Abstract
Complex II (succinate-ubiquinone reductase; SQR) is a mitochondrial respiratory chain enzyme that is directly involved in the TCA cycle. Complex II exerts a reverse reaction, fumarate reductase (FRD) activity, in various species such as bacteria, parasitic helminths and shellfish, but the existence of FRD activity in humans has not been previously reported. Here, we describe the detection of FRD activity in human cancer cells. The activity level was low, but distinct, and it increased significantly when the cells were cultured under hypoxic and glucose-deprived conditions. Treatment with phosphatase caused the dephosphorylation of flavoprotein subunit (Fp) with a concomitant increase in SQR activity, whereas FRD activity decreased. On the other hand, treatment with protein kinase caused an increase in FRD activity and a decrease in SQR activity. These data suggest that modification of the Fp subunit regulates both the SQR and FRD activities of complex II and that the phosphorylation of Fp might be important for maintaining mitochondrial energy metabolism within the tumor microenvironment.
Keywords: respiratory chain, complex II, succinate, fumarate, phosphorylation
Introduction
Mitochondria are important organelles that produce ATP. Under aerobic conditions, ATP is synthesized by oxidative phosphorylation via the respiratory chain and ATP synthase. One of the enzyme complexes in the respiratory chain, complex II, functions as succinate-ubiquinone reductase (SQR; EC 1.3.5.1) and is also an important enzyme in the tricarboxylic acid (TCA) cycle in mammalian mitochondria. Complex II consists of four nuclear-encoded subunits: a flavoprotein (Fp) and an iron-sulfur protein (Ip), which together comprise the catalytic portion of the enzyme complex, and two hydrophobic cytochrome b subunits (CybL and CybS). Several mutations in the three subunit genes (SDHB, SDHC and SDHD) other than Fp have been reported in phaeochromocytoma and paraganglioma,1)–3) suggesting that these genes might act as tumor suppressor genes. We previously found two Fp isoforms in human complex II and reported that some cancer cells exhibit different expression patterns of Fp isoforms.4),5) These observations suggest that complex II, including Fp, might be related to tumorigenesis.
Warburg reported that cancers are largely dependent on glycolysis for their energy production, even under conditions where sufficient oxygen is available.6) The reasons and mechanisms for this Warburg effect have attracted much attention but remain largely unknown. The hypoxic condition of cancer tissues is regarded as one of the main reasons for the increase in glycolysis. The importance of the expressions of genes by hypoxia-inducible factor-1 (HIF-1) related to tumorigenesis, and the malignant progression of cancer is widely accepted.7)
In hypovascular tumors, representatively pancreatic cancer, the situation seems to be more complicated. Not only oxygen, but also nutrients (including glucose) are scarce in such environments.8) Recently, we analyzed the profiles of many metabolites, including glucose and several amino acids, in cancer tissues and found that energy metabolism in cancer tissue is quite unique.9) The deprivation of glucose was clearly shown in cancer tissues, indicating that an increase in glycolysis alone cannot explain how cancer cells maintain their required energy levels.
Under hypoxic conditions, complex II sometimes exhibits a different function in bacteria (e.g., Rhodoferax fermentans) and parasitic helminths (e.g., Ascaris suum) as well as lower marine eukaryotes.10),11) Under such conditions, complex II catalyzes the reduction of fumarate, which is the reverse of the reaction catalyzed by SQR, as the final step enzyme in the NADH-fumarate reductase system.10) In mammalian cells, succinate accumulation under hypoxic conditions suggests that mammalian complex II may also be capable of reducing fumarate and playing a role in oxygen homeostasis.12)–14) Moreover, the mutation of the CybS subunit in complex II has been reported to lead to the stability of HIF-1α.15) Thus, complex II might function as a fumarate reductase during adaptation to a hypoxic environment to ensure the maintenance of homeostasis. Our recent study investigating the cancer metabolome also supports this idea, since succinate, fumarate and malate were present at higher levels in cancer tissues than in normal tissues.9)
Escherichia coli has two different genes, sdh and frd, whose products are involved in complex II and are regulated by environmental changes.16) On the other hand, the same enzyme may catalyze both SQR and FRD activities in mammalian mitochondria, since the frd gene has not been identified in mammalian genomes. How does mammalian complex II change between its two activities? In this regard, the role of phosphorylation in the regulation of the respiratory complexes should be examined.
The 18-kDa iron-protein fraction (IP) subunit of complex I (NADH-ubiquinone reductase) encoded by the nuclear ndufs4 gene is reportedly phosphorylated by a cAMP-dependent protein kinase, and this phosphorylation is responsible for regulating the activity of this enzyme complex.17),18) In fact, the phosphorylation of the Fp subunit has also been reported in mammalian complex II.19)–22) In the present paper, we first confirmed the existence of FRD activity in cancer cells. Then, we demonstrated that the phosphorylation of Fp is related to the activity of complex II. Finally, we observed an increase in the FRD activity of complex II when cancer cells were cultured under hypoxic and glucose-deprived conditions, which mimic the tumor microenvironment.
Materials and methods
Cell cultures
Human colorectal cancer cells (DLD-1), human pancreatic cancer cells (Panc-1, CAPAN-1, and PSN-1), and human hepatocellular carcinoma cells (HepG2) were cultured in DMEM (GIBCO BRL, USA), human colorectal cancer cells (HT-29) were cultured in McCoy’s 5A (GIBCO BRL, USA), and human dermal fibroblast cells (HDF; TOYOBO, Japan) were cultured in DMEM/F12 (GIBCO BRL, USA) supplemented with 10% heat-inactivated fetal bovine serum (Tissue Culture Biologicals, USA) and antibiotics in 75 cm2 tissue culture flasks (CORNING, USA) of 5% CO2 in the air at 37 °C. All cells except for HDF were purchased from ATCC, USA. For glucose depletion, glucose-free DMEM (Sigma, USA) with 10% heat-inactivated dialyzed fetal bovine serum was used instead of the normal medium. For hypoxia, the cells were cultured in an atmosphere of 5% CO2, 1% O2 at 37 °C.
Preparation of mitochondria from culture cells
Cultured cells (approximately 108 cells) were harvested and suspended in mitochondrial preparation buffer containing 250 mM sucrose, 20 mM HEPES, 3 mM EDTA and 1 mM sodium malonate at pH7.5. The cells were homogenized using a power-driven Potter-Elvehjem homogenizer with Teflon pestle. The homogenate was centrifuged at 600 × g for 15 min to pellet the cell debris and nuclei. The supernatant was centrifuged at 14,300 × g for 20 min to obtain the mitochondrial pellet. The pellet was then suspended and centrifuged at 14,300 × g for 20 min to obtain the final mitochondrial pellet.23) The mitochondrial pellet was suspended in mitochondrial preparation buffer without 1 mM sodium malonate. The mitochondria were solubilized with 2.5% (w/v) sucrose monolaurate (Iwai, Japan) as a detergent, protease inhibitor cocktails (Sigma, USA), 1 mM sodium malonate, and phosphatase inhibitors I and II (Sigma, USA) and were stirred on ice for one hour. After centrifugation at 72,000 × g for 20 min at 4 °C, the supernatant was subjected to two-dimensional gel electrophoresis and enzyme assays (SQR specific activities (nmol/min mg protein) of prepared solubilized mitochondria were 132.6 ± 2.6 in DLD-1, 211.3 ± 6.3 in HT-29, 77.8 ± 17.3 in PSN-1, 113.2 ± 17.0 in CAPAN-1, 70.1 ± 13.6 in Panc-1, 133.5 ± 11.1 in HepG2 and 141.2 ± 9.4 in HDF).
Two-dimensional polyacrylamide gel electrophoresis and western blotting
For two-dimensional (2-D) gel electrophoresis, a linear immobilized pH gradient (4–7 L, IPG, 13 cm; GE Healthcare, UK) was initially used for isoelectric point gel electrophoresis. After hydrating the gel strips, 9.2 M urea, 1% (w/v) sucrose monolaurate, 0.6% (w/v) DTT, 2% (v/v) IPG buffer, a small amount of Orange G, the solubilized samples and a pI marker (BDH, UK; Range 5.65 ∼ 8.3) were added. The voltage program was 50 μA/strip at 300 V for 4 h on step-n-hold, 1,000 V for 1 h on gradient, 5,000 V for 2.5 h on gradient, and 5,000 V for 1.5 h on step-n-hold using an IPGphor isoelectric focusing unit (GE Healthcare, UK). The strips were equilibrated with SDS-PAGE running buffer, and SDS-PAGE was performed using 12.5% polyacrylamide gels at 10 mA per gel. The gels were removed from the glass plates and subjected to western blotting. The proteins were transferred to a nitrocellulose membrane (Whatman, UK) at 80 V in 25 mM Tris, 192 mM glycine, and 20% (v/v) isopropanol at 4 °C. The membrane was incubated with 1,000-fold diluted anti-bovine Fp monoclonal antibody (2E3; Molecular Probes, USA) in TBS containing 0.05% (v/v) Tween 20 and 0.5% skim milk overnight at 4 °C. Detection using an Immobilon Western was performed according to the manufacturer’s protocol (Millipore, USA).
Treatments with phosphatase and protein kinase
For the phosphatase treatment, the solubilized mitochondria in Antarctic Phosphatase buffer containing a protease inhibitor cocktail were treated with Antarctic Phosphatase (BioLabs, USA) at 37 °C for 2 h. For the absence of phosphatase (control), phosphatase inhibitors I and II were added. For the protein kinase treatment, solubilized mitochondria were treated with the protein kinase A catalytic subunit from bovine heart (P2645; Sigma, USA), protease inhibitor cocktail, phosphatase inhibitors, 8 mM MgCl2 and 70 μM ATP at 30 °C for 2 h.24)
Measurement of enzyme activities
SQR activity was measured in the presence of ubiquinone-1 (Coenzyme Q1) (Sigma, USA) using a millimolar extinction coefficient of 15 at 278 nm for ubiquinone-1.25) FRD activity was measured as the change in absorbance produced by the oxidation of reduced methyl viologen using a millimolar extinction coefficient of 6 at 550 nm in an anaerobic cuvette containing 10 mM β-D-glucose, 10 units of glucose oxidase, and 26 units of catalase.11)
Results
In mammalian mitochondria, complex II acts as succinate-ubiquinone reductase (SQR). Recently, several groups have speculated on the presence of a reverse reaction of complex II, fumarate reductase (FRD), in mammalian cells, although no direct evidence of FRD activity in mammalian complex II has been reported.9),12)–14) In the present report, we measured the two activities of complex II, SQR and FRD. The human cancer cells that had been cultured under ordinary conditions had relatively low FRD/SQR ratios in their mitochondria: 0.023 ± 0.004 in DLD-1, 0.016 ± 0.004 in HT-29, 0.063 ± 0.007 in PSN-1, 0.047 ± 0.007 in CAPAN-1, 0.066 ± 0.010 in Panc-1, and 0.028 ± 0.002 in HepG2. The mitochondria of human dermal fibroblast cells also had a distinct but low FRD activity, FRD/SQR ratio being 0.011 ± 0.002. Thus, distinct FRD activity was found in human mitochondria, although the activity was lower than that in A. suum (1.12 ± 0.250). The pancreatic cancer cells had higher FRD/SQR ratios than the colorectal cancer cells or the hepatoma cells in the present study. The reason why the FRD/SQR ratios differed in these cells is not presently clear. Of interest, the cancer cells had higher FRD activity levels than the normal fibroblast cells.
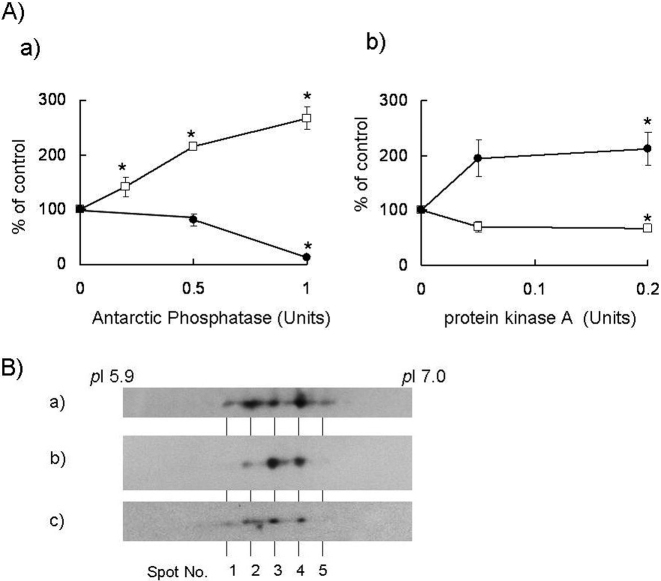
Fp, which is a catalytic subunit of complex II and the site of succinate/fumarate conversion, is known to undergo phosphorylation.19)–22) However, the relationship between the phosphorylation status of Fp and the function of complex II is not known. Therefore, we investigated whether the SQR and FRD activities of complex II could be regulated by the phosphorylation of Fp. Using two-dimensional gel electrophoresis, the Fp proteins were separated to five spots with different pI values (Fig. 1B). The phosphorylation of Fp was detected using a western blot analysis with anti-phosphorylated-amino acid antibodies and a phosphoprotein gel staining analysis (Pro-Q phosphoprotein gel stain; Molecular Probes, USA) (data not shown). We next examined whether complex II activity was correlated with the phosphorylation status of the Fp subunit. After treatment with Antarctic Phosphatase, which dephosphorylates proteins non-specifically, the SQR activity increased in a dose-dependent manner (Fig. 1A). Meanwhile, treatment with the protein kinase A catalytic subunit, which is known to be localized in the matrices of mitochondria,17),18) increased the FRD activity level (Fig. 1B), whereas treatment with phosphatase decreased the FRD activity level (Fig. 1A). The increase in the spot intensity of the Fp proteins at spot No. 3 by treatment with phosphatase was resulted from an increase in the dephosphorylation status of the Fp proteins, whereas the decrease in the spot intensity of Fp proteins with spot No. 4 and 5 by treatment with protein kinase resulted in an increase in the phosphorylation status of the Fp proteins (Fig. 1B). The total amounts of Fp proteins were almost the same after treatment with phosphatase and kinase. Therefore, these data showed that protein kinase and phosphatase treatments caused changes in the phosphorylation and dephosphorylation statuses of the Fp proteins, respectively. These data are consistent with the idea that a low-phosphorylation status of the Fp subunit activates SQR activity while simultaneously inactivating FRD activity in complex II.
Fig. 1.
Effects of phosphatase and protein kinase on complex II. A) Effects of phosphatase and protein kinase on SQR and FRD activities in DLD-1 mitochondria. a) SQR and FRD activities after treatment with Antarctic Phosphatase in solubilized DLD-1 mitochondria. b) SQR and FRD activities after treatment with the protein kinase A catalytic subunit in solubilized DLD-1 mitochondria. The open squares show the ratio of SQR activity versus the control value, while the closed circle show the ratio of FRD activity versus the control in the absence of phosphatase/kinase. All data were expressed as the means ± S.D. of three experiments. Statistically significant differences with respect to the control value (p < 0.05) are shown by an asterisk (Student’s t-test). B) Separation of Fp proteins. The detection of Fp was performed using an anti-bovine heart Fp monoclonal antibody after two-dimensional gel electrophoresis in solubilized DLD-1 mitochondria. a) Untreated, b) treated with 0.5 Units Antarctic Phosphatase, and c) treated with 0.2 Units protein kinase A.
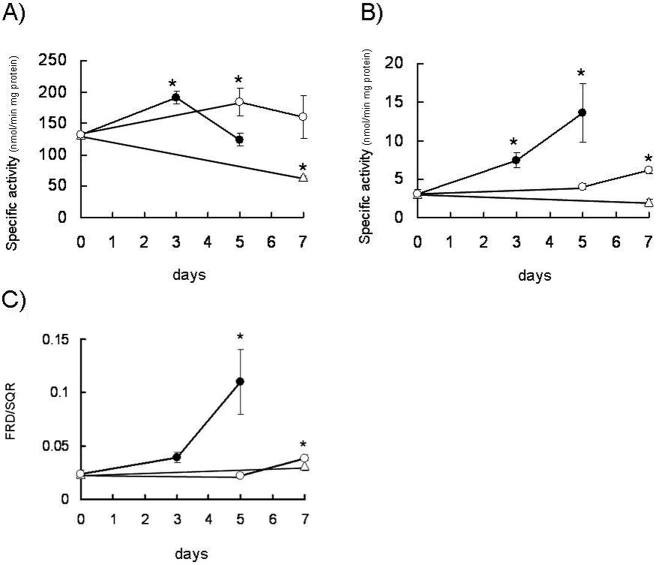
Next, we examined whether the SQR and FRD activities of complex II change under different culture conditions. Figure 2 shows the changes in the activities of SQR and FRD when the cells were cultured in a low nutrient and hypoxic environment. The SQR activities were slightly decreased after long-term culture in a tumor-mimicking microenvironment (hypoxic and glucose-depleted conditions; Fig. 2A). Although FRD activity was approximately 40-fold lower than SQR activity under normal conditions, FRD activity increased in cells cultured under hypoxic and glucose-depleted conditions (Fig. 2B); the FRD/SQR ratio also increased under these conditions (Fig. 2C). Although the SQR activities seemed to be unchanged or activated in hypoxia, the FRD activities increased. Since several phosphorylation sites are probably involved in the regulation of SQR and FRD activities, the activity changes might not occur at the same time. The phosphorylation of Fp in various cancer cells was almost the same, as spot Nos. 2, 3 and 4 were the main spots separated by two-dimensional gel electrophoresis. Moreover, the quantity of phosphorylated Fp proteins was slightly increased in cells cultured under glucose-deprived conditions (data not shown). Thus, these data suggest that the function of human complex II changes from succinate dehydrogenase to fumarate reductase under the tumor microenvironment through the phosphorylation of Fp subunit.
Fig. 2.
Complex II activities in mitochondria from DLD-1 under tumor-mimicking microenvironmental conditions. A) SQR activities, B) FRD activities, and C) ratios of FRD/SQR. The closed circles show the enzyme activities in solubilized mitochondria from DLD-1 cultured under hypoxic and glucose-depleted conditions. The open circles show the enzyme activities in solubilized mitochondria from DLD-1 cultured under hypoxic conditions. The open triangles show the enzyme activities in solubilized mitochondria from DLD-1 cultured under glucose-depleted conditions. All the data were expressed as the means ± S.D. of three experiments. Statistically significant differences with respect to the baseline values (p < 0.05) are shown by an asterisk (Student’s t-test).
Discussion
A novel function of complex II in the maintenance of oxygen homeostasis in mammalian mitochondria has been proposed.12)–14) Weinberg et al. showed that succinate accumulated under a hypoxic condition induced by acute ischemia and speculated that complex II might operate as fumarate reductase under such environmental conditions.12) In the present report, we directly measured the reduction of fumarate by human complex II. Moreover, 1% oxygen and glucose deprivation from the growth medium caused an increase in FRD activity and a decrease in SQR activity in cancer cells. In the case of normal tissue, which is distinct from cancer tissue, elaborate physiological responses protect the cells from chronic nutrient deprivation and hypoxic conditions. Thus, the increase in FRD activity was related to the tolerance to hypoxia and glucose starvation in tumor tissues, where uncontrolled tumor cell proliferation and inadequate angiogenesis result in a chronically insufficient blood supply.
Succinate formation by complex II is the final step of the NADH-fumarate reductase system in the anaerobic helminth A. suum.10) Since we showed that human complex II exhibits fumarate reductase activity, the NADH-fumarate reductase system probably also functions in human mitochondria. We measured and detected the activity of NADH-fumarate reductase in human mitochondria, and the activities of these enzyme complexes increased in long-term cultures of cancer cells under hypoxic and glucose-depleted conditions (data not shown). In this system, NADH is reduced by NADH-ubiquinone reductase (complex I) and an electron is transferred to complex II via ubiquinone; finally, succinate is produced by fumarate reductase. Complex I functions as a proton pump and forms a transmembrane electrochemical proton gradient, driving ATP synthesis through ATP synthase (complex V). Therefore, in cancer cells under hypoxic and glucose-depleted conditions, ATP can be synthesized by the NADH-fumarate reductase system, enabling cancer cells to survive under severe environmental conditions.
Phosphatase and protein kinase treatments led to changes in the activity levels of complex II with concomitant changes in the phosphorylation status of the Fp subunit. When Fp was dephosphorylated, the SQR activity increased and the FRD activity decreased. Conversely, when Fp was phosphorylated, the SQR activity decreased and the FRD activity increased. The phosphorylation of mitochondrial respiratory chain enzymes has been demonstrated using proteome analyses.19)–21) However, few reports have focused on the physiological significance of the phosphorylation of these proteins. In complex I (NADH-ubiquinone reductase), the phosphorylation of the 18-kDa IP subunit has been reported, and the activity of complex I is known to be regulated by the phosphorylation status of this subunit.17),18) In complex IV (cytochrome c oxidase) as well, the phosphorylation of subunits I, II and Vb have been reported, and the activity of cytochrome c oxidase is also regulated by its phosphorylation status.26) In the present report, we showed that the phosphorylation status of the Fp subunit in complex II caused a change in the levels of the SQR and FRD enzymatic activities.
Although we showed that Fp is phosphorylated, the exact site of phosphorylation is unknown. We detected the phosphorylation of Fp at Tyr, Ser and Thr residues using western blotting with anti-phosphorylated amino acid antibodies (data not shown). In a database analysis, the two human Fp sequences (FpI and FpII) showed several phosphorylation motifs including that for cAMP-dependent protein kinase, and two complex IIs constructed with FpI and FpII isoforms had both SQR and FRD activities (data not shown). Thus, the SQR and FRD activities are produced by the same subunit complex encoded by the same genes, with the only difference being the phosphorylation status of the Fp subunit. Recently, Salvi et al. reported that Y543 and Y604 in the Fp subunit are phosphorylated by Fgr tyrosine kinase, a member of the Src kinase family.22) We constructed cells with an over-expressing mutated Fp, in which the amino acid residues of the putative phosphorylation sites were substituted in a manner so that they would not be phosphorylated, and the activities of complex II were measured. The A606 Fp, (not A604 Fp) over-expressing cells, in which a mutant Fp having a substitution at Tyr 606 to Ala was over-expressed, showed approximately 1.3-fold higher SQR activity than the wild-type Fp over-expressing cells cultured under ordinary conditions; thus, the Y606 site might be important for the activity of complex II.
We showed that glucose and oxygen starvations, which are regarded as mimicking the tumor microenvironment, decreased the SQR activity and increased the FRD activity of complex II. These activity changes are thought to be caused by the phosphorylation of the Fp subunit. Nutrient and oxygen availabilities in tumors are now widely accepted as regulating growth and tumor characteristics.27) Moreover, some cancer cells, such as pancreatic cancer cells, exhibit a resistance to glucose starvation through the activation of PKB/Akt and AMPK.28),29) PKB/Akt is involved in the regulation of glycolysis and tumorigenesis.30),31) Since the activations of PKB/Akt and mTOR are related to tumorigenesis and metastasis, they have been investigated as targets for novel cancer therapies.32),33) Likewise, several phosphatases and kinases are related to and/or regulate cell survival and environmental adaptation. Little information is available as to how and which kinases and phosphatases phosphorylate Fp. Thus, the phosphorylation–dephosphorylation of the Fp subunit in complex II might be regulated directly or indirectly by the activations of kinases and phosphatases as a result of environmental changes, and the resulting changes in the activities of complex II might advance for maintaining homeostasis.
In this report, we showed that human complex II has two activities, SQR and FRD, and that these activities change in response to a tumor-mimicking microenvironment. These observations are important for determining the role of complex II in a wide variety of human diseases, including cancer. This functional change in complex II might be an effective target for future medical research and novel therapies.
Acknowledgments
This work was supported in part by a Research Resident Fellowship award from the Foundation for the Promotion of Cancer Research (Japan) of the 3rd Term Comprehensive 10-Year Strategy for Cancer Control, and by a Grant-in-Aid for Creative Scientific Research (18GS0314) from the Japan Society for the Promotion of Science, and for Scientific Research on Priority Areas (18073004) and Targeted Proteins Research Program from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Abbreviations:
- SQR:
succinate-ubiquinone reductase
- FRD:
fumarate reductase
- Fp:
flavoprotein subunit of complex II
- TCA cycle:
tricarboxylic acid cycle
References
- 1).Favier, J., Briere, J.J., Strompf, L., Amar, L., Filali, M., Jeunemaitre, X.et al. (2005) Hereditary paraganglioma/pheochromocytoma and inherited succinate dehydrogenase deficiency. Horm. Res. 63, 171–179 [DOI] [PubMed] [Google Scholar]
- 2).Pawl, C., Bausch, B., Neumann, H.P.H. (2005) Mutations of the SDHB and SDHD genes. Familial Cancer 4, 49–54 [DOI] [PubMed] [Google Scholar]
- 3).Muller, U., Troidl, C. and Niemann, S. (2005) SDHC mutations in hereditary paraganglioma/pheochromocytoma. Familial Cancer 4, 9–12 [DOI] [PubMed] [Google Scholar]
- 4).Tomitsuka, E., Goto, Y., Taniwaki, M. and Kita, K. (2003) Direct evidence for expression of Type II flavoprotein subunit in human complex II (succinate-ubiquinone reductase). Biochem. Biophys. Res. Com. 311, 774–779 [DOI] [PubMed] [Google Scholar]
- 5).Tomitsuka, E., Hirawake, H., Goto, Y., Taniwaki, M., Harada, S. and Kita, K. (2003) Direct evidence for two distinct forms of the flavoprotein subunit of human mitochondrial complex II (succinate-ubiquinone reductase). J. Biochem. 134, 191–195 [DOI] [PubMed] [Google Scholar]
- 6).Warburg, O. (1956) On respiratory impairment in cancer cells. Science 124, 269–270 [PubMed] [Google Scholar]
- 7).Semenza, G.L. (2007) HIF-1 mediates the Warburg effect in clear cell renal carcinoma. J. Bioenerg. Biomembr. 39, 231–234 [DOI] [PubMed] [Google Scholar]
- 8).Brown, J.M. and Wilson, W.R. (2004) Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 4, 437–447 [DOI] [PubMed] [Google Scholar]
- 9).Hirayama, A., Kami, K., Sugimoto, M., Sugawara, M., Toki, N., Onozuka, H.et al. (2009) Quantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time-of-flight mass spectrometry. Cancer Res. 69, 4918–4925 [DOI] [PubMed] [Google Scholar]
- 10).Kita, K., Hirawake, H., Miyadera, H., Amino, H and Takeo, S. (2002) Role of complex II in anaerobic respiration of the parasite mitochondria from Ascaris suum and Plasmodium falciparum. Biochim. Biophys. Acta 1553, 123–139 [DOI] [PubMed] [Google Scholar]
- 11).Miyadera, H., Hiraishi, A., Miyoshi, H., Samamoto, K., Mineki, R., Murayama, K.et al. (2003) Complex II from phototrophic purple bacterium Rhofoferax fermentans displays rhodoquinol-fumarate reductase activity. Eur. J. Biochem. 270, 1863–1874 [DOI] [PubMed] [Google Scholar]
- 12).Weinberg, J.M., Venkatachalam, M.A., Roeser, N.F. and Nissim, I. (2000) Mitochondrial dysfunction during hypoxia/reoxygenation and its correction by anaerobic metabolism of citric acid cycle intermediate. Proc. Natl. Acad. Sci. USA 97, 2826–2831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Selak, M.A., Armour, S.M., MacKenzie, E.D., Boulahbel, H., Watson, D.G., Mansfield, K.D.et al. (2005) Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer Cell 7, 77–85 [DOI] [PubMed] [Google Scholar]
- 14).Henrich, H., Paddenberg, R., Haberberger, R.V., Scholz, A., Gruss, M., Hempelmann, G.et al. (2004) Hypoxic increase in nitric oxide generation of rat sensory neurons requires activation of mitochondrial complex II and voltage-gated calcium channels. Neuroscience 128, 337–345 [DOI] [PubMed] [Google Scholar]
- 15).Pollard, P.J., Briere, J.J., Barwell, J., Barclay, E., Wortham, N.C., Hunt, T.et al. (2005) Accumulation of Krebs cycle intermediates and over-expression of HIF1α in tumours which result from germline FH and SDH mutations. Hum. Mol. Genet. 14, 2231–2239 [DOI] [PubMed] [Google Scholar]
- 16).Hagerhall, C. (1997) Succinate: quinone oxidoreductases. Variations on a conserved theme. Biochim. Biophys. Acta 1320, 107–141 [DOI] [PubMed] [Google Scholar]
- 17).Papa, S., Sardanelli, A.M., Scacco. S., Petruzzella, V., Technikova-Dobrova, Z., Vergari, R.et al. (2002) The NADH: ubiquinone oxidoredactase (Complex I) of the mammalian respiratory chain and the cAMP cascade. J. Bioenerg. Biomembr. 34, 1–10 [DOI] [PubMed] [Google Scholar]
- 18).Papa, S., Scacco. S., Sardanelli, A.M., Petruzzella, V., Vergari, R., Signorile, A.et al. (2002) Complex I and the cAMP cascade in human physiopathology. Biosci. Rep. 22, 3–15 [DOI] [PubMed] [Google Scholar]
- 19).Schulenberg, B., Aggeler, R., Beechem, J.M., Capaldi, R.A. and Patton, W.F. (2003) Analysis of steady-state protein phosphorylation in mitochondria using novel fluorescent phosphosensor dye. J. Biol. Chem. 278, 27251–27255 [DOI] [PubMed] [Google Scholar]
- 20).Murray, J., Marusich, M.F., Capaldi, R.A. and Aggeler, R. (2004) Focused proteomics: monoclonal antibody-based isolation of the oxidative phosphorylation machinery and detection of phosphoproteins using a fluorescent phosphoprotein gel stain. Electrophoresis 25, 2520–2525 [DOI] [PubMed] [Google Scholar]
- 21).Hopper, R., Carroll, S., Aponte, A.M., Johnson, D.T., French, S., Shen, R.-F.et al. (2006) Mitochondrial matrix phosphoproteome: effect of extra mitochondrial calcium. Biochemistry 45, 2524–2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Salvi, M., Morrice, N.A., Brunati, A.M. and Toninello, A. (2007) Identification of the flavoprotein of succinate dehydrogenase and aconitase as in vitro mitochondrial substrates of Fgr tyrosine kinase. FEBS Lett. 581, 5579–5585 [DOI] [PubMed] [Google Scholar]
- 23).Kler, R.S., Jackson, S., Bartlett, K., Bindoff, L.A., Eaton, S., Pourfarzan, M.et al. (1991) Quantitation of Acyl-CoA and acylcarnitine esters accumulated during abnormal mitochondria fatty acid oxidation. J. Biol. Chem. 266, 22932–22938 [PubMed] [Google Scholar]
- 24).Sardanelli, A.M., Technikova-Dobrova, Z., Scacco, S.C., Speranza, F. and Papa, S. (1995) Characterization of proteins phosphorylated by the cAMP-dependent protein kinase of bovine heart mitochondria. FEBS Lett. 377, 470–474 [DOI] [PubMed] [Google Scholar]
- 25).Kita, K., Vibat, C.R.T., Meinharde, S., Guest, J.R. and Gennis, R.B. (1989) One-step purification from Escherichia coli of complex II (succinate: ubiquinone oxidoreductase) associated with succinate-reducible cytochrome b556, J. Biol. Chem. 264, 2672–2677 [PubMed] [Google Scholar]
- 26).Lee, I., Bender, E. and Kadenbach, B. (2002) Control of mitochondrial membrane potential and ROS formation by reversible phosphorylation of cytochrome c oxidase. Mol. Cell Biochem. 234/235, 63–70 [PubMed] [Google Scholar]
- 27).Shaw, R.J. and Cantley, L.C. (2006) Ras, PI(3)K and mTOR signaling controls tumor cell growth. Nature 441, 424–431 [DOI] [PubMed] [Google Scholar]
- 28).Izuishi, K., Kato, K., Ogura, T., Kinoshita, T. and Esumi, H. (2000) Remarkable tolerance of tumor cells to nutrient deprivation: possible new biochemical target for cancer therapy. Cancer Res. 60, 6201–6207 [PubMed] [Google Scholar]
- 29).Esumi, H., Izuishi, K., Kato, K., Hashimoto, K., Kurashima, Y., Kishimoto, A.et al. (2002) Hypoxia and nitric oxide treatment confer tolerance to glucose starvation in a 5’-AMP-activared protein kinase-dependent manner. J. Biol. Chem. 277, 32791–32798 [DOI] [PubMed] [Google Scholar]
- 30).Elstrom, R.L., Bauer, D.E., Buzzai, M., Karnauskas, R., Harris, M.H., Plas, D.R.et al. (2004) Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 64, 3892–3899 [DOI] [PubMed] [Google Scholar]
- 31).Robey, R.B. and Hay, N. (2005) Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene 25, 4683–4696 [DOI] [PubMed] [Google Scholar]
- 32).Guertin, D.A. and Sabatini, D.M. (2007) Defining the role of mTOR in cancer. Cancer Cell 12, 9–22 [DOI] [PubMed] [Google Scholar]
- 33).Seeliger, H., Guba, M., Kleespies, A., Jauch, K.-W. and Bruns, C.J. (2007) Role of mTOR in solid tumor systems: a therapeutical target against primary tumor growth, metastasis, and angiogenesis. Cancer Metast. Rev. 26, 611–621 [DOI] [PubMed] [Google Scholar]