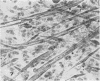
Abstract
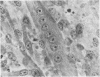
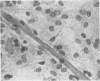
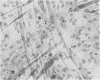
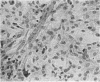
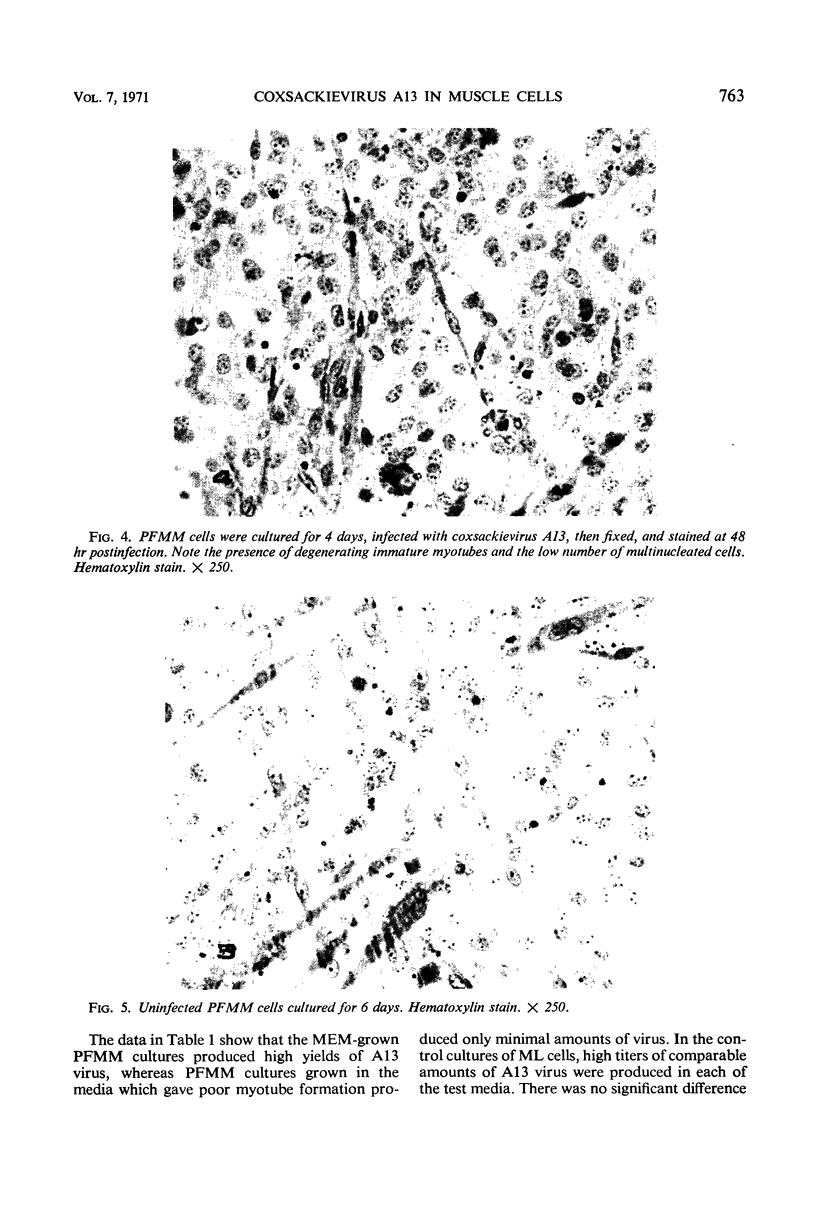
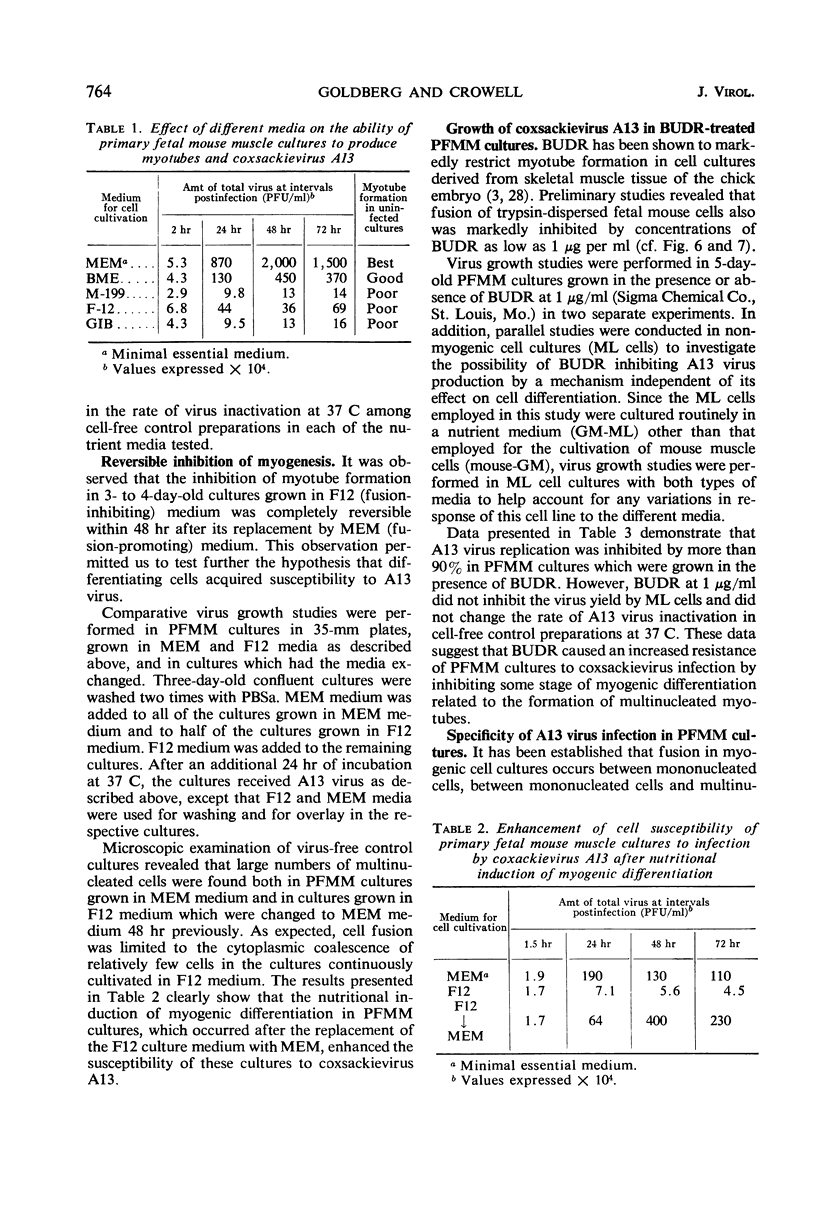
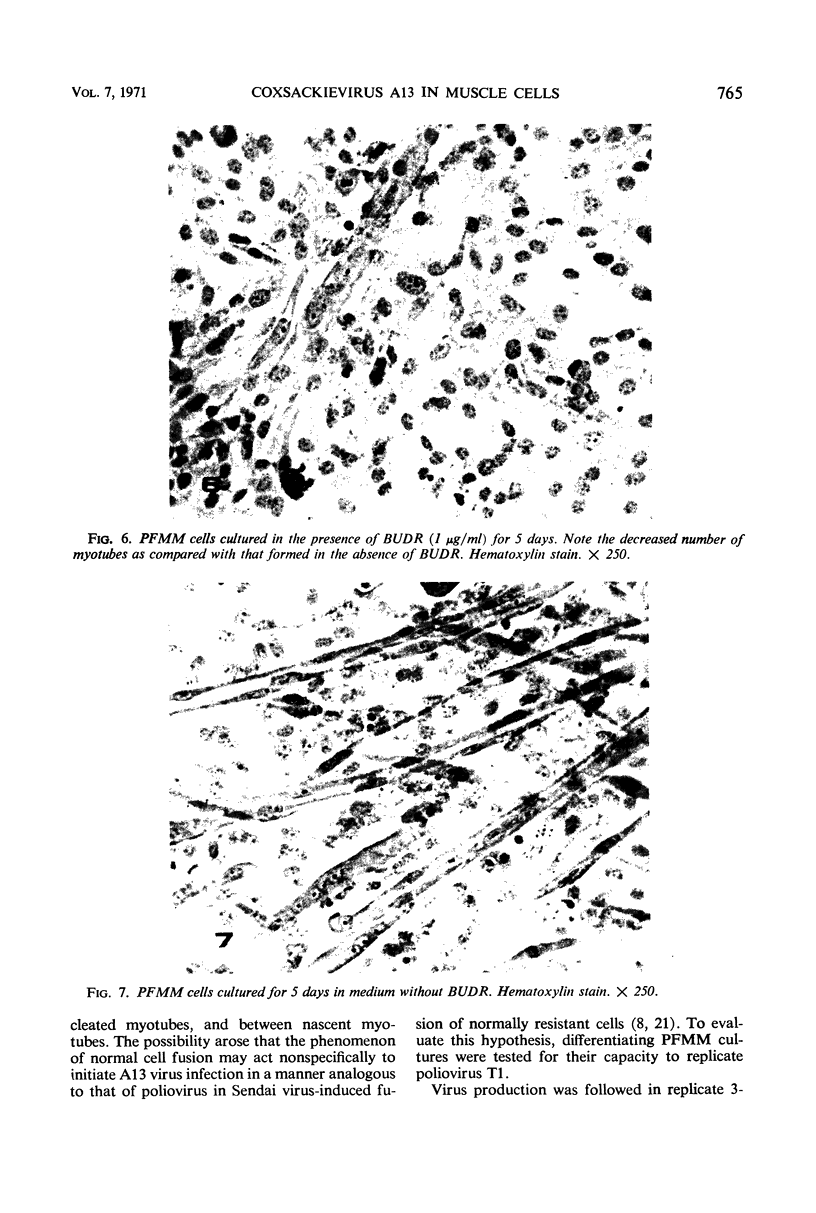
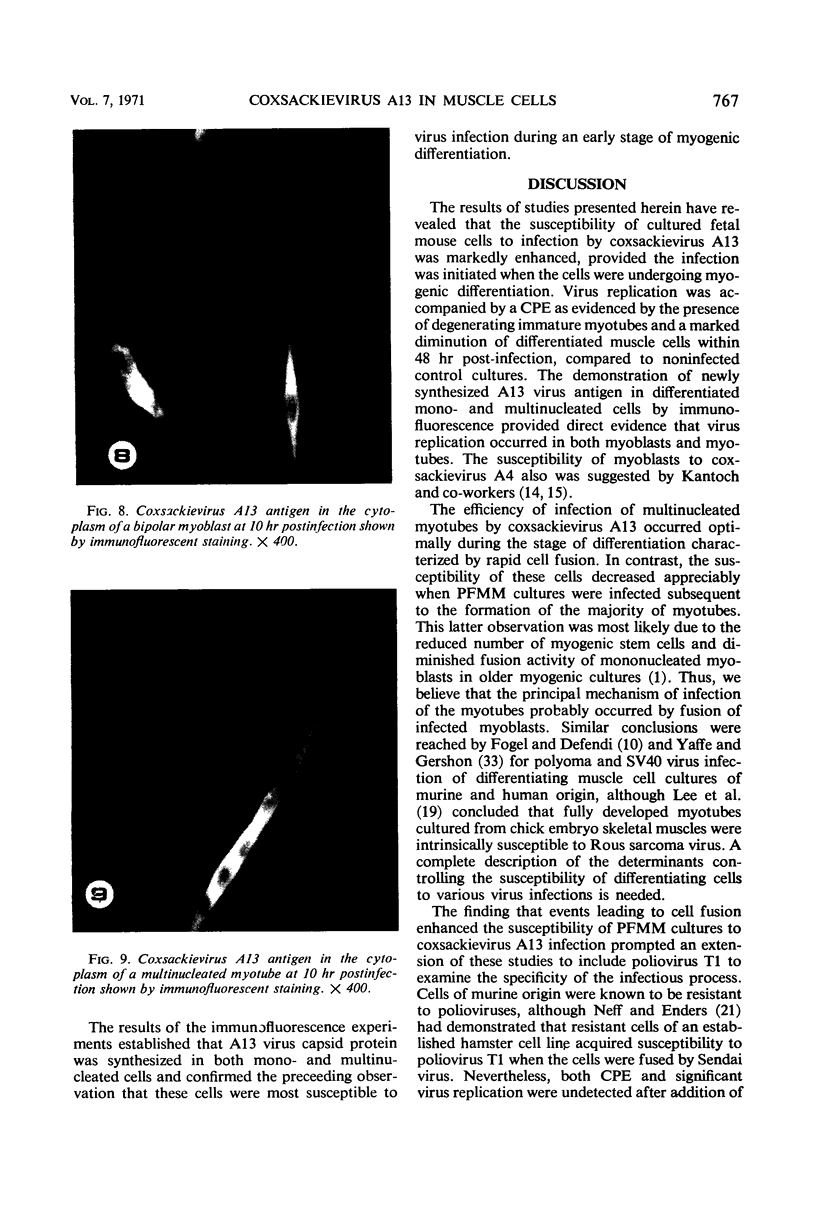
The interaction of coxsackievirus A13 with differentiating muscle cells, cultured from tissues of the fetal mouse, was studied. Cultures infected at that stage of myogenic differentiation characterized by the rapid formation of multinucleated myotubes produced maximum virus titers of over 107 plaque-forming units. Virus-induced cytopathic effect was characterized by a marked diminution in the number of multinucleated cells. The susceptibility of these cultures decreased appreciably when infection was initiated after the majority of the myotubes had formed. The demonstration of newly synthesized A13 virus antigen by immunofluorescence provided direct evidence that A13 virus replication occurred both in myoblasts and myotubes. The synthesis of A13 virus was markedly depressed in muscle cultures in which the formation of multinucleated cells was inhibited by BUDR or by fusion-inhibiting media. After reversal of this inhibition, the cultures acquired the increased susceptibility to A13 virus characteristic of cells undergoing myogenic differentiation. In contrast to the results obtained with coxsackievirus A13, the primary fetal mouse muscle cultures were resistant to poliovirus T1. It is suggested that changes in the surfaces of developing muscle cells may coincide with the formation and disappearance of specific virus receptors and thereby regulate the cell susceptibility to coxsackievirus A13.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bischoff R., Holtzer H. Mitosis and the processes of differentiation of myogenic cells in vitro. J Cell Biol. 1969 Apr;41(1):188–200. doi: 10.1083/jcb.41.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAME P. E., CROWELL R. L. STUDIES OF RESISTANCE OF FETAL MOUSE TISSUES IN CULTURE TO COXSACKIE GROUP A VIRUSES. Virology. 1964 Aug;23:542–552. doi: 10.1016/0042-6822(64)90238-7. [DOI] [PubMed] [Google Scholar]
- Crowell R. L. Specific cell-surface alteration by enteroviruses as reflected by viral-attachment interference. J Bacteriol. 1966 Jan;91(1):198–204. doi: 10.1128/jb.91.1.198-204.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Nutrition needs of mammalian cells in tissue culture. Science. 1955 Sep 16;122(3168):501–514. doi: 10.1126/science.122.3168.501. [DOI] [PubMed] [Google Scholar]
- Enders J. F., Holloway A., Grogan E. A. Replication of poliovirus I in chick embryo and hamster cells exposed to sendai virus. Proc Natl Acad Sci U S A. 1967 Mar;57(3):637–644. doi: 10.1073/pnas.57.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel M. Synthesis of an antigenic material (RAM) in rat cells cultured in vitro and in rat organs in vivo. Exp Cell Res. 1965 Nov;40(2):365–382. doi: 10.1016/0014-4827(65)90270-3. [DOI] [PubMed] [Google Scholar]
- Geme J. W., Jr, Toyama P. S., Brumbaugh J. L., Dietz M. E. Incompetent relationship between coxsackievirus A6 ribonucleic acid and mouse cells. Proc Soc Exp Biol Med. 1967 May;125(1):58–62. doi: 10.3181/00379727-125-32012. [DOI] [PubMed] [Google Scholar]
- Goldberg R. J., Gravell M., Crowell R. L. Evidence for the susceptibility of primary fetal mouse cells in culture to coxsackievirus A13. Proc Soc Exp Biol Med. 1969 Nov;132(2):743–748. doi: 10.3181/00379727-132-34301. [DOI] [PubMed] [Google Scholar]
- HAM R. G. CLONAL GROWTH OF MAMMALIAN CELLS IN A CHEMICALLY DEFINED, SYNTHETIC MEDIUM. Proc Natl Acad Sci U S A. 1965 Feb;53:288–293. doi: 10.1073/pnas.53.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J., HOYER B. H. Early stages of enterovirus infection. Cold Spring Harb Symp Quant Biol. 1962;27:101–112. doi: 10.1101/sqb.1962.027.001.013. [DOI] [PubMed] [Google Scholar]
- KANTOCH M., KUCZKOWSKA B. STUDIES ON SUSCEPTIBILITY OF MOUSE MUSCLE CELLS TO COXSACKIE A4 VIRUS INFECTION. Arch Immunol Ther Exp (Warsz) 1964;12:358–369. [PubMed] [Google Scholar]
- KITIYAKARA A., ANGEVINE D. M. A STUDY OF THE PATTERN OF POSTEMBRYONIC GROWTH OF M. GRACILIS IN MICE. Dev Biol. 1963 Dec;8:322–340. doi: 10.1016/0012-1606(63)90033-2. [DOI] [PubMed] [Google Scholar]
- KONIGSBERG I. R. The differentiation of cross-striated myofibrils in short term cell culture. Exp Cell Res. 1960 Nov;21:414–420. doi: 10.1016/0014-4827(60)90273-1. [DOI] [PubMed] [Google Scholar]
- KUNIN C. M. CELLULAR SUSCEPTIBILITY TO ENTEROVIRUSES. Bacteriol Rev. 1964 Dec;28:382–390. doi: 10.1128/br.28.4.382-390.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kańtoch M., Siemińska A. Studies on the susceptibility of mouse muscle cultures to Coxsackie A-4 viruses in vitro. Arch Immunol Ther Exp (Warsz) 1965;13(4):413–421. [PubMed] [Google Scholar]
- Lee H. H., Kaighn M. E., Ebert J. D. Induction of thymidine-3H incorporation in multinucleated myotubes by Rous sarcoma virus. Int J Cancer. 1968 Jan 15;3(1):126–136. doi: 10.1002/ijc.2910030115. [DOI] [PubMed] [Google Scholar]
- MORGAN J. F., MORTON H. J., PARKER R. C. Nutrition of animal cells in tissue culture; initial studies on a synthetic medium. Proc Soc Exp Biol Med. 1950 Jan;73(1):1–8. doi: 10.3181/00379727-73-17557. [DOI] [PubMed] [Google Scholar]
- Neff J. M., Enders J. F. Poliovirus replication and cytopathogenicity in monolayer hamster cell cultures fused with beta propiolactone-inactivated Sendai virus. Proc Soc Exp Biol Med. 1968 Jan;127(1):260–267. doi: 10.3181/00379727-127-32668. [DOI] [PubMed] [Google Scholar]
- Okazaki K., Holtzer H. An analysis of myogenesis in vitro using fluorescein-labeled antimyosin. J Histochem Cytochem. 1965 Nov-Dec;13(8):726–739. doi: 10.1177/13.8.726. [DOI] [PubMed] [Google Scholar]
- SLATER E. A., SYVERTON J. T. The cultivation of Coxsackie virus. Proc Soc Exp Biol Med. 1950 Jul;74(3):509–510. doi: 10.3181/00379727-74-17955. [DOI] [PubMed] [Google Scholar]
- ST GEME J. W., Jr BEHAVIOR OF COXSACKIE A6 VIRUS IN MURINE CELLS: FAILURE OF NEWER TECHNIQUES TO DETECT MULTIPICATION IN VITRO. J Bacteriol. 1964 Apr;87:969–970. doi: 10.1128/jb.87.4.969-970.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOCKDALE F. E., HOLTZER H. DNA synthesis and myogenesis. Exp Cell Res. 1961 Sep;24:508–520. doi: 10.1016/0014-4827(61)90450-5. [DOI] [PubMed] [Google Scholar]
- STOCKDALE F., OKAZAKI K., NAMEROFF M., HOLTZER H. 5-BROMODEOXYURIDINE: EFFECT ON MYOGENESIS IN VITRO. Science. 1964 Oct 23;146(3643):533–535. doi: 10.1126/science.146.3643.533. [DOI] [PubMed] [Google Scholar]
- STULBERG C. S., SCHAPIRA R., EIDAM C. R. Comparative characteristics of two Coxsackie viruses in tissue culture. J Immunol. 1954 Jan;72(1):107–111. [PubMed] [Google Scholar]
- YAFFE D., FELDMAN M. THE EFFECT OF ACTINOMYCIN D ON HEART AND THIGH MUSCLE CELLS GROWN IN VITRO. Dev Biol. 1964 Jun;9:347–366. doi: 10.1016/0012-1606(64)90030-2. [DOI] [PubMed] [Google Scholar]
- YAFFE D., FELDMAN M. THE FORMATION OF HYBRID MULTINUCLEATED MUSCLE FIBERS FROM MYOBLASTS OF DIFFERENT GENETIC ORIGIN. Dev Biol. 1965 Apr;11:300–317. doi: 10.1016/0012-1606(65)90062-x. [DOI] [PubMed] [Google Scholar]
- Yaffe D., Gershon D. Multinucleated muscle fibres: induction of DNA synthesis and mitosis by polyoma virus infection. Nature. 1967 Jul 22;215(5099):421–424. doi: 10.1038/215421a0. [DOI] [PubMed] [Google Scholar]