SUMMARY
Calorie restriction (CR) extends lifespan and ameliorates age-related pathologies in most species studied; yet the mechanisms underlying these effects remain unclear. Using mouse skeletal muscle as a model, we show that CR acts in part by enhancing the function of tissue-specific stem cells. Even short-term CR significantly enhanced stem cell availability and activity in the muscle of young and old animals, in concert with an increase in mitochondrial abundance and induction of conserved metabolic and longevity regulators. Moreover, CR enhanced endogenous muscle repair and CR initiated in either donor or recipient animals improved the contribution of donor cells to regenerating muscle following transplant. These studies indicate that metabolic factors play a critical role in regulating stem cell function and that this regulation can influence the efficacy of recovery from injury and the engraftment of transplanted cells.
Calorie restriction (CR) is a dietary intervention that extends lifespan and delays, prevents, or reduces the severity of age-related pathologies in many species and tissues (Ahmet et al., 2005; Hursting et al., 2003; Russell and Kahn, 2007). Indeed, age-matched animals maintained on a reduced calorie diet throughout life show fewer malignancies, enhanced cognitive and motor function, and a lower incidence of diabetes, as compared to control animals allowed food ad libitum. Prior studies in the rodent hematopoietic system (Ertl et al., 2008) suggest that lifelong calorie restriction may maintain stem cell function, which normally declines with age, but whether short-term CR in otherwise healthy young animals might enhance the function of tissue stem cells has not been addressed. Moreover, the effect of CR in recipient animals on the efficiency of stem cell engraftment remains unknown. Answers to both these questions may help to improve the targeting and transplant of stem cells for therapy and illuminate normal metabolic regulators of tissue stem cell function and the age-related factors that typically impair this function (Rossi et al., 2008).
To assess the effects of short-term CR on tissue stem cell activity, we focused on skeletal muscle, a well-characterized tissue that normally undergoes robust repair in response to injury. Muscle regenerative activity is mediated by a specialized population of precursor cells known as satellite cells (Mauro, 1961), which reside adjacent to myofibers. Satellite cells are activated by muscle injury to divide and differentiate, regenerating damaged tissue and restoring muscle function (reviewed in (Wagers and Conboy, 2005)). The satellite cell pool contains self-renewing muscle stem cells, which can be isolated using antibody staining and fluorescence-activated cell sorting (FACS). A number of different marker combinations have been used for satellite cell isolation (Kuang et al., 2007; Montarras et al., 2005; Sacco et al., 2008; Sherwood et al., 2004; Tanaka et al., 2009); here, we identify satellite cells as CD45−Sca1−Mac1−CXCR4+β1-integrin+ myofiber-associated cells (Figures 1A and S2A), based on our previous analyses demonstrating that this marker combination yields a substantial enrichment of satellite cells that matches or exceeds that seen with other approaches (Montarras et al., 2005; Sacco et al., 2008). In particular, >95% of sorted CD45−Sca1−Mac1−CXCR4+β1-integrin+ cells stain for the canonical satellite cell transcription factor Pax7 (Figure 1D and (Cerletti et al., 2008a)), and <5% express MyoD (Cerletti et al., 2008a). CD45−Sca1−Mac1−CXCR4+β1-integrin+ satellite cells also co-express other reported satellite cell markers, including CD34 (100% CD34+; data not shown), Syndecan-4 (100% Syndecan-4+; (Cerletti et al., 2008a)), M-cadherin (100% M-cadherin+; (Cerletti et al., 2008a)), and alpha7-integrin (95% alpha7-integrin+; (Jang et al., 2011)). Most importantly, however, CD45−Sca1−Mac1−CXCR4+β1-integrin+ cells exhibit robust, clonal myogenic activity in vitro and in vivo (Cerletti et al., 2008a; Cerletti et al., 2008b), replenish the satellite cell compartment upon intramuscular transplantation (Cerletti et al., 2008a), and are completely devoid of fibrogenic or adipogenic potential as assessed by in vitro differentiation and transplant assays (Hettmer et al., 2011; Schulz et al., 2011; Tan et al., 2011). Thus, CD45−Sca1−Mac1−CXCR4+β1-integrin+ cells display phenotypic and functional properties consistent with a highly enriched population of adult muscle satellite cells.
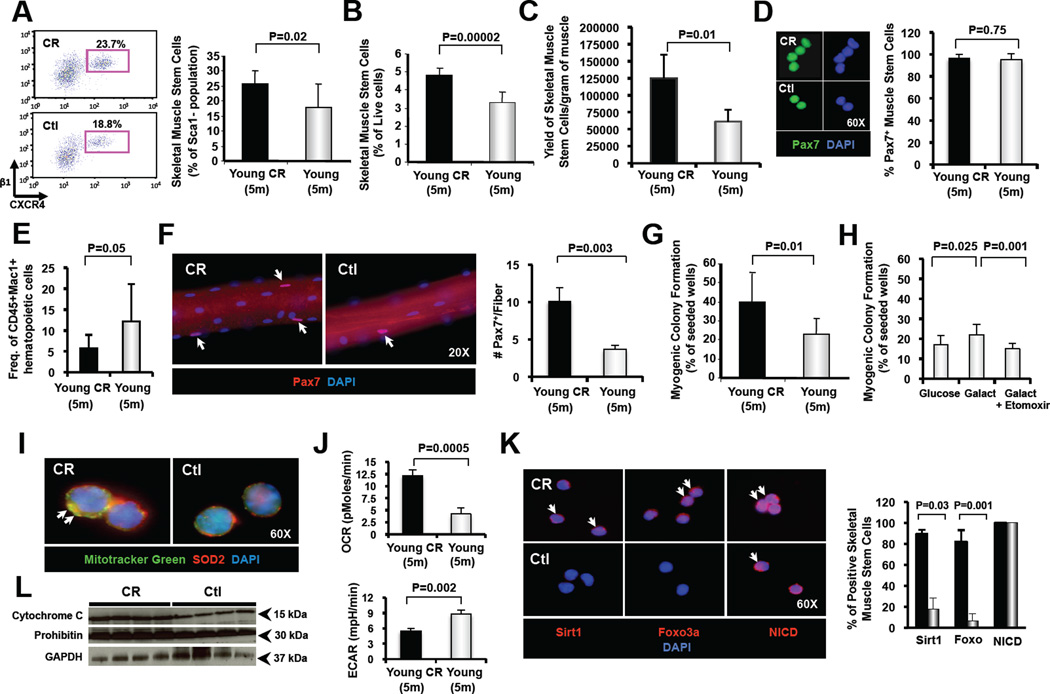
Figure 1. Skeletal muscle stem cell frequency and function are enhanced in CR-treated muscle.
(A) Representative flow cytometric analysis of satellite cells from young (5 months of age) CR-treated and control C57BL/6 mice. Plots shown depict CXCR4 and β1-integrin staining of Sca1−CD45−Mac1− cells previously gated also by scatter and vital dyes. Bar chart at right shows the frequency (mean ± SD) of CXCR4+β1-integrin+ satellite cells among CD45−Sca1−Mac1− myogenic (non-hematopoietic and non-fibrogenic (Joe et al., 2010; Sherwood et al., 2004; Uezumi et al., 2010)) cells harvested from skeletal muscle. Data compiled from analysis of n=7 CR-treated and n=10 control mice. (B) Average frequency (mean ± SD) of CD45−Sca1−Mac1−CXCR4+β1-integrin+ satellite cells among total live (propidium iodide-; calcein blue+) mononuclear cells harvested from skeletal muscle was determined by flow cytometry (as in A, data representative of n=7 CR-treated mice and n=4 control mice). (C) Yield of CD45−Sca1−Mac1−CXCR4+β1-integrin+ satellite cells per gram of muscle following sorting from CR-treated or control muscle. Data compiled from n=5–7 sorts per group. (D) Immunofluorescence staining for Pax7 in freshly sorted CD45−Sca1−Mac1−CXCR4+β1-integrin+ satellite cells from CR-treated and control mice shows equivalent percentage of positive-staining cells (right). Data represent analysis of 100 cells per group from CR-treated (black bars) or Ctl (grey bars) mice. (E) Frequency (mean ± SD) of Sca-1−CD45+Mac1+ inflammatory cells in muscle was determined by flow cytometry (CR-treated, black bars; Ctl, grey bars; n=8 mice per condition). (F) Immunofluorescence staining of Pax7+ satellite cells on single, isolated myofibers prepared from young CR-treated and control mice. Numbers of Pax7+ cells per fiber were quantified by counting >20 fibers from each of 4 CR-treated and 4 control mice (total of 80–160 fibers per experimental condition). Data represent mean ± SEM. (G, H) Clonal myogenesis assays of double-sorted satellite cells from young control or CR-treated mice. Data represent mean ± SD and reflect the percent of wells seeded with 1 satellite cell that contained a myogenic colony at day 5–6. Data are compiled from clonal assays of 4–7 individual mice per group, and report comparison of cells from control vs. CR-treated mice in glucose-containing medium (G) or from control mice only cultured in media containing glucose, galactose, or galactose and etoxomir, an inhibitor of mitochondrial energy production (H). (I) Immunofluorescence images showing staining with Mitotracker Green (green) and SOD2 (red). Nuclei were marked by DAPI staining (blue). CR-treated satellite cells showed increased mitochondrial abundance. (J) Oxygen consumption rate (OCR, pMoles/min, top panel) and glycolytic activity, measured by extracellular acidification rate (ECAR, mpH/min, bottom panel), of satellite cells isolated from CR or control mice were measured using a Seahorse Bioscience extracellular flux analyzer (XF24). (K) Immunofluorescence staining for Sirt1, Foxo3a and activated Notch1 (assessed by staining for the Notch intracellular domain (NICD), which is generated by cleavage of Notch receptor following ligand binding) in freshly sorted CD45−Sca1−Mac1−CXCR4+β1-integrin+ satellite cells from CR-treated and control mice. Percentage of positive-staining cells is shown at right for 100 cells analyzed from CR-treated (black bars) or Ctl (grey bars) mice. (L) Western blot analysis for the mitochondrial proteins cytochrome c and prohibitin in total protein lysate from satellite cells harvested from CR-treated or control mice (n=4 mice per group). GAPDH served as a loading control. See also Figures S1 and S2.
To examine the effect of CR on the overall frequency and myogenic activity of skeletal muscle stem cells, we purified these cells from young C57BL/6 mice, raised initially on control diet and then switched to CR at 2 months of age, or from aged C57BL/6 mice in which CR was initiated at 18 months of age. In both cases, after 12 weeks of CR (1 week at 20% restriction and 11 weeks at 40% (Figure S1A), animals were sacrificed for FACS analysis and myogenesis assays to compare the frequency and activity of satellite cells in their muscles with those of age-matched controls. Strikingly, both young and aged animals (now at 5 or 21 months of age, respectively) exposed to short-term CR showed significantly increased frequencies (Figures 1A,B and S2A,B) of Pax7-expressing (Figure 1D) satellite cells as detected by FACS analysis. The total yield of satellite cells (per gram of muscle harvested) that could be recovered after cell sorting was also increased for CR muscle (Figures 1C and S2C). These CR-induced increases in satellite cell frequency were confirmed by staining for Pax7+ nuclei on isolated myofibers, which demonstrated an ~3 fold increase in the number of Pax7+ cells per myofiber in young mice (n=4, Figure 1F) and an ~2-fold increase in aged mice (n=4, Figure S2E).
Satellite cells from CR-treated animals also exhibited significantly enhanced myogenic function in ex vivo colony forming assays (~50–60% increase in the number of cells capable of initiating myogenic colony formation, n=7 (young) or n=4 (aged); Figures 1G and S2F). In both young and aged CR-treated mice, this enhanced myogenic function was accompanied by an increased fraction of satellite cells expressing the conserved longevity/metabolic regulators Sirt1 and Foxo3a (Figures 1K and S2G). In addition, CR-treated satellite cells in aged mice specifically, exhibited restoration of activation-induced Notch signaling (Figure S2G), a critical age-regulated determinant of myogenic function (Conboy et al., 2005; Conboy and Rando, 2002). Thus, short-term CR, initiated either in youth or in old age, exerts a profound effect on muscle stem cells, altering their gene expression profile, enhancing their endogenous availability, and promoting their myogenic activity.
Given that skeletal muscle of CR-treated animals typically shows increases in fatty acid oxidation and mitochondrial energy production (Anderson and Weindruch, 2010), we hypothesized that the mechanism by which CR increases satellite cell colony-forming capacity might relate to alterations in mitochondrial bioenergetics. To test this notion, we produced similar metabolic reprogramming in vitro by culturing satellite cells from control animals in media containing galactose instead of glucose, thereby forcing the cultured cells to utilize mitochondrial oxidative phosphorylation for energy production (Marroquin et al., 2007). Galactose culture of satellite cells from control fed mice significantly enhanced myogenic colony formation, and this increase in myogenic activity was blocked in the presence of etomoxir (Figure 1H), a specific inhibitor of mitochondrial fatty acid oxidation (Baht and Saggerson, 1989). In addition, comparison of the overall function of mitochondria in satellite cells from control and CR-treated mice revealed a significant increase in oxygen consumption rate in CR-treated satellite cells (Figure 1J). Conversely, glycolytic lactate production was dramatically decreased in CR satellite cells (Figure 1J), suggesting that under CR, satellite cells rely more on oxidative phosphorylation than glycolysis for energy production. Finally, immunofluorescence staining for mitochondria (Figure 1I) and Western blotting for mitochondrial proteins (Figure 1L) revealed an increase in mitochondrial abundance in satellite cells from CR mice. Thus, consistent with reports on CR-induced metabolic reprogramming in whole organs (reviewed in (Anderson and Weindruch, 2010)), CR induces in satellite cells substantial alterations in mitochondrial mass and function, which promote oxidative metabolism. Moreover, an in vitro intervention (galactose) that likewise enhances mitochondrial energy production recapitulates the beneficial effects of CR on satellite cells, implicating modulation of mitochondrial bioenergetics as a likely mechanism by which CR enhances satellite cell frequency and activity in skeletal muscle.
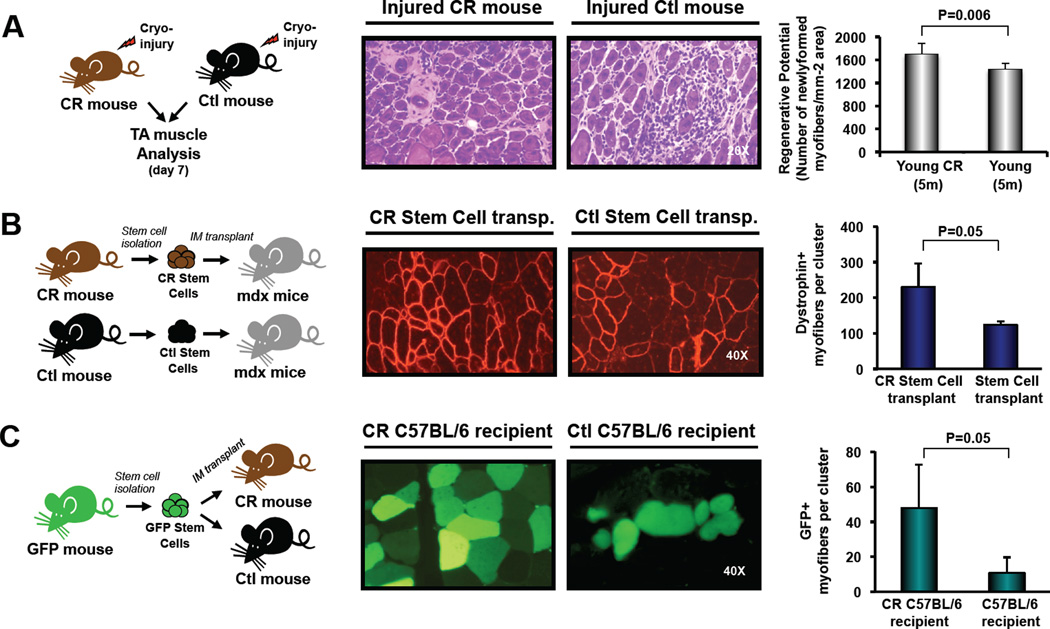
The increased frequency and function of satellite cells within the muscle of CR-treated animals suggests that this simple dietary intervention may hold promise for enhancing the therapeutic efficacy of muscle stem cells in the context of muscle disease or dysfunction. To test this possibility, we analyzed the regenerative activity following injury of endogenous satellite cells in CR or control mice, and the engraftment potential of donor satellite cells harvested from short-term CR or control fed mice and transplanted into the muscles of recipient mdx mice, a well-studied model of Duchenne Muscular Dystrophy (Sicinski et al., 1989). Remarkably, previously injured tibialis anterior (TA) muscles from CR mice exhibited improved regenerative capacity, indicated by a higher density of newly formed myofibers in comparison to control mice at 7 days after injury (n=7 (CR) or n=6 (Ctl); Figure 2A). Moreover, transplant of satellite cells from CR-treated donors generated almost twice as many donor-derived (dystrophin-expressing) myofibers as an equal number of satellite cells from control mice (n=4 (CR) or n=3 (Ctl) recipient mice; Figure 2B), signifying a substantial enhancement of transplantation efficiency and in vivo regenerative potential of muscle stem cells harvested from donors subjected to short-term CR.
Figure 2. Enhanced muscle repair activity of satellite cells following CR-treatment.
Experimental design for each study is shown at left. (A) TA muscles of young CR-treated or control C57BL/6 mice were freeze-injured and harvested for histology 7 days after injury. Muscle regeneration was quantified on hematoxylin/eosin (H&E) tissue sections as the mean number of centrally nucleated myofibers per mm2 (n=7 CR and n=6 Ctl, mean ± SD), and indicate an ~15% increase in the number of newly formed fibers in the regenerated tissue of CR mice. These findings are consistent with other studies (McCarthy et al., 2011; Murphy et al., 2011) indicating a correlative, but not necessarily quantitative, relationship between the endogenous content of muscle satellite cells (increased ~2-fold in CR-treated mice, Figure 1C,F) and the number of regenerating myofibers after injury. (B) 8000 double-sorted satellite cells from CR-treated (n=4) or control (n=3) mice were injected intramuscularly into mdx recipients injured 1 day previously by injection of cardiotoxin (CDTX) into the same TA muscle. Transverse cryosections of muscles receiving CR-treated (left) or control (right) satellite cells were stained with anti-dystrophin antibody (red; middle panels). Dystrophin normally is lacking in mdx muscle (Sicinski et al., 1989), and therefore serves as a marker of donor cell contributions to muscle regeneration. Data are presented as the mean (± SD) number of dystrophin+ myofibers found in each engrafted muscle. (C) 5000 double-sorted GFP-expressing satellite cells from control mice were injected into the TA of CR- or control-treated C57BL/6 mice (n=4 per group), injured 1 day previously by CTDX injection. Transverse cryosections from muscles harvested 4 weeks after transplant were analyzed for GFP expression by direct epifluorescence. Data are presented as the mean (± SD) number of GFP+ myofibers (green; middle panels) found in each engrafted muscle. Data were considered statistically significant at p<0.05 and all p-values were calculated by Student’s t-test. Note that overall levels of engraftment into mdx recipients are superior to those in wild-type recipients (compare (B) to (C)), likely due to differences in endogenous muscle stability, regenerative activity, and host muscle stem cell number (Cerletti et al., 2008a).
Interestingly, the beneficial effects of CR were not limited to the stem cell donor. In a complementary experiment, we transplanted fluorescently labeled (GFP+) satellite cells from ad lib fed mice into CR-treated C57BL/6 mice, in which CR was initiated at 10 weeks of age, or into age-matched controls. CR was continued for an additional 4 weeks after transplant. Strikingly, despite receiving the same donor cells as control fed recipients, when sacrificed for analysis, CR recipients showed ~4-fold greater engraftment of myofibers (n=4 mice per group, Figure 2C), demonstrating increased contribution of transplanted cells to muscle regeneration under CR conditions. We hypothesize that the beneficial impact of CR in the host for satellite cell engraftment relates at least in part to the anti-inflammatory effects of CR. Consistent with previous studies showing reduced inflammation in CR-treated animals (reviewed in (Fontana, 2009)), CR mice in our experiments exhibited a significant reduction in the frequency of inflammatory cells in muscle as compared to controls (Figures 1E and S2D). Interestingly, it has been demonstrated that cell transplantation into the muscle of immunocompetent animals leads frequently to acute cell death. In the case of myoblast transplant, 95–99% of transferred cells may die within days of transplant, and this death has been proposed to result from an innate immune/inflammatory response mounted by the host (Tremblay and Guerette, 1997)). Thus, we predict that the increased engraftment seen in CR recipients may be explained by improved survival of the transplanted cells in CR muscle as opposed to control muscle due to a CR-induced decrease in the inflammatory response.
In summary, the data presented here demonstrate that reduced calorie intake rapidly alters the physiology and function of muscle stem cells, such that even short-term CR, initiated late in life (18mo, Figure S2), has significant beneficial effects in enhancing myogenic activity. Preliminary experiments using even older mice (27 mo. at time of sacrifice) indicate that short-term CR induces similar increases in muscle stem cell frequency in these animals as well (data not shown). These beneficial effects are associated with a dramatic metabolic reprogramming that favors oxidative over glycolytic metabolism and is linked to alterations in pivotal regulators of mitochondrial mass and function, including the NAD-dependent protein deacetylase SIRT1. Likely in conjunction with other critical nutrient sensors such as AMPK, a key regulator of mitochondrial biogenesis in response to energy deprivation (Canto and Auwerx, 2009; Zong et al., 2002), SIRT1 may regulate mitochondrial metabolism in CR-treated satellite cells through activation of PGC-1α (Gerhart-Hines et al., 2007) and FOXO (Jacobs et al., 2008)). Importantly, the enhanced myogenic function of satellite cells in the CR environment significantly accelerates endogenous repair and improves the capacity of donor muscle stem cells to productively engraft transplant recipients. These results support the notion that both cell-autonomous and non-autonomous factors, particularly metabolic regulators, contribute to the detrimental effects of aging on muscle regenerative function and therefore represent candidate targets for therapeutic intervention.
Supplementary Material
HIGHLIGHTS.
CR increases skeletal muscle stem cell frequency in young and aged mice.
Muscle stem cells from CR-treated mice show increased abundance of mitochondria.
CR improves muscle regeneration and enhances stem cell transplant efficiency.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Joslin’s HSCI/DERC Flow Cytometry Core (NIH Award Number P30DK036836) and the HSCRB Flow Cytometry Core at Harvard for excellent flow cytometry support. This work was funded in part by grants from the Harvard Stem Cell Institute, NIH (1RO1 AG033053, and 1P30 AG031679) and Glenn Foundation to AJW, grants from the NIA (AG032375), Glenn Foundation, Ellison Medical Foundation and MDA to MH, and an NSF graduate research fellowship to LF. Content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or other funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahmet I, Wan R, Mattson MP, Lakatta EG, Talan M. Cardioprotection by intermittent fasting in rats. Circulation. 2005;112:3115–3121. doi: 10.1161/CIRCULATIONAHA.105.563817. [DOI] [PubMed] [Google Scholar]
- Anderson RM, Weindruch R. Metabolic reprogramming, caloric restriction and aging. Trends Endocrinol Metab. 2010;21:134–141. doi: 10.1016/j.tem.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baht HS, Saggerson ED. Effect of noradrenaline on triacylglycerol synthesis in rat brown adipocytes. Biochem J. 1989;258:369–373. doi: 10.1042/bj2580369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerletti M, Jurga S, Witczak CA, Hirshman MF, Shadrach JL, Goodyear LJ, Wagers AJ. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell. 2008a;134:37–47. doi: 10.1016/j.cell.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerletti M, Shadrach JL, Jurga S, Sherwood R, Wagers AJ. Regulation and function of skeletal muscle stem cells. Cold Spring Harb Symp Quant Biol. 2008b;73:317–322. doi: 10.1101/sqb.2008.73.054. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell. 2002;3:397–409. doi: 10.1016/s1534-5807(02)00254-x. [DOI] [PubMed] [Google Scholar]
- Ertl RP, Chen J, Astle CM, Duffy TM, Harrison DE. Effects of dietary restriction on hematopoietic stem-cell aging are genetically regulated. Blood. 2008;111:1709–1716. doi: 10.1182/blood-2007-01-069807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L. Neuroendocrine factors in the regulation of inflammation: excessive adiposity and calorie restriction. Experimental gerontology. 2009;44:41–45. doi: 10.1016/j.exger.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. The EMBO journal. 2007;26:1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettmer S, Liu J, Miller CM, Lindsay MC, Sparks CA, Guertin DA, Bronson RT, Langenau DM, Wagers AJ. Sarcomas induced in discrete subsets of prospectively isolated skeletal muscle cells. Proc Natl Acad Sci U S A. 2011;108:20002–20007. doi: 10.1073/pnas.1111733108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu Rev Med. 2003;54:131–152. doi: 10.1146/annurev.med.54.101601.152156. [DOI] [PubMed] [Google Scholar]
- Jacobs KM, Pennington JD, Bisht KS, Aykin-Burns N, Kim HS, Mishra M, Sun L, Nguyen P, Ahn BH, Leclerc J, et al. SIRT3 interacts with the daf-16 homolog FOXO3a in the mitochondria, as well as increases FOXO3a dependent gene expression. International journal of biological sciences. 2008;4:291–299. doi: 10.7150/ijbs.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang YC, Sinha M, Cerletti M, Dall'osso C, Wagers AJ. Skeletal Muscle Stem Cells: Effects of Aging and Metabolism on Muscle Regenerative Function. Cold Spring Harb Symp Quant Biol. 2011 doi: 10.1101/sqb.2011.76.010652. [DOI] [PubMed] [Google Scholar]
- Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nature cell biology. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marroquin LD, Hynes J, Dykens JA, Jamieson JD, Will Y. Circumventing the Crabtree effect: replacing media glucose with galactose increases susceptibility of HepG2 cells to mitochondrial toxicants. Toxicol Sci. 2007;97:539–547. doi: 10.1093/toxsci/kfm052. [DOI] [PubMed] [Google Scholar]
- Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011;138:3657–3666. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, Buckingham M. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132:681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- Russell SJ, Kahn CR. Endocrine regulation of ageing. Nat Rev Mol Cell Biol. 2007;8:681–691. doi: 10.1038/nrm2234. [DOI] [PubMed] [Google Scholar]
- Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TJ, Huang TL, Tran TT, Zhang H, Townsend KL, Shadrach JL, Cerletti M, McDougall LE, Giorgadze N, Tchkonia T, et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci U S A. 2011;108:143–148. doi: 10.1073/pnas.1010929108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood RI, Christensen JL, Conboy IM, Conboy MJ, Rando TA, Weissman IL, Wagers AJ. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119:543–554. doi: 10.1016/j.cell.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science. 1989;244:1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- Tan KY, Eminli S, Hettmer S, Hochedlinger K, Wagers AJ. Efficient generation of iPS cells from skeletal muscle stem cells. PLoS One. 2011;6:e26406. doi: 10.1371/journal.pone.0026406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka KK, Hall JK, Troy AA, Cornelison DD, Majka SM, Olwin BB. Syndecan-4-expressing muscle progenitor cells in the SP engraft as satellite cells during muscle regeneration. Cell Stem Cell. 2009;4:217–225. doi: 10.1016/j.stem.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay JP, Guerette B. Myoblast Transplantation: a Brief Review of the Problems and of Some Solutions. Basic Appl Myol. 1997;7:221–230. [Google Scholar]
- Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nature cell biology. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- Wagers AJ, Conboy IM. Cellular and molecular signatures of muscle regeneration: current concepts and controversies in adult myogenesis. Cell. 2005;122:659–667. doi: 10.1016/j.cell.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002;99:15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.