Abstract
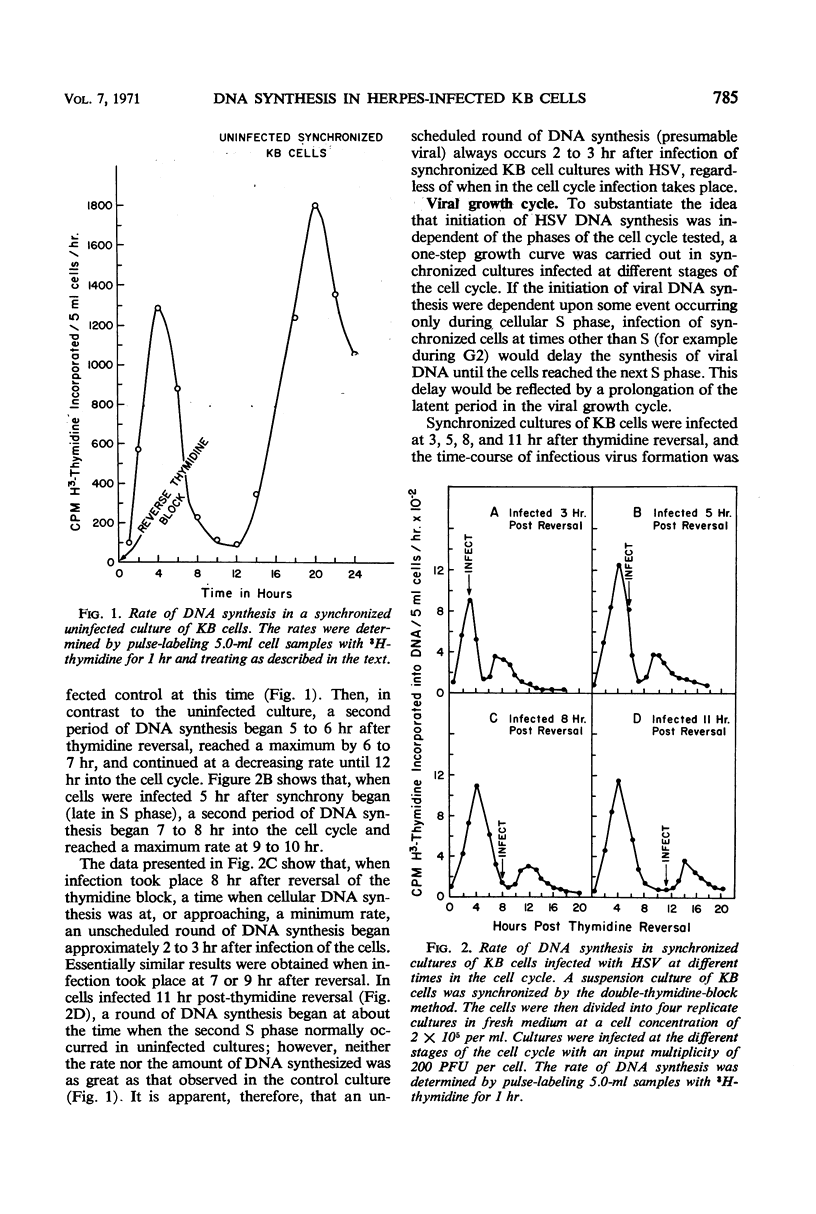
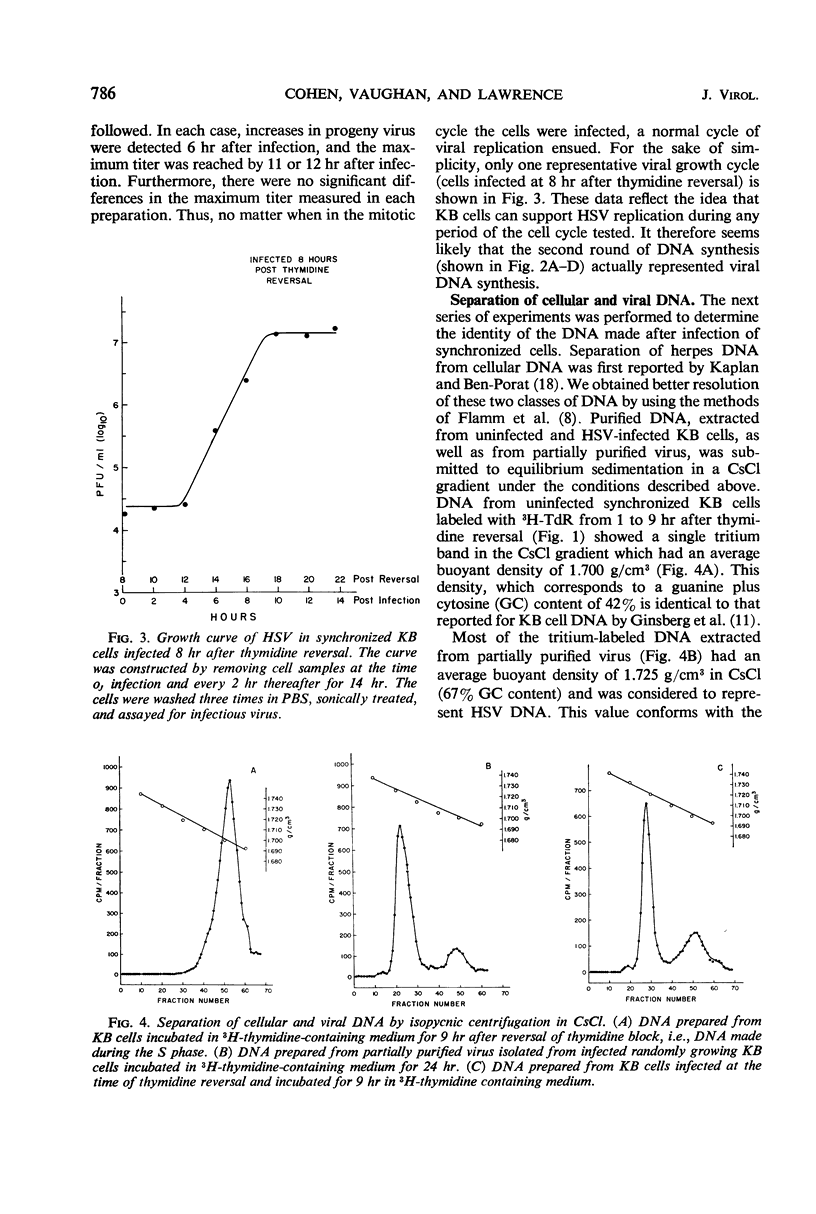
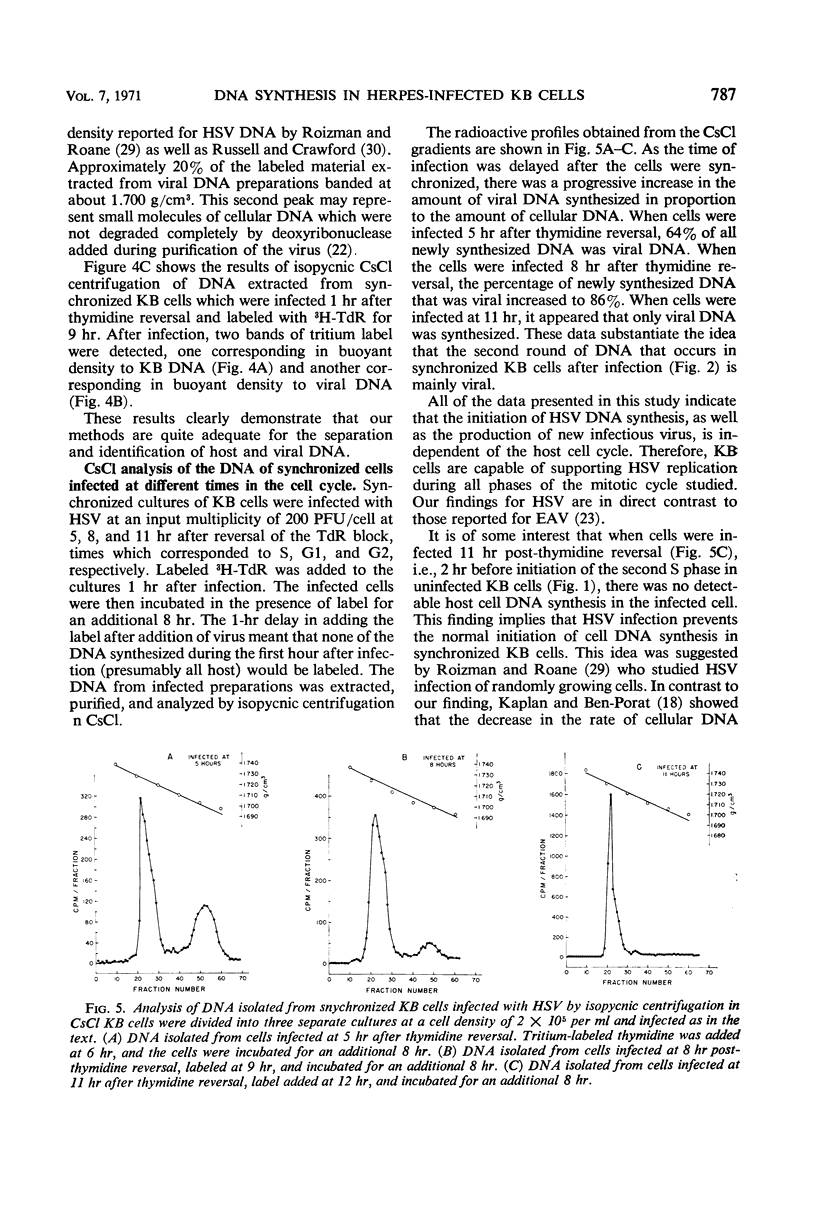
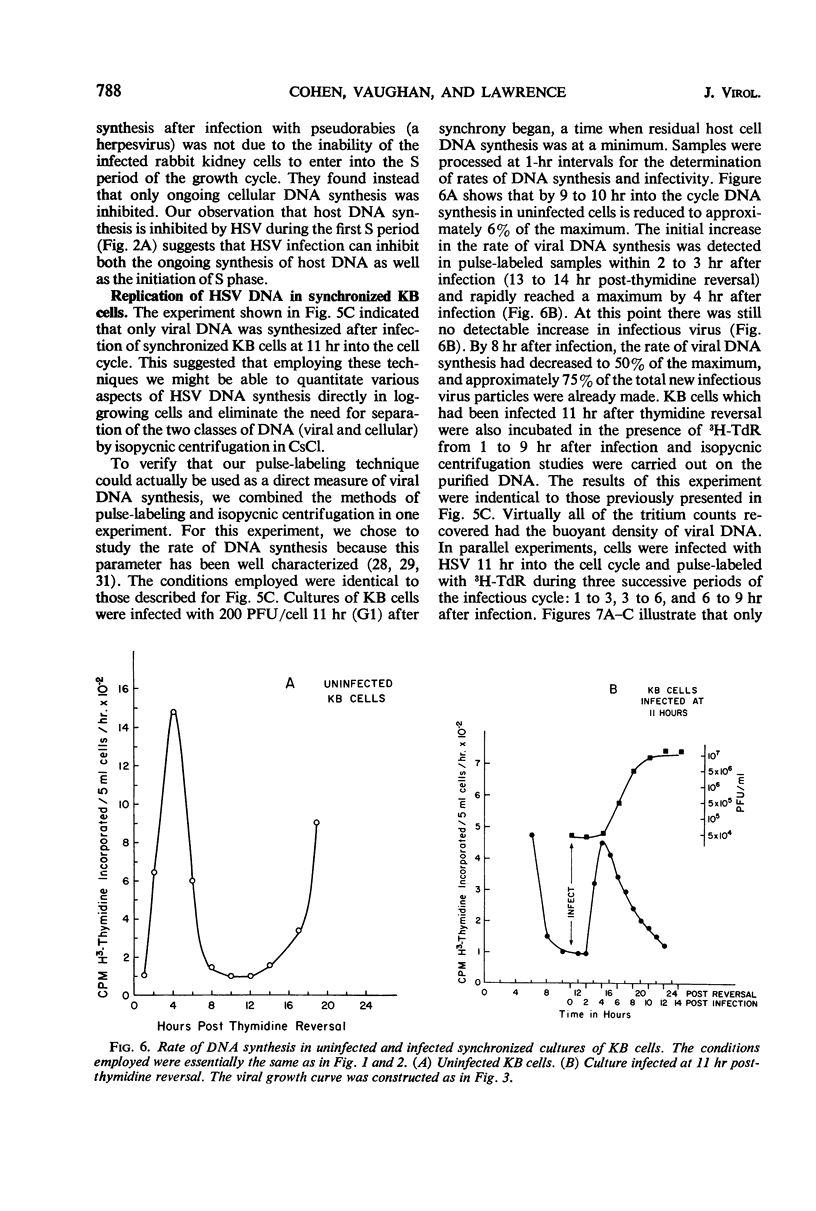
We examined the patterns of host cell and virus deoxyribonucleic acid (DNA) synthesis in synchronized cultures of KB cells infected at different stages of the cell cycle with herpes simplex virus (HSV). We found that the initiation of HSV DNA synthesis, we well as the production of new infectious virus, is independent of the S, G1, and G2 phases of the mitotic cycle of the host cell. This is in contrast to data previously found with equine abortion virus. Because HSV replicates independently of the cell cycle, we were able to establish conditions that would permit the study of rates of HSV DNA synthesized in logarithmically growing cells in the virtual absence of cellular DNA synthesis. This eliminates the need for separation of viral and cellular DNA by isopycnic centrifugation in CsCl. We found that HSV DNA synthesis was initiated between 2 to 3 hr after infection. The rate of DNA synthesis increased rapidly, reaching a maximum 4 hr after infection, and decreased to 50% of maximum by 8 hr. Evidence is also presented which suggests that HSV infection can inhibit both the ongoing synthesis of host DNA as well as the initiation of the S phase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEN-PORAT T., KAPLAN A. S. The synthesis and fate of pseudorabies virus DNA in infected mammalian cells in the stationary phase of growth. Virology. 1963 Jun;20:310–317. doi: 10.1016/0042-6822(63)90120-x. [DOI] [PubMed] [Google Scholar]
- BOOTSMA D., BUDKE L., VOS O. STUDIES ON SYNCHRONOUS DIVISION OF TISSUE CULTURE CELLS INITIATED BY EXCESS THYMIDINE. Exp Cell Res. 1964 Jan;33:301–309. doi: 10.1016/s0014-4827(64)81035-1. [DOI] [PubMed] [Google Scholar]
- Becker Y., Dym H., Sarov I. Herpes simplex virus DNA. Virology. 1968 Oct;36(2):184–192. doi: 10.1016/0042-6822(68)90135-9. [DOI] [PubMed] [Google Scholar]
- Bello L. J. Synthesis of DNA-like RNA in synchronized cultures of mammalian cells. Biochim Biophys Acta. 1968 Mar 18;157(1):8–15. doi: 10.1016/0005-2787(68)90258-x. [DOI] [PubMed] [Google Scholar]
- Buchan A., Watson D. H., Dubbs D. R., Kit S. Serological study of a mutant of herpesvirus unable to stimulate thymidine kinase. J Virol. 1970 Jun;5(6):817–818. doi: 10.1128/jvi.5.6.817-818.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAIRNS J. The initiation of vaccinia infection. Virology. 1960 Jul;11:603–623. doi: 10.1016/0042-6822(60)90103-3. [DOI] [PubMed] [Google Scholar]
- DARLINGTON R. W., RANDALL C. C. The nucleic acid content of equine abortion virus. Virology. 1963 Mar;19:322–327. doi: 10.1016/0042-6822(63)90071-0. [DOI] [PubMed] [Google Scholar]
- Flanagan J. F. Virus-specific ribonucleic acid synthesis in KB cells infected with herpes simplex virus. J Virol. 1967 Jun;1(3):583–590. doi: 10.1128/jvi.1.3.583-590.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAHAM A. F. Symposium on the biology of cells modified by viruses or antigens. III. Physiological conditions for studies of viral biosynthesis in mammalian cells. Bacteriol Rev. 1959 Dec;23(4):224–231. doi: 10.1128/br.23.4.224-231.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN M., PINA M. BIOCHEMICAL STUDIES ON ADENOVIRUS MULTIPLICATION, VI. PROPERTIES OF HIGHLY PURIFIED TUMORIGENIC HUMAN ADENOVIRUSES AND THEIR DNA. Proc Natl Acad Sci U S A. 1964 Jun;51:1251–1259. doi: 10.1073/pnas.51.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodheart C. R. Herpesviruses and cancer. JAMA. 1970 Jan 5;211(1):91–96. [PubMed] [Google Scholar]
- Groyon R. M., Kniazeff A. J. Vaccinia virus infection of synchronized pig kidney cells. J Virol. 1967 Dec;1(6):1255–1264. doi: 10.1128/jvi.1.6.1255-1264.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge L. D., Scharff M. D. Effect of adenovirus on host cell DNA synthesis in synchronized cells. Virology. 1969 Apr;37(4):554–564. doi: 10.1016/0042-6822(69)90273-6. [DOI] [PubMed] [Google Scholar]
- JAHMIAS A. J., KIBRICK S., ROSAN R. C. VIRAL LEUKOCYTE INTERRELATIONSHIPS. I. MULTIPLICATION OF A DNA VIRUS--HERPES SIMPLEX--IN HUMAN LEUKOCYTE CULTURES. J Immunol. 1964 Jul;93:69–74. [PubMed] [Google Scholar]
- KAPLAN A. S., BEN-PORAT T. The pattern of viral and cellular DNA synthesis in pseudorabies virus-infected cells in the logarithmic phase of growth. Virology. 1963 Feb;19:205–214. doi: 10.1016/0042-6822(63)90010-2. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R. Acquisition of thymidine kinase activity by herpes simplex-infected mouse fibroblast cells. Biochem Biophys Res Commun. 1963 Apr 2;11:55–59. doi: 10.1016/0006-291x(63)90027-5. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R. BIOCHEMISTRY OF VACCINIA-INFECTED MOUSE FIBROBLASTS (STRAIN L-M). IV. 3H-THYMIDINE UPTAKE INTO DNA OF CELLS EXPOSED TO COLD SHOCK. Exp Cell Res. 1963 Aug;31:397–406. doi: 10.1016/0014-4827(63)90016-8. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J., HSU T. C. DELETION OF THYMIDINE KINASE ACTIVITY FROM L CELLS RESISTANT TO BROMODEOXYURIDINE. Exp Cell Res. 1963 Aug;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- Knowland J. S. Polyacrylamide gel electrophoresis of nucleic acids synthesised during the early development of Xenopus laevis Daudin. Biochim Biophys Acta. 1970 Apr 15;204(2):416–429. doi: 10.1016/0005-2787(70)90162-0. [DOI] [PubMed] [Google Scholar]
- Lawrence W. C. Evidence for a relationship between equine abortion (herpes) virus deoxyribonucleic acid synthesis and the S phase of the KB cell mitotic cycle. J Virol. 1971 Jun;7(6):736–748. doi: 10.1128/jvi.7.6.736-748.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPP F., HSU T. C. VIRUSES AND MAMMALIAN CHROMOSOMES. IV. REPLICATION OF HERPES SIMPLEX VIRUS IN DIPLOID CHINESE HAMSTER CELLS. Virology. 1965 Mar;25:401–411. doi: 10.1016/0042-6822(65)90061-9. [DOI] [PubMed] [Google Scholar]
- ROIZMAN B., ROANE P. R., Jr THE MULTIPLICATION OF HERPES SIMPLEX VIRUS. II. THE RELATION BETWEEN PROTEIN SYNTHESIS AND THE DUPLICATION OF VIRAL DNA IN INFECTED HEP-2 CELLS. Virology. 1964 Feb;22:262–269. doi: 10.1016/0042-6822(64)90011-x. [DOI] [PubMed] [Google Scholar]
- ROIZMAN B. Virus infection of cells in mitosis. I. Observations on the recruitment of cells in karyokinesis into giant cells induced by herpes simplex virus and bearing on the site of virus antigen formation. Virology. 1961 Apr;13:387–401. doi: 10.1016/0042-6822(61)90269-0. [DOI] [PubMed] [Google Scholar]
- RUSSELL W. C., CRAWFORD L. V. SOME CHARACTERISTICS OF THE DEOXYRIBONUCLEIC ACID FROM HERPES SIMPLEX VIRUS. Virology. 1963 Nov;21:353–361. doi: 10.1016/0042-6822(63)90196-x. [DOI] [PubMed] [Google Scholar]
- Roizman B. The herpesviruses--a biochemical definition of the group. Curr Top Microbiol Immunol. 1969;49:3–79. [PubMed] [Google Scholar]
- SALZMAN N. P., SHATKIN A. J., SEBRING E. D., MUNYON W. On the replication of vaccinia virus. Cold Spring Harb Symp Quant Biol. 1962;27:237–244. doi: 10.1101/sqb.1962.027.001.023. [DOI] [PubMed] [Google Scholar]
- STOKER M. G., NEWTON A. The effect of herpes virus on HeLa cells dividing parasynchronously. Virology. 1959 Apr;7(4):438–448. doi: 10.1016/0042-6822(59)90072-8. [DOI] [PubMed] [Google Scholar]
- Waubke R., Zur Hausen H., Henle W. Chromosomal and autoradiographic studies of cells infected with herpes simplex virus. J Virol. 1968 Oct;2(10):1047–1054. doi: 10.1128/jvi.2.10.1047-1054.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]



