Abstract
Although the relationship between muscle strength and exercise capacity has been demonstrated in dermatomyositis without lung dysfunction, little is known about the association between exercise capacity and interstitial lung disease in dermatomyositis. Eleven patients with dermatomyositis with interstitial lung disease without the manifestation of muscle weakness and 12 patients with idiopathic interstitial pneumonia underwent the 6-minute walk test (6MWT). PaO2, creatine kinase, percentage predicted 6MWT distance (6MWD%), and SpO2 at rest were similar between patients. Percentage predicted vital capacity, carbon monoxide diffusing capacity (DLCO%), and SpO2 after 6MWT were higher and exertional dyspnea was lower in patients with dermatomyositis than in patients with idiopathic interstitial pneumonia. SpO2 after 6MWT was positively correlated with 6MWD% in patients with dermatomyositis, while DLCO% and PaO2 were positively correlated with 6MWD% in patients with idiopathic interstitial pneumonia. Lung dysfunction in dermatomyositis might not be a major limitation factor in exercise capacity.
Keywords: dermatomyositis, interstitial lung disease, exercise capacity, idiopathic interstitial pneumonia
Introduction
Dermatomyositis is an autoimmune disease characterized by myogenic changes and skin eruptions.1 Various autoantibodies have been found in dermatomyositis, and some of them preferably affect lung tissue, resulting in interstitial lung disease.2–4 For example, it is known that rapidly progressive interstitial lung disease likely accompanies typical skin lesions by anti-CADM-140 antibodies and requires intensive treatment with medication. On the other hand, interstitial lung disease is also found in dermatomyositis with anti-aminoacyl-tRNA synthetase antibodies or some other autoantibodies showing better prognosis.5
Exercise capacity in dermatomyositis may decline due to complex factors including muscle weakness by myositis, lung dysfunction, and medication, especially corticosteroids. Peak oxygen uptake in myositis patients with normal spirometry values was reported to be lower than that of healthy people, correlating with peak isometric torque of knee extensors.6 Moreover, aerobic training was shown to improve muscle strength, quality of life, and oxygen uptake in myositis patients.7 Thus, since the relationship between muscle strength and exercise capacity has been demonstrated, exercise training was suggested to be a beneficial treatment for patients with dermatomyositis exhibiting muscle weakness. However, exercise capacity in patients with dermatomyositis with interstitial lung disease has not been examined. Interstitial lung disease in systemic sclerosis was observed to affect exercise capacity as measured by the 6-minute walk test (6MWT).8 Therefore, interstitial lung disease in dermatomyositis seems likely to reduce exercise capacity.
Idiopathic interstitial pneumonia is a typical interstitial lung disease without muscle complications. In idiopathic pulmonary fibrosis, one of subtypes of idiopathic interstitial pneumonia, 6MWT distance (6MWD) was correlated with percentage predicted carbon monoxide diffusing capacity (DLCO%) and gender.9 Exercise training could also improve exercise capacity and quality of life in patients with idiopathic pulmonary fibrosis.10 Our hypothesis was that if dermatomyositis patients with interstitial lung disease were not affected by the manifestation of muscle weakness, their exercise capacity would not differ from that of patients with idiopathic interstitial pneumonia concerning lung dysfunction. The present study aimed to compare limitation factors for exercise capacity between patients with interstitial lung disease accompanied by dermatomyositis and patients with idiopathic interstitial pneumonia retrospectively.
Materials and Methods
Subjects were consecutive adult patients referred to the Rehabilitation Division of our university hospital between 2008 and 2011. Thirty-nine patients with dermatomyositis were diagnosed by physical findings, and their autoantibodies were examined. Interstitial lung disease was found in 20 of 39 patients by examinations that included the pulmonary function test, high resolution CT, and/or open lung biopsy. Exclusion criteria for the present study were proximal muscle weakness in the extremities of less than 4 in manual muscle testing, experience of muscular training due to muscle weakness, and neoplasm. Therefore, 11 of 20 patients were assigned to this study (Table 1). All patients were diagnosed with nonspecific interstitial pneumonia for interstitial lung disease. The anti-CADM-140 antibody was found in three patients, anti-aminoacyl-tRNA synthetase antibody (EJ and Jo-1) in five, anti U1-RNP antibody in one, and unknown with negative anti-aminoacyl-tRNA synthetase antibody in two. Twenty-one patients with idiopathic interstitial pneumonia visited our division, and nine of them were excluded because of severe respiratory symptoms during walking, a history of paralytic disease of the central nervous system, and having had exercise training for exertional dyspnea. Six of the remaining 12 were diagnosed with idiopathic pulmonary fibrosis and six were diagnosed with nonspecific interstitial pneumonia.
Table 1.
Patient characteristics.
| Dermatomyositis (n = 11) | Idiopathic interstitial pneumonia (n = 12) | P | |
|---|---|---|---|
| Gender (f/m) | 7/4 | 4/8 | 0.22 |
| Age (ys) | 58.3 ± 10.1 | 64.6 ± 12.6 | 0.20 |
| Height (cm) | 159 ± 6 | 163 ± 7 | 0.15 |
| Weight (kg) | 58 ± 13 | 61 ± 12 | 0.64 |
| Duration after diagnosis (ys) | 2.2 ± 2.7 | 1.7 ± 1.8 | 0.60 |
| Vital capacity (% predicted) | 89 ± 20 | 74 ± 14 | 0.048* |
| DLCO (% predicted) | 58 ± 15 | 41 ± 12 | 0.0070* |
| PaO2 (mmHg) | 83 ± 8 | 78 ± 11 | 0.25 |
| SpO2 at rest (%) | 96 ± 3 | 97 ± 2 | 0.75 |
| SpO2 after 6MWT (%) | 94 ± 4 | 88 ± 6 | 0.0084* |
| Borg score after 6MWT | 2.7 ± 1.6 | 5.2 ± 1.6 | 0.0030* |
| 6MWD (% predicted) | 80 ± 19 | 89 ± 16 | 0.25 |
| Creatine kinase (U/l) | 169 ± 207 | 104 ± 55 | 0.33 |
| Corticosteroids (yes/no) | 5/6 | 2/10 | 0.19 |
Notes: Values are mean ± standard deviation.
P < 0.05.
Abbreviations: DLCO, carbon monoxide diffusing capacity; 6MWT, 6-minute walk test; 6MWD, 6-minute walk distance.
The following clinical information was collected retrospectively from all patients by reviewing their medical records: gender, age, morphological measurements, duration after diagnosis, dose of corticosteroids, percentage predicted vital capacity (VC%), DLCO%, PaO2 of blood gas analysis, serum creatine kinase for myogenic activity, oxygen saturation (SpO2) at rest and after 6MWT, Borg dyspnea score (0 to 10 category-ratio scale) at the end of 6MWT, and percentage predicted 6-minute walk distance (6MWD%) correcting 6MWD by gender, age, and morphological measurements.11 Evaluations of all patients were performed at the first visit to our facility to be referred for consultation on further treatments. They completed 6MWT without leg pain or leg discomfort. A maintenance dose of corticosteroids had been prescribed for five patients with dermatomyositis and two patients with idiopathic pulmonary fibrosis before these evaluations, while the remaining patients did not received corticosteroids. The protocol for the study was approved by the human ethics committee of our university, conforming to the provisions of the Declaration of Helsinki.
Statistical analysis
JMP 8 software (SAS Institute, Inc., Cary, NC) was used for statistical analysis. Descriptive statistics (means, standard deviation) were performed on all dependent variables. The t test served to compare patients with dermatomyositis to those with idiopathic interstitial pneumonia. Fisher exact probability test was used for comparison of gender and number of patients with corticosteroid medication. A simple linear correlation coefficient was determined to estimate the correlation between 6MWD% and collected parameters. Moreover, a comparison of 6MWD% on corticosteroid medication was performed for each disease; P < 0.05 was considered significant.
Results
The characteristics of patients with dermatomyositis and idiopathic interstitial pneumonia were similar for gender, age, height, weight, duration after diagnosis, and corticosteroid medication, as shown in Table 1. Patients were also similar with respect to PaO2, but VC% and DLCO% of lung function were significantly lower in patients with idiopathic interstitial pneumonia than in patients with dermatomyositis (P = 0.048 and 0.007, respectively). Creatine kinase in patients with dermatomyositis was as low as that in patients with idiopathic interstitial pneumonia. There were no significant differences in 6MWD% and SpO2 at rest between patients with dermatomyositis and those with idiopathic interstitial pneumonia, whereas SpO2 after 6MWT was significantly higher and the Borg dyspnea score was significantly lower in patients with dermatomyositis than in patients with idiopathic interstitial pneumonia (P = 0.008 and 0.003, respectively).
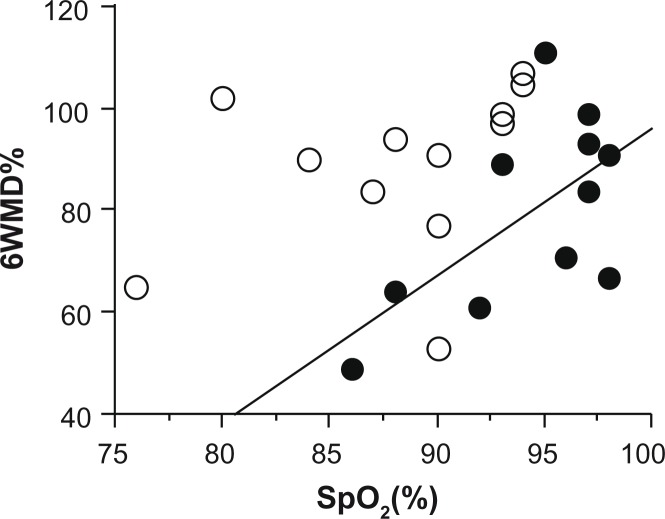
A positive correlation between 6MWD% and SpO2 after 6MWT was observed in patients with dermatomyositis (R2 = 0.40, P = 0.038) (Table 2). In patients with idiopathic interstitial pneumonia, 6MWD% was positively correlated with DLCO% and PaO2 (R2 = 0.41, P = 0.035 and R2 = 0.45, P = 0.018, respectively). The Borg dyspnea score and creatine kinase were not related to 6MWD% in patients with either disease. As the correlation between 6MWD% and SpO2 after 6MWT was not significant in patients with idiopathic interstitial pneumonia (R2 = 0.14, P = 0.23), Figure 1 shows the regression line for patients with dermatomyositis. Values of 6MWD% in patients with dermatomyositis were lower than those in patients with idiopathic interstitial pneumonia considering SpO2 after 6MWT. Therefore, 6MWD% was low in patients with dermatomyositis without a severe reduction in SpO2.
Table 2.
Correlation between 6MWD% and measured parameters.
|
Dermatomyositis (n = 11)
|
Idiopathic interstitial pneumonia (n = 12)
|
|||
|---|---|---|---|---|
| R2 | P | R2 | P | |
| Duration after diagnosis (ys) | 0.34 | 0.061 | 0.010 | 0.75 |
| Vital capacity (% predicted) | 0.28 | 0.096 | 0.055 | 0.46 |
| DLCO (% predicted) | 0.20 | 0.16 | 0.41 | 0.035* |
| PaO2 (mmHg) | 0.24 | 0.15 | 0.45 | 0.018* |
| SpO2 at rest (%) | 0.32 | 0.070 | 0.12 | 0.26 |
| SpO2 after 6MWT (%) | 0.40 | 0.038* | 0.14 | 0.23 |
| Borg score after 6MWT | 0.0002 | 0.97 | 0.08 | 0.43 |
| Creatine kinase (U/l) | 0.077 | 0.41 | 0.17 | 0.18 |
Note:
P < 0.05.
Abbreviations: DLCO, carbon monoxide diffusing capacity; 6MWT, 6-minute walk test; 6MWD, 6-minute walk distance.
Figure 1.
Correlation between 6MWD% and SpO2 after the 6-minute walk test.
Notes: Closed circles represent patients with dermatomyositis (n = 11) and open circles represent those with idiopathic interstitial pneumonia (n = 12). The regression line is for patients with dermatomyositis (R2 = 0.40, P = 0.038). No line is inserted for patients with idiopathic interstitial pneumonia because of no correlation between parameters.
Patients with dermatomyositis taking corticosteroids showed significantly lower 6MWD% than those not taking corticosteroids (P = 0.026) (Table 3). The same tendency was found in patients with idiopathic interstitial pneumonia, though only two patients were taking corticosteroids, which was too small to show significance.
Table 3.
Comparison of 6MWD% in patients taking and not taking corticosteroid medication.
| Dermatomyositis | Idiopathic interstitial pneumonia | |
|---|---|---|
| Corticosteroids | ||
| Yes | 66 ± 15 (n = 5) | 65 ± 17 (n = 2) |
| No | 91 ± 15 (n = 6) | 93 ± 12 (n = 10) |
| P | 0.026* | 0.23 |
Note:
P < 0.05.
Abbreviation: 6MWD, 6-minute walk distance.
Discussion
The present study showed that gender, age, morphological measurements, and duration after diagnosis were similar in patients with dermatomyositis and those with idiopathic interstitial pneumonia. However, VC%, DLCO%, and SpO2 after 6MWT were significantly higher in patients with dermatomyositis than in patients with idiopathic interstitial pneumonia, despite similar 6MWD%. Contradicting our hypothesis, the results suggested that patients with dermatomyositis who were less affected by pulmonary function could manage to walk the same distance as patients with idiopathic interstitial pneumonia.
Additionally, the Borg dyspnea score was significantly lower in patients with dermatomyositis after 6MWT than in patients with idiopathic interstitial pneumonia. No relationship was seen between the Borg dyspnea score and 6MWD% in patients with dermatomyositis, which suggests that perceived dyspnea may not be the limitating factor for exercise capacity in patients with dermatomyositis even with interstitial lung disease. The Borg dyspnea score after 6MWT has been reported to be 3.0 in patients with pulmonary fibrosis, including those with nonidiopathic disease,12 and 2.77 in patients with systemic sclerosis with interstitial lung disease.13 In our study, the Borg dyspnea score of 2.7 in patients with dermatomyositis was similar to the Borg dyspnea score in patients with interstitial lung disease.
In patients with idiopathic interstitial pneumonia, 6MWD% was correlated with DLCO% and PaO2. This result was supported by previous observations showing significant relationships between 6MWD and DLCO%, percent predicted forced vital capacity, resting AaPO2, St. George’s Respiratory Questionnaire,14 and the Medical Research Council scale15 in patients with idiopathic pulmonary fibrosis. Moreover, a decrease in SpO2 during 6MWT was one of the prognostic factors for patients with idiopathic interstitial pneumonia.16 Thus, the parameters affecting 6MWD in patients with idiopathic interstitial pneumonia were baseline characteristics reflecting pulmonary dysfunction.
Similar to dermatomyositis, the relationship between pulmonary symptoms and 6MWD in patients with systemic sclerosis has not been fully clarified. The dyspnea index in patients with systemic sclerosis with interstitial lung disease was one of the variables significantly associated with 6MWD.8 Stronger correlations between measures of lung disease and 6MWD were also observed in patients with systemic sclerosis by removing those with lower extremity pain.9 However, patients with systemic sclerosis have varying degrees of musculoskeletal and peripheral vascular involvement, which reduce exercise capacity without pulmonary impairment.17 These results make it difficult to define the limitations to 6MWD in patients with systemic sclerosis due to various complications.
As one of the parameters limiting 6MWD%, the value of SpO2 after 6MWT was positively correlated with 6MWD% in patients with dermatomyositis; however, SpO2 was not lower than that in patients with idiopathic interstitial pneumonia. Minimum SpO2 during 6MWT in patients with systemic sclerosis with interstitial lung disease was also shown to be significantly higher than that in patients with idiopathic pulmonary fibrosis.9 One of the explanations for these results may be that myositis prevented patients from walking under muscular hypoxic conditions, such that 6MWD% depended upon SpO2 more precisely that in patients with idiopathic interstitial pneumonia. Future studies regarding muscle histology, muscle oxidative enzyme capacity, and other relevant factors to prove the relationship in myositis are necessary.18
Another parameter limiting 6MWD% was corticosteroid medication in patients with dermatomyositis. No significant difference in creatine kinase between patients with either disease implied well controlled myopathy by medication. The Fisher exact probability test showed no difference for the comparison of the number of patients with corticosteroid medication, although the dose was different. Two patients with idiopathic interstitial pneumonia were prescribed 4 mg and 5 mg of prednisolone a day, although for one, 4 mg daily for months was necessary for months to suppress the clinical symptoms of dermatomyositis. For one other patients, the dose necessary to achieve this was 6 mg daily, and for three patients, the dose was 20 mg daily.
Generally, chronic steroid myopathy is exceedingly rare with daily doses of <7.5 mg prednisolone.19 Normal serum aldolase and creatine kinase with increased creatinuria are considered suggestive of steroid myopathy; however, we did not examine creatinuria. Therefore, we could not distinguish the status of reduced 6MWD% in patients with dermatomyositis from steroid myopathy. In the present study, two patients with idiopathic interstitial pneumonia also showed low values of 6MWD% despite low doses of corticosteroids. In patients with pulmonary sarcoidosis, 6MWD did not differ regarding corticosteroids treatment, but the dose was not discussed.20 As there was no statistically clear evidence of the dose needed for steroid myopathy because of the muscle condition of the underlying disease,19 it was difficult to infer that reduced 6MWD% was caused by steroid myopathy, but some possibility of this relationship remained.
Our present study is limited by the small number of patients, even though statistically significant differences were found between patients with dermatomyositis and those with idiopathic interstitial pneumonia regarding pulmonary factors (P < 0.01) as shown in Table 1. There is a need for larger studies to further examine the parameters affecting exercise capacity in patients with dermatomyositis using methods such as multivariate regression analysis. Many patients with dermatomyositis were excluded from our study due neoplasm with the p155/140 antibody4 because chemotherapy and/or surgical treatment added further complications to patients. The variety of antibodies in dermatomyositis made it difficult for this kind of study to establish the parameters affecting symptoms. Meta-analysis is required for this purpose.
In conclusion, our study demonstrated exercise intolerance in patients with dermatomyositis with interstitial lung disease without the manifestation of muscle weakness. Parameters correlated with 6MWD%, that is, SpO2 after 6MWT and corticosteroids medication, in patients with dermatomyositis were different from parameters correlated with idiopathic interstitial pneumonia. As the parameters for lung function could not be proven to affect 6MWD% in patients with dermatomyositis, myopathy may be a possible factor in exercise capacity even if it is asymptomatic. However, further investigations are needed to confirm this association.
Footnotes
Author Contributions
Conceived and designed the experiments, analyzed the data, and wrote the manuscript: FS. Agree with manuscript results and conclusions and contributed to the writing of the manuscript: NM. Both authors reviewed and approved of the final manuscript.
Funding
Author(s) disclose no funding sources.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
Author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication. The external blind peer reviewers report no conflicts of interest.
References
- 1.Callen JP, Wortmann RL. Dermatomyositis. Clin Dermatol. 2006;24:363–73. doi: 10.1016/j.clindermatol.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Ikeda N, Takahashi K, Yamaguchi Y, Inasaka M, Kuwana M, Ikezawa Z. Analysis of dermatomyositis-specific autoantibodies and clinical characteristics in Japanese patients. J Dermatol. 2011;38:973–9. doi: 10.1111/j.1346-8138.2011.01262.x. [DOI] [PubMed] [Google Scholar]
- 3.Hamaguchi Y, Kuwana M, Hoshino K, et al. Clinical correlations with dermatomyositis-specific autoantibodies in adult Japanese patients with dermatomyositis. Arch Dermatol. 2011;147:391–8. doi: 10.1001/archdermatol.2011.52. [DOI] [PubMed] [Google Scholar]
- 4.Saketkoo LA, Ascherman DP, Cottin V, Christopher-Stine L, Danoff SK, Oddis CV. Interstitial lung disease in idiopathic inflammatory myopathy. Curr Rheumatol Rev. 2010;6:108–9. doi: 10.2174/157339710791330740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mukae H, Ishimoto H, Sakamoto N, et al. Clinical differences between interstitial lung disease associated with clinically amyopathic dermatomyositis and classic dermatomyositis. Chest. 2009;136:1341–7. doi: 10.1378/chest.08-2740. [DOI] [PubMed] [Google Scholar]
- 6.Wiesinger GF, Quittan M, Nuhr M, et al. Aerobic capacity in adult dermatomyositis/polymyositis patients and healthy controls. Arch Phys Med Rehabil. 2000;81:1–5. doi: 10.1016/s0003-9993(00)90212-0. [DOI] [PubMed] [Google Scholar]
- 7.de Salles Painelli V, Gualano B, Artioli GG, et al. The possible role of physical exercise on the treatment of idiopathic inflammatory myopathies. Autoimmunity Rev. 2009;8:355–9. doi: 10.1016/j.autrev.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Villalba WO, Sampaio-Barros PD, Pereira MC, et al. Six-minute walk test for the evaluation of pulmonary disease severity in scleroderma patients. Chest. 2007;131:217–22. doi: 10.1378/chest.06-0630. [DOI] [PubMed] [Google Scholar]
- 9.Garin MC, Highland KB, Silver RM, Strange C. Limitations to the 6-minute walk test in interstitial lung disease and pulmonary hypertension in scleroderma. J Rheumatol. 2009;36:330–6. doi: 10.3899/jrheum.080447. [DOI] [PubMed] [Google Scholar]
- 10.Nishiyama O, Kondoh Y, Kimura T, et al. Effects of pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis. Respirology. 2008;13:394–9. doi: 10.1111/j.1440-1843.2007.01205.x. [DOI] [PubMed] [Google Scholar]
- 11.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158:1384–7. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 12.Jastrzebski D, Gumola A, Gawlik R, Kozielski J. Dyspnea and quality of life in patients with pulmonary fibrosis after six weeks of respiratory rehabilitation. J Physiol Pharmacol. 2006;57(Suppl 4):139–48. [PubMed] [Google Scholar]
- 13.Buch MH, Denton CP, Furst DE, et al. Submaximal exercise testing in the assessment of interstitial lung disease secondary to systemic sclerosis: reproducibility and correlations of the 6-min walk test. Ann Rheum Dis. 2007;66:169–73. doi: 10.1136/ard.2006.054866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.du Bois RM, Weycker D, Albera C, et al. Six-minute-walk test in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:1231–7. doi: 10.1164/rccm.201007-1179OC. [DOI] [PubMed] [Google Scholar]
- 15.Manali ED, Lyberopoulos P, Triantafillidou C, et al. MRC chronic Dyspnea Scale: Relationships with cardiopulmonary exercise testing and 6-minute walk test in idiopathic pulmonary fibrosis patients: a prospective study. BMC Pulmonary Med. 2010;10:32–41. doi: 10.1186/1471-2466-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lama VN, Flaherty KR, Toews GB, et al. Prognostic value of desaturation during a 6-minute walk test in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2003;168:1084–90. doi: 10.1164/rccm.200302-219OC. [DOI] [PubMed] [Google Scholar]
- 17.de Oliveira NC, dos Santos Sabbag LM, Ueno LM, et al. Reduced exercise capacity in systemic sclerosis patients without pulmonary involvement. Scand J Rheumatol. 2007;36:458–61. doi: 10.1080/03009740701605889. [DOI] [PubMed] [Google Scholar]
- 18.Wagner PD. Skeletal muscles in chronic obstructive pulmonary disease: deconditioning, or myopathy? Respirology. 2006;11:681–6. doi: 10.1111/j.1440-1843.2006.00939.x. [DOI] [PubMed] [Google Scholar]
- 19.Da Silva JAP, Jacobs JWG, Kirwan JR, et al. Safety of low dose glucocorticoid treatment in rheumatoid arthritis: published evidence and prospective trial data. Ann Rheum Dis. 2006;65:285–93. doi: 10.1136/ard.2005.038638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alhamad EH, Shaik SA, Idrees MM, Alanezi MO, Isnani AC. Outcome measures of the 6 minute walk test: relationships with physiologic and computed tomography findings in patients with sarcoidosis. BMC Pulmonary Med. 2010;10:42–8. doi: 10.1186/1471-2466-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]