Abstract
The biomarker potential of using various lipids fractions for predicting risk of acute myocardial infarction (AMI) is controversial. We therefore compared the lipid profiles, including serum total cholesterol (TC), low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL) and triglycerides (TG), in 67 AMI patients. Patients included 28 STEMI (ST-elevated myocardial infarction) patients, 39 NSTEMI (non-ST-elevated myocardial infarction) patients and 25 patients with chest pain. Control group included 54 age- and gender-matched normal subjects. We also studied the correlation between lipid profile and systemic inflammation in these subjects. There were significant decreases in TC, LDL and HDL levels in both STEMI and NSTEMI patients as compared to normal subjects; however, patients with chest pain did not show any significant change in these lipids. Serum TG levels did not differ significantly among the study groups. There were significant increases in serum high-sensitive C-reactive protein (hs-CRP) levels in STEMI and NSTEMI patients, as compared to control group. Serum hs-CRP showed significant inverse correlation with HDL; however, hs-CRP was not correlated with TC, LDL, and TG. In conclusion, our findings suggest that reduction in serum TC does not prevent the risk of AMI, whereas a decrease in serum HDL and increase in hs-CRP strongly predisposes the risky individuals to an AMI event. We emphasize the importance of HDL and CRP measurements for the assessment of a combined lipid-inflammation risk factor that could be a useful predictor of high risk individuals, as well as a prognostic marker in AMI patients.
Keywords: acute myocardial infarction, lipid profile, inflammation, biomarker
Introduction
The pathogenesis of acute myocardial infarction (AMI) is multifactorial; however, several studies have implicated impaired lipid metabolism as one of the crucial factors in the development of this disease. Kumar et al1 observed significantly higher total cholesterol (TC) and triglyceride (TG) levels and lower high-density lipoprotein cholesterol (HDL) levels in AMI patients. In a series of 50 male AMI patients, serum low-density lipoprotein cholesterol (LDL) levels and the ratio of LDL to HDL were not significantly different among the two groups; however, serum HDL levels were significantly decreased in AMI group.2 The risk of AMI was associated with an increase in LDL and a decrease in HDL, in both Asians and non-Asians.3 Lower concentrations of serum HDL and higher serum TG were found to be independent risk factors, while serum LDL was not associated with AMI.4 Woo et al5 observed higher mean TC, LDL, and TG, as well as lower mean HDL in AMI patients; high HDL was among the protective factors. Lehto et al6 did not find any difference in mean serum TC levels between the AMI patients and controls, whereas mean HDL was significantly lower in the AMI group. The cardiac marker, troponin T, has been positively correlated with TC, LDL, and TG and negatively correlated with HDL.7
The management of dyslipidemia after myocardial infarction is an important aspect of post-myocardial infarction care.8 Using the bivariate and multivariate Cox proportional hazards analysis to identify independent predictors of subsequent major adverse coronary events (hospitalization for AMI or acute coronary syndrome), it was found that HDL predicted major adverse coronary events, which in turn provided support for interventions targeting HDL for cardiovascular risk reduction.9 Another Cox proportional hazards model has revealed that plasma HDL—but not LDL—predicts the risk of recurrent cardiovascular events over the ensuing 16 weeks. Even LDL reduction does not account for the clinical risk reduction with atorvastatin treatment after acute coronary syndrome (ACS).10 Li et al11 have shown that the cases with low HDL level had rates of AMI events and CHD mortality similar to those of the entire group, including hyperlipidemia. However, AMI attacks and deaths decreased significantly at the normal and high HDL levels, indicating that protective effect of HDL against coronary artery disease is more prominent in people with low lipid level.
The above literature clearly indicates an important role of lipids metabolism in AMI. However, the biomarker value of various components of lipids profile is not clear due to conflicting findings in various studies. We therefore compared the lipid profiles of AMI and chest pain patients with respect to normal subjects. In addition, we also studied the role of inflammation in AMI and evaluated its correlation with lipid profiles.
Patients and Methods
This prospective study was conducted on 67 AMI patients and 25 patients with chest pain admitted to Prince Sultan Cardiac Center and King Khalid University Hospital, Riyadh, Saudi Arabia. The AMI patients were classified into STEMI (ST-elevated myocardial infarction, N = 28) and NSTEMI (non-ST-elevated myocardial infarction, N = 39). Mean ages of STEMI, NSTEMI, and chest pain patients were 56.21 ± 12.65 years, 61.98 ± 10.83 years, and 53.83 ± 13.75 years, respectively (Table 1). We also included 54 age- and gender-matched controls for comparison.
Table 1.
Characteristics of subjects.
| Age (y) | Male/female ratio | Smoking (%) | BMI (kg/m2) | |
|---|---|---|---|---|
| Control | 54.85 ± 10.14 | 3.6 | 28 | 27.64 ± 3.78 |
| STEMI | 56.21 ± 12.65 | 4.8 | 41 | 28.97 ± 4.80 |
| NSTEMI | 61.98 ± 10.83 | 3.3 | 24 | 28.81 ± 5.04 |
| Chest pain | 53.83 ± 13.75 | 2.9 | 26 | 32.27 ± 9.63 |
The diagnosis of myocardial infarction required the presence at least two of the following criteria: (i) history of characteristic prolonged (≥30 minutes) pain or discomfort, (ii) creatine kinase (CK) levels exceeding twice the upper limit of normal (or CK—MB ≥ 50% of total CK); (iii) presence of new Q waves or new abnormal ST—T features.12 Patients with STEMI were classified on the basis of the following criteria: (i) continuous chest pain upon presentation, refractory to nitrates, and lasting ≥ 30 minutes; (ii) ST-segment elevation of ≥0.2 mV in ≥2 contiguous precordial leads, or ≥0.1 mV in ≥2 contiguous limb leads, or new left bundle branch block on admission electrocardiogram; (iii) presentation within the first 12 hours from index pain. Patients with NSTEMI were required to have angina-like chest pain at rest in the last 24 hours lasting ≥ 5 minutes, with associated ST segment depression of ≥0.1 mV in ≥2 contiguous leads upon presentation.13 The patient exclusion criteria included recent surgery, active infection, chronic inflammatory diseases, significant hepatic, or renal dysfunction and malignancy. The protocol of this study was approved by our Institutional Review Board (IRB) for human studies and all the patients signed informed consent.
Venous blood was collected after overnight fasting from all the subjects in serum separator Vaccutainer tubes. The levels of serum total cholesterol, lipoproteins, triglycerides, and hs-CRP were analyzed using an Autoanalyzer (Roche, Germany). The data were evaluated by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test using SPSS statistical package version 17. Pearson’s and Spearman’s tests were used for correlation analysis of continuous and categorical variables respectively. P values < 0.05 were considered as statistically significant.
Results
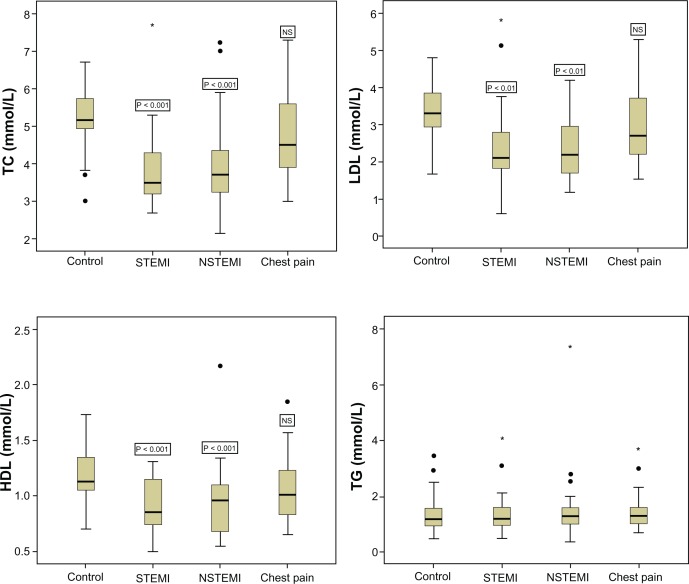
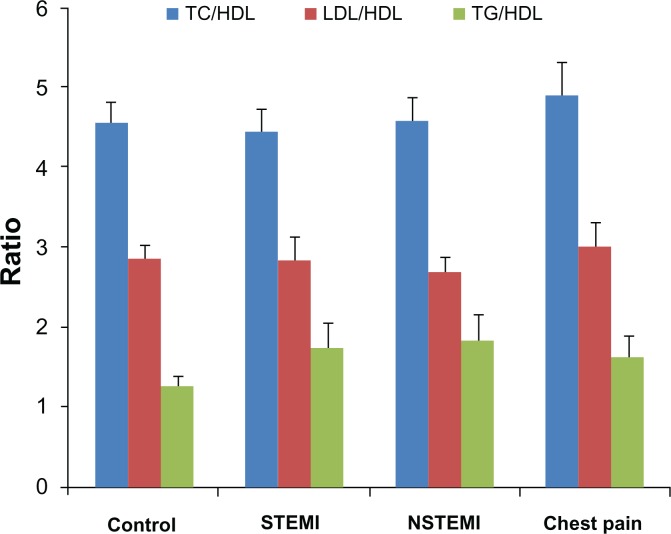
The characteristics of patients and controls are summarized in Table 1. The family history of associated diseases was noted for hypertension (3/28 STEMI, 1/39 NSTEMI and 5/25 chest pain) and CAD and hypertension (1 STEMI, 2 NSTEMI and 2 chest pain). There were significant decreases in TC (ANOVA F = 13.848, P = 0.000), LDL (ANOVA F = 6.520, P = 0.000), and HDL (ANOVA F = 6.027, P = 0.001) levels in both STEMI and NSTEMI patients, as compared to normal subjects; however, patients with chest pain did not show any significant change in these lipids (Table 2 and Fig. 1). Serum TG levels did not differ significantly among STEMI, NSTEMI, or chest pain patients, as compared to control group (ANOVA F = 0.061, P = 0.980) (Table 2 and Fig. 1). The commonly used informative lipids ratios—including TC/HDL, LDL/HDL, and TG/HDL—did not differ significantly among different groups (Fig. 2).
Table 2.
Lipid profiles of control and patient groups.
| TC | LDL | HDL | TG | |
|---|---|---|---|---|
| Control | 5.185 ± 0.136 | 3.298 ± 0.161 | 1.194 ± 0.052 | 1.422 ± 0.127 |
| STEMI | 3.769 ± 0.203** | 2.418 ± 0.237* | 0.896 ± 0.050** | 1.399 ± 0.160 |
| NSTEMI | 3.901 ± 0.184** | 2.336 ± 0.125* | 0.921 ± 0.049** | 1.476 ± 0.183 |
| Chest pain | 4.767 ± 0.267 | 2.973 ± 0.231 | 1.058 ± 0.071 | 1.491 ± 0.173 |
Notes: Values are mean ± SEM, mmol/l.
P < 0.01 and
P < 0.001 versus control group using Dunnett’s multiple comparison test.
Figure 1.
Box plots showing lipid profile (TC, LDL, HDL and TG) in normal subjects and patients with AMI (STEMI and NSTEMI) and chest pain.
Notes: Values are median 25%–75% interquartile; outliers and extremes are shown as filled circles and stars respectively. The P values are against control group using Dunnett’s test.
Figure 2.
Ratios of TC, LDL and TG to HDL in controls and different patient groups.
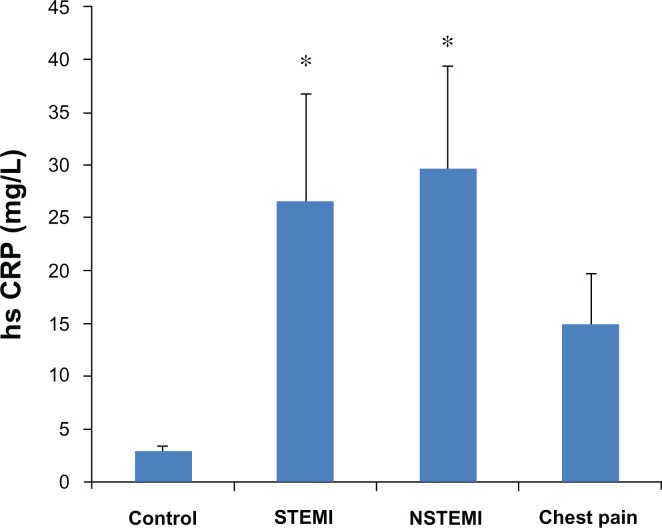
There were significant increases in serum hs-CRP levels in STEMI and NSTEMI patients as compared to control group (ANOVA F = 3.213, P = 0.025) (Fig. 3). Serum hs-CRP showed significant inverse correlation with HDL (R = −0.193, P = 0.042). However, hs-CRP was not correlated with TC (R = −0.010, P = 0.910), LDL (R = 0.065, P = 0.497) and TG (R = −0.031, P = 0.733).
Figure 3.
Serum hs-CRP in different groups.
Note: *P < 0.05 versus control group using Dunnett’s test.
Age was significantly and inversely correlated with TC (R = −0.144, P = 0.025) and LDL (R = −0.164, P = 0.015) and directly correlated with hs-CRP (R = 0.193, P = 0.022) (Table 3). There was no significant correlation between age and HDL and TG. Body mass index, gender, and smoking did not show any significant correlation with lipids or hs-CRP (Table 3). We did not observe any significant correlation between systolic blood pressure and lipids or hs-CRP; however, diastolic blood pressure showed significant correlation with TC (R = 0.198, P = 0.013) and LDL (R = 0.162, P = 0.046) but not with HDL, TG or hs-CRP (Table 3).
Table 3.
Correlations between subjects’ characteristics and biochemical parameters.
| TC | LDL | HDL | TG | hs-CRP | |
|---|---|---|---|---|---|
| Age | R = −0.144 | R = −0.164 | R = −0.071 | R = −0.034 | R = 0.193 |
| P = 0.025* | P = 0.015* | P = 0.293 | P = 0.596 | P = 0.022* | |
| Gender | R = −0.029 | R = −0.108 | R = −0.008 | R = −0.043 | R = 0.071 |
| P = 0.657 | P = 0.105 | P = 0.909 | P = 0.501 | P = 0.401 | |
| Smoking | R = 0.076 | R = 0.019 | R = −0.028 | R = 0.108 | R = 0.135 |
| P = 0.370 | P = 0.808 | P = 0.727 | P = 0.163 | P = 0.231 | |
| BMI | R = −0.034 | R = −0.097 | R = 0.035 | R = 0.012 | R = −0.072 |
| P = 0.644 | P = 0.202 | P = 0.645 | P = 0.876 | P = 0.505 | |
| BP (systolic) | R = 0.133 | R = 0.095 | R = 0.116 | R = −0.011 | R = −0.175 |
| P = 0.095 | P = 0.242 | P = 0.154 | P = 0.895 | P = 0.185 | |
| BP (diastolic) | R = 0.198 | R = 0.162 | R = 0.158 | R = 0.024 | R = −0.230 |
| P = 0.013* | P = 0.046* | P = 0.052 | P = 0.766 | P = 0.079 |
Note:
Statistically significant.
Discussion
Our results showed significant decreases in TC, LDL, and HDL levels in AMI patients (Table 2). Gorecki et al14 observed higher levels of TC and LDL in patients with complicated versus those with uncomplicated clinical course of infarction, suggesting higher levels of these biomarkers during the first 24 hours of AMI have a strong negative prognostic value. Akosah et al15 reported acceptable or optimal LDL levels in a series of 183 young adults experiencing AMI. Gaziano et al8 found that the mean TC and LDL levels were significantly lower while in-hospital than levels 2 to 3 months later; however, from a clinical perspective, in-hospital levels can be used to guide decisions regarding lipid-lowering therapy, which can begin in the immediate post-MI setting. In a series of 34 AMI patients, on the first day after admission there were significant decreases in TC (14.1%) and LDL (14.4%), as well as insignificant decreases in HDL (9.3%) and TG (19.5%).16 On the basis of lipid profile data of 97 patients with NSTEMI and unstable angina, it has been concluded that HDL level (as opposed to LDL and TG) adds prognostic value to the prediction of in-hospital recurrent events during non-ST-elevation acute coronary syndromes.17 Rosoklija et al18 have followed-up the HDL cholesterol levels in STEMI patients from 24 hours to 3 months and concluded that the optimal times for determining the HDL level are the first 24 hours of the actual event; this is due to the fact that in the first 24 hours there is a relevant decrease of the HDL cholesterol level in the blood. The cause of reduced serum lipids in AMI patients in our study is not clear. It could be related to dietary modifications or due to metabolic changes during acute crisis. Nevertheless, the lipids data negate the general conception that dietary lipids restrictions may prevent the risk of AMI.
High serum levels of HDL are associated with reduced risk for the development of atherosclerotic disease. HDL particles are believed to be antiatherogenic, secondary to their capacity to drive reverse cholesterol transport and antagonize pathways of inflammation, thrombosis, and oxidation. The majority of patients in both the primary and secondary prevention settings continue to experience significant residual risk for acute cardiovascular events, even when their LDL cholesterol is lowered aggressively via a combination of lifestyle modification and pharmacologic intervention.19 Al Aqeel et al20 have observed that HDL appears to be the main lipid risk factor in patients presenting with AMI in Kuwaiti patients, suggesting that primary prevention strategies should focus on treatment modalities that increase HDL. In an in-vivo mouse model of myocardial ischemia/reperfusion, it has been observed that HDL and its sphingolipid component, sphingosine-1-phosphate, dramatically attenuated the infarct size, suggesting a rapid therapeutic elevation of plasma HDL levels may be beneficial in patients at high risk of acute myocardial ischemia.21 There is an increased focus on targeting and treating low serum levels of HDL in an effort to further reduce risk for cardiovascular events, including myocardial infarction.19
In our study, none of the lipids ratios were found to be informative in prediction of AMI (Fig. 2). On the contrary, LDL/HDL ratio has been suggested to be an independent predictor for AMI in the Japanese rural population.22 Goswami et al23 have suggested that the apo-B/apo-AI ratio is a better discriminator of CAD risk in the atherosclerosis-prone population than any of the conventional lipid ratios such as TC/HDL or LDL/HDL. Karthikeyan et al3 have also found the strongest association occurs between ApoB/ApoA1 and the risk of AMI. McQueen et al24 have observed the highest population-attributable risk (PAR) with the ratio of ApoB/ApoA1 (54%) compared to the PAR associated with the ratios of LDL/HDL (37%) and TC/HDL (32%).
We observed significant increase in hs-CRP levels in AMI patients (Fig. 3), which is in agreement with earlier reports.25–27 hs-CRP is a sensitive marker of inflammation and a potential independent predictor of cardiovascular disease as it may play a role in the development of atherosclerosis; additionally, it also adversely affects mortality.28 Suzuki et al29 have found nearly equivalent systemic and coronary levels of hs-CRP in AMI patients. They also observed a positive correlation between systemic hs-CRP and coronary plaque area, suggesting an important link between systemic and coronary levels of inflammation. Their observations are also associated with vulnerable coronary morphology in the development of acute coronary syndromes.29 Higher baseline hs-CRP level showed significant association with 12-month all-cause mortality, independent of other prognostic markers, in overweight or obese AMI patients.30 The findings of a cross sectional study of 188 patients with STEMI suggested that plasma hs-CRP could be useful for prediction of development of heart failure in AMI patients.31 Elevated serum hs-CRP levels in patients with AMI < 6 hours may portend vulnerable plaque rupture.32 The patients with persistent, severe, treatment-unresponsive unstable angina (UA) had significantly higher CRP levels and a higher incidence of clinical events than patients with treatment-responsive UA, suggesting an important prognostic value of plasma CRP.33 However, there was no correlation between plasma CRP and TC or HDL in UA patients.33 A significant inverse correlation between hs-CRP and HDL (but not with other lipid fractions), as observed in our study, indicates the co-existence of inflammation and impaired lipid metabolism in AMI patients. The correlation data showed that serum HDL was not affected by age, gender, BMI, smoking, and blood pressure (Table 3). Although the levels of hs-CRP were directly correlated with age, hs-CRP was not influenced by gender, BMI, smoking, and blood pressure (Table 3).
Conclusion
Our findings suggest that reduction in serum cholesterol does not prevent the risk of AMI. There was a significant increase in systemic inflammation in AMI patients, inversely correlated with HDL levels, suggesting an important role of inflammatory mediators in AMI. Thus, a decrease in serum HDL and increase in hs-CRP strongly predispose the risky individuals to the event of AMI. We emphasize the importance of HDL and hs-CRP measurements in the assessment of a combined lipido-inflammatory risk factor for the screening of high risk individuals and the prognosis of AMI.
Acknowledgments
This study was supported by the National Plan for Science and Technology (NPST) Program by King Saud University Project Number 08-BIO571-02. We are thankful to Dr. Shahid Habib and Dr. Abdulrahman Al Moghairy for their clinical observations. We would also like to acknowledge the technical assistance of Adnan Ali Khan and the nursing staff of the King Khalid University Hospital and the Prince Sultan Cardiac Center (Riyadh) for sample collection and patient care.
Footnotes
Author Contributions
Conceived and designed the experiments: HAK, ASA, SHS. Analyzed the data: HAK. Wrote the first draft of the manuscript: HAK. Contributed to the writing of the manuscript: HAK, ASA, SHS. Agree with manuscript results and conclusions: HAK, ASA, SHS. Jointly developed the structure and arguments for the paper: HAK, ASA, SHS. Made critical revisions and approved final version: HAK, ASA, SHS. All authors reviewed and approved of the final manuscript.
Funding
A grant from National Plan for Science and Technology (NPST) Program by King Saud University Project Number 08-BIO571-02.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.Kumar A, Nagtilak S, Sivakanesan R, Gunasekera S. Cardiovascular risk factors in elderly normolipidemic acute myocardial infarct patients-a case controlled study from India. Southeast Asian J Trop Med Public Health. 2009;40:581–92. [PubMed] [Google Scholar]
- 2.Kulsoom B, Nazrul SH. Association of serum C-reactive protein and LDL:HDL with myocardial infarction. J Pak Med Assoc. 2006;56:318–22. [PubMed] [Google Scholar]
- 3.Karthikeyan G, Teo KK, Islam S, et al. Lipid profile, plasma apolipoproteins, and risk of a first myocardial infarction among Asians: an analysis from the INTERHEART Study. J Am Coll Cardiol. 2009;53:244–53. doi: 10.1016/j.jacc.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 4.Tokuda Y. Risk factors for acute myocardial infarction among Okinawans. J Nutr Health Aging. 2005;9:272–6. [PubMed] [Google Scholar]
- 5.Woo J, Ho SC, Wong SL, et al. Lipids, lipoproteins and other coronary risk factors in Chinese male survivors of myocardial infarction. Int J Cardiol. 1993;39:195–202. doi: 10.1016/0167-5273(93)90038-i. [DOI] [PubMed] [Google Scholar]
- 6.Lehto S, Palomaki P, Miettinen H, et al. Serum cholesterol and high density lipoprotein cholesterol distributions in patients with acute myocardial infarction and in the general population of Kuopio province, eastern Finland. J Intern Med. 1993;233:179–85. doi: 10.1111/j.1365-2796.1993.tb00671.x. [DOI] [PubMed] [Google Scholar]
- 7.Nayak SB, Pinto Pereira LM, Boodoo S, et al. Association of troponin T and altered lipid profile in patients admitted with acute myocardial infarction. Arch Physiol Biochem. 2010;116:21–7. doi: 10.3109/13813450903397638. [DOI] [PubMed] [Google Scholar]
- 8.Gaziano JM, Hennekens CH, Satterfield S, et al. Clinical utility of lipid and lipoprotein levels during hospitalization for acute myocardial infarction. Vasc Med. 1999;4:227–31. doi: 10.1177/1358836X9900400404. [DOI] [PubMed] [Google Scholar]
- 9.Koro CE, Bowlin SJ, Stump TE, Sprecher DL, Tierney WM. The independent correlation between high-density lipoprotein cholesterol and subsequent major adverse coronary events. Am Heart J. 2006;151:755, e1–6. doi: 10.1016/j.ahj.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Olsson AG, Schwartz GG, Szarek M, et al. High-density lipoprotein, but not low-density lipoprotein cholesterol levels influence short-term prognosis after acute coronary syndrome: results from the MIRACL trial. Eur Heart J. 2005;26:890–6. doi: 10.1093/eurheartj/ehi186. [DOI] [PubMed] [Google Scholar]
- 11.Li JZ, Chen ML, Wang S, Dong J, Zeng P, Hou LW. Apparent protective effect of high density lipoprotein against coronary heart disease in the elderly. Chin Med J. 2004;117:511–5. [PubMed] [Google Scholar]
- 12.Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined—a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959–69. doi: 10.1016/s0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- 13.Braunwald E. Unstable angina: a classification. Circulation. 1989;80:410–4. doi: 10.1161/01.cir.80.2.410. [DOI] [PubMed] [Google Scholar]
- 14.Gorecki A, Bednarz B, Jaxa-Chamiec T, et al. Lipid profile during the first 24 hours after myocardial infarction has significant prognostic value. Kardiol Pol. 2004;60:229–36. [PubMed] [Google Scholar]
- 15.Akosah KO, Cerniglia RM, Havlik P, Schaper A. Myocardial infarction in young adults with low-density lipoprotein cholesterol levels ≤ 100 mg/dL: clinical profile and 1-year outcomes. Chest. 2001;120:1953–8. doi: 10.1378/chest.120.6.1953. [DOI] [PubMed] [Google Scholar]
- 16.Pfohl M, Schreiber I, Liebich HM, Häring HU, Hoffmeister HM. Upregulation of cholesterol synthesis after acute myocardial infarction-is cholesterol a positive acute phase reactant? Atherosclerosis. 1999;142:389–93. doi: 10.1016/s0021-9150(98)00242-1. [DOI] [PubMed] [Google Scholar]
- 17.Correia LC, Rocha MS, Esteves JP. HDL-cholesterol level provides additional prognosis in acute coronary syndromes. Int J Cardiol. 2009;136:307–14. doi: 10.1016/j.ijcard.2008.05.067. [DOI] [PubMed] [Google Scholar]
- 18.Rosoklija A, Georgievska-Ismail L, Dzekova-Stojkova S. Tracking the changes in the HDL cholesterol levels at different time intervals after acute myocardial infraction. Prilozi. 2004;25:67–82. [PubMed] [Google Scholar]
- 19.Davidson MH, Toth PP. High-density lipoprotein metabolism: potential therapeutic targets. Am J Cardiol. 2007;100:n32–40. doi: 10.1016/j.amjcard.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Al Aqeel A, Mojiminiyi OA, Al Dashti R, Al Ozairi ES. Differences in physician compliance with guideline on lipid profile determination within 24 h after acute myocardial infarction. Med Princ Pract. 2005;14:41–5. doi: 10.1159/000081922. [DOI] [PubMed] [Google Scholar]
- 21.Theilmeier G, Schmidt C, Herrmann J, et al. High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation. 2006;114:1403–9. doi: 10.1161/CIRCULATIONAHA.105.607135. [DOI] [PubMed] [Google Scholar]
- 22.Yokokawa H, Yasumura S, Tanno K, et al. Serum low-density lipoprotein to high-density lipoprotein ratio as a predictor of future acute myocardial infarction among men in a 2.7-year cohort study of a Japanese northern rural population. J Atheroscler Thromb. 2011;18:89–98. doi: 10.5551/jat.5215. [DOI] [PubMed] [Google Scholar]
- 23.Goswami B, Rajappa M, Mallika V, Kumar S, Shukla DK. Apo-B/apo-AI ratio: a better discriminator of coronary artery disease risk than other conventional lipid ratios in Indian patients with acute myocardial infarction. Acta Cardiol. 2008;63:749–55. doi: 10.2143/AC.63.6.2033393. [DOI] [PubMed] [Google Scholar]
- 24.McQueen MJ, Hawken S, Wang X, et al. Lipids, lipoproteins, and apo-lipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet. 2008;372:224–33. doi: 10.1016/S0140-6736(08)61076-4. [DOI] [PubMed] [Google Scholar]
- 25.Blancke F, Claeys MJ, Jorens P, et al. Systemic inflammation and reperfusion injury in patients with acute myocardial infarction. Mediators Inflamm. 2005;2005:385–9. doi: 10.1155/MI.2005.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo Y, Jiang D, Wen D, Yang J, Li L. Changes in serum interleukin-6 and high-sensitivity C-reactive protein levels in patients with acute coronary syndrome and their responses to simvastatin. Heart Vessels. 2004;19:257–62. doi: 10.1007/s00380-004-0776-6. [DOI] [PubMed] [Google Scholar]
- 27.Auer J, Berent R, Lassnig E, Eber B. C-reactive protein and coronary artery disease. Jpn Heart J. 2002;43:607–19. doi: 10.1536/jhj.43.607. [DOI] [PubMed] [Google Scholar]
- 28.Koenig W, Khuseyinova N, Baumert J, Meisinger C. Prospective study of high-sensitivity C-reactive protein as a determinant of mortality: results from the MONICA/KORA Augsburg Cohort Study, 1984–1998. Clin Chem. 2008;54:335–42. doi: 10.1373/clinchem.2007.100271. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki M, Saito M, Nagai T, Saeki H, Kazatani Y. Systemic versus coronary levels of inflammation in acute coronary syndromes. Angiology. 2006;57:459–63. doi: 10.1177/0003319706290742. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed K, Jeong MH, Chakraborty R, et al. Prognostic impact of baseline high-sensitivity C-reactive protein in patients with acute myocardial infarction undergoing percutaneous coronary intervention based on body mass index. Korean Circ J. 2012;42:164–72. doi: 10.4070/kcj.2012.42.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nozari Y, Geraiely B. Correlation between the serum levels of uric acid and HS-CRP with the occurrence of early systolic failure of left ventricle following acute myocardial infarction. Acta Med Iran. 2011;49:531–5. [PubMed] [Google Scholar]
- 32.Yip HK, Wu CJ, Chang HW, et al. Levels and values of serum high-sensitivity C-reactive protein within 6 hours after the onset of acute myocardial infarction. Chest. 2004;126:1417–22. doi: 10.1378/chest.126.5.1417. [DOI] [PubMed] [Google Scholar]
- 33.Li JJ, Jiang H, Huang CX, et al. Elevated level of plasma C-reactive protein in patients with unstable angina: its relations with coronary stenosis and lipid profile. Angiology. 2002;53:265–72. doi: 10.1177/000331970205300303. [DOI] [PubMed] [Google Scholar]