and C. Tape for anti-huTACE antibodies and M. Vukelic, Y. Chinenov, E. Mogollon, A. Lee, and R. Gupte for technical assistance. Funding was provided by the NIH (R01 GM64750, to C.P. Blobel; AR38889, to J.E. Salmon; AR050401, to L.B. Ivashkiv; DFG LA2558/3-1, AvH SKA2010, to P.A. Lang; and AR061430 to K.-H. Park-Min).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2013;123(2):928–932. doi:10.1172/JCI66168.
See the related Commentary beginning on page 560.
References
- 1.Adrain C, Zettl M, Christova Y, Taylor N, Freeman M. Tumor necrosis factor signaling requires iRhom2 to promote trafficking and activation of TACE. Science. 2012;335(6065):225–228. doi: 10.1126/science.1214400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McIlwain DR, et al. iRhom2 regulation of TACE controls TNF-mediated protection against Listeria and responses to LPS. Science. 2012;335(6065):229–232. doi: 10.1126/science.1214448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siggs OM, et al. iRhom2 is required for the secretion of mouse TNFalpha. Blood. 2012;119(24):5769–5771. doi: 10.1182/blood-2012-03-417949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black R, et al. A metalloprotease disintegrin that releases tumour-necrosis factor-a from cells. Nature. 1997;385(6618):729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 5.Horiuchi K, et al. Cutting edge: TNF-a-converting enzyme (TACE/ADAM17) inactivation in mouse myeloid cells prevents lethality from endotoxin shock. J Immunol. 2007;179(5):2686–2689. doi: 10.4049/jimmunol.179.5.2686. [DOI] [PubMed] [Google Scholar]
- 6.Moss ML, et al. Cloning of a disintegrin metalloproteinase that processes precursor tumour-recrosis factor-a. Nature. 1997;385(6618):733–736. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- 7.Peschon JJ, et al. An essential role for ectodomain shedding in mammalian development. Science. 1998;282(5392):1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 8.Blobel CP. ADAMs: key players in EGFR-signaling, development and disease. Nat Rev Mol Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- 9.Blaydon DC, et al. Inflammatory skin and bowel disease linked to ADAM17 deletion. N Engl J Med. 2011;365(16):1502–1508. doi: 10.1056/NEJMoa1100721. [DOI] [PubMed] [Google Scholar]
- 10.Franzke CW, et al. Epidermal ADAM17 maintains the skin barrier by regulating EGFR ligand-dependent terminal keratinocyte differentiation. J Exp Med. 2012;209(6):1105–1119. doi: 10.1084/jem.20112258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chalaris A, et al. Critical role of the disintegrin metalloprotease ADAM17 for intestinal inflammation and regeneration in mice. J Exp Med. 2010;207(8):1617–1624. doi: 10.1084/jem.20092366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandl K, et al. MyD88 signaling in nonhematopoietic cells protects mice against induced colitis by regulating specific EGF receptor ligands. Proc Natl Acad Sci U S A. 2010;107(46):19967–19972. doi: 10.1073/pnas.1014669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji H, et al. Critical roles for interleukin 1 and tumor necrosis factor alpha in antibody-induced arthritis. J Exp Med. 2002;196(1):77–85. doi: 10.1084/jem.20020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitaura H, et al. Marrow stromal cells and osteoclast precursors differentially contribute to TNF-alpha-induced osteoclastogenesis in vivo. J Immunol. 2004;173(8):4838–4846. doi: 10.4049/jimmunol.173.8.4838. [DOI] [PubMed] [Google Scholar]
- 15.Ji H, et al. Arthritis critically dependent on innate immune system players. Immunity. 2002;16(2):157–168. doi: 10.1016/S1074-7613(02)00275-3. [DOI] [PubMed] [Google Scholar]
- 16.Maretzky T, Zhou W, Huang XY, Blobel CP. A transforming Src mutant increases the bioavailability of EGFR ligands via stimulation of the cell-surface metalloproteinase ADAM17. Oncogene. 2011;30(5):611–618. doi: 10.1038/onc.2010.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tape CJ, et al. Cross-domain inhibition of TACE ectodomain. Proc Natl Acad Sci U S A. 2011;108(14):5578–5583. doi: 10.1073/pnas.1017067108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durandy A, et al. Intravenous immunoglobulins--understanding properties and mechanisms. Clin Exp Immunol. 2009;158(suppl 1):2–13. doi: 10.1111/j.1365-2249.2009.04022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baumann U, et al. A codominant role of Fc gamma RI/III and C5aR in the reverse Arthus reaction. J Immunol. 2000;164(2):1065–1070. doi: 10.4049/jimmunol.164.2.1065. [DOI] [PubMed] [Google Scholar]
- 20.Hopken UE, Lu B, Gerard NP, Gerard C. Impaired inflammatory responses in the reverse arthus reaction through genetic deletion of the C5a receptor. J Exp Med. 1997;186(5):749–756. doi: 10.1084/jem.186.5.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu X, et al. Sensitization of IFN-gamma Jak-STAT signaling during macrophage activation. Nat Immunol. 2002;3(9):859–866. doi: 10.1038/ni828. [DOI] [PubMed] [Google Scholar]
- 22.Maretzky T, et al. Migration of growth factor-stimulated epithelial and endothelial cells depends on EGFR transactivation by ADAM17. Nat Commun. 2011;2:229. doi: 10.1038/ncomms1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choe JY, Crain B, Wu SR, Corr M. Interleukin 1 receptor dependence of serum transferred arthritis can be circumvented by toll-like receptor 4 signaling. J Exp Med. 2003;197(4):537–542. doi: 10.1084/jem.20021850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antoniv TT, Ivashkiv LB. Dysregulation of interleukin-10-dependent gene expression in rheumatoid arthritis synovial macrophages. Arthritis Rheum. 2006;54(9):2711–2721. doi: 10.1002/art.22055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.