Summary
Increases in cytosolic Ca2+ concentration ([Ca2+]c) mediated by inositol (1,4,5)-trisphosphate [Ins(1,4,5)P3, hereafter InsP3] regulate activities that include division, contraction and cell death. InsP3-evoked Ca2+ release often begins at a single site, then regeneratively propagates through the cell as a Ca2+ wave. The Ca2+ wave consistently begins at the same site on successive activations. Here, we address the mechanisms that determine the Ca2+ wave initiation site in intestinal smooth muscle cells. Neither an increased sensitivity of InsP3 receptors (InsP3R) to InsP3 nor regional clustering of muscarinic receptors (mAChR3) or InsP3R1 explained the selection of an initiation site. However, examination of the overlap of mAChR3 and InsP3R1 localisation, by centre of mass analysis, revealed that there was a small percentage (∼10%) of sites that showed colocalisation. Indeed, the extent of colocalisation was greatest at the Ca2+ wave initiation site. The initiation site might arise from a selective delivery of InsP3 from mAChR3 activity to particular InsP3Rs to generate faster local [Ca2+]c increases at sites of colocalisation. In support of this hypothesis, a localised subthreshold ‘priming’ InsP3 concentration applied rapidly, but at regions distant from the initiation site, shifted the wave to the site of the priming. Conversely, when the Ca2+ rise at the initiation site was rapidly and selectively attenuated, the Ca2+ wave again shifted and initiated at a new site. These results indicate that Ca2+ waves initiate where there is a structural and functional coupling of mAChR3 and InsP3R1, which generates junctions in which InsP3 acts as a highly localised signal by being rapidly and selectively delivered to InsP3R1.
Key words: Ca2+ waves, InsP3, Smooth muscle
Introduction
Activities which include blood flow through vascular blood vessels, peristaltic motion in the gastrointestinal tract and rhythmic contractions of the uterus during labour are all regulated by contraction of smooth muscle. Transient increases in the cytosolic Ca2+ concentration ([Ca2+]c) provide the trigger for contraction but also affect other different, at times contradictory, processes including cell growth, division and apoptosis. Ca2+ may regulate so many complex behaviours at least in part by the cell restricting the location of increases in [Ca2+]c to specific sites in the cell by the distribution of various Ca2+ signalling proteins. Under certain (as yet ill-defined) conditions, the Ca2+ signal may be propagated through the cell to extend the reach of the signal and enable Ca2+ to encode for many cellular processes.
[Ca2+]c increases occur via Ca2+ influx through voltage-gated and receptor-operated Ca2+ channels on the plasma membrane or release from inositol (1,4,5)-trisphosphate [Ins(1,4,5)P3, hereafter InsP3] receptors (InsP3R) and ryanodine receptors (RyR) present on the sarcoplasmic reticulum (SR), an internal Ca2+ store (Chalmers et al., 2007). The generation of an InsP3-mediated Ca2+ signal occurs via extracellular activation (e.g. agonists) of G protein coupled receptors present on the plasma membrane. Activation leads to phospholipase C β (PLC-β) catalysed formation of InsP3, a diffusible messenger that, with Ca2+, activates InsP3R.
Interestingly, although a rapidly diffusing (diffusion co-efficient = 300 µm2 s−1) messenger presumably with free access throughout the cell, InsP3 may in some circumstances act as a local rather than global signal to generate specificity in receptor function. Some explanation for the local nature of the signal is found in the observation that certain receptors colocalize with InsP3R to form a local signalling complex (Delmas et al., 2002; Hur et al., 2005; Lur et al., 2011; Yuan et al., 2005). For example, while muscarinic and bradykinin receptors each stimulate PLC, only bradykinin receptors co-immunoprecipitate with, and activate, InsP3R to evoke Ca2+ release (Delmas et al., 2002). The arrangement enables PLC activation by muscarinic and bradykinin receptors to evoke different cellular responses. The positioning of active InsP3R near the plasma membrane provides a mechanism to facilitate agonist activation, acting via InsP3, to target specific types of cellular response, i.e. by generating Ca2+ rises in specific regions of the cell (Smith et al., 2009). Additional local specificity is achieved by clustering surface receptors in certain regions on the plasma membrane, which gives rise to areas with increased sensitivity to extracellular stimuli (Thomason et al., 2002). Together the clustering of surface receptors and subplasma membrane location of InsP3R permits agonists that activate G protein coupled receptors to generate differences in which signalling pathways are subsequently activated and direct, specific associations between signalling proteins located at the plasma membrane and the SR membrane to occur (Hur et al., 2005; Yuan et al., 2005). These arrangements enable selective changes in [Ca2+]c and cell performance to occur from messengers which are normally thought to evoke activity throughout the cell.
Under some conditions the reach of a local Ca2+ signal may be selectively extended by the initial, Ca2+ rise being propagated through the cell. The cell normally retains careful control of the signal reach by regulating InsP3 distribution (McCarron et al., 2004). In addition, in some cells with particular arrangements in their gross cell structure, such as hippocampal neurons, exocrine acinar cells and hepatocytes, the Ca2+ wave initiation site and direction of travel of the wave appears linked to the expression and distribution of InsP3R subtype (Hernandez et al., 2007; Jacob et al., 2005; Takemura et al., 1999), presumably to maintain selectivity in responses. For example, although InsP3R1 were expressed throughout the cell, InsP3-mediated Ca2+ waves initiated from the apical region where InsP3R2 (hepatocytes) and InsP3R3 (ocular ciliary polarised epithelial cells) were expressed (Hirata et al., 1999; Rooney et al., 1990).
Although many other cell types such as smooth muscle do not display any apparent structural polarity they too have sites where Ca2+ waves preferentially initiate. The mechanisms which contribute to the sites at which a Ca2+ wave initiate in these cells are unknown and addressed in the present study. Local differences in the sensitivity to InsP3 did not explain the wave initiation site. Nor did the expression patterns of mAChR3 and InsP3R1 indicate any apparent regional receptor clustering. Yet, dual labelling of InsP3R1 and mAChR3 revealed a small percentage of colocalization (∼10%) of the receptors. This colocalisation was greater at sites where Ca2+ waves initiated. The wave initiation site may arise from the rapid delivery of InsP3 to particular InsP3R. In support, the wave initiation site could be altered either by locally increasing [InsP3] at a location distant to the initiation site or by rapidly buffering the [Ca2+]c increase at the initiation site. This work demonstrates that the initiation site of a Ca2+ wave is not dependent on the density or expression of InsP3R present at that location but is dependent on the close proximity of mAChR and InsP3R and rapid delivery of InsP3 to particular InsP3R.
Results
InsP3-meditated Ca2+ waves initiate at a single location
In voltage-clamped single colonic smooth muscle cells depolarization (−70 to +10 mV) activated an inward current and transient increase in [Ca2+]c (Fig. 1Aa,Ab). The [Ca2+]c increase initiated at the same time throughout all regions of the cell presumably due to the concerted opening of voltage-gated Ca2+ channels across the plasma membrane (McCarron et al., 2008). In the same cell, the muscarinic acetylcholine receptor agonist carbachol (100 µM), applied by hydrostatic pressure ejection, evoked InsP3-mediated Ca2+ release (Fig. 1Ba,Bb). Carbachol was applied in a Ca2+-free solution so that the Ca2+ response was only due to Ca2+ release from the SR. InsP3R activity is required for the initiation of carbachol-evoked Ca2+ waves. In support, the InsP3R inhibitor 2-aminoethoxydiphenyl borate (2-APB) blocked the carbachol-evoked Ca2+ wave. The [Ca2+]c increased by 1.24±0.22 F/F0 in control and by 0.14±0.04 F/F0 after 2-APB (10 µM) preincubation, respectively (n = 5; P<0.01; not shown). Neither RyR activity nor SR store content are affected by 2-APB in this cell type supporting the selectivity of action of 2-APB at InsP3R (McCarron et al., 2002).
Fig. 1.
Depolarisation and carbachol-evoked increases in [Ca2+]c. (A,B) Depolarisation (−70 mV to +10 mV; Ae) activated a voltage-dependent Ca2+ current (Ad) to cause an increase in [Ca2+]c (Aa,Ab) that occurred at the same time throughout the cell. In contrast, carbachol (CCh; Bc) evoked an increase in [Ca2+]c that began in one region (region i) and propagated away from that site (Ba,Bb). The [Ca2+]c images (Aa,Ba) are derived from the time points indicated by the corresponding numbers in Aa and Ba. [Ca2+]c changes in Aa and Ba are represented by colour; blue is low and red is high [Ca2+]c. Changes in the fluorescence ratio with time (Ab,Bb) are derived from 3×3 pixel boxes from the second panel in Aa; regions i to v are drawn as larger yellow circles to facilitate visualisation. A brightfield image of the cell is shown (Aa; left panel); the whole-cell patch electrode is on the left side. The carbachol-containing puffer pipette is located to the right of the cell outside the field of view. The inward current evoked during carbachol application (Bd) might arise from activation of Ca2+-activated Cl− channels or non-specific cation channels (Inoue and Isenberg, 1990; Lee et al., 1993), and was not studied further here.
Carbachol-evoked Ca2+ release initiated typically at one site (Fig. 1Aa, panel 1, region i) and propagated the length of the cell as a Ca2+ wave (Fig. 1Ba,Bb). Interestingly, subsequent applications of carbachol at ∼90 sec intervals evoked Ca2+ release of similar magnitude which initiated from the same location each time. There was no correlation between the location of agonist application and site of Ca2+ wave initiation (data not shown). Nor was the initiation site located preferentially at the nucleus or site of patch electrode attachment (data not shown). In cells that were not voltage-clamped, repetitive applications of carbachol also evoked Ca2+ waves, each of which initiated at the same single site (data not shown). The amplitude of the Ca2+ wave varied in different regions of the cell, often there was greater release near the nucleus (McCarron et al., 2010). The mechanisms that may contribute to the occurrence of an ‘eager’ site of Ca2+ wave initiation were examined first by determining InsP3R and mAChR expression patterns and then their relative functional contributions to wave initiation.
Distribution of proteins involved in InsP3-mediated Ca2+ release
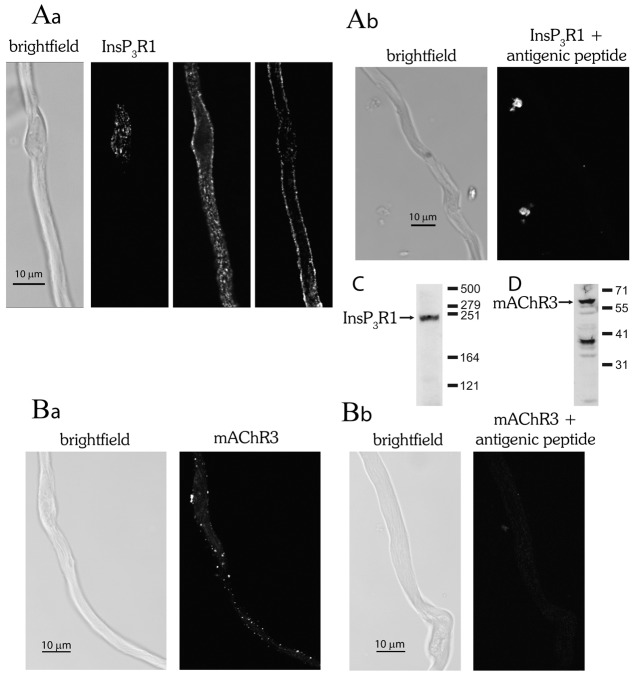
The distribution of InsP3R and mAChR was examined by fluorescence immunocytochemistry in single smooth muscle cells. The InsP3R type 1 isoform (InsP3R1) is highly expressed in colonic smooth muscle, whereas InsP3R type 2 (InsP3R2) and InsP3R type 3 (InsP3R3) are less abundant (Dr S. Currie, personal communication). Confocal images of labelled smooth muscle cells show InsP3R1 were extensively distributed near to the cell surface (Fig. 2Aa). Each punctate region is presumably a cluster of InsP3R. The distribution appeared approximately uniform along the cell and there did not appear to be any region of the cell with a greater expression.
Fig. 2.
Distribution of the muscarinic acetylcholine receptor type 3 (mAChR3) isoform and InsP3R type 1 (InsP3R1) isoform in a single smooth muscle cell. (A,B) InsP3R1 was labelled using a monoclonal antibody specific for the type 1 isoform (Abnova; no. H00003708-M01) and visualised using a fluorescently conjugated secondary antibody (Molecular Probes; A11001). A brightfield image (Aa; far left panel) of a single cell from which confocal images of InsP3R1 are shown at three different planes of focus (Aa; left panel, top of cell; middle panel, bottom of cell; right panel, middle of cell). The confocal images show InsP3R1 expression near the plasma membrane and that InsP3R clusters are distributed along the length of the cell. (Ba) mAChR3 was labelled using a polyclonal antibody specific for the type 3 isoform (AbCam; no. AB41169) and visualised using a fluorescently conjugated secondary antibody (Molecular Probes; no. T269) and shows punctate labelling of the cell surface. A brightfield image of the same cell is also shown (Ba; left panel). (Ab,Bb) Control experiments in which the antibody was first preabsorbed using the partial recombinant protein (H00003708-Q01 for InsP3R1 or antigenic peptide for mAChR3) that was used to produce the antibody failed to show labelling, demonstrating specificity of binding. Control experiments with secondary antibodies only are not shown. The resolution of each pixel was 80 nm×80 nm in the x-y dimension in accordance with Nyquist sampling. (C) Western blot probed with anti-InsP3R1 primary (Abnova; H00003708-M01) and anti-mouse-HRP secondary antibodies, which shows a single clear band for InsP3R1 at the anticipated molecular mass. Although the InsP3R1 monomer has a molecular mass of 313 kDa, previous reports (Diaz-Muñoz et al., 2008; Fissore et al., 1999) show the protein running in SDS-PAGE with an apparent molecular mass of 230–260 kDa, which corresponds well with the blot. (D) Western blot probed with anti-mAChR3 primary (AbCam; AB41169) and anti-rabbit-HRP secondary antibodies. The molecular mass of mAChR3 is 66 kDa and a strong band that corresponds to this mass occurs. The remaining bands might be proteolytic degradation products.
The mAChR type2 (mAChR2) and type 3 (mAChR3) isoforms are present on the plasma membrane of colonic smooth muscle (Sawyer and Ehlert, 1998; Zhang et al., 1991). Although the mAChR2 outnumbers mAChR3 3∶1, the contractile response arises predominantly from mAChR3 activation of PLC-β to evoke InsP3-mediated Ca2+ release (Zhang and Buxton, 1991; Zhang et al., 1991). Selective localisation of mAChR3 in one or few regions of the plasma membrane, could cause a greater local agonist-evoked InsP3 production to determine the site of Ca2+ wave initiation. mAChR expression was punctate but mAChR were expressed at various locations along the cell. Any of these sites could be putative Ca2+ wave initiation sites (Fig. 2Ba), i.e. a particular expression of mAChR does not appear to explain the wave initiation site. In control experiments, pre-absorption of either the InsP3R1 or mAChR3 antibody with the immunogen (2∶1) prevented binding as shown by an absence of fluorescence (Fig. 2Ab,Bb). Non-specific fluorescence was not detected in experiments using only secondary antibody (not shown). Western blots probed with the anti-InsP3R1 primary antibody show a single clear band (Fig. 2C). Although the InsP3R1 monomer has a molecular weight of 313 kDa, previous reports (Diaz-Muñoz et al., 2008; Fissore et al., 1999) with western blots show the protein running with an apparent molecular weight of 230–260 kDa in accord with the mass observed. In a western blot probed with anti-mAChR3 primary antibody, a clear band occurs near the expected molecular weight for mAChR3 (66 kDa) (Fig. 2D). The remaining bands may be proteolytic degradation products.
From these results it appears that gross structural differences in expression of InsP3R1 and mAChR3 do not determine the site of Ca2+ wave initiation.
Does the InsP3R cluster size or sensitivity determine the site of Ca2+ wave initiation?
Although the expression pattern of InsP3R1 does not immediately suggest that differences in distribution of the receptor may determine the site of Ca2+ wave initiation there may be regional differences in Ca2+ sensitivity of InsP3R to InsP3 itself or in InsP3R cluster size to account for the initiation site (Shuai et al., 2006; Smith and Parker, 2009; Swillens et al., 1999). To examine this possibility the local Ca2+ response to a fixed [InsP3] was measured at different locations throughout the cell and compared to the site of initiation of a carbachol-evoked Ca2+ wave. In the representative cell (Fig. 3), carbachol (100 µM) evoked a Ca2+ wave which initiated at a single location (region iii) and propagated the length of the cell (Fig. 3Aa,b). The Ca2+ response was not uniform in all regions of the cell and varied from 1.13 F/F0 at the site of initiation to 2.67 F/F0 (region i) near the nucleus. Next, the location-dependent differences in the sensitivity to InsP3 itself was measured in the same cell. A fixed concentration of caged InsP3 was photolyzed at one minute intervals in 20 µm regions (Fig. 3Ba,b, arrow). The magnitude of InsP3-mediated Ca2+ release at each site (regions i–vi) was compared to the Ca2+ release during a carbachol-evoked Ca2+ wave. In this cell (Fig. 3), the greatest InsP3-evoked Ca2+ release occurred at region i, some 50 µm from the site of wave initiation (region iii). The amplitude of InsP3-mediated Ca2+ release was 1.02 F/F0 (region iii) at the site where the carbachol-evoked Ca2+ wave initiated and 2.78 F/F0 at region i. Similar results were obtained with four other cells (Fig. 3C). With one exception, in each case the site where InsP3-evoked the greatest Ca2+ response was not the location of initiation of the carbachol-evoked Ca2+ wave. These results indicate that the site specificity of agonist-evoked Ca2+ wave initiation is neither due to a greater sensitivity nor increased number of active InsP3R at that location.
Fig. 3.
The site of initiation of a carbachol-evoked [Ca2+]c wave does not correlate with the region of greatest InsP3 sensitivity. (A,B) Carbachol (CCh, Ac) evoked an increase in [Ca2+]c (Aa,b) that began at a single site (arrow, Aa, panel 2; region iii shown in panel 1) and propagated from there in either direction (arrows, Aa, panel 3). The [Ca2+] rises from the regions shown in Aa1 are plotted in Ab. Localised photolysis of caged InsP3 (Bb; arrow) at different sites (regions i to vi; Aa1) along the same cell increased [Ca2+]c locally. [Ca2+]c then passively diffused from the site of release. Interestingly, the InsP3-evoked [Ca2+]c increase (Ba,b; region iii) at the site where a carbachol-evoked wave initiated (Ab; region iii, green line) was modest when compared with the amplitude of that measured at other sites. The greatest InsP3-evoked [Ca2+]c increase occurred at region i (black line) some 50 µm from the site of wave initiation. The [Ca2+]c images (Aa,Ba) are derived from the time points indicated by the corresponding numbers in (Ab,Bb). [Ca2+]c changes in Aa and Ba are represented by colour; blue is low and red is high [Ca2+]c. Changes in the fluorescence ratio with time (Ab,Bb) are derived from 3×3 pixel boxes in the second panel in Aa; regions i to vi are drawn as larger yellow circles to facilitate visualisation. A brightfield image of the cell is shown (Aa; left panel); the whole-cell electrode can be seen (left side). The carbachol-containing puffer pipette is located to the right of the cell outside the field of view. (C) Summarised data from five different cells. The Ca2+ response at site of greatest InsP3-evoked Ca2+ release and that at the wave initiation site of individual cells are shown using connected lines.
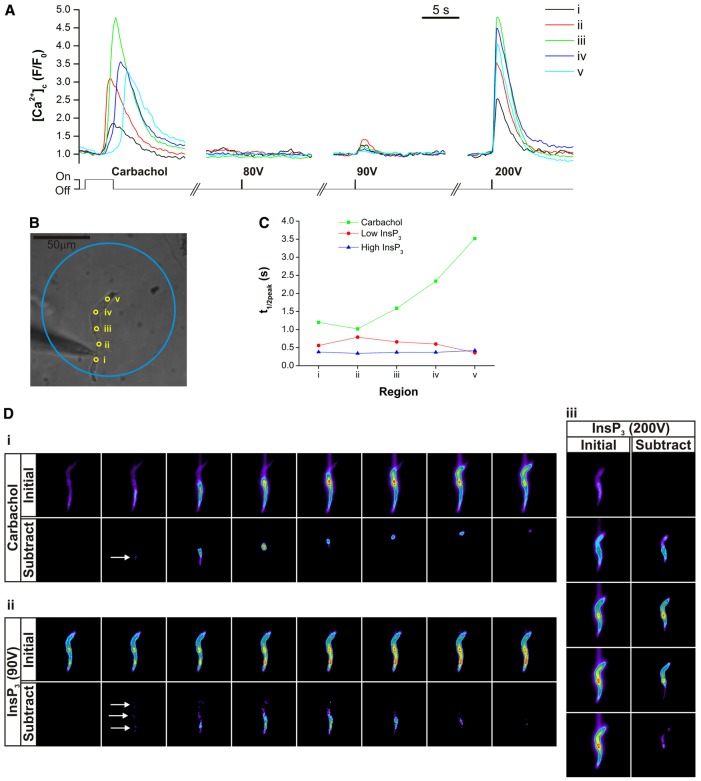
Further support for this proposal is found in a separate series of experiments in which subthreshold [InsP3] was applied globally throughout the cell to determine the site of greatest sensitivity and to compare that site with the region of wave initiation. To do this a larger diameter (1.25 mm core-cladding diameter in place of 0.2 mm) fibre optic cable was used for coupling of the xenon flashlamp used for photolysis to the microscope. With a ×40 objective, the area of illumination was ∼115 µm in diameter (as determined by coupling the fibre optic cable to a fibre optic illuminator and capturing a brightfield image). The charging voltage applied to the photolysis system's capacitors, which controls the intensity of the output light pulse, was increased in steps from a sub-threshold level where no Ca2+ rise occurred to the voltage where a clear, but submaximal, Ca2+ response was first detected (‘Low InsP3’, in the region of 50–90 V). The voltage was then further increased to a level where the maximal Ca2+ response was obtained (‘High InsP3’, typically around 200 V). The results show (Fig. 4) that, while the response varies somewhat throughout the cell to subthreshold [InsP3], the most sensitive region was not the wave initiation site (Fig. 4). The data was examined several ways. First, five regions of interest were defined along the cell within illumination zone being spaced at ∼18 µm intervals. The temporal series of mean intensity values for each region were normalised to the local baseline for each event to produce F/F0 values (Fig. 4A). For each event (CCh or flash photolysis), the time from the first rise in Ca2+ observed (for whichever region was the first to respond) to the point where the [Ca2+]c reached half its peak F/F0 value (t1/2peak) was measured for each region and plotted versus position (Fig. 4C). The rate of rise provides a measure of sensitivity to InsP3 and was not greatest at the site of wave initiation (5 out of 5 cells).
Fig. 4.
CCh evoked a Ca2+ wave, and global uncaging of InsP3 caused [Ca2+]c to rise simultaneously throughout the cell. (A) Carbachol evoked a Ca2+ wave that initiated from a single site (region 2) and propagated from there as a wave. Low and high levels of InsP3 uncaging, by varying the voltage used for photolysis (80 V, 90 V and 200 V; see Materials and Methods), resulted in [Ca2+]c increasing almost simultaneously throughout the cell. Regions i–v correspond to those in brightfield image (B). (C) Whereas the amplitude of the transient increased with voltage, the time of activation in each region was approximately similar for InsP3 as revealed by the rise times (t1/2 to peak). On the other hand, for CCh, the t1/2 to peak increased with distance from the initiation site. (D) Example frames showing the Ca2+ increase (‘Initial’) during the CCh evoked wave (i) and submaximal (90 V; ii) and maximal (200 V; iii) InsP3. A sequential subtraction was also performed on the data stacks (‘Subtract’), where the pixel intensity values for each frame were subtracted from the values of the image two frames ahead to enable clear visualisation of the regions where the first change in [Ca2+]c occurred. The CCh-evoked wave (i) initiation site is shown with an arrow on the subtracted data set. With subthresold voltage (90 V; ii), InsP3 release began at three separate areas (arrows). As it is a significantly longer event, the images in the carbachol wave sequence (i) are each three frames apart, whereas the images for the InsP3 events (ii, iii) are a single frame apart. We found similar results in four additional cells.
To help visualise the localised [Ca2+]c changes in response to CCh and InsP3 a sequential subtraction process was also performed on the entire image stack (Fig. 4D). In this case, pixel by pixel intensity values for each frame were subtracted from the values of the image two frames ahead. This subtraction enabled clear visualisation of areas where there where the first changes in [Ca2+]c occurred to subthrehold InsP3 (Fig. 4, dii arrows), which could be compared with the site of wave initiation (Fig. 4di, arrow). In 5 out of 5 cells [Ca2+]c rose simultaneously at several sites throughout the cell. Again the results suggest that the most sensitive sites are not the region of wave initiation (n = 5).
Sites of greater local [InsP3] determined by InsP3R and mAChR colocalisation
While sensitivity to InsP3 does not offer an explanation for the site of wave initiation it is possible that there are regions of the cell where there are greater [InsP3] increases to activate InsP3R. In these regions InsP3R presumably would open sooner and create faster local Ca2+ rises. Sites of faster local rises in Ca2+ may occur in regions of increased proximity of mAChR3 and InsP3R1. The proximity of InsP3R1 and mAChR3 to each other was examined by immunocytochemistry and quantified using ImageJ analysis software and JACoP (Just Another Colocalisation Plugin) (Bolte and Cordelières, 2006). Three-dimensional objects were created from the confocal image z stacks and the centre of mass of each object determined (Fig. 5Aa,Ab,Ba,Bb). Object-based colocalisation and the centre of mass method enables quantification where there are differences in protein expression, as was the case for InsP3R1 and mAChR3. InsP3R1 and mAChR3 colocalisation was quantified by determining the number of centres from one image that overlapped with objects from the other image (Fig. 5Bc). In the cell in Fig. 5, 15.8% of objects throughout the stack colocalised (18 out of 114 objects detected) for mAChR3 and 8.9% of objects (17 out of 191 objects detected) for InsP3R1 (Fig. 5). The images shown are from plane 33 of a 59-plane z-stack, each image was acquired at 150-nm intervals in the axial direction. Similar results were obtained with five other cells; the average colocalisation for mAChR3 was 13.2±2.9% and for InsP3R1 was 9.0±2.3% (n = 6). These experiments suggest that despite widespread distribution of InsP3R and mAChR there are only a few sites where colocalisation of the receptors occurs. At these sites InsP3R may be exposed to faster [InsP3] increases as compared to other sites in the cell to explain the site of Ca2+ wave initiation.
Fig. 5.
Colocalisation of InsP3R1 and mAChR3 in colonic smooth muscle. (A,B) Immunofluorescence labelling of mAChR3 and InsP3R1 in freshly isolated colonic myocytes was visualised by using confocal scanning microscopy. A single plane from an image stack where mAChR3 (Aa) was labelled using a polyclonal antibody specific for the type 3 isoform (AbCam; AB41169) and visualised using a fluorescently conjugated secondary antibody (TMRE; Molecular Probes). (Ba) InsP3R1 was labelled using a monoclonal antibody specific for the type 1 isoform (Abnova; H00003708-M01) and visualised using a fluorescently conjugated secondary antibody (Alexa Fluor 488; Molecular Probes). Colocalisation was quantified using image analysis software ImageJ and the plugin JACoP to examine object-based colocalisation. Briefly, the image stacks were smoothed using a 3×3 median filter and an image mask was applied. Three-dimensional objects were created using the 3D object counter plugin from the regions of the images with fluorescence above a threshold value (Ab,Bb). The centre of mass of each object was then determined. InsP3R1 and mAChR3 colocalisation was quantified by determining the number of centres from one image that were colocalised with objects from the other image (Bc). In the above experiment, 15.8% of the objects colocalised (18 out of 114 objects detected) for mAChR3 and 8.9% of the objects (17 out of 191 objects detected) in the z-stack for InsP3R1. The pixel size was 40 nm×40 nm, which is the minimum justifiable pixel size for the objective and emission wavelengths used. The images shown are from plane 33 of a 59-plane z-stack; each image was taken at 150-nm intervals. A brightfield image of the same cell is also shown (Ac).
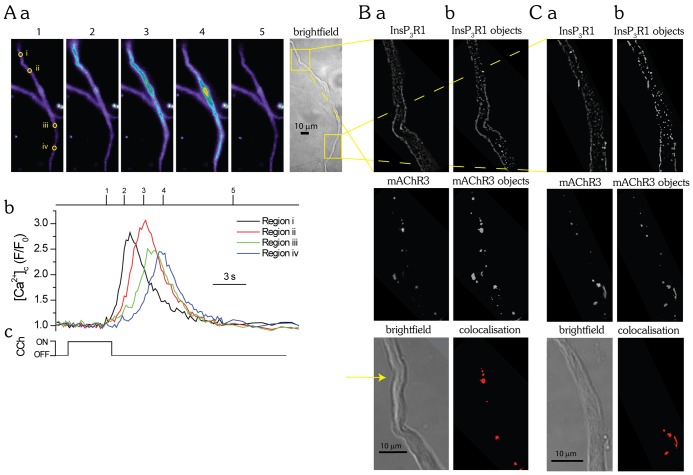
To further explore whether there was a greater extent of InsP3R and mAChR3 colocalisation where the Ca2+ wave initiated, this site was compared to another separate site in the same cell. First, the carbachol-evoked InsP3-mediated Ca2+ wave initiation site was established (Fig. 6A). Once this site was determined the cells were fixed and prepared as before for immunocytochemistry. In these experiments to identify each cell a coverslip with an etched grid was used. InsP3R1 and mAChR3 colocalisation was quantified, in the same cell, at the site of Ca2+ wave initiation and at another distant region (Fig. 6B). In these experiments 6±2% of objects colocalised for InsP3R1 with mAChR3 (n = 3) at the site of Ca2+ wave initiation. At the region distant from the site of wave initiation 3±2% of objects for InsP3R1 colocalised with mAChR3 (n = 3). Thus, there was substantially increased overlap of InsP3R and mAChR3 at the site of Ca2+ wave initiation.
Fig. 6.
The amount of colocalisation of InsP3R1 and mAChR3 is greater at sites of Ca2+ wave initiation. (A–C) Carbachol (CCh, 4 s; Ac) applied by a puffer pipette evoked an increase in [Ca2+]c in the form of a Ca2+ wave (Aa,Ab) that initiated from a single site (Aa; region i) and propagated away from that site. The Ca2+ wave always initiated from the same site during subsequent carbachol applications. The extent of InsP3R1 and mAChR3 colocalisation was then assessed in the same cell at the site of Ca2+ wave initiation and compared with that in another region of the cell (Aa; yellow boxes on the brightfield image). To do this, the cell was fixed, prepared for immunocytochemistry, labelled with anti-InsP3R1 monoclonal and anti-mAChR3 polyclonal antibodies and visualised using a fluorescently conjugated secondary antibody with confocal microscopy (Ba,Ca; top and middle panels). Colocalisation was quantified using image analysis software ImageJ and the plugin JACoP to examine object-based colocalisation. Briefly, the image stacks were smoothed using a 3×3 median filter and an image mask applied. Three-dimensional objects were created using the 3D object counter plugin from the regions of the images with fluorescence above a threshold value (Bb,Cb; top and middle panels). The centre of mass of each object was then determined. InsP3R1 and mAChR3 colocalisation was quantified by determining the number of centres from one image that were colocalised with objects from the other image (Bb, Cb; bottom panel). In the above experiment 9.0% of the objects colocalised (9 out of 100 objects detected) at the initiation site and 4.6% colocalised (7 out of 152 objects detected) at the other site. The images shown are from plane 45 (B, initiation site) and plane 34 (C, other site) of 70- and 50-plane z-stacks, respectively; each image is taken at 150-nm intervals. Brightfield images of the same cell are shown (Ba, Ca; lower panel); the initiation site is indicated (Ba; yellow arrow).
The site of Ca2+ wave initiation is dependent on a rapid local Ca2+ increase
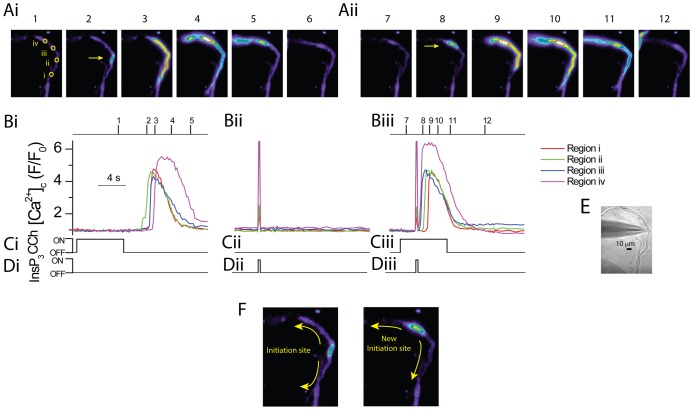
The experiments thus far raise the possibility that the site for wave initiation arises from overlap of mAChR3 and InsP3R1 to generate regions with a more rapid [InsP3] increase. In a first series of experiments to test this possibility [InsP3] was artificially, rapidly increased at a site which was distinct from the usual site of wave initiation. To do this, caged InsP3 was locally photolyzed, releasing a subthreshold [InsP3] during carbachol application. In the first part of the experiment carbachol (100 µM; Fig. 7Ci) was applied to verify the Ca2+ wave initiation site (region ii in Fig. 7Ai frame 1, yellow arrow in Fig. 7Ai frame 2); the wave then propagated the length of the cell (Fig. 7Ai,Bi). Caged InsP3 was next photoreleased locally at a site distant (50 µm) from that of wave initiation (20 µm diameter; region iv in Fig. 7Ai), using an empirically determined amount which did not trigger any SR Ca2+ release (Fig. 7Bii). Finally, carbachol was again applied (∼90 seconds later) but this time subthreshold InsP3 was photolysed before the Ca2+ wave could initiate. Upon photorelease of caged InsP3 a Ca2+ wave initiated (yellow arrow in Fig. 7Aii frame 8) at the site of photolysis (region iv shown in Fig. 7Ai) and propagated from there (Fig. 7Aii,Biii). These results support the proposal that the site of wave initiation occurs in regions of the greatest [InsP3] increase in which the photolyzed InsP3 adds to that produced by carbachol, thereby raising InsP3 concentration over the threshold for wave initiation to occur (n = 4).
Fig. 7.
The site of Ca2+ wave initiation moves to the region where subthreshold [InsP3] was rapidly and locally applied. (A–D) Carbachol (CCh, 6 s; Ci) applied via a puffer pipette evoked an increase in [Ca2+]c in the form of a Ca2+ wave (Ai,Bi). The wave initiated at a central site (region ii, Ai, panel 1) and propagated from there in either direction (Ai). Caged InsP3 (Dii), at an empirically determined concentration that was just subthreshold did not produce an increase in [Ca2+]c when focally photoreleased (Bii) ∼90 seconds later in a 20 µm region at a site distant from the carbachol-evoked Ca2+ wave initiation site (region iv in Ai panel 1; note the flash artefact shown in Bii). In the next part of the experiment, carbachol was again applied but this time the subthreshold caged InsP3 was locally photoreleased (Diii) 3 seconds after the start of the carbachol application (Ciii) and before the Ca2+ wave initiated. In this case, the site of initiation was altered and the Ca2+ wave began (Aii,Biii) at the site where [InsP3] rapidly increased (region iv in Ai, panel 1). The [Ca2+]c images (Ai and Aii) are derived from the time points indicated by the corresponding numerals in Bi and Biii. [Ca2+]c changes in Ai and Aii are represented by colour; blue is low and red is high [Ca2+]c. Changes in the fluorescence ratio with time (Bi,Bii,Biii) are derived from 3×3 pixel boxes in regions i to iv in A, panel 1. The regions are drawn as larger yellow circles to facilitate visualisation. (E) Brightfield image of the cell; the whole-cell electrode can be seen (left side). The carbachol-containing puffer pipette is located to the right of the cell outside the field of view. (F) Direction of Ca2+ wave from the initiation site either before (left panel) or after (right panel) photolysis of subthreshold InsP3 (n = 4).
In the next series of experiments to determine the contribution of the local [Ca2+]c increase to the specificity of the initiation site, the Ca2+ rise was selectively attenuated only at the initiation site by local photorelease of the caged Ca2+ buffer diazo-2.
First, control experiments were carried out to confirm the effectiveness of diazo-2 in buffering [Ca2+]c in small, restricted locations of the cell during membrane depolarisation and voltage-dependent Ca2+ entry (ICa) (Fig. 8). A voltage-clamped colonic myocyte depolarised from −70 mV to +10 mV activated ICa and produced transient, uniform, reproducible elevations in [Ca2+]c throughout the cell (Fig. 8Aa,b). One minute later the cell was again depolarised but this time the caged Ca2+ chelator diazo-2 (100 µM) was photoreleased to buffer the Ca2+ in one small (20 µm) region of the cell 100 ms prior to the depolarisation. Photorelease of diazo-2 selectively attenuated the Ca2+ rise at the site of photolysis whereas the [Ca2+]c increases at other locations were unaffected (Fig. 8Aa,e). In another experiment on this same cell diazo-2 was photoreleased 800 ms after membrane depolarisation and it rapidly buffered the [Ca2+]c increase at that location as shown by an instantaneous decrease in [Ca2+]c (Fig. 8Ba,e). Again, other locations throughout the cell were unaffected. These experiments confirm diazo-2 rapidly and effectively buffers local [Ca2+]c increases after photolysis.
Fig. 8.
Photolysis of the caged Ca2+ buffer diazo-2 rapidly attenuates the [Ca2+]c rise at the location of photolysis during depolarisation-evoked [Ca2+]c increases. (A,B) Depolarisation (−70 mV to +10 mV; Ac,Bc) activated a voltage-dependent Ca2+ current (ICa; Ad,Bd) to evoke approximately reproducible increases in [Ca2+]c (∼60-second interval) (Ab,Bb). Next, the depolarization protocol was repeated (Ae,Af,Ag) but the caged Ca2+ buffer, diazo-2, was photoreleased (Ae; arrow) in a small region of the cell immediately (100 ms) before the second depolarising pulse (Af; right panel). The [Ca2+]c increase at the site of photolysis (Aa,Ae; region ii, red line) was attenuated while [Ca2+]c increases in other regions of the cell (regions i and iii) were unaffected. In another experiment, the depolarisation protocol was repeated in the same cell (B) but this time diazo-2 was photoreleased (Be; arrow) 800 ms after the second depolarising pulse. The [Ca2+]c increase was attenuated at the site of photolysis (Ba,Be; region ii, red line) while [Ca2+]c increases in other regions (regions i and iii) were unaffected. The insets show [Ca2+]c transients from two regions only (for clarity) on an expanded time base. [Ca2+]c images (Aa,Ba) are derived from the time points indicated by the corresponding numerals in Ae and Be. [Ca2+]c changes in Aa and Ba are represented by colour; blue is low and red is high [Ca2+]c. Changes in the fluorescence ratio with time (Ab,Ae,Bb,Be) are derived from 3×3 pixel boxes shown in the far right panel in Aa; regions i to iii are drawn as larger yellow circles to facilitate visualisation. A brightfield image of the cell (Aa; right panel); the whole-cell electrode can be seen (left side) and photolysis spot is indicated.
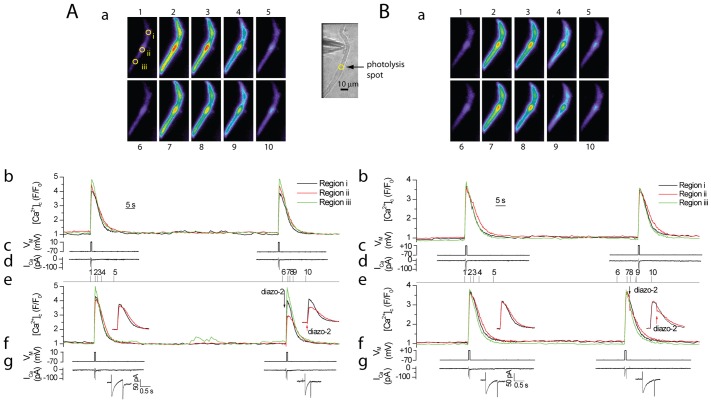
Diazo-2 was next used to determine whether or not the site of Ca2+ wave initiation required regions of faster increases in [Ca2+]c. The InsP3-generating agonist carbachol (100 µM; Fig. 9Ci) produced a Ca2+ wave that initiated at a single location and propagated from that site along the length of the cell (Fig. 9Ai,Bi). There was a latency of 5.3 sec from the start of agonist stimulation to Ca2+ wave initiation (Fig. 9Ai,Bi). In the next part of the experiment (∼90 sec later) caged Ca2+ buffer diazo-2 was photoreleased (Fig. 9Dii) at the wave initiation site after carbachol application (Fig. 9Cii) but before the Ca2+ wave initiated. Photolysis of diazo-2 prevented the increase in [Ca2+]c at the wave initiation site and interestingly the carbachol-evoked Ca2+ wave moved from the primary location after a latency of 6.6 sec, to initiate at a secondary location (Fig. 9Aii,Bii). In five other cells diazo-2 effectively prevented the Ca2+ wave from initiating at the primary ‘eager’ location and after a delay generated a secondary location which produced the Ca2+ wave. The average delay from the start of carbachol application to Ca2+ wave initiation under control conditions was 4.23±2.5 ms for waves occurring at the first site and 5.40±2.6 ms at the second site after diazo-2 photolysis (P<0.05) (Fig. 9E). These experiments suggest that a characteristic of the initiation site is a region of most rapid Ca2+ rise. It appears that if the primary ‘eager’ site of Ca2+ wave initiation is compromised the cell maintains the capability to initiate a Ca2+ wave at a new ‘eager’ location. Together, these results suggest that the site of Ca2+ wave initiation arises from an increased rate of rise of Ca2+ at the ‘eager’ location presumably due to faster local [InsP3] increase as a result of colocalisation of InsP3R and mAChR.
Fig. 9.
The site of Ca2+ wave initiation is altered when the [Ca2+]c increase at the initiation site is prevented by using photolysis of the caged buffer diazo-2. (A–D) Carbachol (4 seconds; Ci) applied from a puffer pipette evoked an increase in [Ca2+]c in the form of a Ca2+ wave (Ai,Bi) that initiated from a central site (arrow Ai, panel 2, region iii) and propagated away in either direction. Carbachol (Cii; ∼90 seconds later) was applied again, but this time the caged Ca2+ buffer, diazo-2, was locally photoreleased (Dii) at the site of Ca2+ wave initiation (region ii) 2 seconds after the start of the carbachol application (Cii; note the flash artefact in the [Ca2+]c trace Bii). The Ca2+ wave once again initiated but this time from a different location (arrow Aii, panel 8, region ii) and there was a significant delay in wave initiation (Bii). The [Ca2+]c images (Ai,Aii) are derived from the time points indicated by the corresponding numerals in Bi and Bii. [Ca2+]c changes (Ai,Aii) are represented by colour; blue is low and red is high [Ca2+]c. Changes in the fluorescence ratio with time (Bi,Bii) are derived from 3×3 pixel boxes in panel 1 in Ai; regions i to v are drawn as larger yellow circles to facilitate visualisation. A brightfield image of the cell is also shown (Ai; left panel); the whole-cell electrode can be seen (left side). (E) The time to the initiation of the Ca2+ response after onset of carbachol application was shorter when the Ca2+ wave initiated from the first site as compared with after diazo-2 release and the onset of a second wave initiation site. The average delay was 4.23 seconds before and 5.40 seconds after diazo-2 release (n = 6; P<0.05). Ca2+ wave initiation delay times of individual cells before and after diazo-2 are shown using connected lines.
Discussion
In several cell types a characteristic response to InsP3-generating agonists is a Ca2+ rise that initiates at a small single site then propagates along the length of the cell as a Ca2+ wave (Bootman et al., 1997a; Marchant and Parker, 2001; McCarron et al., 2010; Straub et al., 2000; Thorn et al., 1993). Interestingly, the site at which Ca2+ waves initiate is often fixed and constant during each agonist activation. The present study examined the mechanisms that determine the site of agonist-evoked Ca2+ wave initiation in smooth muscle and the results suggest waves initiate where [InsP3] and [Ca2+]c increases most rapidly as a result of colocalisation of InsP3R1 and mAChR3.
InsP3R were found to be distributed widely throughout the cell in the present study but with increased occurrence at the nucleus and plasma membrane as was also seen in intestinal myocytes (and Zholos, 2004). The InsP3-generating muscarinic receptor, mAChR3, was also approximately uniformly distributed in the plasma membrane. The distribution of each receptor does not explain the site of wave initiation. However, when mAChR3 and InsP3R1 were dual labelled and the distribution quantified by centre of mass analysis, there was a small number of sites which showed co-existence of the two receptors. A 13% overlap between mAChR3 and InsP3R1 and 9% overlap of InsP3R1 and mAChR3 existed. The much higher expression of InsP3R1 than mAChR3 accounts for the difference in percent overlap. This observation raised the possibility that regions of overlap may explain the site of origin of the Ca2+ wave. To examine this possibility a Ca2+ wave was first evoked then immunocytochemistry carried out in the same cell to determine the extent of overlap of InsP3R1 and mAChR3 at the wave initiation site. A significantly greater overlap of the receptors existed at the site of wave initiation than in other regions of the cell. The colocalisation of mAChR3 and InsP3R1 may create regions where InsP3 has little distance to diffuse to activate InsP3R to generate faster and greater Ca2+ release at these sites. This arrangement will produce an effective InsP3 junction in which mAChR3-generated InsP3 is preferentially and rapidly delivered to particular InsP3R. InsP3 acts in these circumstances as a highly localised and targeted signal rather than a diffusible second messenger. Indeed in SH-SY5Y cells the majority of sites of local Ca2+ release (Ca2+ puffs) were within 100 nm of the plasma membrane (Smith et al., 2009). In addition, a Ca2+-activated K+ current was activated by InsP3-evoked Ca2+ release (Hoesch et al., 2004). These results suggest a prevalence of active InsP3R near the plasma membrane.
A prediction from the proposal that waves initiate where InsP3R and mAChR co-localised is that at these sites [InsP3] and [Ca2+]c may increase most rapidly. To test this possibility two experiments were undertaken. In the first, localised increases in [InsP3] at sites distant from the normal initiation site were evoked more rapidly than the [InsP3] increases that occur by the mAChR3 agonist carbachol to short circuit the wave initiation process. [InsP3] was rapidly and locally increased by photolysis of a subthreshold amount of caged InsP3 during application of the InsP3-generating agonist (carbachol). The local photolysis resulted in the Ca2+ wave initiation site shifting to the region of InsP3 release and the wave travelling along a new path. In the second experiment, the [Ca2+]c rise at the site of wave initiation, and only this site, was attenuated using targeted localised photolysis of the caged Ca2+ buffer diazo-2. When the [Ca2+]c was rapidly buffered during carbachol application this again resulted in the Ca2+ wave shifting from the normal site and initiating at a new secondary site after a short delay. There are only a few regions of the cell where mAChR and InsP3R colocalised and if the [Ca2+]c increase at the normal site of Ca2+ wave initiation is compromised then presumably one of these other sites will become the wave initiation site. Together, these results suggest InsP3 derived from activation of particular mAChR3 is selectively delivered to InsP3R via the close apposition of the two receptors to generate the Ca2+ wave initiation site.
Directed, selective communication between processes in cells drives intracellular signalling. Sites of privileged communication also coordinates activities among InsP3R and various proteins involved in intracellular Ca2+ signalling to generate selective responses (Delmas et al., 2002; Hur et al., 2005; Tovey et al., 2008; Yuan et al., 2005). For example, some agonists acting via G protein coupled receptors to release Ca2+ via InsP3R evoke specific responses by being additionally coupled to adenylate cyclase (AC) to create a local signalling complex. Parathyroid hormone-stimulated cyclic adenosine monophosphate (cAMP) formation was delivered locally from AC (AC6) to InsP3R2. This activity selectively sensitised InsP3R2 to the inositide (Tovey et al., 2008). InsP3R may also respond to the redox state of the intracellular store lumen via association with ERp54 protein (Higo et al., 2005). The Ca2+ content of the store may also be communicated to InsP3R via the Ca2+ binding protein calreticulin (Roderick et al., 1998). InsP3R1, PKA and epidermal growth factor receptor (EGFR) may also form a signalling complex to facilitate bradykinin-induced InsP3R1 phosphorylation and sensitisation and Ca2+ release (Hur et al., 2005). Together these studies highlight the significance of local coupling events to global cell signalling procedures.
Signalling complexes may also explain the site of Ca2+ wave initiation. Localised expression of particular proteins sensitise the response to InsP3 and correlate with the wave initiation site in some cells. In cultured rat hippocampal neurons and pheochromocytoma cells the wave initiation site was associated with localised expression of the ER luminal protein chromogranin B, a protein which sensitises InsP3R to InsP3 and phosphatidylinositol-4-phosphate kinase (PIPKIγ), a kinase involved in the production of the InsP3 precursor phosphatidyl inositol 4,5-bisphosphate (PIP2) (Jacob et al., 2005). In HeLa cells local Ca2+ release usually initiated from a single perinuclear Ca2+ puff site although InsP3R were expressed evenly around the nucleus (Thomas et al., 2000). The perinuclear sites produced multiple Ca2+ puffs during prolonged histamine stimulation and often became the site where a Ca2+ wave initiated (Bootman et al., 1997b). The location and frequency of Ca2+ puffs were dependent on [InsP3], where some sites appeared to have a greater sensitivity to InsP3 and Ca2+ release occurred sooner (Bootman et al., 1997b; Marchant and Parker, 2001).
Regional differences in sensitivity to InsP3 do not appear to determine the location of Ca2+ wave initiation in the present study. We examined this possibility by comparing the amplitude of [Ca2+]c increase during local photolysis of caged InsP3, to the magnitude of Ca2+ release during carbachol-evoked Ca2+ waves at several sites in the cell including the initiation site. The magnitude of Ca2+ release during a fixed [InsP3] was not greatest at the site of wave initiation as would have been expected if the sensitivity to InsP3 was greatest here. Indeed, InsP3-evoked Ca2+ increase at the wave initiation site was significantly smaller than for other regions of the cell. These experiments suggest that differences in the sensitivity to InsP3 do not determine the site of Ca2+ wave initiation in smooth muscle. Consistent with our results, others found no difference in the frequency and sensitivity of perinuclear and cytosolic Ca2+ puffs to the [InsP3] stimulation threshold (Smith et al., 2009).
The differences in the above results and in the apparent sensitivities to InsP3 could be explained by different methodological approaches. Thomas and colleagues (Thomas et al., 2000) used agonists to evoke Ca2+ puffs whereas Smith and colleagues (Smith et al., 2009) used a membrane permeant photolabile InsP3 analogue. In the first instance responses to InsP3 produced in microdomains would be measured and in the second photolysis would raise the [InsP3] evenly throughout the cell. The differences in Ca2+ puff characteristics measured at different puff sites could be explained by microdomains of synthesis and diffusion of InsP3 as proposed in the present study.
Nerve released acetylcholine is directed to specific regions of the smooth muscle cell via the close apposition (20 nm) of muscle and nerve at the synapse (Faussone-Pellegrini et al., 1989; Goyal and Chaudhury, 2010; Mitsui and Komuro, 2002; Silva et al., 1968) and presumably would create microdomains of higher concentration of the agonist there. The site of Ca2+ wave initiation in smooth muscle arises from a complex where mAChR3 and InsP3R1 are structurally and functionally coupled to generate junctions in which InsP3 acts as a highly localised signal by being rapidly and selectively delivered to particular InsP3R.
Materials and Methods
Cell isolation
Male guinea-pigs (350–500 g) were humanely killed by cervical dislocation followed by immediate exsanguination in accordance with the guidelines of the Animal (Scientific Procedures) Act UK, 1986. A segment of intact distal colon (∼5 cm) was transferred to oxygenated (95% O2, 5% CO2) physiological saline solution comprising (mM): 118.4 NaCl, 25 NaHCO3, 4.7 KCl, 1.13 NaH2PO4, 1.3 MgCl2, 2.7 CaCl2 and 11 glucose (pH 7.4). Following removal of the mucosa and longitudinal muscle layer from the tissue, single smooth muscle cells, largely from circular muscle, were enzymatically dissociated (McCarron and Muir, 1999). All experiments were carried out at room temperature (20±2°C) unless otherwise noted.
Electrophysiology
Cells were voltage-clamped using conventional tight-seal whole-cell recording methods. The extracellular solution contained (mM): 80 Na glutamate, 40 NaCl, 20 tetraethylammonium chloride (TEA), 1.1 MgCl2, 3 CaCl2, 10 HEPES and 30 glucose (pH 7.4 with NaOH). The pipette solution contained (mM): 85 Cs2SO4, 20 CsCl, 1 MgCl2, 30 HEPES, 3 MgATP, 2.5 pyruvic acid, 2.5 malic acid, 1 NaH2PO4, 5 creatine phosphate, 0.5 guanosine phosphate and 0.025 caged inositol 1,4,5-trisphosphate (InsP3) trisodium salt. Whole-cell currents were measured using an Axopatch 200B (Axon Instruments, Union City, CA), low-pass filtered at 500 Hz (8-pole Bessel filter; Frequency Devices, Haverhill, MA), digitally sampled at 1.5 kHz using a Digidata interface and pClamp (version 8; Axon Instruments) and stored for analysis.
Ca2+ imaging
Single, freshly isolated colonic smooth muscle cells were loaded with the Ca2+-sensitive dye fluo 3 acetoxymethylester (AM) (10 µM) and wortmannin (10 µM; to prevent contraction) for at least 20 min before the start of the experiment. The 20–30 min was sufficient to allow intracellular esterases to hydrolyze the AM moiety. Two-dimensional [Ca2+]c images were obtained using a wide-field digital imaging system (Olson et al., 2010). Single cells were illuminated at 488 nm (bandpass 14 nm) from a monochrometer (Polychrome IV, T.I.L.L. Photonics, Martinsried, Germany) and imaged through an oil-immersion objective (×40 UV 1.3 NA; Nikon UK, Surrey, UK). Excitation light was passed via a fibre-optic guide through a 485 bandpass (15 nm) filter and a fieldstop diaphragm and reflected off a 505 nm long-pass dichroic mirror. Emitted light was guided through a 535 nm barrier filter (bandpass 45 nm) to an intensified, cooled, frame transfer CCD camera (Pentamax Gen IV, Roper Scientific, Trenton, NJ). Ca2+ imaging data were recorded on a personal computer using Metafluor (Molecular Devices, Wokingham, UK). Full-frame images (160×160 pixels), with a pixel size 720 nm at the cell, were acquired at ∼10 frames per second. Electrophysiological measurements and imaging data were synchronized by recording, on pClamp, a transistor transistor logic (TTL) output from the CCD camera, which reported its readout status together with the electrophysiological information.
Agonist application
The muscarinic acetylcholine receptor agonist carbachol (100–250 µM) was applied (2–6 sec) by hydrostatic pressure ejection via a puffer pipette using a picospritzer system. In most experiments carbachol was applied in a Ca2+ free bath solution (containing 1 mM EGTA) but in some a normal bath solution was used with no difference in the results.
Localised flash photolysis
A xenon flashlamp (Rapp Optoelecktronic, Hamburg, Germany) was used to photolyze caged InsP3 or the caged Ca2+ buffer diazo-2. The flashlamp output was passed through a UG-5 filter to select ultraviolet light, focused and merged into the excitation light path through a fibre-optic bundle and long-pass dichroic mirror at the lens part of the epi-illumination attachment of the microscope. The diameter of the fibre optic together with the lens magnification determined the area (spot size ∼20 µm) of photolysis (McCarron and Olson, 2008). The output intensity of the flash lamp was 0.19 mW at the objective lens. The timing of photolysis was recorded using pClamp software by using a TTL output from the flashlamp.
Global uncaging of caged InsP3
For global uncaging of InsP3 a larger diameter fibre optic cable (1.25-mm core-cladding diameter in place of 0.2 mm) was used to couple the xenon flashlamp to the microscope. With this larger fibre optic a ×40 objective, the area of illumination was ∼115 µm in diameter (as determined by coupling the fibre optic cable to a fibre optic illuminator and capturing a brightfield image). Only cells that lay primarily within the illumination zone and whose carbachol initiation site also fell within the zone were used. Although a ×20 objective increased the photolysis illumination area, such that the majority of cells could generally be positioned entirely within the illumination zone, the image resolution obtained with this lens was inadequate for detailed image analysis.
The charging voltage applied to the photolysis system's capacitors, which controls the intensity of the output light pulse, was increased in steps from a sub-threshold level where no Ca2+ rise occurred to the voltage where a clear, but submaximal, Ca2+ response was first detected (‘Low InsP3’, in the region of 50–90 V). The voltage was then further increased to a level where the maximal Ca2+ response was obtained (‘High InsP3’, typically around 200 V).
Immunocytochemistry
Smooth muscle cells were placed on to microscope slides and allowed to settle for 60 min in a humid environment. Cells were fixed (15 min) in 4% (w/v) paraformaldehyde in phosphate buffered saline (PBS) (2.7 mM KCl, 1.5 mM KH2PO4, 137 mM NaCl, 8 mM Na2HPO4·7H2O, pH 7.4), permeabilized with 0.1% Triton X-100 in PBS (10 min), then incubated with primary antibodies (2 hours) in an antibody buffer (150 mM NaCl, 15 mM Na3Citrate, 2% goat serum, 1% BSA, 0.05% Triton-X100). Incubation with secondary antibodies was for 1 hour. InsP3R1 was detected using monoclonal InsP3R1 antibodies (1∶50). mAChR3 was detected using an isoform-specific polyclonal antibody (1∶40). Primary antibodies were visualised by fluorescence confocal microscopy using fluorochrome conjugated secondary antibodies.
The cells were imaged using a Leica SP5 upright confocal microscope (Leica Microsystems UK, Milton Keynes, UK). The excitation beam was produced by an argon (488 nm) or HeNe (543 and 643 nm) laser and delivered to the sample via an oil immersion objective lens (HCX PL APO×63 1.40 NA). Emitted fluorescence was captured using acousto-optical tuneable filters coupled to a PMT controlled by Leica imaging software. Full frame images (246 µm×246 µm) with a pixel size of 240.5 nm at the cell were acquired. Z stacks were acquired as 3 x–y image averages at 150-nm intervals. The scanner angle was further adjusted to obtain images at 6× magnification and a pixel size of 40 nm×40 nm.
Western blotting
Tissue was frozen in liquid N2 immediately following dissection and homogenised (200 mg tissue per ml) in either RIPA buffer or in a Triton-based lysis buffer [20 mM Tris Base, 150 mM NaCl, 1% Triton X-100, 5 mM EDTA (pH 7.4)], both containing protease inhibitors, by grinding with eppendorf-tube pestles and repeatedly vortexing. Homogenates were clarified by centrifugation (5 min at 13,200 rpm) and the supernatant aliquoted into 100 µl volumes and stored at −80°C. Prior to running a western blot, the lysate was diluted 4-fold in lysis buffer. For each lane to be run, a sample was prepared by adding 5 µl×5-PLB [40 ml glycerol (18%), 120 ml 10% SDS (5.5%), 50 ml 1 M Tris (pH 6.5) (0.23 M), 50 mg Bromophenol Blue (0.023%), 10 ml β-mercaptoethanol (4.5%)] to an eppendorf containing 20 µl diluted lysate (i.e each lane contained lysate from ∼1 mg tissue). The samples were then heated at 99°C for 5 min and pulse centrifuged then loaded into polyacrylamide gels (7% for InsP3R blot, 10% for mAChR), along with 10 µl HiMark Prestained HMW Protein Standard, and run at 200 V for 1–2 h (2 h for 7% gel, 1 h for 10% gel) using a 0.25 M Glycine, 0.025 M Tris, 0.1% SDS running buffer. The proteins were then transferred to a nitrocellulose membrane by electroblotting for 1.5–2 h (1.5 h for mAChR blot, 2 h for InsP3R blot), using a Bio-Rad Mini Trans-Blot cell with a limiting voltage/current of 100 V/400 mA and a 25 mM Tris, 192 mM Glycine, 20% MeOH, pH 8.0 transfer buffer. After electroblotting, the membrane was removed and blocked in a 5% nonfat milk powder solution in PBS overnight at 4°C. After two 5 min washes in PBS-T (0.05% Tween in PBS), the membrane was probed with a 1∶500 primary antibody dilution (rabbit polyclonal anti-mAChR3, and mouse monoclonal anti-InsP3R1) in 5% milk powder in PBS-T for 1 h. After three 5 min and two 15 min washes, the membrane was incubated in a 1∶1000 secondary antibody dilution (anti-mouse-HRP and anti-rabbit-HRP) in 5% milk powder in PBS-T for 1 h followed by two 5 min and two 10 min washes. All washes and antibody incubations were carried out at room temperature on an orbital shaker (∼50 rpm). The protein bands were then detected by enhanced chemiluminescence using Pierce's ECL Western Blotting Substrate and imaged using an X-OMAT system.
Data analysis
[Ca2+]c images were analysed using the program Metamorph 7.1.3 (Molecular Devices, Wokingham, UK). Changes in fluorescence were expressed as ratios (F/F0 or ΔF/F0) of fluorescence counts (F) relative to baseline (control) values (taken as 1) before stimulation (F0). Peak height was subtracted from the average baseline value derived from the 100 frames before carbachol application or flash photolysis of caged InsP3. The delay in the onset of the Ca2+ wave after application of carbachol was measured as the time from the start of carbachol application for [Ca2+]c to increase by 10% F/F0 of the peak amplitude. Colocalisation of InsP3R1 and mAChR3 was quantified using ImageJ v1.44 analysis software (Rasband, 1997-2011) and JACoP v2.0 (Just Another Colocalisation Plugin) (Bolte and Cordelières, 2006) to determine object based colocalisation. Briefly, the confocal image stacks were smoothed using a 3×3 median filter and an image mask applied. Three-dimensional objects were created using the 3D object counter plugin from the regions of the images with fluorescence above a threshold value. The centre of mass of each object was then determined. InsP3R1 and mAChR3 colocalisation was quantified by determining the number of centres from one image that were colocalised with objects from the other image.
Summarised results are expressed as means ± s.e.m. of n cells. A paired or unpaired Student's t-test was applied to the raw data, as appropriate; P<0.05 was considered significant.
Global InsP3 photolysis analysis
The raw fluorescence data was analysed using Metamorph. First the data was smoothed using a 3-frame rolling average applied to the sequence of captured images so that the small [Ca2+]c increases with submaximal InsP3 could be resolved. Five regions of interest were then defined along the cell within the illumination zone, one of which was at the CCh wave initiation site, with the others being spaced at ∼18 µm intervals. The temporal series of mean intensity values for each region were exported to the software Microcal Origin, in which the data was normalised to the local baseline for each event to produce F/F0 values. For each event, the time from the first rise in [Ca2+]c observed (for whichever region was the first to respond) to the point where the [Ca2+]c reached half its peak F/F0 value (t1/2peak) was measured for each region and plotted versus position.
Using Metamorph, a sequential subtraction process was also performed on the rolling average sequences, where the pixel intensity values for each frame were subtracted from the values of the image two frames ahead. This enabled clear visualisation of the region where the first changes in [Ca2+]c occurred and, in the case of CCh, of wave progression.
Drugs and chemicals
Concentrations in the text refer to the salts, where appropriate. Fluo-3 AM, diazo-2 tetrapotassium salt, goat anti-rabbit and goat anti-mouse secondary antibodies (Alexa 488, TMRE, Alexa 647) and HiMark Prestained HMW Protein Standard were purchased from Invitrogen (Paisley, UK) and caged InsP3-trisodium salt from SiChem GmbH (Bremen, Germany). Anti-ITPR1 antibody (Abnova, USA) was purchased from Stratech (Suffolk, UK). Anti-mAChR3 antibody was purchased from Chemicon (Watford, UK) and AbCam (Cambridge, UK). RIPA buffer and ECL Western Blotting Substrate were purchased from Pierce (Thermo Fisher Scientific, Northumberland, UK). All other reagents were purchased from Sigma (Poole, UK).
Footnotes
Funding
This work was funded by the Wellcome Trust [grant number 092292/Z/10/Z]; and the British Heart Foundation [grant numbers PG/08/066, PG/11/70/29086]. Deposited in PMC for immediate release.
References
- Bolte S., Cordelières F. P. (2006). A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224, 213–232 10.1111/j.1365-2818.2006.01706.x [DOI] [PubMed] [Google Scholar]
- Bootman M., Niggli E., Berridge M., Lipp P. (1997a). Imaging the hierarchical Ca2+ signalling system in HeLa cells. J. Physiol. 499, 307–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman M. D., Berridge M. J., Lipp P. (1997b). Cooking with calcium: the recipes for composing global signals from elementary events. Cell 91, 367–373 10.1016/S0092-8674(00)80420-1 [DOI] [PubMed] [Google Scholar]
- Chalmers S., Olson M. L., MacMillan D., Rainbow R. D., McCarron J. G. (2007). Ion channels in smooth muscle: regulation by the sarcoplasmic reticulum and mitochondria. Cell Calcium 42, 447–466 10.1016/j.ceca.2007.05.010 [DOI] [PubMed] [Google Scholar]
- Delmas P., Wanaverbecq N., Abogadie F. C., Mistry M., Brown D. A. (2002). Signaling microdomains define the specificity of receptor-mediated InsP(3) pathways in neurons. Neuron 34, 209–220 10.1016/S0896-6273(02)00641-4 [DOI] [PubMed] [Google Scholar]
- Díaz–Muñoz M., de la Rosa Santander P., Juárez–Espinosa A. B., Arellano R. O., Morales–Tlalpan V. (2008). Granulosa cells express three inositol 1,4,5-trisphosphate receptor isoforms: cytoplasmic and nuclear Ca2+ mobilization. Reprod. Biol. Endocrinol. 6, 60 10.1186/1477-7827-6-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faussone–Pellegrini M. S., Pantalone D., Cortesini C. (1989). An ultrastructural study of the smooth muscle cells and nerve endings of the human stomach. J. Submicrosc. Cytol. Pathol. 21, 421–437 [PubMed] [Google Scholar]
- Fissore R. A., Longo F. J., Anderson E., Parys J. B., Ducibella T. (1999). Differential distribution of inositol trisphosphate receptor isoforms in mouse oocytes. Biol. Reprod. 60, 49–57 10.1095/biolreprod60.1.49 [DOI] [PubMed] [Google Scholar]
- Gordienko D. V., Zholos A. V. (2004). Regulation of muscarinic cationic current in myocytes from guinea-pig ileum by intracellular Ca2+ release: a central role of inositol 1,4,5-trisphosphate receptors. Cell Calcium 36, 367–386 10.1016/j.ceca.2004.02.021 [DOI] [PubMed] [Google Scholar]
- Goyal R. K., Chaudhury A. (2010). Mounting evidence against the role of ICC in neurotransmission to smooth muscle in the gut. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G10–G13 10.1152/ajpgi.00426.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez E., Leite M. F., Guerra M. T., Kruglov E. A., Bruna–Romero O., Rodrigues M. A., Gomes D. A., Giordano F. J., Dranoff J. A., Nathanson M. H. (2007). The spatial distribution of inositol 1,4,5-trisphosphate receptor isoforms shapes Ca2+ waves. J. Biol. Chem. 282, 10057–10067 10.1074/jbc.M700746200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higo T., Hattori M., Nakamura T., Natsume T., Michikawa T., Mikoshiba K. (2005). Subtype-specific and ER lumenal environment-dependent regulation of inositol 1,4,5-trisphosphate receptor type 1 by ERp44. Cell 120, 85–98 10.1016/j.cell.2004.11.048 [DOI] [PubMed] [Google Scholar]
- Hirata K., Nathanson M. H., Burgstahler A. D., Okazaki K., Mattei E., Sears M. L. (1999). Relationship between inositol 1,4,5-trisphosphate receptor isoforms and subcellular Ca2+ signaling patterns in nonpigmented ciliary epithelia. Invest. Ophthalmol. Vis. Sci. 40, 2046–2053 [PubMed] [Google Scholar]
- Hoesch R. E., Weinreich D., Kao J. P. (2004). Localized IP3-evoked Ca2+ release activates a K+ current in primary vagal sensory neurons. J. Neurophysiol. 91, 2344–2352 10.1152/jn.01008.2003 [DOI] [PubMed] [Google Scholar]
- Hur E. M., Park Y. S., Huh Y. H., Yoo S. H., Woo K. C., Choi B. H., Kim K. T. (2005). Junctional membrane inositol 1,4,5-trisphosphate receptor complex coordinates sensitization of the silent EGF-induced Ca2+ signaling. J. Cell Biol. 169, 657–667 10.1083/jcb.200411034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R., Isenberg G. (1990). Acetylcholine activates nonselective cation channels in guinea pig ileum through a G protein. Am. J. Physiol. 258, C1173–C1178 [DOI] [PubMed] [Google Scholar]
- Jacob S. N., Choe C. U., Uhlen P., DeGray B., Yeckel M. F., Ehrlich B. E. (2005). Signaling microdomains regulate inositol 1,4,5-trisphosphate-mediated intracellular calcium transients in cultured neurons. J. Neurosci. 25, 2853–2864 10.1523/JNEUROSCI.4313-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. K., Bayguinov O., Sanders K. M. (1993). Role of nonselective cation current in muscarinic responses of canine colonic muscle. Am. J. Physiol. 265, C1463–C1471 [DOI] [PubMed] [Google Scholar]
- Lur G., Sherwood M. W., Ebisui E., Haynes L., Feske S., Sutton R., Burgoyne R. D., Mikoshiba K., Petersen O. H., Tepikin A. V. (2011). InsP3receptors and Orai channels in pancreatic acinar cells: co-localization and its consequences. Biochem. J. 436, 231–239 10.1042/BJ20110083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant J. S., Parker I. (2001). Role of elementary Ca2+ puffs in generating repetitive Ca2+ oscillations. EMBO J. 20, 65–76 10.1093/emboj/20.1.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarron J. G., Muir T. C. (1999). Mitochondrial regulation of the cytosolic Ca2+ concentration and the InsP3-sensitive Ca2+ store in guinea-pig colonic smooth muscle. J. Physiol. 516, 149–161 10.1111/j.1469-7793.1999.149aa.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarron J. G., Olson M. L. (2008). A single luminally continuous sarcoplasmic reticulum with apparently separate Ca2+ stores in smooth muscle. J. Biol. Chem. 283, 7206–7218 10.1074/jbc.M708923200 [DOI] [PubMed] [Google Scholar]
- McCarron J. G., Craig J. W., Bradley K. N., Muir T. C. (2002). Agonist-induced phasic and tonic responses in smooth muscle are mediated by InsP(3). J. Cell Sci. 115, 2207–2218 [DOI] [PubMed] [Google Scholar]
- McCarron J. G., MacMillan D., Bradley K. N., Chalmers S., Muir T. C. (2004). Origin and mechanisms of Ca2+ waves in smooth muscle as revealed by localized photolysis of caged inositol 1,4,5-trisphosphate. J. Biol. Chem. 279, 8417–8427 10.1074/jbc.M311797200 [DOI] [PubMed] [Google Scholar]
- McCarron J. G., Anderson K. I., Wright A. J., Girkin J. M. (2008). Simultaneous Imaging of subplasma membrane and bulk average Ca2+ concentrations in single smooth muscle cells. J. Vasc. Res. 45, 1–11417898542 [Google Scholar]
- McCarron J. G., Chalmers S., MacMillan D., Olson M. L. (2010). Agonist-evoked Ca2+ wave progression requires Ca2+ and IP3. J. Cell. Physiol. 224, 334–344 10.1002/jcp.22103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui R., Komuro T. (2002). Direct and indirect innervation of smooth muscle cells of rat stomach, with special reference to the interstitial cells of Cajal. Cell Tissue Res. 309, 219–227 10.1007/s00441-002-0592-1 [DOI] [PubMed] [Google Scholar]
- Olson M. L., Chalmers S., McCarron J. G. (2010). Mitochondrial Ca2+ uptake increases Ca2+ release from inositol 1,4,5-trisphosphate receptor clusters in smooth muscle cells. J. Biol. Chem. 285, 2040–2050 10.1074/jbc.M109.027094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband W. S. (1997–2011). ImageJ. US National Institutes of Health, Bethesda, Maryland, USA: http://imagej.nih.gov/ij/ [Google Scholar]
- Roderick H. L., Llewellyn D. H., Campbell A. K., Kendall J. M. (1998). Role of calreticulin in regulating intracellular Ca2+ storage and capacitative Ca2+ entry in HeLa cells. Cell Calcium 24, 253–262 10.1016/S0143-4160(98)90049-5 [DOI] [PubMed] [Google Scholar]
- Rooney T. A., Sass E. J., Thomas A. P. (1990). Agonist-induced cytosolic calcium oscillations originate from a specific locus in single hepatocytes. J. Biol. Chem. 265, 10792–10796 [PubMed] [Google Scholar]
- Sawyer G. W., Ehlert F. J. (1998). Contractile roles of the M2 and M3 muscarinic receptors in the guinea pig colon. J. Pharmacol. Exp. Ther. 284, 269–277 [PubMed] [Google Scholar]
- Shuai J., Rose H. J., Parker I. (2006). The number and spatial distribution of IP3 receptors underlying calcium puffs in oocytes. Biophys. J. 91, 4033–4044 10.1529/biophysj.106.088880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva D. G., Farrell K. E., Smith G. C. (1968). Ultrastructural and histochemical studies on the innervation of the mucous membrane of the mouse colon. Anat. Rec. 162, 157–176 10.1002/ar.1091620204 [DOI] [PubMed] [Google Scholar]
- Smith I. F., Parker I. (2009). Imaging the quantal substructure of single IP3R channel activity during Ca2+ puffs in intact mammalian cells. Proc. Natl. Acad. Sci. USA 106, 6404–6409 10.1073/pnas.0810799106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I. F., Wiltgen S. M., Parker I. (2009). Localization of puff sites adjacent to the plasma membrane: functional and spatial characterization of Ca2+ signaling in SH-SY5Y cells utilizing membrane-permeant caged IP3. Cell Calcium 45, 65–76 10.1016/j.ceca.2008.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub S. V., Giovannucci D. R., Yule D. I. (2000). Calcium wave propagation in pancreatic acinar cells: functional interaction of inositol 1,4,5-trisphosphate receptors, ryanodine receptors, and mitochondria. J. Gen. Physiol. 116, 547–560 10.1085/jgp.116.4.547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swillens S., Dupont G., Combettes L., Champeil P. (1999). From calcium blips to calcium puffs: theoretical analysis of the requirements for interchannel communication. Proc. Natl. Acad. Sci. USA 96, 13750–13755 10.1073/pnas.96.24.13750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura H., Yamashina S., Segawa A. (1999). Millisecond analyses of Ca2+ initiation sites evoked by muscarinic receptor stimulation in exocrine acinar cells. Biochem. Biophys. Res. Commun. 259, 656–660 10.1006/bbrc.1999.0818 [DOI] [PubMed] [Google Scholar]
- Thomas D., Lipp P., Tovey S. C., Berridge M. J., Li W., Tsien R. Y., Bootman M. D. (2000). Microscopic properties of elementary Ca2+ release sites in non-excitable cells. Curr. Biol. 10, 8–15 10.1016/S0960-9822(99)00258-4 [DOI] [PubMed] [Google Scholar]
- Thomason P. A., Wolanin P. M., Stock J. B. (2002). Signal transduction: receptor clusters as information processing arrays. Curr. Biol. 12, R399–R401 10.1016/S0960-9822(02)00885-0 [DOI] [PubMed] [Google Scholar]
- Thorn P., Lawrie A. M., Smith P. M., Gallacher D. V., Petersen O. H. (1993). Local and global cytosolic Ca2+ oscillations in exocrine cells evoked by agonists and inositol trisphosphate. Cell 74, 661–668 10.1016/0092-8674(93)90513-P [DOI] [PubMed] [Google Scholar]
- Tovey S. C., Dedos S. G., Taylor E. J., Church J. E., Taylor C. W. (2008). Selective coupling of type 6 adenylyl cyclase with type 2 IP3 receptors mediates direct sensitization of IP3 receptors by cAMP. J. Cell Biol. 183, 297–311 10.1083/jcb.200803172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z., Cai T., Tian J., Ivanov A. V., Giovannucci D. R., Xie Z. (2005). Na/K-ATPase tethers phospholipase C and IP3 receptor into a calcium-regulatory complex. Mol. Biol. Cell 16, 4034–4045 10.1091/mbc.E05-04-0295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. B., Buxton I. L. (1991). Muscarinic receptors in canine colonic circular smooth muscle. II. Signal transduction pathways. Mol. Pharmacol. 40, 952–959 [PubMed] [Google Scholar]
- Zhang L. B., Horowitz B., Buxton I. L. (1991). Muscarinic receptors in canine colonic circular smooth muscle. I. Coexistence of M2 and M3 subtypes. Mol. Pharmacol. 40, 943–951 [PubMed] [Google Scholar]