Abstract
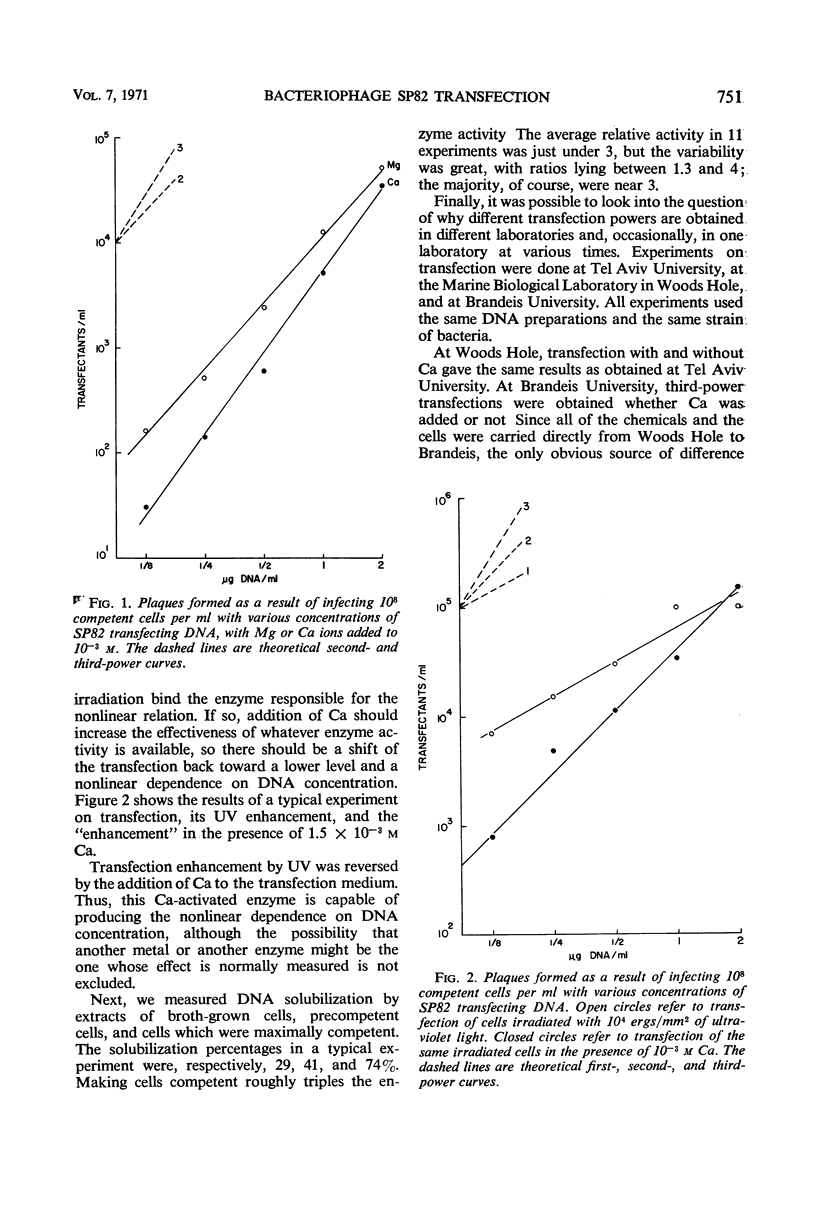
Extracts of competent cells of Bacillus subtilis exhibited nuclease activity on radioactively labeled cell deoxyribonucleic acid (DNA). The activity was not decreased when Mg was omitted from the reaction mixture but was decreased to zero by addition of ethylenediaminetetraacetic acid. Of the other metals tested, only Ca increased nuclease activity more than Mg. Addition of 1.5 × 10−3m Ca to transfection mixtures increased the nonlinearity of the relationship between number of transfectants and DNA concentration. The inferred role of the Ca-activated nuclease was checked by showing that the ultraviolet enhancement of transfection is reversed by addition of Ca. It was also shown, by testing in three different laboratories, that the Ca ion in water is the likely source of the different nonlinear relationships found in different laboratories.
Full text
PDF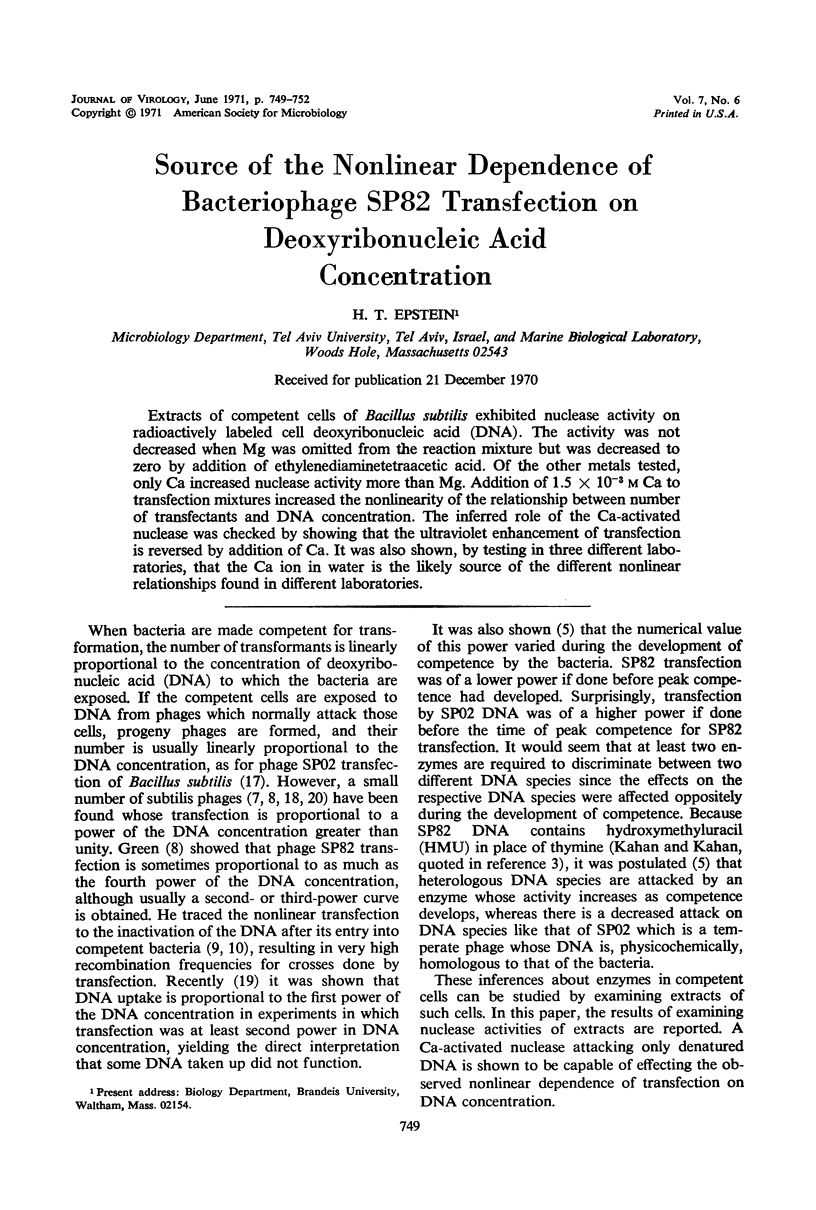


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer W. F. Recombination and integration in Bacillus subtilis transformation: involvement of DNA synthesis. J Mol Biol. 1965 Dec;14(2):534–557. doi: 10.1016/s0022-2836(65)80203-0. [DOI] [PubMed] [Google Scholar]
- Cahn F. H., Fox M. S. Fractionation of transformable bacteria from ocompetent cultures of Bacillus subtilis on renografin gradients. J Bacteriol. 1968 Mar;95(3):867–875. doi: 10.1128/jb.95.3.867-875.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein H. T. Factors affecting bacterial competence for transfection and transfection enhancement. Bacteriol Rev. 1968 Dec;32(4 Pt 1):313–319. [PMC free article] [PubMed] [Google Scholar]
- Epstein H. T., Mahler I. Mechanisms of enhancement of SP82 transfection. J Virol. 1968 Jul;2(7):710–715. doi: 10.1128/jvi.2.7.710-715.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein H. T. Transfection enhancement by ultraviolet irradiation. Biochem Biophys Res Commun. 1967 Apr 20;27(2):258–262. doi: 10.1016/s0006-291x(67)80071-8. [DOI] [PubMed] [Google Scholar]
- FOELDES J., TRAUTNER T. A. INFECTIOUS DNA FROM A NEWLY ISOLATED B. SUBTILIS PHAGE. Z Vererbungsl. 1964 Apr 10;95:57–65. doi: 10.1007/BF00898184. [DOI] [PubMed] [Google Scholar]
- GREEN D. M. INFECTIVITY OF DNA ISOLATED FROM BACILLUS SUBTILIS BACTERIOPHAGE, SP82. J Mol Biol. 1964 Dec;10:438–451. doi: 10.1016/s0022-2836(64)80065-6. [DOI] [PubMed] [Google Scholar]
- Hadden C., Nester E. W. Purification of competent cells in the Bacillus subtilis transformation system. J Bacteriol. 1968 Mar;95(3):876–885. doi: 10.1128/jb.95.3.876-885.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javor G. T., Tomasz A. An autoradiographic study of genetic transformation. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1216–1222. doi: 10.1073/pnas.60.4.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCarthy C., Nester E. W. Macromolecular synthesis in newly transformed cells of Bacillus subtilis. J Bacteriol. 1967 Jul;94(1):131–140. doi: 10.1128/jb.94.1.131-140.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKAZAKI T., KORNBERG A. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. XV. PURIFICATION AND PROPERTIES OF A POLYMERASE FROM BACILLUS SUBTILIS. J Biol Chem. 1964 Jan;239:259–268. [PubMed] [Google Scholar]
- OKUBO S., STRAUSS B., STODOLSKY M. THE POSSIBLE ROLE OF RECOMBINATION IN THE INFECTION OF COMPETENT BACILLUS SUBTILIS BY BACTERIOPHAGE DEOXYRIBONUCLEIC ACID. Virology. 1964 Dec;24:552–562. doi: 10.1016/0042-6822(64)90207-7. [DOI] [PubMed] [Google Scholar]
- Okubo S., Romig W. R. Comparison of ultraviolet sensitivity of Bacillus subtilis bacteriophage SPO2 and its infectious DNA. J Mol Biol. 1965 Nov;14(1):130–142. doi: 10.1016/s0022-2836(65)80235-2. [DOI] [PubMed] [Google Scholar]
- Oostindier-Braaksma F., Epstein H. T. DNA fixation and development of transformability and transfectability in Bacillus subtilis. Mol Gen Genet. 1970;108(1):23–27. doi: 10.1007/BF00343180. [DOI] [PubMed] [Google Scholar]
- REILLY B. E., SPIZIZEN J. BACTERIOPHAGE DEOXYRIBONUCLEATE INFECTION OF COMPETENT BACILLUS SUBTILIS. J Bacteriol. 1965 Mar;89:782–790. doi: 10.1128/jb.89.3.782-790.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. N., Pitale M. P. Competence and deoxyribonucleic acid uptake in Bacillus subtilis. J Bacteriol. 1968 Mar;95(3):864–866. doi: 10.1128/jb.95.3.864-866.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somma S., Polsinelli M. Quantitive autoradiographic study of competence and deoxyribonucleic acid incorporation in Bacillus subtilis. J Bacteriol. 1970 Mar;101(3):851–855. doi: 10.1128/jb.101.3.851-855.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevethia M. J., Mandel M. Nature of the ethylenediaminetetraacetic acid requirement for transformation of Bacillus subtilis with single-stranded deoxyribonucleic acid. J Bacteriol. 1970 Mar;101(3):844–850. doi: 10.1128/jb.101.3.844-850.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


