Summary
Macrophages migrate to sites of insult during normal inflammatory responses. Integrins guide such migration, but the transmission of signals from integrins into the requisite cytoskeletal changes is poorly understood. We have discovered that the hematopoietic adaptor protein Skap2 is necessary for macrophage migration, chemotaxis, global actin reorganization and local actin reorganization upon integrin engagement. Binding of phosphatidylinositol [3,4,5]-triphosphate to the Skap2 pleckstrin-homology (PH) domain, which relieves its conformational auto-inhibition, is critical for this integrin-driven cytoskeletal response. Skap2 enables integrin-induced tyrosyl phosphorylation of Src-family kinases (SFKs), Adap, and Sirpα, establishing their roles as signaling partners in this process. Furthermore, macrophages lacking functional Sirpα unexpectedly have impaired local integrin-induced responses identical to those of Skap2−/− macrophages, and Skap2 requires Sirpα for its recruitment to engaged integrins and for coordinating downstream actin rearrangement. By revealing the positive-regulatory role of Sirpα in a Skap2-mediated mechanism connecting integrin engagement with cytoskeletal rearrangement, these data demonstrate that Sirpα is not exclusively immunoinhibitory, and illuminate previously unexplained observations implicating Skap2 and Sirpα in mouse models of inflammatory disease.
Key words: Cytoskeleton, Inflammation, Integrin, Macrophage, Migration
Introduction
To participate in inflammatory responses, macrophages and their precursors must migrate to perceived sites of infection or insult (Geissmann et al., 2010; Hume, 2006). These cells rely on cues from the local environment delivered through integrins, receptors critical for cell-matrix and cell-cell interactions (Berton and Lowell, 1999; Hynes, 2002). Inflammatory stimuli can induce “inside-out” signals that control integrin cell surface expression and affinity for extracellular ligands. In turn, upon ligand binding, integrins initiate “outside-in” signals that control multiple downstream pathways required for cell adhesion, migration, and chemotaxis, all key components of innate and adaptive immune responses (Berton and Lowell, 1999; Gahmberg et al., 1998). These integrin-mediated responses also depend on the macrophage's ability to remodel its cytoskeleton, thereby allowing changes in cell shape, motility, and directionality (Vicente-Manzanares and Sánchez-Madrid, 2004; Worthylake and Burridge, 2001). Despite their central role in macrophage function, the mechanisms by which integrin signals lead to cytoskeletal reorganization remain unclear.
In leukocytes, adaptor proteins participate in integrin-driven signaling. One such protein, Src kinase-associated phosphoprotein 2 (Skap2, a.k.a. Skap-hom), interacts with Adhesion and Degranulation-Promoting Adapter Protein (Adap, a.k.a. Fyb), an established integrin-responsive protein (Kasirer-Friede et al., 2007). Moreover, integrin engagement induces tyrosyl phosphorylation of Skap2 and Adap (Timms et al., 1999). The structurally similar Skap1 is expressed specifically in T cells and also binds Adap. However, Skap2 is expressed broadly in lymphoid and myeloid cells (Kouroku et al., 1998; Liu et al., 1998; Marie-Cardine et al., 1998) and cannot substitute for Skap1 in regulating T cell inside-out signals (Griffiths et al., 2001; Jo et al., 2005; Moog-Lutz et al., 2001), suggesting that Skap2 may perform a distinct role during integrin signaling. Studies have suggested an adhesive/migratory role for Skap2 in B cells and dendritic cells, but have provided no mechanism or relationship to integrin engagement or cytoskeleton modulation (Reinhold et al., 2009; Togni et al., 2005). Indeed, how Skap2 functions at the molecular level is not well understood in any biological system.
Skap2 also binds the transmembrane protein Signal Regulatory Protein-α (Sirpα, a.k.a. SHPS-1), which is generally regarded as an immune inhibitory receptor (Barclay, 2009; Kharitonenkov et al., 1997; Veillette et al., 1998). Like Skap2, Sirpα is tyrosyl phosphorylated in response to integrin engagement in macrophages (Inagaki et al., 2000; Johansen and Brown, 2007; Motegi et al., 2003; Timms et al., 1999). Importantly, Skap2- and Sirpα-deficient mice are resistant to autoimmune processes, such as experimental autoimmune encephalomyelitis (EAE) and collagen-induced arthritis, which serve as models for human disease (multiple sclerosis and rheumatoid arthritis) that have integrin-dependent components (Okuzawa et al., 2008; Ransohoff, 2007; Togni et al., 2005; Tomizawa et al., 2007). Indeed integrins have emerged as important therapeutic targets in multiple sclerosis, rheumatoid arthritis, and other autoimmune diseases, such as inflammatory bowel disease (Parikh et al., 2012; Peters et al., 2011; Ransohoff, 2007; Rutgeerts et al., 2009). Thus, determining whether Skap2 and Sirpα regulate macrophage responses to integrin signals could significantly enhance our mechanistic understanding of inflammatory responses and diseases, thereby facilitating the identification of new therapeutic targets. Here, we find that Skap2 and Sirpα drive the transduction of integrin-evoked signals that lead to the cytoskeletal rearrangement required for macrophage migration.
Results
Skap2 is required for macrophage migration, chemotaxis, and spreading
To determine whether Skap2 is required for migration, we tested the ability of bone marrow-derived macrophages (BMMs) from Skap2−/− mice to migrate into scratches introduced into densely populated cultures. Skap2−/− BMMs exhibited a pronounced migration defect in this assay (Fig. 1A). Likewise, these cells showed decreased migratory responses driven by M-CSF or the chemokines CCL2 and CXCL4 in transwell assays (Fig. 1B–D). Skap2−/− BMMs also spread poorly on glass, which binds BMMs predominantly through their β2 integrins (Brown, 1991; Yakubenko et al., 2002), and impaired BMM spreading was accompanied by reduced F-actin content as measured by phalloidin staining (Fig. 1E,F). Together, these results suggest a mechanism in which Skap2 is necessary for eliciting the cytoskeletal changes that drive macrophage motility.
Fig. 1.
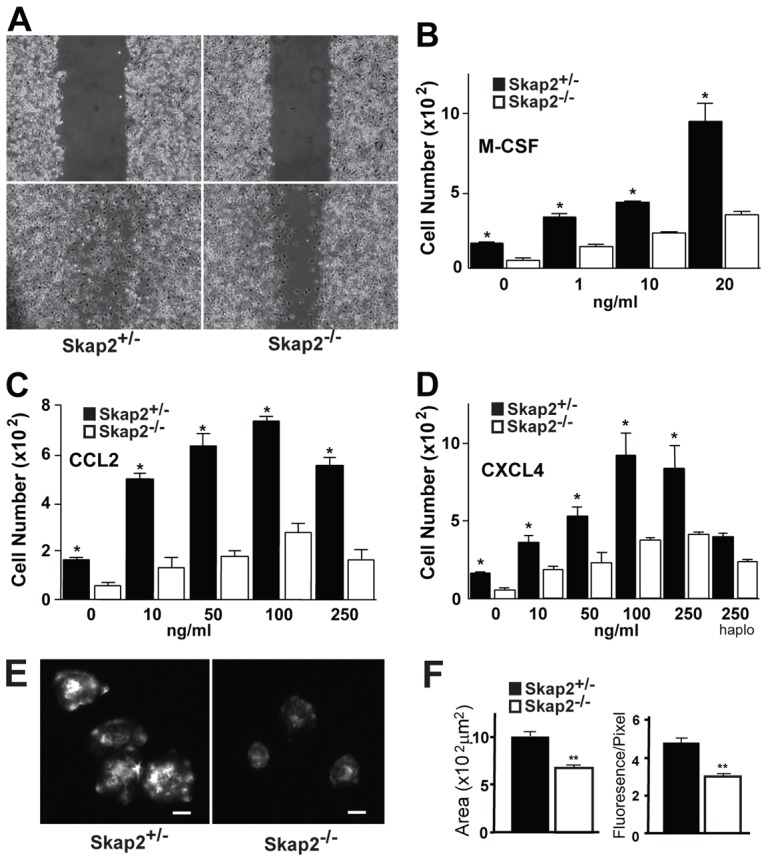
Skap2-deficient BMMs exhibit defective migration, spreading, and actin polymerization. (A) Scratches were introduced into confluent cultures of Skap2+/− or Skap2−/− BMMs (upper panels). After 8 hours (lower panels), migration across the scratch wounds was impaired in cells lacking Skap2. (B–D) Chemotaxis of Skap2+/− and Skap2−/− BMMs in response to cytokine M-CSF (B) and chemokines CCL2 (C) and CXCL4 (D) in transwell migration assays. In D, “haplo” denotes haplotaxis conditions where equal concentrations of CXCL4 are in both chambers. For B–D, data are presented as mean ± S.E.M., n = 3, *P<0.01 compared to Skap2−/− under the same condition. (E) Skap2+/− or Skap2−/− BMMs were suspended in DMEM for 3 hours, plated for 30 minutes on glass coverslips, then fixed and stained for F-actin with rhodamine-labeled phalloidin. Scale bar: 10 µm. (F) Quantification of plating-induced spreading area from (E) in µm2 (left) and relative fluorescence intensity per pixel for rhodamine-labeled phalloidin (right) for Skap2+/− and Skap2−/− BMMs. Data presented as mean ± S.E.M., n = 30, **P<0.001 compared to Skap2+/− BMMs.
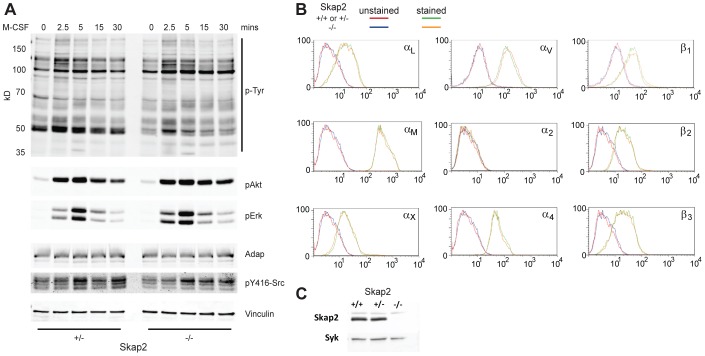
The spreading and migratory defects were observed in the absence of chemokine/cytokine gradients, suggesting that processes downstream of integrin engagement, not cytokine or chemokine detection per se, were affected by the loss of Skap2. Indeed, Skap2−/− BMMs proliferate normally in response to M-CSF and GM-CSF (Togni et al., 2005), and M-CSF-induced tyrosyl phosphorylation was not globally perturbed by Skap2 deficiency (Fig. 2A). Although lysates from M-CSF-stimulated Skap2−/− BMMs showed the absence of a ∼50 kD phosphotyrosyl band that was observed in M-CSF-treated Skap2+/− BMMs, this band primarily corresponded to Skap2 itself (Bourette et al., 2005), as demonstrated by immunodepletion experiments (supplementary material Fig. S1). Skap2−/− BMMs also had normal integrin expression, so their defective migration and spreading were not due to decreased integrin availability (Fig. 2B). Furthermore, flow cytometric analysis with the activation-specific antibody 9EG7 showed similar levels of basal and phorbol 12-myristate 13-acetate (PMA)-evoked β1 integrin activation in WT and Skap2−/− BMMs, suggesting that inside-out integrin activation was not impaired in these cells (supplementary material Fig. S2).
Fig. 2.
Skap2 is not required for M-CSF-induced signaling and does not affect integrin expression in macrophages. (A) Adherent Skap2+/− and Skap2−/− BMMs treated with M-CSF for the indicated times were lysed, electrophoresed, and immunoblotted for phosphotyrosine (p-Tyr) and phosphorylated Akt, Erk2, and Src, with total Adap and Vinculin as loading controls. (B) Flow cytometric analysis was performed on Skap2-replete (WT, except Skap2+/− for αV and β1) or Skap2−/− BMMs using antibodies against the indicated cell surface integrins. (C) Skap2+/+ and Skap2+/− BMMs express equal amounts of Skap2 protein; Syk is a loading control. For all panels, representative results from two independent experiments are shown.
Notably, Skap2+/− BMM responses were identical to those of wild-type (WT, Skap2+/+) BMMs in our assays; this was likely due to the role Adap plays in binding and stabilizing Skap1 and Skap2 against proteolysis (Huang et al., 2005; Kasirer-Friede et al., 2007), thereby limiting Skap2's intracellular expression (Togni et al., 2005), which was equivalent in WT and Skap2+/− BMMs (Fig. 2C). Indeed, immunodepletion of Skap2 from lysates of Skap2+/− cells also resulted in depletion of Adap (Timms et al., 1999; supplementary material Fig. S1), suggesting that the level of protein produced by a single Skap2 allele was able to saturate the available Adap.
Skap2 is required for integrin-dependent actin cytoskeletal rearrangement
Because Skap2 is crucial for macrophage migration, chemotaxis, and spreading, we hypothesized that it is required for integrin-dependent actin cytoskeletal rearrangement. After spreading on glass, Skap2+/− BMMs developed pronounced actin ruffles, or curvilinear accumulations of polymerized F-actin (Fig. 3A). By contrast, Skap2−/− BMMs developed fewer and shorter ruffles (Fig. 3B). In Skap2+/− BMMs, Skap2 colocalized with ruffles preferentially at the leading edges and apical portions of the cells (Fig. 3C), often within punctate structures associated with the edges of ruffles. Trails of subjacent actin were observed to emanate from the ruffles' leading edges (supplementary material Movie 1).
Fig. 3.
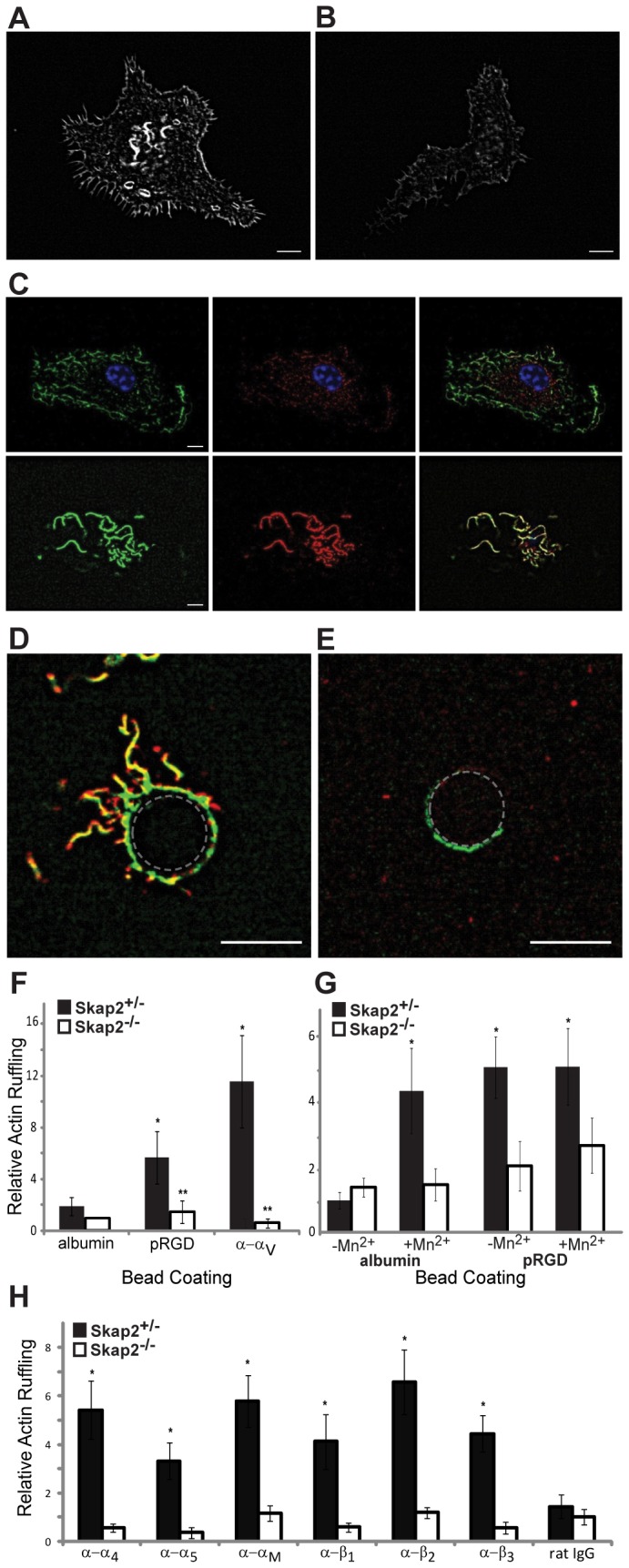
Skap2 is required for integrin-dependent actin cytoskeletal rearrangement. (A) Confocal micrograph of a typical phalloidin-stained Skap2+/− BMM plated on glass, demonstrating curvilinear actin ruffles. (B) Skap2−/− BMMs typically exhibit less actin ruffle formation. (C) At the plane of the nucleus (top panels, with blue nucleus in focus), a Skap2+/− BMM has minimal cortical actin (green) and cytosolic Skap2 (red), without significant colocalization (yellow, in merge); in contrast, at the apical surface (bottom panels), Skap2 colocalizes strongly with cortical actin ruffles. See also supplementary material Movie 1. (D) Upon binding to a Skap2+/− BMM for 20 minutes, a polystyrene bead (denoted by dotted outline) coated with anti-αV integrin antibodies recruits actin ruffles (green) colocalized with Skap2 (red). See also supplementary material Movie 2. (E) This response is markedly reduced in Skap2−/− BMMs. Scale bar: 10 µm. (F) Quantified actin ruffling responses, measured as average pixel intensity in an 8-µm annulus surrounding beads coated with integrin-engaging or control proteins, are shown for BMMs of both genotypes. Data are normalized against the albumin/Skap2−/− condition as Relative Actin Ruffling and presented as mean ± S.E.M., n = 10 per condition, *P<0.05 compared to albumin-coated beads on the same cells, **P<0.01 compared to same beads on Skap2+/− BMMs. (G) Quantified actin ruffling responses, induced by binding to polystyrene beads coated with either albumin or polyRGD, is shown for Skap2+/− and Skap2−/− BMMs with or without treatment with 1 mM Mn2+. Data are normalized against the Mn2+/albumin/Skap2+/− condition and presented as mean ± S.E.M., n = 10 per condition, *P<0.05 compared to albumin-coated beads, no Mn2+, on the same cells. (H) Quantified actin ruffling responses to subtype-specific integrin-engaging beads are shown for BMMs of both genotypes. Data are normalized against the rat IgG/Skap2−/− condition and presented as mean ± S.E.M., n = 10 per condition, *P<0.01 compared to the same beads on Skap2−/− BMMs.
Cell adhesion, migration, and chemotaxis are complex processes that integrate changes in integrin modulation and engagement, cytoskeletal reorganization, cell polarization, and cell shape through interconnected signaling pathways (Berzat and Hall, 2010; Chen et al., 2003; Jones, 2000). In the face of such complexity, it is advantageous to study integrin-mediated events through spatially and temporally focused approaches in model systems that isolate and control receptor engagement in order to measure local, early responses. Therefore, we employed a bead assay that incorporated integrin ligands and monoclonal antibodies to examine further the role of Skap2 in integrin-mediated cytoskeletal rearrangement. Polystyrene beads were coated with polyRGD (pRGD) (Alenghat et al., 2009; Miyamoto et al., 1995), which binds to a broad range of integrins, including β1, β2, and β3 (Plow et al., 2000). Skap2+/− and Skap2−/− BMMs bound these beads similarly, consistent with their equivalent levels of surface integrin expression (Fig. 2B). However, whereas pRGD beads evoked pronounced actin ruffling in Skap2+/− BMMs (Fig. 3D), Skap2−/− BMMs generated markedly less ruffling in proximity to the beads (Fig. 3E,F). This cytoskeletal response occurred within 20 minutes of integrin engagement, and beads coated with a control protein (albumin) failed to elicit ruffling in BMMs of either genotype (Fig. 3F). Finally, a monoclonal antibody directed against the extracellular portion of αV integrin, which, by clustering integrins, evokes signaling, yielded similar results (Fig. 3F). As with the ruffles seen in plated cells, Skap2 was concentrated at the leading edges of these integrin-induced actin structures (supplementary material Movie 2).
Manganese (Mn2+), which activates integrins non-selectively (Dransfield et al., 1992), enhanced the cytoskeletal response to control beads in Skap2-replete cells, but had no detectable additional effect on local integrin-mediated ruffling in response to pRGD-coated beads in either Skap2+/− or Skap2−/− cells (Fig. 3G). Moreover, the requirement for Skap2 for integrin-induced actin ruffling was generalizable across several other α and β integrins found on macrophages (Fig. 3H). From these studies, we concluded that Skap2 plays a general and essential role in integrin-mediated actin rearrangements in BMMs.
Skap2 structural domains determine integrin-induced signaling
Structural and functional studies have shown that unstimulated Skap2 exists in an auto-inhibited state, wherein the pleckstrin-homology (PH) domain binds to a four-helix bundle created by the interaction of N-terminal dimerization domains (DM) of Skap2 monomers, and that this auto-inhibitory interaction is relieved upon phosphatidylinositol [3,4,5]-triphosphate (PIP3) binding (Swanson et al., 2008). To probe the role of specific Skap2 domains in regulating integrin-mediated cytoskeletal responses, we reconstituted Skap2−/− BMMs with Skap2 mutants that either disrupt the auto-inhibitory DM/PH domain interaction (D129K) or impair PIP3 binding (R140M) (Swanson et al., 2008). WT Skap2 rescued the deficient actin ruffling of Skap2−/− BMMs (Fig. 4A). However, expression of the R140M mutant in Skap2−/− BMMs failed to rescue integrin-induced ruffling (Fig. 4A). Furthermore, expression of this mutant, which dimerizes with normal Skap2 in Skap2-replete BMMs (Swanson et al., 2008), completely prevented the normal response in a dominant negative fashion (Fig. 4B). Preventing PIP3 production with the PI3K inhibitor GSK2126458 (Leung et al., 2011) also ablated the ruffling response (supplementary material Fig. S3A). By contrast, superimposing the D129K mutation on R140M (D129K/R140M) led to hyperactive actin polymerization beyond the level generated by WT Skap2 (Fig. 4A), and even generated a significant response to control beads (likely through non-specific integrin interactions with polystyrene (Brown, 1991; Yakubenko et al., 2002). This hyperactive response occurred despite the inability of this mutant to bind PIP3 (as a consequence of the R140M mutation), indicating that the auto-inhibitory DM/PH domain interaction is a critical regulator of Skap2 action. Consistent with this conclusion, mutation of D129K alone also evoked hyperactive actin polymerization (supplementary material Fig. S3B). These data indicated that relief of auto-inhibition via PIP3 binding to the Skap2 PH domain is critical for Skap2 function in integrin-mediated cytoskeletal reorganization in BMMs; they further showed that the normal auto-inhibitory interaction – between the Skap2 PH and DM domains – is required for regulating the integrin-driven actin response in these cells.
Fig. 4.
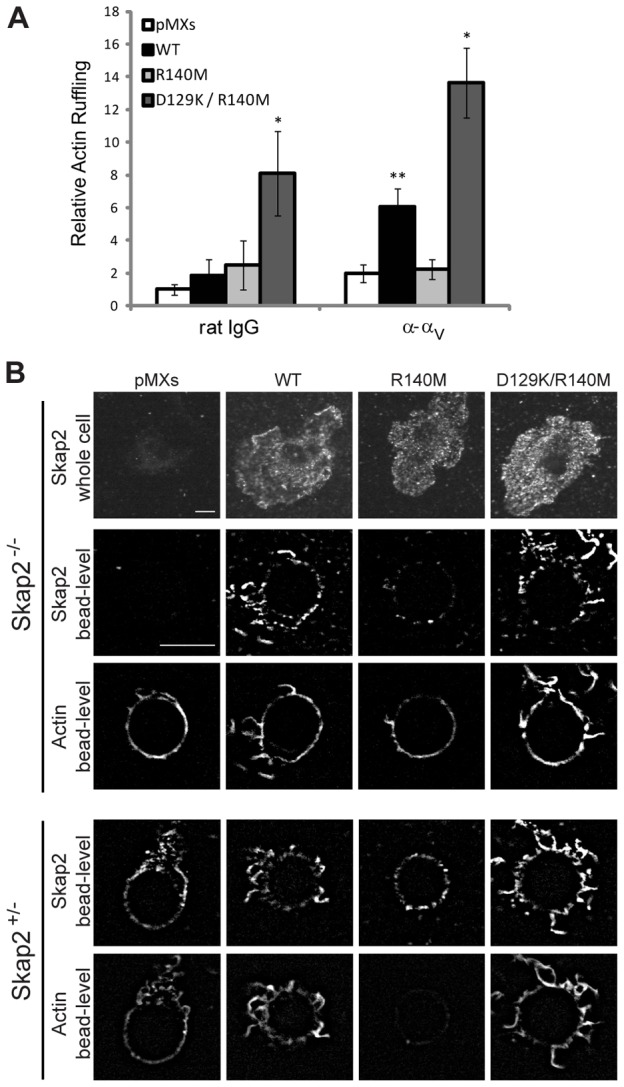
Integrin-induced cytoskeletal reorganization requires a functional Skap2 PH domain. (A) Actin ruffling induced by beads coated with rat IgG (control) or anti-αV bound to Skap2−/− BMMs infected with empty vector (pMXs), WT Skap2 (WT), or Skap2 mutants with an R140M substitution or with combined D129K and R140M substitutions. Data are presented as mean ± S.E.M., n = 10 per condition, *P<0.01 compared to pMXs, **P<0.05 compared to pMXs. (B) Skap2−/− and Skap2+/− BMMs infected with empty vector (pMXs), WT Skap2 (WT), or Skap2 mutants with an R140M substitution or with combined D129K and R140M substitutions were bound to polystyrene beads coated with antibodies directed against αV integrin, and stained for actin (phalloidin) and for Skap2. n = 10 per condition. Scale bar: 10 µm.
Skap2 directs a subset of integrin-induced Sirpα-associated signaling events
The observed defects in integrin mobility, cytoskeletal rearrangement, and migration in Skap2−/− BMMs led us to investigate the effect of Skap2 deficiency on integrin-stimulated signaling. BMMs were plated onto fibrinogen-coated surfaces in order to trigger integrin (primarily β2 and β3) activation. Plating-induced protein tyrosyl phosphorylation was globally decreased in the absence of Skap2, consistent with a defect in an early event in integrin signal transduction (Fig. 5A). Indeed, activation of SFK was abrogated in the absence of Skap2 (Fig. 5A). Although there was a mild decrement in the residual low level of SFK activation seen in suspended Skap2−/− cells compared with control cells, this did not appear to affect the basal integrin activation state (supplementary material Fig. S2). Notably, Mn2+ restored global integrin-evoked tyrosyl phosphorylation as well as SFK activation in Skap2−/− BMMs (Fig. 5A); this is significant because Mn2+ was unable to restore integrin-induced actin ruffling in these cells (Fig. 3G). Therefore, the Mn2+-induced tyrosyl-phosphorylated proteins in Skap2-deficient cells likely represent participants in pathways distinct from those leading to the Skap2-dependent actin ruffling response. Likewise, whereas basal Syk phosphorylation was mildly increased in Skap2−/− cells, plating-induced tyrosyl phosphorylation of Syk, an established integrin effector required for cytoskeletal rearrangement (Vines et al., 2001), was not affected significantly by the absence of Skap2, and, like SFK activity, was amplified by Mn2+ treatment in both Skap2+/− and Skap2−/− cells (Fig. 5B). These results demonstrate that, while activated integrins can stimulate SFKs and Syk, even forced activation of these proteins with Mn2+ was insufficient to drive actin rearrangement in the absence of Skap2.
Fig. 5.
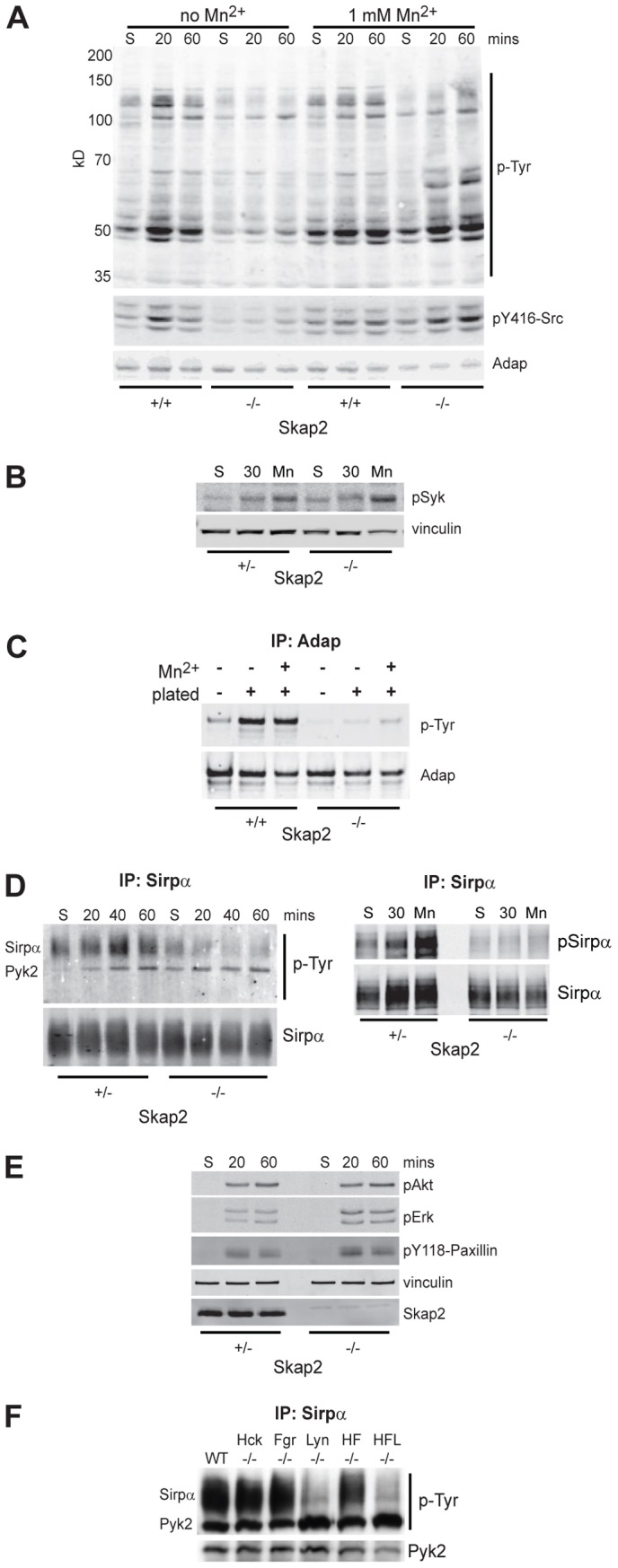
Skap2 is required for specific integrin-induced signaling events. (A) Skap2+/+ and Skap2−/− BMMs in suspension (S) or plated on fibrinogen for 20 or 60 minutes, either in the absence (left six lanes) or presence (right six lanes) of 1 mM Mn2+, were lysed, electrophoresed and immunoblotted for phosphotyrosine (p-Tyr; upper panel), Src pY416 (middle panel), and Adap as a loading control (lower panel). (B) Skap2+/− and Skap2−/− BMMs were kept in suspension or plated on fibrinogen for 30 minutes in the absence or presence of Mn2+, lysed, and immunoblotted for pSyk and vinculin as a loading control. Baseline quantified pSyk/vinculin is ∼2-fold higher in Skap2−/− cells. Plating increases levels ∼2-fold and Mn2+ increases levels ∼5-fold in both genotypes. (C) Skap2+/+ and Skap2−/− BMMs were kept in suspension or plated on fibrinogen for 30 minutes in the absence or presence of Mn2+, lysed, immunoprecipitated for Adap, and immunoblotted for phosphotyrosine (pTyr; upper panel) and Adap (lower panel). Baseline quantified pTyr/Adap is ∼5-fold higher in Skap2+/− cells. Plating increases levels ∼5-fold in Skap2+/− and ∼1.2-fold in Skap2−/− cells. Mn2+ increases levels ∼6-fold in Skap2+/− cells and 3-fold in Skap2−/− cells. (D) Sirpα was immunoprecipitated from lysates of BMMs in suspension (S) or after plating for the indicated times on fibrinogen, and phosphotyrosine (p-Tyr) was analyzed by immunoblotting (left panels), with prominent bands corresponding to Sirpα and Pyk2. Similar lysates of suspended, plated, or 1 mM Mn2+-treated BMMs (right panels) were probed for Sirpα phosphorylation. (E) Akt, Erk, and Paxillin phosphorylation were probed in Skap2+/− and Skap2−/− BMMs in suspension (S) or after plating for the indicated times on fibrinogen. (F) BMMs from Hck−/−, Fgr−/−, and Lyn−/−, as well as Hck/Fgr double knockout (HF−/−) and Hck/Fgr/Lyn triple knockout (HFL−/−) mice, were lysed after plating on fibrinogen, subjected to immunoprecipitation for Sirpα, and immunoblotted for p-Tyr and Pyk2. All panels are representative of at least three independent experiments.
Adap, a known binding partner of Skap2, failed to become tyrosyl phosphorylated in response to integrin engagement in Skap2−/− BMMs; moreover, in contrast to the SFK and Syk results, Mn2+-induced Adap phosphorylation was minimal and failed to reach the levels of Adap phosphorylation found in control cells (Fig. 5C). Therefore, although Skap2 participates in inside-out integrin activation of SFKs, activation of a specific Skap2 signaling partner (Adap), along with downstream actin remodelling, is driven primarily through Skap2-mediated outside-in signaling. Similar results were obtained when BMMs were plated on fibronectin (not shown), which primarily engages β1 and β3 integrins, again indicating that Skap2 propagates signals downstream of multiple integrins.
We have shown previously that the transmembrane receptor Sirpα forms complexes with Skap2 and Pyk2 independently of Sirpα tyrosyl phosphorylation and Shp1/2 binding (Timms et al., 1999). Furthermore, inhibition of Sirpα function can lead to defects in cell migration (Inagaki et al., 2000; Motegi et al., 2003). Whereas both Sirpα and Sirpα-associated Pyk2 became tyrosyl phosphorylated in Skap2+/− BMMs plated on integrin ligands, plating-induced phosphorylation of Sirpα was minimal in Skap2−/− BMMs (Fig. 5D). By contrast, activation of Pyk2, another binding partner of Sirpα (Timms et al., 1999), was not affected by Skap2 deficiency. As with Adap, Sirpα phosphorylation, which occurs within the immunoreceptor tyrosine-based inhibitory motifs (ITIMs), was not salvaged by Mn2+ treatment of Skap2−/− cells (Fig. 5D), suggesting that this event, but not Pyk2 activation, is part of a Skap2-dependent outside-in pathway. This model is consistent with the finding that Skap2 and Pyk2 associate with separate pools of Sirpα (Timms et al., 1999), and with our observation that plating-induced activation of Akt, Erk, and Paxillin (all Pyk2 effectors) was not altered in Skap2−/− BMMs (Fig. 5E) (Blaukat et al., 1999; Williams and Ridley, 2000). Interestingly, Lyn−/− BMMs showed a similar defect in Sirpα phosphorylation with normal Pyk2 phosphorylation upon plating-induced integrin engagement (Fig. 5F; supplementary material Fig. S4), implicating Lyn as the primary SFK involved downstream of Skap2 in pathways leading to Sirpα phosphorylation (Hibbs et al., 2002).
Sirpα also is necessary for the Skap2-dependent cytoskeletal response
Skap2 binds to Sirpα and directs its integrin-dependent phosphorylation, but does not control other aspects of integrin-dependent Sirpα signaling (see above). These results led us to further probe Sirpα's role in integrin-induced cytoskeletal rearrangement. As in Skap2−/− cells, BMMs from mice homozygous for a Sirpα mutant lacking its cytoplasmic tail [SirpαΔ/Δ (Inagaki et al., 2000)] exhibited deficient actin ruffling in response to integrin ligation by beads coated with either pRGD or α–αV (Fig. 6A). Like Skap2+/− BMMs, Sirpα+/Δ BMM responses were similar to that seen for WT cells. Furthermore, just as in Skap2-deficient cells, the response to integrin ligation was not augmented significantly by Mn2+ in SirpαΔ/Δ BMMs (Fig. 6B). The normal response of Sirpα+/Δ cells suggested that the mutant Sirpα fragment did not interfere with the function of endogenous wild-type Sirpα. To test this further, we identified two shRNA sequences that depleted Sirpα expression to ∼25% of wild-type levels, as demonstrated by immunofluorescence microscopy and by immunoblotting (Fig. 6C). Importantly, cytoskeletal rearrangements in response to bead-based integrin engagement exhibited similar impairment to that seen in the SirpαΔ/Δ BMMs (Fig. 6D). These results show that the Sirpα mutation behaves as a null allele for these integrin-dependent functions, and, along with the Skap2-dependent phosphorylation of Sirpα (Fig. 5D), support a cooperative mechanism between Skap2 and Sirpα in driving the actin response.
Fig. 6.
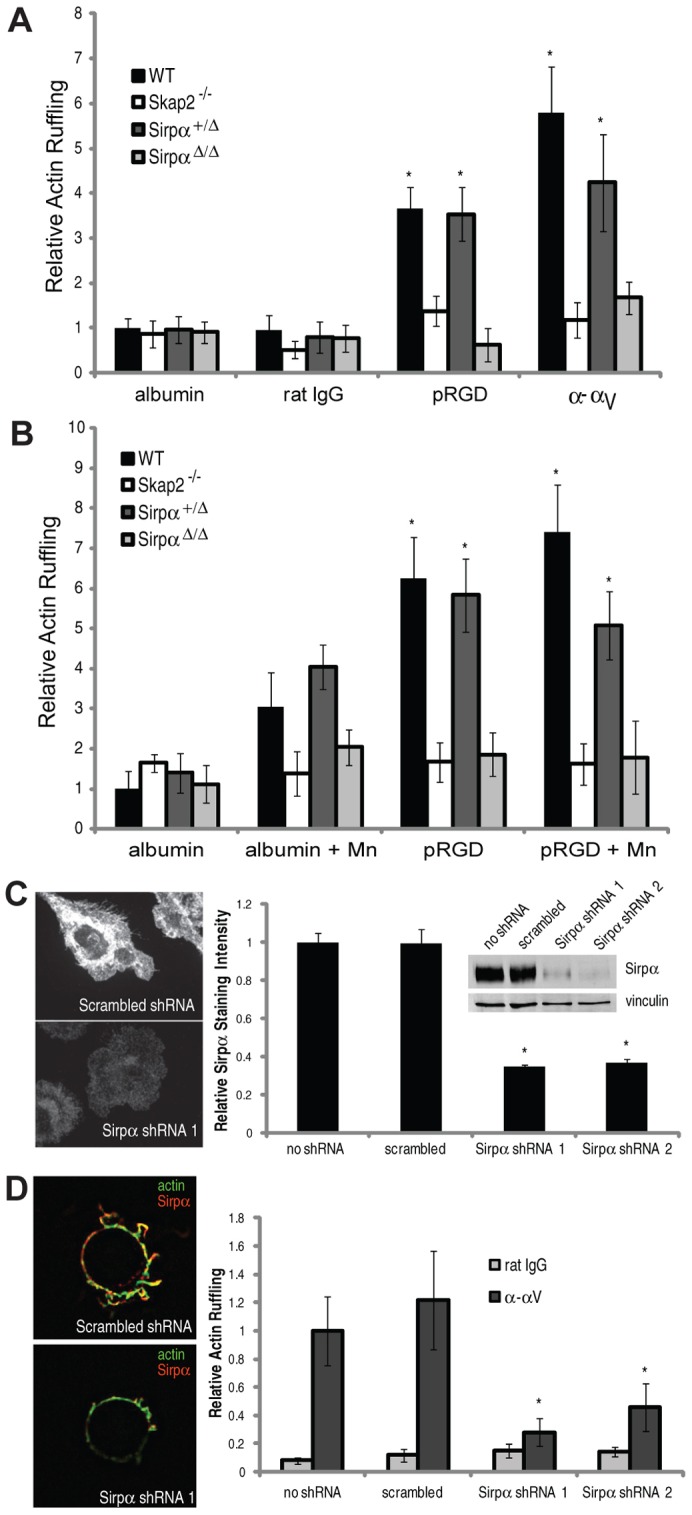
Integrin-induced cytoskeletal reorganization is also Sirpα-dependent. (A) Actin ruffling induced by beads coated with albumin, rat polyclonal IgG, pRGD, or anti-αV bound to WT, Skap2−/−, Sirpα+/Δ, and SirpαΔ/Δ BMMs. (B) Actin ruffling induced by beads coated with albumin or pRGD with or without 1 mM Mn2+. For A and B, data are presented as mean ± S.E.M., n = 10 per condition, *P<0.01 compared to either homozygous mutant cell under same conditions. (C) Immunofluorescence staining for Sirpα in representative WT BMMs treated with scrambled versus Sirpα shRNA, along with quantified relative staining intensity in untreated cells and cells treated with scrambled shRNA versus the two different Sirpα shRNA. Inset shows western analysis of cell lysates of the same cell populations, blotted for Sirpα. (D) Immunofluorescence staining for actin and Sirpα in response to αV-directed beads on WT BMMs treated with indicated shRNA, along with quantified actin ruffling induced by these beads. Data are presented as mean ± S.E.M., n = 10 per condition, *P<0.05 compared to scrambled shRNA and untreated conditions.
To test this possibility further, we quantified the recruitment of these signaling proteins to local sites of integrin ligation. Skap2 recruitment to integrin-directed beads was impaired in SirpαΔ/Δ cells; by contrast, Sirpα recruitment to engaged integrins was not significantly reduced in Skap2−/− BMMs (Fig. 7A). Indeed, in these cells, the Sirpα staining pattern showed small accumulations that were deficient in actin (Fig. 7A). As expected, Adap, which binds stoichiometrically to Skap2, was not recruited to sites of integrin engagement in Skap2−/− BMMs (Fig. 7A). To further test the relationship between Sirpα and Skap2, we determined whether the constitutively active D129K/R140M variant of Skap2 could bypass the requirement for Sirpα to rescue the phenotype rendered by Sirpα deficiency. Unlike Skap2-deficient or Skap2-replete BMMs, SirpαΔ/Δ BMMs expressing D129K/R140M Skap2 could not mount a hyperactive cytoskeletal response to integrin stimulation (Fig. 7B). Together, these data demonstrate that Sirpα is required to recruit the Skap2/Adap complex to sites of integrin engagement and, in turn, that recruited Skap2/Adap directs both Sirpα ITIM phosphorylation and downstream actin polymerization.
Fig. 7.
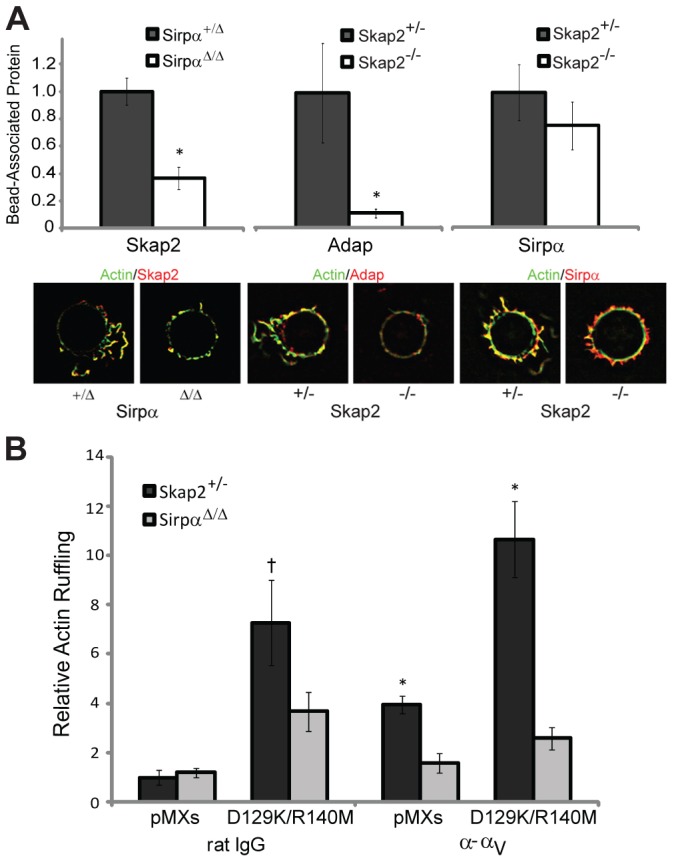
Sirpα and Skap2 cooperate at sites of integrin engagement to drive cytoskeletal rearrangement. (A) Quantified Skap2 recruitment to α–αV beads bound to Sirpα+/Δ and SirpαΔ/Δ BMMs, and Adap and Sirpα recruitment to α–αV beads bound to Skap2+/− and Skap2−/− BMMs. Data are normalized to the respective heterozygous condition and presented as mean ± S.E.M., n = 10–20 per condition, * P<0.05. Representative beads, co-stained for actin, are shown. (B) Actin ruffling induced by beads coated with rat polyclonal IgG or anti-αV, bound to Skap2+/− and SirpαΔ/Δ BMMs infected with empty vector (pMXs) or D129K/R140M Skap2. Data are presented as mean ± S.E.M., n = 10–15 per condition; † P = 0.1 compared to SirpαΔ/Δ and P<0.01 compared to pMXs under the same conditions; * P<0.01 compared to SirpαΔ/Δ under the same conditions.
Discussion
This study uncovers a previously unrecognized signaling mechanism required for integrin-mediated signal transduction leading to cytoskeletal reorganization (Fig. 8). Specifically, we show that Skap2 and Sirpα, the latter previously regarded mainly as an inhibitory receptor, are both required for driving integrin-induced cytoskeletal reorganization in macrophages. Our results further indicate that these proteins act in concert – and in proximity to integrins – to transmit signals necessary for the early steps of a novel pathway required for cell migration. Notably, this pathway utilizes a distinct mechanism from IgG- and complement-mediated phagocytosis, which are not affected by the absence of Skap2 and are hyperactive in the absence of Sirpα (Okazawa et al., 2005; Togni et al., 2005). Whereas SFK activation is defective in plated Skap2−/− cells, forcing integrins into an active conformation (with Mn2+) stimulates both Syk and SFK activation. However, Mn2+ treatment does not restore Adap or Sirpα phosphorylation or integrin-stimulated actin ruffling. Therefore, Syk and SFKs are necessary (Vines et al., 2001), but not sufficient, for integrin-stimulated cytoskeletal rearrangement; their activation via integrin engagement does not obviate the need for Skap2 as a major early mediator downstream of PI3K but upstream of Adap and Sirpα phosphorylation. Skap2 signaling may also be involved in feedback regulation triggered by downstream events including branched actin formation (Coppolino et al., 2001); such a mechanism could be responsible for the Skap2-dependent, plating-induced SFK activation we observed.
Fig. 8.

Skap2 and Sirpα in integrin signaling. A Sirpα/Skap2/Adap signaling module drives actin cytoskeleton reorganization downstream of integrin engagement in macrophages.
Interestingly, Adap is regarded as a driver of inside-out signaling, such as that stimulated by antigen receptor engagement (Wang et al., 2009). However, in our experiments, β1 integrin activation is not dependent on Skap2, the major Adap binding protein. We also find that Mn2+, which promotes the general activation of integrins, thus bypassing inside-out pathways, is unable to drive normal actin cytoskeletal responses in Skap2−/− BMMs. In these experiments, Mn2+ treatment does restore the tyrosyl phosphorylation of selected proteins, consistent with rescue of early integrin functions. Therefore, while we do not exclude the possibility of subtle differences in inside-out signaling in Skap2−/− BMMs not detectable by our assays, defects in inside-out integrin signaling pathways are not likely the cause of defective actin ruffling in these cells. Rather, it is clear that additional, Skap2-dependent processes are required to couple early integrin-evoked phosphorylation events to downstream cytoskeletal rearrangements. We propose a model in which a key outside-in integrin-stimulated signal requires the Sirpα/Skap2/Adap complex for actin remodelling in macrophages.
Skap2 is recruited to the plasma membrane by binding Sirpα through a ‘back-side’ interaction with its C-terminal SH3 domain (Timms et al., 1999). Accordingly, the PH domain is not required to localize Skap2 to the membrane. Instead, interaction between the PH and dimerization domains of Skap2 imposes auto-inhibition that is relieved upon PIP3 binding (Swanson et al., 2008). Consistent with these findings, we report that a Skap2 variant unable to bind PIP3 (R140M) fails to promote integrin-stimulated actin polymerization. Because functional Skap2 homodimerizes, the dominant-negative effect of this variant is likely due to the mutant Skap2 forming hemi-complexes with endogenous Skap2. However, when coupled with a mutation that perturbs the auto-inhibitory binding interface between the PH and dimerization domains, the resulting variant drives hyperactive actin polymerization in response to integrin ligation and in the absence of PIP3 binding. Taken together, these data place Skap2 as an early mediator – just downstream of PI3K but upstream of SFK, Adap and Sirpα – in pathways leading to integrin-driven actin cytoskeletal rearrangement, and also identify the key role of the PH domain in coupling Skap2 activation to phosphoinositide generation.
Prior studies of Skap2, which was discovered as a SFK-associated protein, have provided little mechanistic insight into Skap2 function, aside from its implication in adhesion/migration events (Black et al., 2000; Reinhold et al., 2009; Timms et al., 1999; Togni et al., 2005). The present work provides a mechanistic context in which Skap2 drives integrin-stimulated cytoskeletal rearrangement. As further support for Skap2's role in normal macrophage biology, it is important to note that the Yersinia pestis virulence factor, YopH, targets the Skap2/ADAP complex for dephosphorylation in macrophages as part of its immunosuppressive strategy to interfere with normal adhesion-mediated signaling (Black et al., 2000).
Sirpα is a member of the so-called immune inhibitory receptor family. In addition to binding Skap2, it also binds Shp1/2, and many studies have focused on Shp1/2 interactions with Sirpα's ITIMs to drive negative-regulatory signals (Fujioka et al., 1996). However, we find that both Sirpα and Skap2 are required to transmit signals from ligand-stimulated integrins and to promote local cytoskeletal rearrangement in macrophages. Although there is prior evidence that Sirpα ligation can stimulate nitric oxide pathways (Adams et al., 1998), our finding that Sirpα contributes to integrin outside-in signaling comes as an unexpected and surprising finding, as it demonstrates that Sirpα acts not only as an inhibitory receptor but also as a facilitator of the Skap2-containing pathways downstream of integrin engagement, with significant impact on the cytoskeleton. The findings that Sirpα is recruited to sites of integrin ligation and that a constitutively hyperactive Skap2 mutant cannot salvage actin ruffling in SirpαΔ/Δ BMMs support a mechanism in which Skap2, Adap, and Sirpα cooperate in a single signaling module.
Our results have important implications for understanding the role of Skap2 in inflammatory disorders. For example, Skap2- and Sirpα-deficient mice are resistant to EAE, a mouse model for multiple sclerosis (MS) (Reinhold et al., 2009; Togni et al., 2005; Tomizawa et al., 2007). Both EAE and MS progression depend on antigen-presenting cells such as macrophages, and integrins are an established therapeutic target in MS (Ransohoff, 2007). Additionally, Sirpα-deficient mice are resistant to rheumatoid arthritis, which is partially macrophage-dependent (Okuzawa et al., 2008; Tanaka et al., 2008). We therefore suspect that both Skap2 and Sirpα play important roles in other settings of acute and chronic inflammation, particularly in highly integrin-dependent and macrophage-centric processes such as atherosclerosis and inflammatory bowel disease (Parikh et al., 2012; Rutgeerts et al., 2009; Travis et al., 2007). These roles, along with finer details of downstream signaling promoted by Sirpα and Skap2, are a focus of current and future investigation.
Finally, Sirpα is the receptor for CD47 (Jiang et al., 1999), which constitutes a “don't eat me” signal on both healthy cells and malignant cells evading immune surveillance (Jaiswal et al., 2009; Oldenborg et al., 2001; Takenaka et al., 2007). Although this CD47-driven inhibition has been attributed to the ability of Sirpα to recruit Shp1 to downregulate signals leading to phagocytosis (Oldenborg et al., 2001), our data lead us to speculate that, in addition, CD47 on opposing membranes may interfere with the Sirpα/Skap2 mechanism described here to alter the integrin-mediated cytoskeletal rearrangement necessary for the mechanics of phagocytosis. Therefore, along with the insights the present findings provide on mechanisms of inflammation, our results also could have implications for cancer biology and macrophage-mediated maintenance of homeostasis.
In summary, we have shown that integrin signaling in macrophages involves Sirpα acting via the Skap2/Adap adaptor molecules. Our study significantly changes the current view of integrin outside-in signal transduction in these cells and also provides new insights that could lead to the identification of novel therapeutic targets to regulate both immune surveillance and inflammatory disease.
Materials and Methods
Antibodies and reagents
Anti-pY416 Src, anti-pY118 Paxillin, and anti-pSirpα were obtained from Cell Signaling Technologies. Anti-Pyk2 was purchased from Santa Cruz. Anti-pY352 Syk was purchased from BioSource. 4G10 was a gift from Dr Tom Roberts. Anti-Sirpα and Anti-Adap were purchased from Millipore. Anti-Skap2 was purchased from Proteintech Group. The following integrin antibodies were used in bead assays: RMV7 anti-αV and 9EG7 anti-β1 from BD Biosciences, R1-2 anti-α4, 5H10-27 anti-α5, M1-70 anti-αM, M18/2 anti-β2, and 2C9.G2 anti-β3 from Biolegend. pRGD and rat polyclonal IgG were purchased from Sigma-Aldrich.
Mice, cell culture, and retroviral gene transduction
Skap2−/− mice (Balb/c) were previously described (Togni et al., 2005), and were maintained under pathogen-free conditions and used at 8–12 weeks. BMMs were differentiated ex vivo as described (Tushinski et al., 1982), and analyzed after 7 days in culture. HEK293T/17 cells were maintained in 10% FCS and 10% CO2 at 37°C. Retroviruses were produced by co-transfecting pEcoPAK (Clonetech) and pMXs-Puro constructs bearing the inserts indicated in the figure legends into HEK293T/17 cells, using polyethylenimine (Polysciences) (Godbey et al., 2000; Swanson et al., 2008). For shRNA knockdown experiments, virus was produced in 296FT cells by co-transfection of the shRNA hairpin in Mission pLKO.1, pCMVD8.9 and p-CMV-VSV-G. shRNA vectors (Sigma-Aldrich) targeted either CTGTCTAACTTCATCCCGAGTT (shRNA 1) or TGGTTCAAAGATGGGCAAGAA (shRNA 2). Viruses were harvested 36 hours post-infection and used to infect bone marrow cultures, and cells were then differentiated into macrophages in the presence of 2 µM puromycin. All studies were approved by the Institutional Animal Care and Use Committee of Beth Israel Deaconess Medical Center and Harvard Medical School.
Cell migration assays
For modified Boyden-chamber assays, BMMs were suspended in growth media and 5×104 cells were placed in transwell chambers (Neuro Probe) with 8-µm pore size and the indicated concentrations of chemokine in the bottom chamber. For chemotaxis to M-CSF, cells were suspended in media containing 10% fetal calf serum. After four hours of incubation, cells were fixed in ethanol, the cells on the upper surface were removed, and the remaining cells on the underside of the membrane were stained with crystal violet and counted subsequent to photomicroscopy at 200×. For wound healing assays, BMMs were plated onto Petri dishes at confluence in growth media and then scratch wounds were introduced into the cultures. The resulting wounds were photographed at 200×, then incubated at 37°C for eight hours and re-photographed.
Bead preparation and binding
10-µm diameter carboxylated polystyrene beads (Polysciences) were washed twice in PBS and mixed with 2 µg of antibody against the specific integrin, rat polyclonal IgG, human serum albumin, or polyRGD overnight at 4°C. This mixture was then washed twice with PBS and blocked with 1% albumin in PBS for 30 minutes. Non-confluent cells plated on No. 1.5 glass coverslips were placed on ice, and beads were added (∼10 beads/cell) and allowed to settle onto the cell surface for 10 minutes before returning to 37°C for 20 minutes. For PI3K inhibition, 5 µg/ml GSK2126458 was incubated with cells for 30 minutes prior to bead binding. Cells were washed three times with PBS and then fixed and stained as described below.
Biochemical and FACS analysis
Cells were detached from polystyrene plates by incubation for 30 minutes in Versene (1.5 mM KH2PO4, 8 mM Na2HPO4, 2.7 mM KCl, 120 mM NaCl, 1 mM glucose, 0.8 mM EDTA) at 4°C. Cells were harvested into DMEM (GibcoBRL), split into equivalent pools and kept in suspension for 2 hours with gentle agitation at room temperature. Equivalent numbers of cells were plated onto polystyrene plates coated with 10 µg/ml bovine fibrinogen for the indicated times and then lysed in Nonidet P-40 (NP-40) lysis buffer (2% NP-40, 100 mM Tris-HCl [pH 7.4], 300 mM NaCl, 200 µM pervanadate, protease inhibitor cocktail [final concentrations, 10 µg/ml leupeptin, 1 µg/ml aprotinin, 1 µg/ml pepstatin, 1 µg/ml antipain]). A sample was also kept in suspension and collected by centrifugation and lysed in lysis buffer. Whole cell extracts were prepared by washing cells in phosphate buffered saline (PBS) and lysing them in NP-40 lysis buffer. Lysates were clarified in a microcentrifuge at 4°C for 10 minutes and protein concentrations in the resulting supernatant were determined using a bicinchoninic acid protein assay reagent kit (Pierce) or Bradford reagent (Biorad). Immunoprecipitations were performed by adding the indicated antibodies and protein A-Sepharose beads to lysates and incubating at 4°C for 2 hr. Immune complexes were washed with lysis buffer, resolved by SDS-PAGE, and transferred onto Immobilon-FL membranes (Millipore). Immunoblots were blocked with 5% BSA or 5% milk in TBS with 0.05% Triton X-100 (TBST) for 1 hr, incubated for 1 hr with primary antibodies in TBST, washed three times for 10 min each in TBST, then incubated for 1 hr with IR 680-labeled anti-mouse IgG (Invitrogen) or IR 800-labeled anti-rabbit IgG (Rockland, Gilbertsville, PA) and detected using the Li-Cor Odyssey fluorescence reader (Lincoln, NB). FACS analysis for basal integrin expression was performed with the indicated antibodies, and at least 5000 cells per condition were analyzed using FACSCalibur (BD Biosciences). Integrin activation was measured by incubating suspended cells with antibody for 15 minutes at 37°C in the presence or absence of 1.5 nM PMA (Lenter et al., 1993), washing three times, labeling with anti-rat secondary antibody, and performing FACS analysis.
Microscopy
Cells were fixed in 4% PFA, 25 mM PIPES pH 6.8, 129 mM KCl, 20% sucrose, 5 mM EDTA, and permeabilized in 0.05% Triton X-100 in PBS, pH 7.4. For immunofluorescence, cells were stained with phalloidin (Invitrogen), conjugated to either Alexa488 or Alexa568, and rabbit anti-Skap2 (Upstate-Millipore) in 1×PBS containing 3% BSA. Bound antibodies were visualized using Alexa568-conjugated anti-rabbit IgG (Invitrogen) and observed under oil immersion using a Zeiss Axiovert 200 M microscope with a 63×Plan-Apochromat objective with a numerical aperture of 1.4. Confocal images were obtained with a Yokogawa spinning disk on a Nikon Ti inverted microscope equipped with a 60×Plan Apo NA 1.4 objective lens. Fluorescence was excited by 488 nm and 568 nm lines from a 100 mW Melles Griot argon krypton laser. Z-stacks of images 0.5 µm apart, centred at the bead equator, were acquired with a Hamamatsu ORCA ER cooled CCD camera controlled with MetaMorph 7 software. Images were analyzed using the iterative deconvolution program in the Axiovision 4.5 and Metamorph software packages. Using the raw deconvolved images, actin ruffles were quantified in an 8 µm annulus around the bead (excluding the bead itself) at the z-level with largest bead diameter (equator). Brightness and contrast were adjusted on displayed images (identically for compared image sets) using MetaMorph 7 and Adobe Photoshop software.
Statistical testing of differences was performed using the 2-tailed Student's t-test.
Supplementary Material
Acknowledgments
We thank Lewis C. Cantley for his generous support of K.D.S. Microscopy was performed at the Nikon Imaging Center at Harvard Medical School. We are grateful to Yongmei Hu, Bin Zheng and Gregory Finn for their technical help. F.J.A. and K.D.S. designed research, performed research, analyzed data, and wrote the paper. N.T.R. performed research and analyzed data. Q.J.B. and L.I.P. performed research, analyzed data, and assisted in writing the paper. C.A.L., D.E.G., and B.G.N. helped design research and assisted in writing the paper. There are no conflicts of interest to report.
Footnotes
Funding
This work was supported by an American Heart Association Postdoctoral Fellowship to F.J.A. and by the National Institutes of Health [grant numbers NIH T32 HL07604-24 to F.J.A., NIH F31 GM78720 to Q.J.B., NIH R01 HL032854 to D.E.G., NIH GM041890 to L.C.C., NIH R37 CA49132 and R01 CA114945 to B.G.N., and R56AI085131 to K.D.S.]. B.G.N. is a Canada Research Chair, Tier 1, and is partially supported by the Ontario Ministry of Health and the Princess Margaret Hospital Foundation. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.111260/-/DC1
References
- Adams S., van der Laan L. J., Vernon–Wilson E., Renardel de Lavalette C., Döpp E. A., Dijkstra C. D., Simmons D. L., van den Berg T. K. (1998). Signal-regulatory protein is selectively expressed by myeloid and neuronal cells. J. Immunol. 161, 1853–1859 [PubMed] [Google Scholar]
- Alenghat F. J., Tytell J. D., Thodeti C. K., Derrien A., Ingber D. E. (2009). Mechanical control of cAMP signaling through integrins is mediated by the heterotrimeric Galphas protein. J. Cell. Biochem. 106, 529–538 10.1002/jcb.22001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay A. N. (2009). Signal regulatory protein alpha (SIRPalpha)/CD47 interaction and function. Curr. Opin. Immunol. 21, 47–52 10.1016/j.coi.2009.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton G., Lowell C. A. (1999). Integrin signaling in neutrophils and macrophages. Cell. Signal. 11, 621–635 10.1016/S0898-6568(99)00003-0 [DOI] [PubMed] [Google Scholar]
- Berzat A., Hall A. (2010). Cellular responses to extracellular guidance cues. EMBO J. 29, 2734–2745 10.1038/emboj.2010.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black D. S., Marie–Cardine A., Schraven B., Bliska J. B. (2000). The Yersinia tyrosine phosphatase YopH targets a novel adhesion-regulated signalling complex in macrophages. Cell. Microbiol. 2, 401–414 10.1046/j.1462-5822.2000.00061.x [DOI] [PubMed] [Google Scholar]
- Blaukat A., Ivankovic–Dikic I., Grönroos E., Dolfi F., Tokiwa G., Vuori K., Dikic I. (1999). Adaptor proteins Grb2 and Crk couple Pyk2 with activation of specific mitogen-activated protein kinase cascades. J. Biol. Chem. 274, 14893–14901 10.1074/jbc.274.21.14893 [DOI] [PubMed] [Google Scholar]
- Bourette R. P., Thérier J., Mouchiroud G. (2005). Macrophage colony-stimulating factor receptor induces tyrosine phosphorylation of SKAP55R adaptor and its association with actin. Cell. Signal. 17, 941–949 10.1016/j.cellsig.2004.11.009 [DOI] [PubMed] [Google Scholar]
- Brown E. J. (1991). Complement receptors and phagocytosis. Curr. Opin. Immunol. 3, 76–82 10.1016/0952-7915(91)90081-B [DOI] [PubMed] [Google Scholar]
- Chen C. S., Alonso J. L., Ostuni E., Whitesides G. M., Ingber D. E. (2003). Cell shape provides global control of focal adhesion assembly. Biochem. Biophys. Res. Commun. 307, 355–361 10.1016/S0006-291X(03)01165-3 [DOI] [PubMed] [Google Scholar]
- Coppolino M. G., Krause M., Hagendorff P., Monner D. A., Trimble W., Grinstein S., Wehland J., Sechi A. S. (2001). Evidence for a molecular complex consisting of Fyb/SLAP, SLP-76, Nck, VASP and WASP that links the actin cytoskeleton to Fcgamma receptor signalling during phagocytosis. J. Cell Sci. 114, 4307–4318 [DOI] [PubMed] [Google Scholar]
- Dransfield I., Cabañas C., Craig A., Hogg N. (1992). Divalent cation regulation of the function of the leukocyte integrin LFA-1. J. Cell Biol. 116, 219–226 10.1083/jcb.116.1.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka Y., Matozaki T., Noguchi T., Iwamatsu A., Yamao T., Takahashi N., Tsuda M., Takada T., Kasuga M. (1996). A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol. Cell. Biol. 16, 6887–6899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahmberg C. G., Valmu L., Fagerholm S., Kotovuori P., Ihanus E., Tian L., Pessa–Morikawa T. (1998). Leukocyte integrins and inflammation. Cell. Mol. Life Sci. 54, 549–555 10.1007/s000180050183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F., Manz M. G., Jung S., Sieweke M. H., Merad M., Ley K. (2010). Development of monocytes, macrophages, and dendritic cells. Science 327, 656–661 10.1126/science.1178331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbey W. T., Barry M. A., Saggau P., Wu K. K., Mikos A. G. (2000). Poly(ethylenimine)-mediated transfection: a new paradigm for gene delivery. J. Biomed. Mater. Res. 51, 321–328 [DOI] [PubMed] [Google Scholar]
- Griffiths E. K., Krawczyk C., Kong Y. Y., Raab M., Hyduk S. J., Bouchard D., Chan V. S., Kozieradzki I., Oliveira–Dos–Santos A. J., Wakeham A.et al. (2001). Positive regulation of T cell activation and integrin adhesion by the adapter Fyb/Slap. Science 293, 2260–2263 10.1126/science.1063397 [DOI] [PubMed] [Google Scholar]
- Hibbs M. L., Harder K. W., Armes J., Kountouri N., Quilici C., Casagranda F., Dunn A. R., Tarlinton D. M. (2002). Sustained activation of Lyn tyrosine kinase in vivo leads to autoimmunity. J. Exp. Med. 196, 1593–1604 10.1084/jem.20020515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Norton D. D., Precht P., Martindale J. L., Burkhardt J. K., Wange R. L. (2005). Deficiency of ADAP/Fyb/SLAP-130 destabilizes SKAP55 in Jurkat T cells. J. Biol. Chem. 280, 23576–23583 10.1074/jbc.M413201200 [DOI] [PubMed] [Google Scholar]
- Hume D. A. (2006). The mononuclear phagocyte system. Curr. Opin. Immunol. 18, 49–53 10.1016/j.coi.2005.11.008 [DOI] [PubMed] [Google Scholar]
- Hynes R. O. (2002). Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 10.1016/S0092-8674(02)00971-6 [DOI] [PubMed] [Google Scholar]
- Inagaki K., Yamao T., Noguchi T., Matozaki T., Fukunaga K., Takada T., Hosooka T., Akira S., Kasuga M. (2000). SHPS-1 regulates integrin-mediated cytoskeletal reorganization and cell motility. EMBO J. 19, 6721–6731 10.1093/emboj/19.24.6721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S., Jamieson C. H., Pang W. W., Park C. Y., Chao M. P., Majeti R., Traver D., van Rooijen N., Weissman I. L. (2009). CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 138, 271–285 10.1016/j.cell.2009.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P., Lagenaur C. F., Narayanan V. (1999). Integrin-associated protein is a ligand for the P84 neural adhesion molecule. J. Biol. Chem. 274, 559–562 10.1074/jbc.274.2.559 [DOI] [PubMed] [Google Scholar]
- Jo E. K., Wang H., Rudd C. E. (2005). An essential role for SKAP-55 in LFA-1 clustering on T cells that cannot be substituted by SKAP-55R. J. Exp. Med. 201, 1733–1739 10.1084/jem.20042577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen M. L., Brown E. J. (2007). Dual regulation of SIRPalpha phosphorylation by integrins and CD47. J. Biol. Chem. 282, 24219–24230 10.1074/jbc.M701565200 [DOI] [PubMed] [Google Scholar]
- Jones G. E. (2000). Cellular signaling in macrophage migration and chemotaxis. J. Leukoc. Biol. 68, 593–602 [PubMed] [Google Scholar]
- Kasirer–Friede A., Moran B., Nagrampa–Orje J., Swanson K., Ruggeri Z. M., Schraven B., Neel B. G., Koretzky G., Shattil S. J. (2007). ADAP is required for normal alphaIIbbeta3 activation by VWF/GP Ib-IX-V and other agonists. Blood 109, 1018–1025 10.1182/blood-2006-05-022301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonenkov A., Chen Z., Sures I., Wang H., Schilling J., Ullrich A. (1997). A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature 386, 181–186 10.1038/386181a0 [DOI] [PubMed] [Google Scholar]
- Kouroku Y., Soyama A., Fujita E., Urase K., Tsukahara T., Momoi T. (1998). RA70 is a src kinase-associated protein expressed ubiquitously. Biochem. Biophys. Res. Commun. 252, 738–742 10.1006/bbrc.1998.9637 [DOI] [PubMed] [Google Scholar]
- Lenter M., Uhlig H., Hamann A., Jenö P., Imhof B., Vestweber D. (1993). A monoclonal antibody against an activation epitope on mouse integrin chain beta 1 blocks adhesion of lymphocytes to the endothelial integrin alpha 6 beta 1. Proc. Natl. Acad. Sci. USA 90, 9051–9055 10.1073/pnas.90.19.9051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung E., Kim J. E., Rewcastle G. W., Finlay G. J., Baguley B. C. (2011). Comparison of the effects of the PI3K/mTOR inhibitors NVP-BEZ235 and GSK2126458 on tamoxifen-resistant breast cancer cells. Cancer Biol. Ther. 11, 938–946 10.4161/cbt.11.11.15527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Kang H., Raab M., da Silva A. J., Kraeft S. K., Rudd C. E. (1998). FYB (FYN binding protein) serves as a binding partner for lymphoid protein and FYN kinase substrate SKAP55 and a SKAP55-related protein in T cells. Proc. Natl. Acad. Sci. USA 95, 8779–8784 10.1073/pnas.95.15.8779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie–Cardine A., Hendricks–Taylor L. R., Boerth N. J., Zhao H., Schraven B., Koretzky G. A. (1998). Molecular interaction between the Fyn-associated protein SKAP55 and the SLP-76-associated phosphoprotein SLAP-130. J. Biol. Chem. 273, 25789–25795 10.1074/jbc.273.40.25789 [DOI] [PubMed] [Google Scholar]
- Miyamoto S., Akiyama S. K., Yamada K. M. (1995). Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science 267, 883–885 10.1126/science.7846531 [DOI] [PubMed] [Google Scholar]
- Moog–Lutz C., Peterson E. J., Lutz P. G., Eliason S., Cavé–Riant F., Singer A., Di Gioia Y., Dmowski S., Kamens J., Cayre Y. E.et al. (2001). PRAM-1 is a novel adaptor protein regulated by retinoic acid (RA) and promyelocytic leukemia (PML)-RA receptor alpha in acute promyelocytic leukemia cells. J. Biol. Chem. 276, 22375–22381 10.1074/jbc.M011683200 [DOI] [PubMed] [Google Scholar]
- Motegi S., Okazawa H., Ohnishi H., Sato R., Kaneko Y., Kobayashi H., Tomizawa K., Ito T., Honma N., Bühring H. J.et al. (2003). Role of the CD47-SHPS-1 system in regulation of cell migration. EMBO J. 22, 2634–2644 10.1093/emboj/cdg278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazawa H., Motegi S., Ohyama N., Ohnishi H., Tomizawa T., Kaneko Y., Oldenborg P. A., Ishikawa O., Matozaki T. (2005). Negative regulation of phagocytosis in macrophages by the CD47-SHPS-1 system. J. Immunol. 174, 2004–2011 [DOI] [PubMed] [Google Scholar]
- Okuzawa C., Kaneko Y., Murata Y., Miyake A., Saito Y., Okajo J., Tomizawa T., Kaneko Y., Okazawa H., Ohnishi H.et al. (2008). Resistance to collagen-induced arthritis in SHPS-1 mutant mice. Biochem. Biophys. Res. Commun. 371, 561–566 10.1016/j.bbrc.2008.04.124 [DOI] [PubMed] [Google Scholar]
- Oldenborg P. A., Gresham H. D., Lindberg F. P. (2001). CD47-signal regulatory protein alpha (SIRPalpha) regulates Fcgamma and complement receptor-mediated phagocytosis. J. Exp. Med. 193, 855–862 10.1084/jem.193.7.855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh A., Leach T., Wyant T., Scholz C., Sankoh S., Mould D. R., Ponich T., Fox I., Feagan B. G. (2012). Vedolizumab for the treatment of active ulcerative colitis: a randomized controlled phase 2 dose-ranging study. Inflamm. Bowel Dis. 18, 1470–1479 10.1002/ibd.21896 [DOI] [PubMed] [Google Scholar]
- Peters M. A., Wendholt D., Strietholt S., Frank S., Pundt N., Korb–Pap A., Joosten L. A., van den Berg W. B., Kollias G., Eckes B.et al. (2012). The loss of α2β1 integrin suppresses joint inflammation and cartilage destruction in mouse models of rheumatoid arthritis. Arthritis Rheum. 64, 1359–1368 10.1002/art.33487 [DOI] [PubMed] [Google Scholar]
- Plow E. F., Haas T. A., Zhang L., Loftus J., Smith J. W. (2000). Ligand binding to integrins. J. Biol. Chem. 275, 21785–21788 10.1074/jbc.R000003200 [DOI] [PubMed] [Google Scholar]
- Ransohoff R. M. (2007). Natalizumab for multiple sclerosis. N. Engl. J. Med. 356, 2622–2629 10.1056/NEJMct071462 [DOI] [PubMed] [Google Scholar]
- Reinhold A., Reimann S., Reinhold D., Schraven B., Togni M. (2009). Expression of SKAP-HOM in DCs is required for an optimal immune response in vivo. J. Leukoc. Biol. 86, 61–71 10.1189/jlb.0608344 [DOI] [PubMed] [Google Scholar]
- Rutgeerts P., Vermeire S., Van Assche G. (2009). Biological therapies for inflammatory bowel diseases. Gastroenterology 136, 1182–1197 10.1053/j.gastro.2009.02.001 [DOI] [PubMed] [Google Scholar]
- Swanson K. D., Tang Y., Ceccarelli D. F., Poy F., Sliwa J. P., Neel B. G., Eck M. J. (2008). The Skap-hom dimerization and PH domains comprise a 3′-phosphoinositide-gated molecular switch. Mol. Cell 32, 564–575 10.1016/j.molcel.2008.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka K., Prasolava T. K., Wang J. C., Mortin–Toth S. M., Khalouei S., Gan O. I., Dick J. E., Danska J. S. (2007). Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat. Immunol. 8, 1313–1323 10.1038/ni1527 [DOI] [PubMed] [Google Scholar]
- Tanaka K., Horikawa T., Suzuki S., Kitaura K., Watanabe J., Gotoh A., Shiobara N., Itoh T., Yamane S., Suzuki R.et al. (2008). Inhibition of Src homology 2 domain-containing protein tyrosine phosphatase substrate-1 reduces the severity of collagen-induced arthritis. J. Rheumatol. 35, 2316–2324 10.3899/jrheum.080369 [DOI] [PubMed] [Google Scholar]
- Timms J. F., Swanson K. D., Marie–Cardine A., Raab M., Rudd C. E., Schraven B., Neel B. G. (1999). SHPS-1 is a scaffold for assembling distinct adhesion-regulated multi-protein complexes in macrophages. Curr. Biol. 9, 927–930 10.1016/S0960-9822(99)80401-1 [DOI] [PubMed] [Google Scholar]
- Togni M., Swanson K. D., Reimann S., Kliche S., Pearce A. C., Simeoni L., Reinhold D., Wienands J., Neel B. G., Schraven B.et al. (2005). Regulation of in vitro and in vivo immune functions by the cytosolic adaptor protein SKAP-HOM. Mol. Cell. Biol. 25, 8052–8063 10.1128/MCB.25.18.8052-8063.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa T., Kaneko Y., Kaneko Y., Saito Y., Ohnishi H., Okajo J., Okuzawa C., Ishikawa–Sekigami T., Murata Y., Okazawa H.et al. (2007). Resistance to experimental autoimmune encephalomyelitis and impaired T cell priming by dendritic cells in Src homology 2 domain-containing protein tyrosine phosphatase substrate-1 mutant mice. J. Immunol. 179, 869–877 [DOI] [PubMed] [Google Scholar]
- Travis M. A., Reizis B., Melton A. C., Masteller E., Tang Q., Proctor J. M., Wang Y., Bernstein X., Huang X., Reichardt L. F.et al. (2007). Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature 449, 361–365 10.1038/nature06110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tushinski R. J., Oliver I. T., Guilbert L. J., Tynan P. W., Warner J. R., Stanley E. R. (1982). Survival of mononuclear phagocytes depends on a lineage-specific growth factor that the differentiated cells selectively destroy. Cell 28, 71–81 10.1016/0092-8674(82)90376-2 [DOI] [PubMed] [Google Scholar]
- Veillette A., Thibaudeau E., Latour S. (1998). High expression of inhibitory receptor SHPS-1 and its association with protein-tyrosine phosphatase SHP-1 in macrophages. J. Biol. Chem. 273, 22719–22728 10.1074/jbc.273.35.22719 [DOI] [PubMed] [Google Scholar]
- Vicente–Manzanares M., Sánchez–Madrid F. (2004). Role of the cytoskeleton during leukocyte responses. Nat. Rev. Immunol. 4, 110–122 10.1038/nri1268 [DOI] [PubMed] [Google Scholar]
- Vines C. M., Potter J. W., Xu Y., Geahlen R. L., Costello P. S., Tybulewicz V. L., Lowell C. A., Chang P. W., Gresham H. D., Willman C. L. (2001). Inhibition of beta 2 integrin receptor and Syk kinase signaling in monocytes by the Src family kinase Fgr. Immunity 15, 507–519 10.1016/S1074-7613(01)00221-7 [DOI] [PubMed] [Google Scholar]
- Wang H., Wei B., Bismuth G., Rudd C. E. (2009). SLP-76-ADAP adaptor module regulates LFA-1 mediated costimulation and T cell motility. Proc. Natl. Acad. Sci. USA 106, 12436–12441 10.1073/pnas.0900510106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. M., Ridley A. J. (2000). Lipopolysaccharide induces actin reorganization and tyrosine phosphorylation of Pyk2 and paxillin in monocytes and macrophages. J. Immunol. 164, 2028–2036 [DOI] [PubMed] [Google Scholar]
- Worthylake R. A., Burridge K. (2001). Leukocyte transendothelial migration: orchestrating the underlying molecular machinery. Curr. Opin. Cell Biol. 13, 569–577 10.1016/S0955-0674(00)00253-2 [DOI] [PubMed] [Google Scholar]
- Yakubenko V. P., Lishko V. K., Lam S. C., Ugarova T. P. (2002). A molecular basis for integrin alphaMbeta 2 ligand binding promiscuity. J. Biol. Chem. 277, 48635–48642 10.1074/jbc.M208877200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.