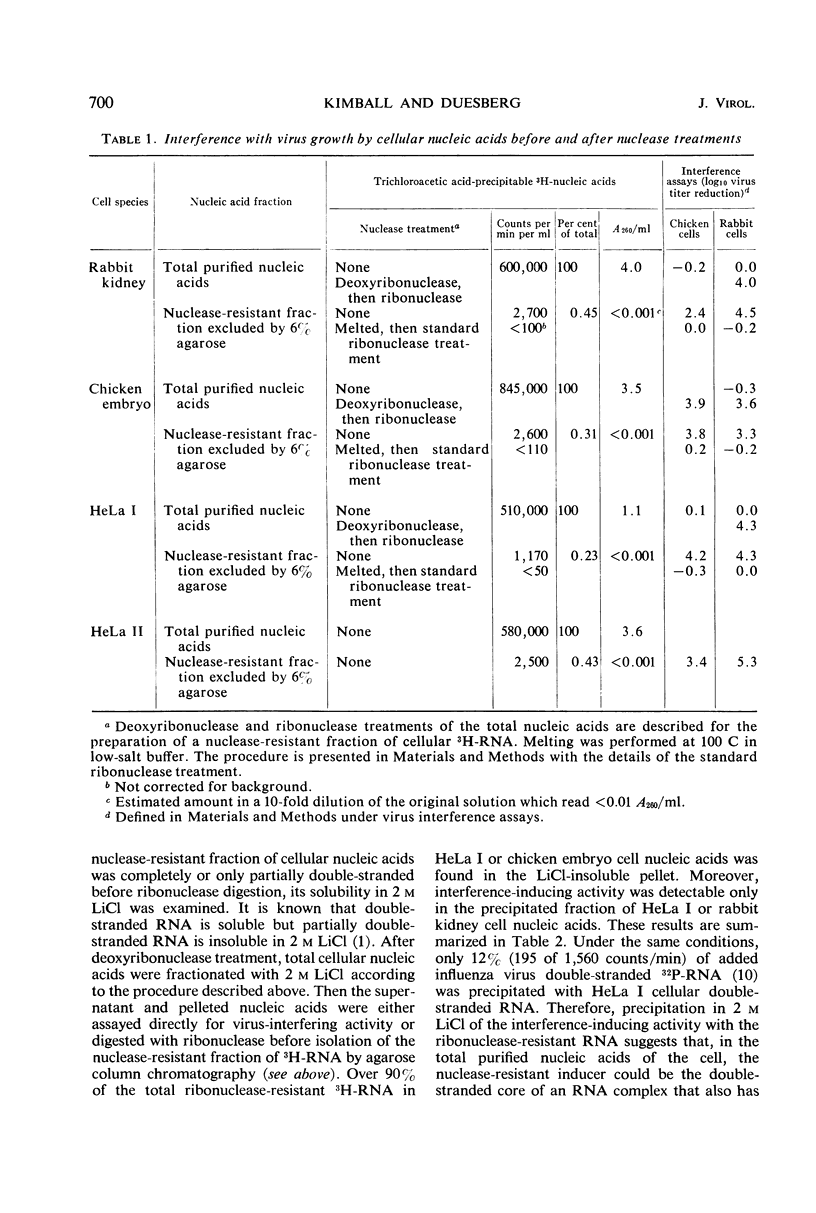
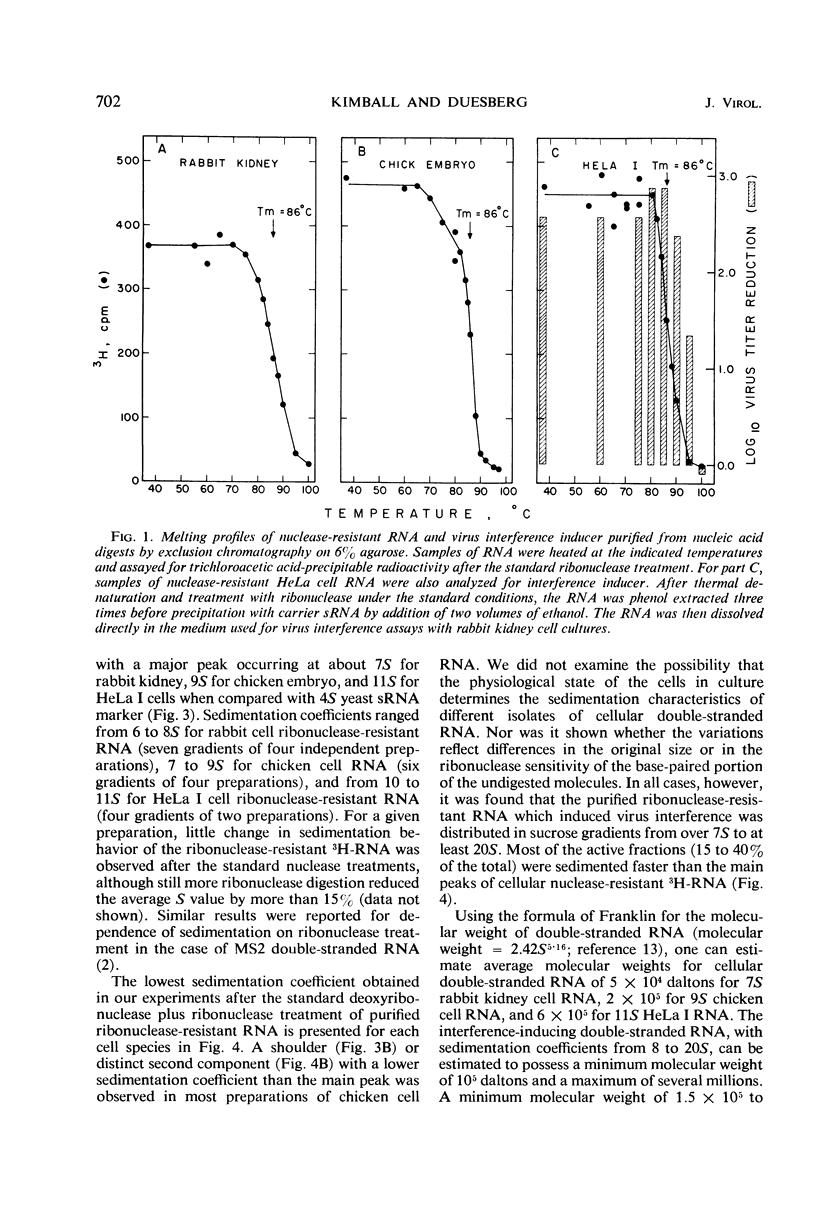
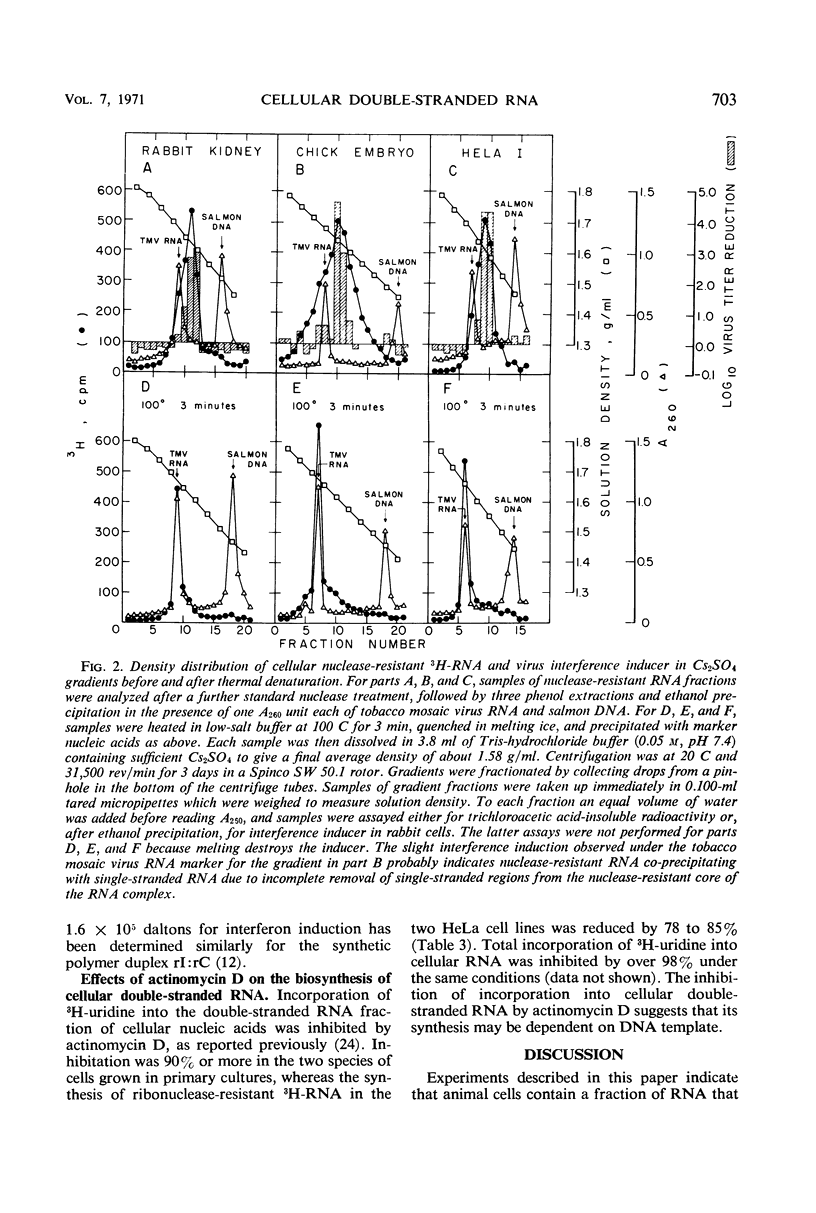
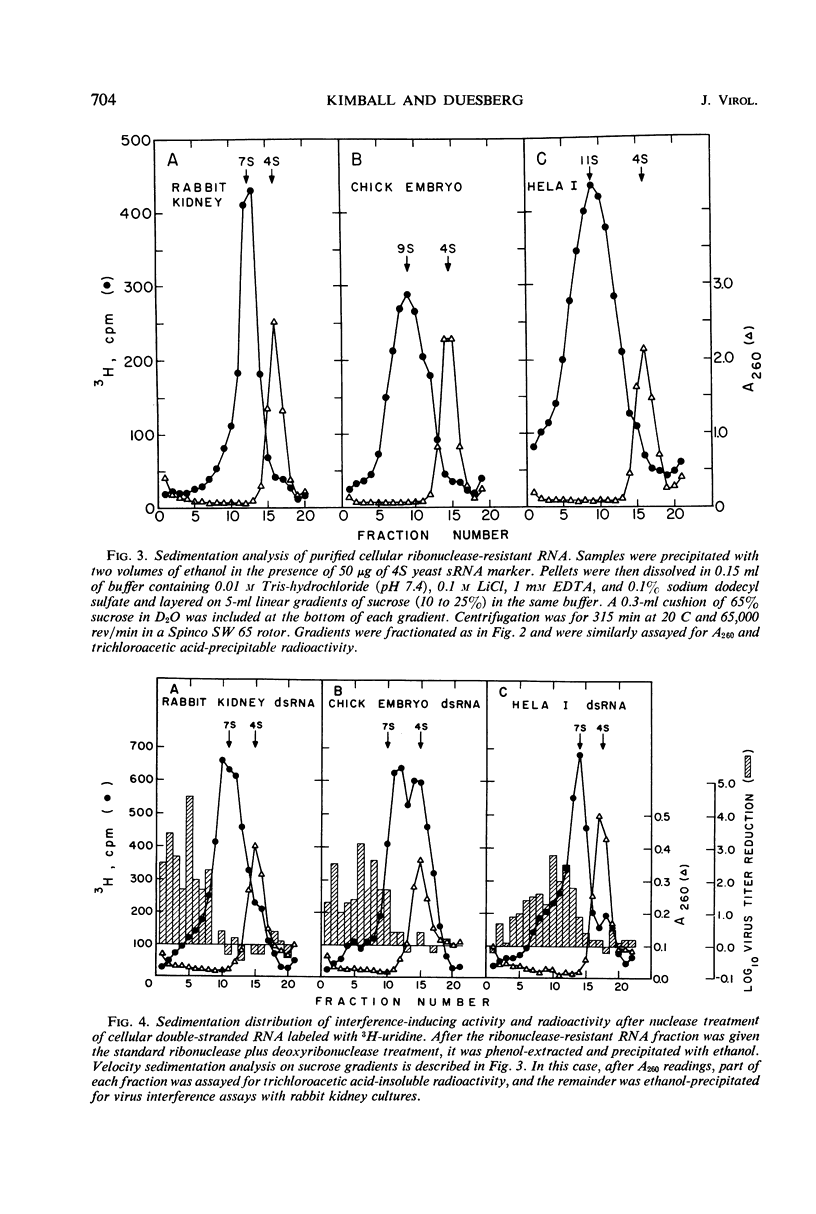
Abstract
Ribonuclease-resistant ribonucleic acid (RNA) was isolated from uridine-labeled cultures of rabbit kidney, chicken embryo, and HeLa cells. This RNA, regardless of its source, was found to induce interference with virus growth in either rabbit kidney or chicken embryo cultures. Nuclease-treated cellular nucleic acids exhibited interference-inducing activity which eluted with a small fraction of RNA in the exclusion volume of a 6% agarose gel column. Besides resistance to ribonucleases, the interference inducer and RNA isolated from partially digested nucleic acids have in common two properties of double-stranded RNA: (i) similar sharp melting profiles were obtained for inducer and ribonuclease-resistant RNA, with Tm dependent on NaCl concentration; (ii) ribonuclease-resistant inducer and RNA banded together in Cs2SO4 density gradients at a density characteristic of known double-stranded RNA. After melting at low ionic strength, the labeled RNA shifted to a higher density and its capacity to inhibit virus replication was lost. Velocity sedimentation analysis of the cellular ribonuclease-resistant RNA indicated that the majority sedimented between 7 and 11S, but only RNA sedimenting at ≧8 to 20S had a high specific activity of interference induction. Without prior ribonuclease treatment, the ribonuclease-resistant RNA can be precipitated with 2 m LiCl and thus appears to exist in purified cellular nucleic acids as part of molecular complexes with both single- and double-stranded regions of RNA. The biosynthesis of cellular double-stranded RNA is inhibited by actinomycin D.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. Purification and properties of poliovirus double-stranded ribonucleic acid. J Mol Biol. 1966 Jul;18(3):421–428. doi: 10.1016/s0022-2836(66)80034-7. [DOI] [PubMed] [Google Scholar]
- Billeter M. A., Weissmann C., Warner R. C. Replication of viral ribonucleic acid. IX. Properties of double-stranded RNA from Escherichia coli infected with bacteriophage MS2. J Mol Biol. 1966 May;17(1):145–173. doi: 10.1016/s0022-2836(66)80101-8. [DOI] [PubMed] [Google Scholar]
- Billiau A., Schome E. Induction of the interferon mechanism by natural RNA. Life Sci. 1970 Jan 22;9(2):69–78. doi: 10.1016/0024-3205(70)90246-8. [DOI] [PubMed] [Google Scholar]
- Colby C., Chamberlin M. J. The specificity of interferon induction in chick embryo cells by helical RNA. Proc Natl Acad Sci U S A. 1969 May;63(1):160–167. doi: 10.1073/pnas.63.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby C., Duesberg P. H. Double-stranded RNA in vaccinia virus infected cells. Nature. 1969 Jun 7;222(5197):940–944. doi: 10.1038/222940a0. [DOI] [PubMed] [Google Scholar]
- De Maeyer E., De Maeyer Guignard J., Montagnier I. Double stranded RNA from rat liver induces interferon in rat cells. Nat New Biol. 1971 Jan 27;229(4):109–110. doi: 10.1038/newbio229109a0. [DOI] [PubMed] [Google Scholar]
- Deusberg P. H., Robinson W. S. On the structure and replication of influenza virus. J Mol Biol. 1967 May 14;25(3):383–405. doi: 10.1016/0022-2836(67)90193-3. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Canaani E. Complementarity between Rous sarcoma virus (RSV) RNA and the in vitro-synthesized DNA of the virus-associated DNA polymerase. Virology. 1970 Nov;42(3):783–788. doi: 10.1016/0042-6822(70)90325-9. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H. Distinct subunits of the ribonucleoprotein of influenza virus. J Mol Biol. 1969 Jun 28;42(3):485–499. doi: 10.1016/0022-2836(69)90237-x. [DOI] [PubMed] [Google Scholar]
- Ebel J. P., Weil J. H., Beck G., Bollack C., Colobert L., Louisot P. Inhibition of the multiplication of Myxovirus and Arbovirus by chemically modified ribonucleic acids from the host cells. Biochem Biophys Res Commun. 1968 Jan 25;30(2):148–152. doi: 10.1016/0006-291x(68)90462-2. [DOI] [PubMed] [Google Scholar]
- Field A. K., Tytell A. A., Lampson G. P., Hilleman M. R. Inducers of interferon and host resistance. II. Multistranded synthetic polynucleotide complexes. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1004–1010. doi: 10.1073/pnas.58.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin R. M. Replication of bacteriophage ribonucleic acid: some physical properties of single-stranded, double-stranded, and branched viral ribonucleic acid. J Virol. 1967 Feb;1(1):64–75. doi: 10.1128/jvi.1.1.64-75.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEIDUSCHEK E. P., MOOHR J. W., WEISS S. B. The secondary structure of complementary RNA. Proc Natl Acad Sci U S A. 1962 Jun 15;48:1078–1086. doi: 10.1073/pnas.48.6.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilleman M. R. Interferon induction and utilization. J Cell Physiol. 1968 Feb;71(1):43–59. doi: 10.1002/jcp.1040710107. [DOI] [PubMed] [Google Scholar]
- ISAACS A., COX R. A., ROTEM Z. Foreign nucleic acids as the stimulus to make interferon. Lancet. 1963 Jul 20;2(7299):113–116. doi: 10.1016/s0140-6736(63)92585-6. [DOI] [PubMed] [Google Scholar]
- ISAACS A. STUDIES ON INTERFERON. Aust J Exp Biol Med Sci. 1965 Jul;43:405–412. doi: 10.1038/icb.1965.74. [DOI] [PubMed] [Google Scholar]
- JENSEN K. E., NEAL A. L., OWENS R. E., WARREN J. INTERFERON RESPONSES OF CHICK EMBRYO FIBROBLASTS TO NUCLEIC ACIDS AND RELATED COMPOUNDS. Nature. 1963 Nov 2;200:433–434. doi: 10.1038/200433a0. [DOI] [PubMed] [Google Scholar]
- KOHLHAGE H., FALKE D. VERMEHRUNGSHEMMUNG DES HERPES-SIMPLEX-VIRUS DURCH RIBONUKLEINSAEUREN. (KURZE MITTEILUNG) Arch Gesamte Virusforsch. 1964;14:404–409. [PubMed] [Google Scholar]
- Louisot P., Colobert L., Bollack C., Weil J. H., Ebel J. P. Inhibition de la multiplication virale à l'aide d'acides ribonucléiques chimiquement modifiés. I. Inhibition de la multiplication de Myxovirus parainfluenzae I (virus Sendai) sur cellules de rein de veau. Biochim Biophys Acta. 1968 Jan 29;155(1):38–50. [PubMed] [Google Scholar]
- Montagnier L. Présence d'un acide ribonucléique en double chaîne dans des cellules animales. C R Acad Sci Hebd Seances Acad Sci D. 1968 Oct 21;267(17):1417–1420. [PubMed] [Google Scholar]
- NYGAARD A. P., HALL B. D. FORMATION AND PROPERTIES OF RNA-DNA COMPLEXES. J Mol Biol. 1964 Jul;9:125–142. doi: 10.1016/s0022-2836(64)80095-4. [DOI] [PubMed] [Google Scholar]
- ROTEM Z., COX R. A., ISAACS A. Inhibition of virus multiplication by foreign nucleic acid. Nature. 1963 Feb 9;197:564–566. doi: 10.1038/197564a0. [DOI] [PubMed] [Google Scholar]
- Rein A., Rubin H. Effects of local cell concentrations upon the growth of chick embryo cells in tissue culture. Exp Cell Res. 1968 Mar;49(3):666–678. doi: 10.1016/0014-4827(68)90213-9. [DOI] [PubMed] [Google Scholar]
- Stollar V., Stollar B. D. Immunochemical measurement of double-stranded RNA of uninfected and arbovirus-infected mammalian cells. Proc Natl Acad Sci U S A. 1970 Apr;65(4):993–1000. doi: 10.1073/pnas.65.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano K., Warren J., Jensen K. E., Neal A. L. Nucleic acid-induced resistance to viral infection. J Bacteriol. 1965 Dec;90(6):1542–1547. doi: 10.1128/jb.90.6.1542-1547.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilcek J., Ng M. H., Friedman-Kien A. E., Krawciw T. Induction of interferon synthesis by synthetic double-stranded polynucleotides. J Virol. 1968 Jun;2(6):648–650. doi: 10.1128/jvi.2.6.648-650.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANKOFSKY S. A., SPIEGELMAN S. The identification of the ribosomal RNA cistron by sequence complementarity. I. Specificity of complex formation. Proc Natl Acad Sci U S A. 1962 Jun 15;48:1069–1078. doi: 10.1073/pnas.48.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]



