Abstract
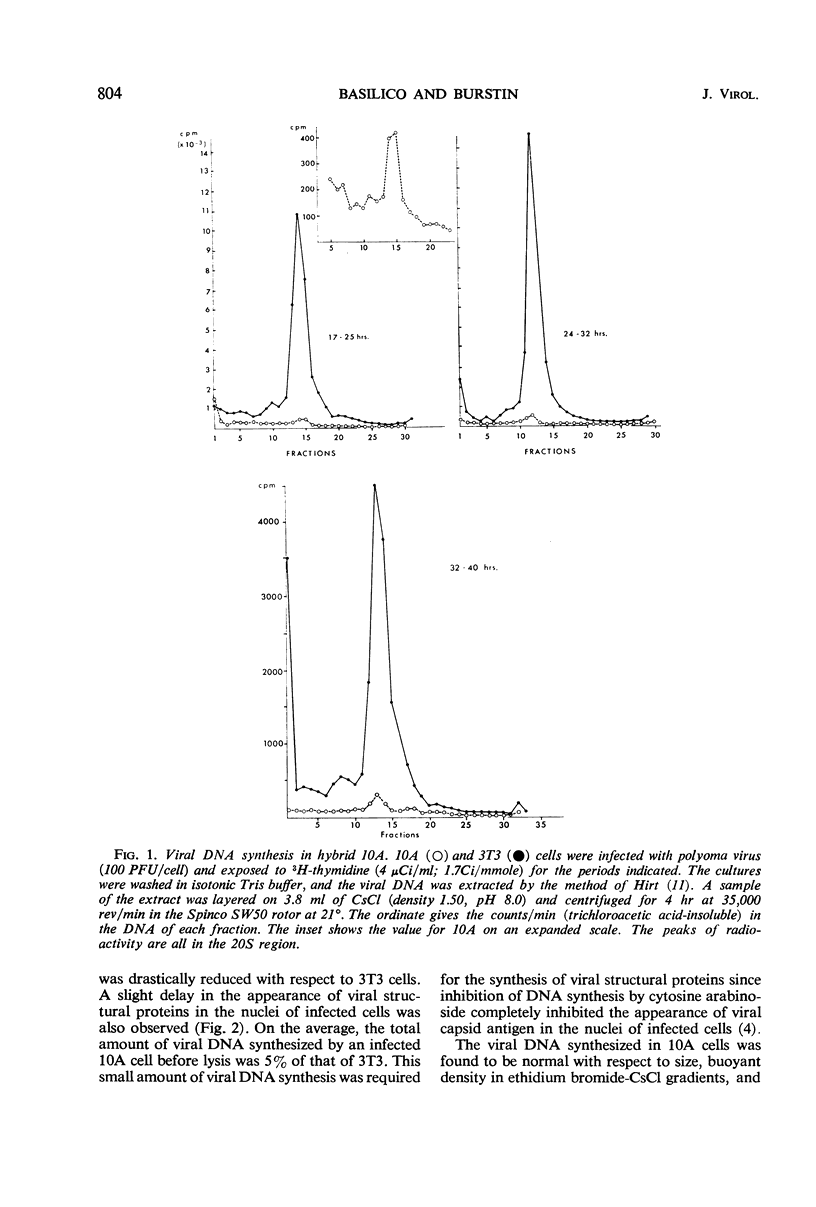
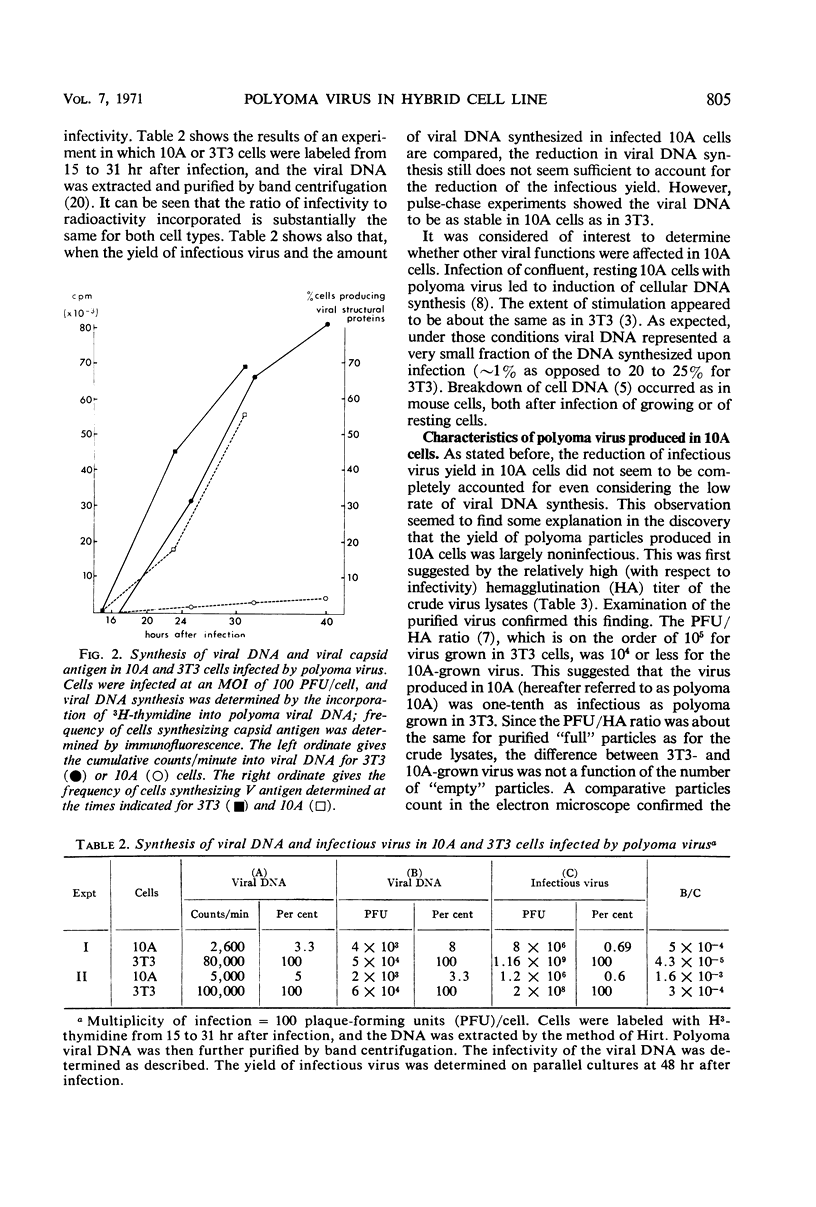
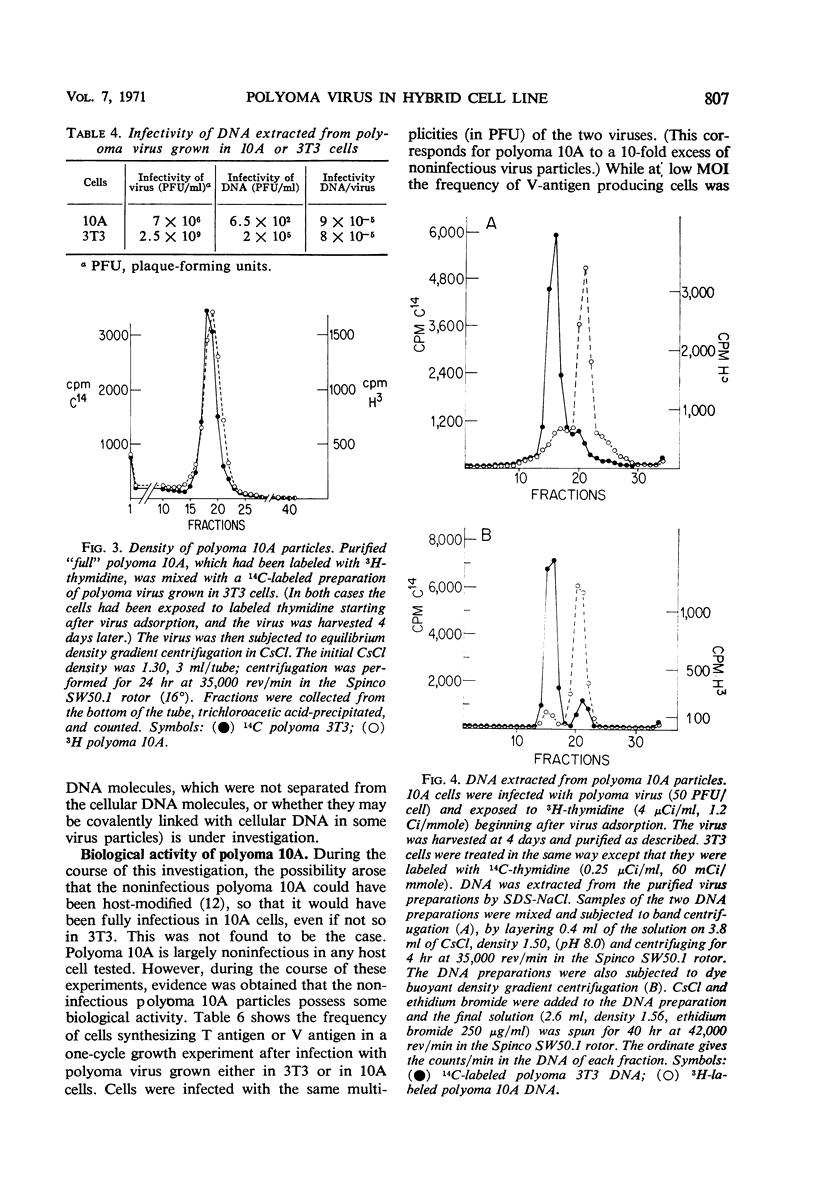
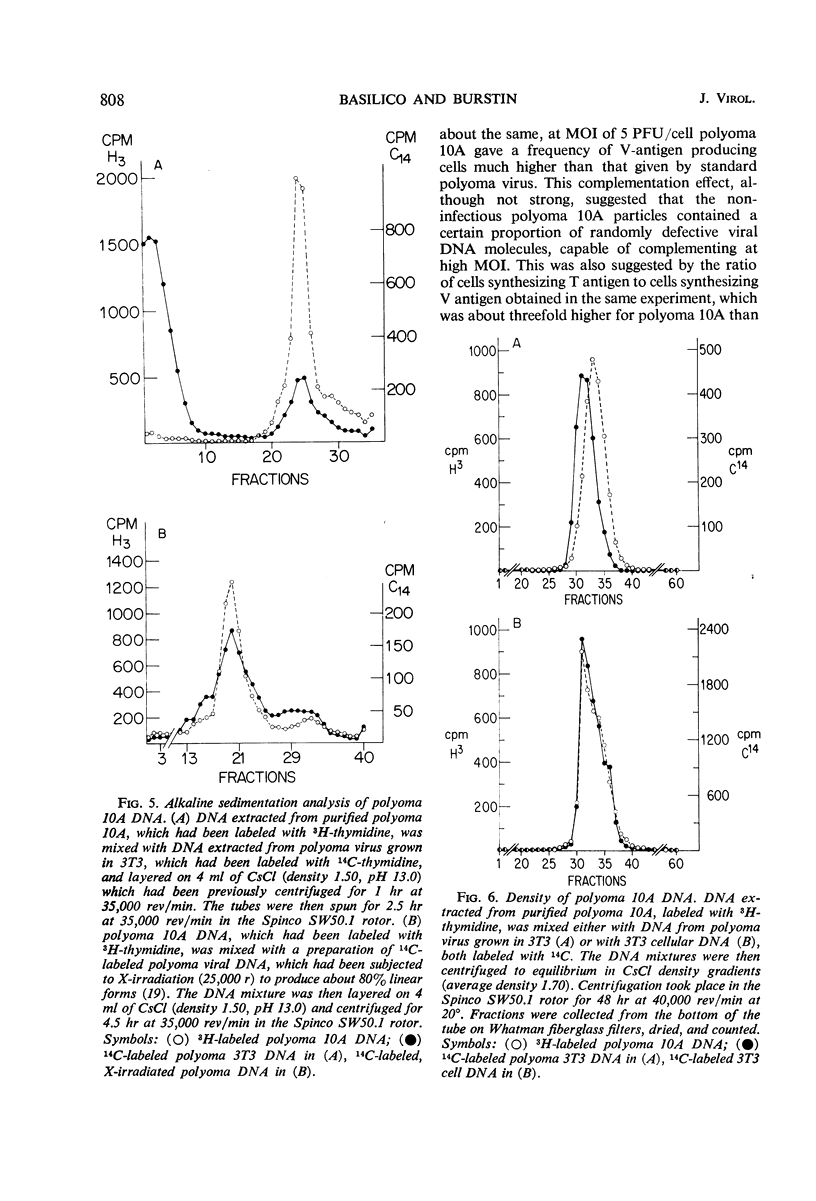
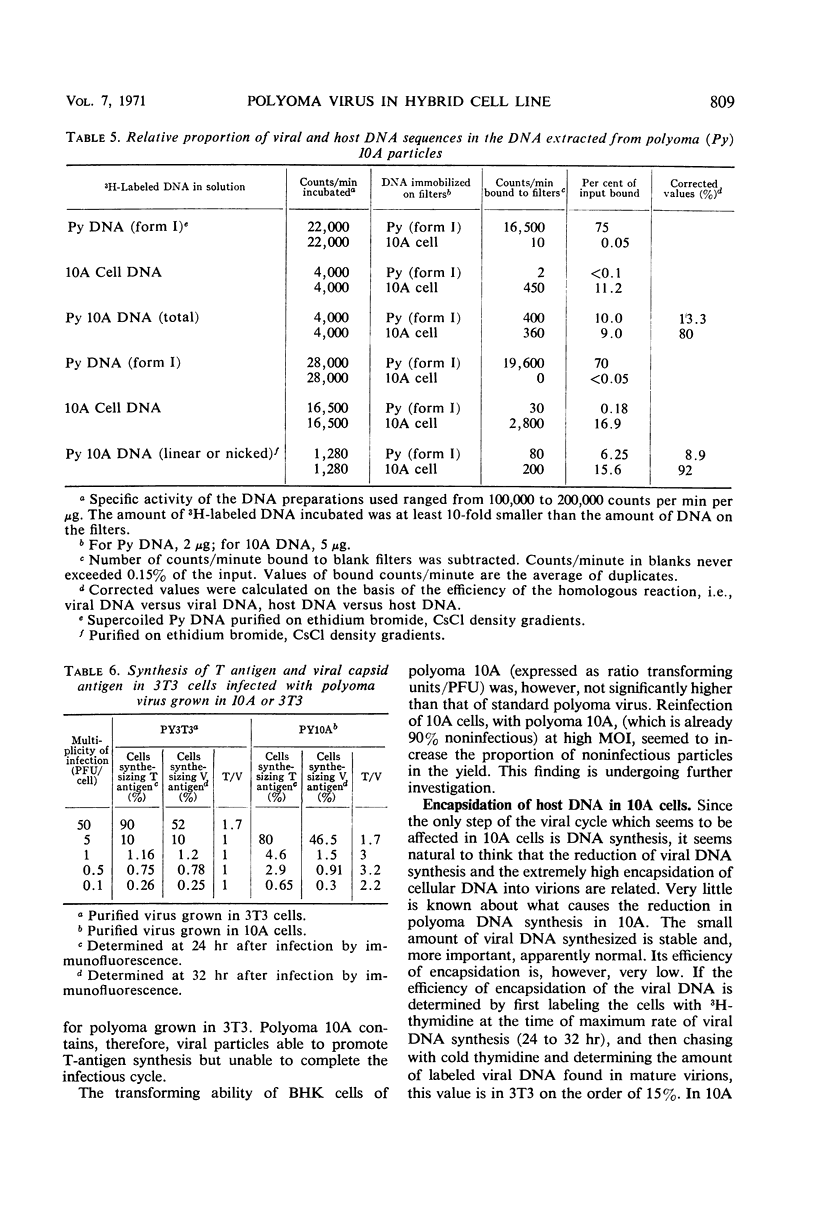
The multiplication of polyoma virus in a mouse-hamster (3T3 × BHK) somatic hybrid line (10A), which, although permissive for viral multiplication, produces very low amounts of virus, has been studied. In this cell line, the efficiency of productive infection is high, but the yield of infectious virus is on the order of 0.5% of that of 3T3 cells. The amount of viral deoxyribonucleic acid (DNA) synthesized by these cells upon infection is about 5% of that of 3T3 cells. An examination of the virus produced in hybrid 10A revealed that it was only one-tenth as infectious as the virus grown in 3T3. Although the viral DNA synthesized in the infected 10A cells is normal, the DNA extracted from purified virus grown in 10A consists of ∼10% of normal, supercoiled polyoma DNA molecules and of ∼90% linear DNA molecules with a sedimentation coefficient of 14 to 16S. These DNA molecules appear to be of cellular origin but contain a limited amount of viral DNA sequences. The host DNA-containing particles are not infectious but appear to possess some biological activity; they give rise to a weak complementation effect, and part of them are able to induce T-antigen synthesis. In addition, the host DNA present in these particles is predominantly that which has been synthesized after infection. The correlation between the block in viral DNA synthesis in this cell line and the abnormal encapsidation of host DNA is discussed.
Full text
PDF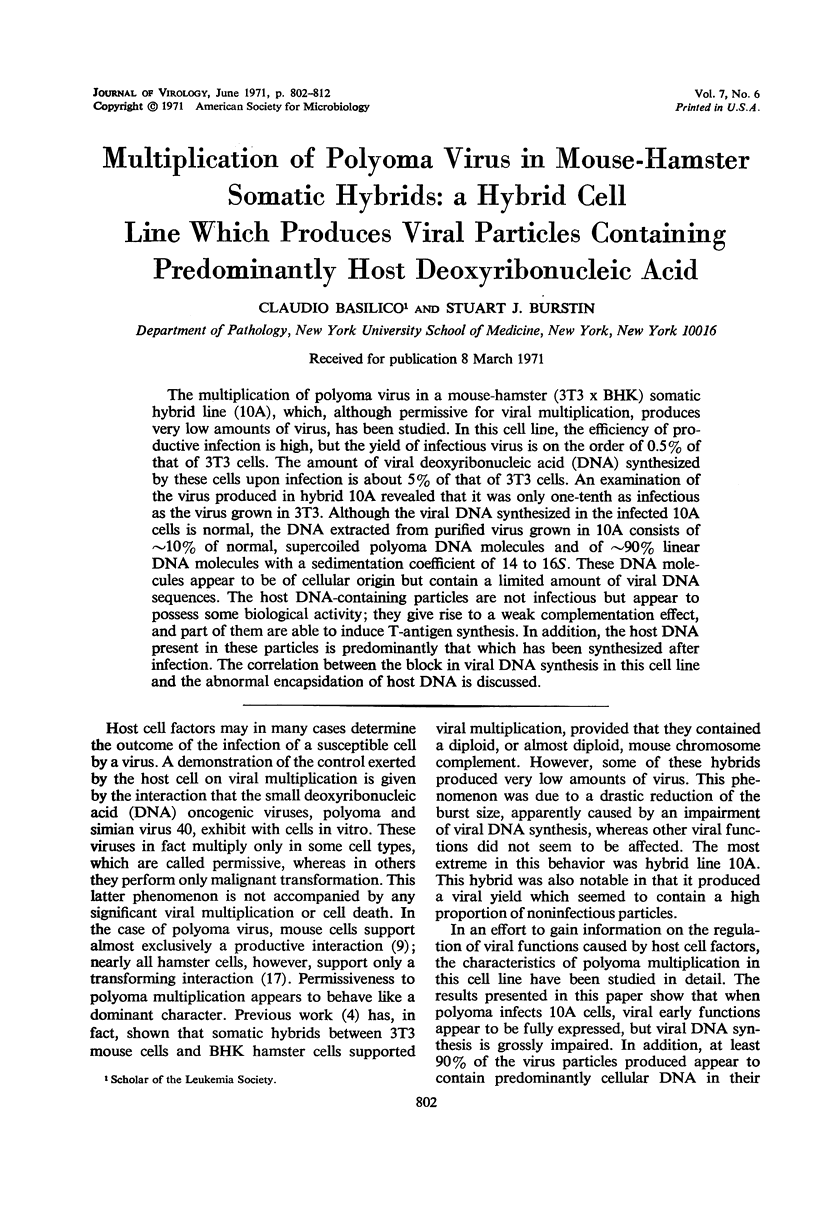





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y., Winocour E., Sachs L., Torten J. Hybridization between SV40 DNA and cellular DNA's. J Mol Biol. 1969 Sep 14;44(2):333–345. doi: 10.1016/0022-2836(69)90179-x. [DOI] [PubMed] [Google Scholar]
- Basilico C., Matsuya Y., Green H. Origin of the thymidine kinase induced by polyoma virus in productively infected cells. J Virol. 1969 Feb;3(2):140–145. doi: 10.1128/jvi.3.2.140-145.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basilico C., Matsuya Y., Green H. The interaction of polyoma virus with mouse-hamster somatic hybrid cells. Virology. 1970 Jun;41(2):295–305. doi: 10.1016/0042-6822(70)90082-6. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., Kaplan A. S. Correlation between replication and degradation of cellular DNA in polyoma virus-infected cells. Virology. 1967 Jul;32(3):457–464. doi: 10.1016/0042-6822(67)90297-8. [DOI] [PubMed] [Google Scholar]
- Branton P. E., Sheinin R. Control of DNA synthesis in cells infected with polyoma virus. Virology. 1968 Dec;36(4):652–661. doi: 10.1016/0042-6822(68)90196-7. [DOI] [PubMed] [Google Scholar]
- DIAMOND L., CRAWFORD L. V. SOME CHARACTERISTICS OF LARGE-PLAQUE AND SMALL-PLAQUE LINES OF POLYOMA VIRUS. Virology. 1964 Feb;22:235–244. doi: 10.1016/0042-6822(64)90008-x. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., HARTWELL L. H., VOGT M. INDUCTION OF CELLULAR DNA SYNTHESIS BY POLYOMA VIRUS. Proc Natl Acad Sci U S A. 1965 Feb;53:403–410. doi: 10.1073/pnas.53.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDDY B. E., STEWART S. E., BERKELEY W. Cytopathogenicity in tissue culture by a tumor virus from mice. Proc Soc Exp Biol Med. 1958 Aug-Sep;98(4):848–851. doi: 10.3181/00379727-98-24205. [DOI] [PubMed] [Google Scholar]
- Frankel F. R. DNA replication after T4 infection. Cold Spring Harb Symp Quant Biol. 1968;33:485–493. doi: 10.1101/sqb.1968.033.01.056. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- LURIA S. E. Host-induced modifications of viruses. Cold Spring Harb Symp Quant Biol. 1953;18:237–244. doi: 10.1101/sqb.1953.018.01.034. [DOI] [PubMed] [Google Scholar]
- Michel M. R., Hirt B., Weil R. Mouse cellular DNA enclosed in polyoma viral capsids (pseudovirions). Proc Natl Acad Sci U S A. 1967 Oct;58(4):1381–1388. doi: 10.1073/pnas.58.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano J. S., McCutchan J. H., Vaheri A. Factors influencing the enhancement of the infectivity of poliovirus ribonucleic acid by diethylaminoethyl-dextran. J Virol. 1967 Oct;1(5):891–897. doi: 10.1128/jvi.1.5.891-897.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOKER M., ABEL P. Conditions affecting transformation by polyoma virus. Cold Spring Harb Symp Quant Biol. 1962;27:375–386. doi: 10.1101/sqb.1962.027.001.035. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VINOGRAD J., BRUNER R., KENT R., WEIGLE J. Band-centrifugation of macromolecules and viruses in self-generating density gradients. Proc Natl Acad Sci U S A. 1963 Jun;49:902–910. doi: 10.1073/pnas.49.6.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez C., Kleinschmidt A. K., Basilico C. Electron microscopic studies of polyoma DNA released in protein monolayers. J Mol Biol. 1969 Jul 28;43(2):317–325. doi: 10.1016/0022-2836(69)90270-8. [DOI] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J., Radloff R., Watson R., Laipis P. The twisted circular form of polyoma viral DNA. Proc Natl Acad Sci U S A. 1965 May;53(5):1104–1111. doi: 10.1073/pnas.53.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIL R. A quantitative assay for a subviral infective agent related to polyoma virus. Virology. 1961 May;14:46–53. doi: 10.1016/0042-6822(61)90130-1. [DOI] [PubMed] [Google Scholar]
- WINOCOUR E. Purification of polyoma virus. Virology. 1963 Feb;19:158–168. doi: 10.1016/0042-6822(63)90005-9. [DOI] [PubMed] [Google Scholar]
- Winocour E. Further studies on the incorporation of cell DNA into polyoma-related particles. Virology. 1968 Apr;34(4):571–582. doi: 10.1016/0042-6822(68)90078-0. [DOI] [PubMed] [Google Scholar]



