Abstract
To assess the role of DNA repair in maintenance of hearing function and neurological integrity, we examined hearing status, neurological function, DNA repair complementation group and history of acute burning on minimal sun exposure in all patients with xeroderma pigmentosum, who had at least one complete audiogram, examined at the National Institutes of Health from 1971 to 2012. Seventy-nine patients, aged 1–61 years, were diagnosed with xeroderma pigmentosum (n = 77) or xeroderma pigmentosum/Cockayne syndrome (n = 2). A total of 178 audiograms were included. Clinically significant hearing loss (>20 dB) was present in 23 (29%) of 79 patients. Of the 17 patients with xeroderma pigmentosum-type neurological degeneration, 13 (76%) developed hearing loss, and all 17 were in complementation groups xeroderma pigmentosum type A or type D and reported acute burning on minimal sun exposure. Acute burning on minimal sun exposure without xeroderma pigmentosum-type neurological degeneration was present in 18% of the patients (10/55). Temporal bone histology in a patient with severe xeroderma pigmentosum-type neurological degeneration revealed marked atrophy of the cochlear sensory epithelium and neurons. The 19-year mean age of detection of clinically significant hearing loss in the patients with xeroderma pigmentosum with xeroderma pigmentosum-type neurological degeneration was 54 years younger than that predicted by international norms. The four frequency (0.5/1/2/4 kHz) pure-tone average correlated with degree of neurodegeneration (P < 0.001). In patients with xeroderma pigmentosum, aged 4–30 years, a four-frequency pure-tone average ≥10 dB hearing loss was associated with a 39-fold increased risk (P = 0.002) of having xeroderma pigmentosum-type neurological degeneration. Severity of hearing loss parallels neurological decline in patients with xeroderma pigmentosum-type neurological degeneration. Audiometric findings, complementation group, acute burning on minimal sun exposure and age were important predictors of xeroderma pigmentosum-type neurological degeneration. These results provide evidence that DNA repair is critical in maintaining neurological integrity of the auditory system.
Keywords: xeroderma pigmentosum, sensorineural hearing loss, neurodegeneration, photosensitivity, DNA repair
Introduction
Patients with xeroderma pigmentosum, a rare autosomal recessive disorder of DNA repair and premature ageing, demonstrate lentiginous skin pigmentation, photosensitivity and a >10 000-fold increased risk of ultraviolet radiation-induced skin cancer (Robbins et al., 1974; Kraemer et al., 1987; Anttinen et al., 2008; Bradford et al., 2011). Although all patients with xeroderma pigmentosum are sensitive to ultraviolet radiation damage, only a portion experience acute burning on minimal sun exposure after only a few minutes in direct sunlight. A subset of patients with xeroderma pigmentosum develops progressive neurological degeneration with features of premature ageing and hearing loss (Robbins et al., 1974, 1991, 2002; Kenyon et al., 1985; Kraemer et al., 1987, 2007; Mamada et al., 1988; Nishigori et al., 1994; Rapin et al., 2000; Lindenbaum et al., 2001; Anttinen et al., 2008; Bradford et al., 2011). DNA damage induced by oxidative metabolism is thought to contribute to the progressive loss of non-dividing neurons in the brain (Brooks et al., 2008). Death typically occurs in the third or fourth decades of life in patients with xeroderma pigmentosum with neurodegeneration (Bradford et al., 2011).
Xeroderma pigmentosum is a heterogeneous disease associated with several different defects in DNA repair. There are seven complementation groups, xeroderma pigmentosum (XP)-A through to XP-G, with defects in nucleotide excision repair (Robbins et al., 1974; Kraemer et al., 2007; Bradford et al., 2011; DiGiovanna and Kraemer 2012). Patients with xeroderma pigmentosum variant have defects in DNA polymerase eta involved in translesional DNA synthesis. Patients with neurodegeneration have mutations in XP-A, XP-B, XP-D, XP-F or XP-G. However, not all patients with mutations in these genes develop neurodegeneration, making prediction of neurological disease clinically difficult.
We investigated the role of DNA repair in the maintenance of auditory and neurological integrity by examining hearing status, complementation group, neurological function and burning phenotype in patients with xeroderma pigmentosum seen at the National Institutes of Health between 1971 and 2012. A secondary goal was to develop a model for predicting neurodegeneration in patients with xeroderma pigmentosum.
Materials and methods
Patients
We conducted a retrospective follow-up study of 84 patients with xeroderma pigmentosum examined at the National Institutes of Health, from 1971 to 2012, who had audiometric data available. Patients were referred to the National Institutes of Health Clinical Centre by outside health care professionals and were enrolled into protocols approved by the National Cancer Institute Institutional Review Board. Written informed consent was obtained from each participant or parents of minor participants. All patients were evaluated by National Cancer Institute dermatologists (J.J.D. and/or K.H.K.) and met clinical criteria for diagnosis of xeroderma pigmentosum or xeroderma pigmentosum/Cockayne syndrome complex, as previously described (Robbins et al., 1974; Kraemer et al., 1987, 2007; Rapin et al., 2000; Lindenbaum et al., 2001). Xeroderma pigmentosum complementation groups and mutations in the xeroderma pigmentosum variant gene were determined using cell fusion, host cell reactivation, western blotting or DNA sequencing (Bradford et al., 2011). Patient data were collected from medical records at the National Institutes of Health Clinical Centre, from records of outside institutions, research records at the National Cancer Institute, previous publications (Bradford et al., 2011) and personal correspondence.
End points
Audiometric analysis
All patients who had an audiometric examination between 1 January 1971 and 18 January 2012 were included in the study. Of the 183 audiograms collected, those with unreliable (poor agreement between speech and pure tones thresholds, notation of minimum response levels instead of actual thresholds, or notation of fair or poor test reliability by the examining audiologist or incomplete audiometric data (tympanometry and/or otoacoustic emissions only) (n = 5) were excluded, resulting in 178 audiograms from 79 patients with xeroderma pigmentosum. Eighty per cent (143/178) of audiometric assessments were conducted at the National Institutes of Health Clinical Centre. We obtained outside audiometric records for review and included these (35 additional audiograms) in our analysis only if the examining audiologist rated reliability as good. To examine the hearing phenotype directly attributable to xeroderma pigmentosum, ears with a history of otological surgery other than myringotomy tube placement (n = 1) were excluded from analysis.
Audiological evaluations at the National Institutes of Health included, when possible, pure-tone air-conduction (0.25–8 kHz) and bone-conduction (0.25–4 kHz) thresholds and speech audiometry using clinical audiometers in double-walled sound suites that met American National Standards Institute criteria (ANSI, 2003, 2004). Masking was used for air and bone conduction testing when appropriate to ensure ear-specific results. Multiple audiograms were available for 34 patients. Duration of audiometric monitoring ranged from a single test (n = 45) to a 39-year period (n = 1). For each subject, we defined the reference audiogram as the most recent complete audiogram.
Type of hearing loss was classified as conductive, sensorineural or mixed based on the three-frequency (0.5/1/2 kHz) pure-tone averages (3F-PTA) for air- and bone-conduction, based on criteria adapted from Mazzoli et al. (2003) and King et al. (2007). When the bone-conduction 3F-PTA was ≤20 dBHL and the air-bone gap was >10 dB, the hearing loss was classified as conductive. When the bone-conduction 3F-PTA was >20 dBHL and the air-bone gap was ≤10 dB, the hearing loss was classified as sensorineural. When the bone-conduction 3F-PTA was >20 dBHL and the air-bone gap was >10 dB, the hearing loss was classified as mixed. When bone-conduction was not tested, hearing loss was classified as sensorineural, but only if tympanometry was normal (Margolis and Heller, 1987), and acoustic stapedial reflexes were present at levels commensurate with the degree of hearing loss (Gelfand et al., 1990). Hearing loss type was classified as unknown when there were insufficient audiological data to categorize the hearing loss as sensorineural, mixed or conductive. Degree of hearing loss was determined using the four-frequency (0.5/1/2/4 kHz) pure-tone averages (4F-PTA) for air-conduction, or in the absence of pure-tone thresholds, degree was based on the speech reception threshold (n = 13). Classifications were as follows: ≤20 dBHL = normal hearing; >20–40 dBHL = mild hearing loss; >40–70 dBHL = moderate hearing loss; >70–95 dBHL = severe hearing loss; and >95 dBHL = profound hearing loss. Pure-tone hearing sensitivity for singe frequencies and the 4F-PTA was analysed using age- and gender-specific reference ranges for hearing levels from a large population of otologically screened adults reported in International Organization for Standardization (ISO) 7029 (International Standard Organization, 2000). Sensory thresholds of the worse hearing ear were determined from bone-conduction thresholds or from air-conduction thresholds, in cases where bone-conduction was not tested and both tympanometry and acoustic reflexes were normal. The sensory thresholds were compared with and plotted against the 5th, 50th and 95th percentile of combined male and female age-matched ISO 7029 data.
Neurological status
Neurological involvement was ranked using the scale of xeroderma pigmentosum-type neurological degeneration developed by Nishigori et al. (1994). The following rankings were assigned at every time point the patient underwent neurological evaluation: 0 = no neurological involvement; −1 = mild mental retardation (IQ > 50), only hyporeflexia; −2 = gait disturbance because of spasticity or ataxia, moderate mental retardation (IQ < 50); −3 = severe mental retardation, and cannot walk, cannot speak and bed resting. If neurological evaluation was not completed at the time of audiometric testing, neurological ranking was assigned from the assessment closest to the date of audiometric evaluation. Patients with neurological involvement atypical of that observed in xeroderma pigmentosum were classified as non-xeroderma pigmentosum-type neurological involvement.
Burning phenotype, skin cancer and other clinical data
Acute burning on minimal sun exposure and other clinical data including presence of skin cancer were based on clinical history, a standard questionnaire administered at the time of the initial National Institutes of Health visit (Kraemer et al., 1994), medical records (including pathology reports of skin and other cancers), patient photographs, imaging reports, research records and published reports (Bradford et al., 2011).
Temporal bone histopathology
Post-mortem temporal bones of an XP-A patient (Patient XP12BE) with neurological disease who died of her progressive neurodegeneration (Ramkumar et al., 2011) were sent to the Otopathology Laboratory at the Massachusetts Eye and Ear Infirmary and Harvard Medical School for histopathological analysis (S.N.M. and J.B.N.).
Statistical analyses
Prism 5.03 GraphPad Software was used for statistical analysis. ANOVA was used to compare the 4F-PTA by degree of neurodegeneration for those patients with xeroderma pigmentosum with neurodegeneration. Fischer’s exact test was used to determine the odds ratio for developing xeroderma pigmentosum-type neurological degeneration. Results with P < 0.05 were considered significant. All statistical tests were two-sided, and the 95% confidence interval was calculated for each test.
Comparison of hearing thresholds over time between the ISO 7029 control patients, xeroderma pigmentosum-type neurological degeneration and the xeroderma pigmentosum without neurological involvement groups was based on regression analyses derived from audiometric thresholds of the worse-hearing ear from the reference audiogram at each of the individual frequencies (0.5/1/2/4/8 kHz). Patients with non-xeroderma pigmentosum-type neurological involvement were not included in the analysis based on the heterogeneity of their neurological disease.
Linear regressions predicting hearing threshold by age were calculated for the xeroderma pigmentosum-type neurological degeneration and xeroderma pigmentosum without neurological involvement groups. One audiogram per patient (the earliest complete audiogram) was used to determine a cross-sectional relationship between age and hearing threshold. Chow tests taking into account the combination of the slope and intercept of these regression lines were used to compare these data against the ISO 7029 50th percentile line of hearing threshold by age. To correct for multiple comparisons, the P-value threshold of 0.05 was divided by 18, yielding a criterion of 0.002 for significance. The 95th percentile prediction interval was calculated for each patient group.
Results
Study demographics
Seventy-seven patients, aged 1–61 years, were diagnosed with xeroderma pigmentosum and two with xeroderma pigmentosum/Cockayne syndrome complex (Table 1). Females comprised 57% (45/79) of the patients. Clinically significant hearing loss (4F-PTA >20 dBHL) was present in 29% (23/79 patients). Acute burning on minimal sun exposure was identified in 40% (32/79). Of those reporting acute burning on minimal sun exposure, 25 were non-Hispanic whites, five were African American, one was Asian and one was of other ethnicity. Skin cancer (basal cell carcinoma, squamous cell carcinoma or melanoma) was diagnosed in 73% (58/79) of the patients by the time of the reference audiogram. Three patients had otitis media at a single audiometric test session, none of which were at the time of the most recent complete (reference) audiogram.
Table 1.
Patients with xeroderma pigmentosum and xeroderma pigmentosum/Cockayne syndrome studied at the National Institutes of Health, from 1971 to 2012
| Total | Gender |
Race/ethnicity |
||||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | NHW | AA | HW | Asian | Other | ||
| Total | 79 | 34 | 45 | 57 | 13 | 4 | 3 | 2 |
| Age at most recent complete audiogram (years) | ||||||||
| Median | 17 | 15 | 19 | 20 | 17 | 7 | 15 | 13 |
| Range | 1–61 | 4–61 | 1–57 | 1–61 | 4–57 | 4–36 | 8–21 | 13–13 |
| Audiometric data | ||||||||
| Number of audiograms | 178 | 69 | 109 | 144 | 24 | 4 | 4 | 2 |
| Clinically significant hearing loss (n)a | 23 | 8 | 15 | 19 | 4 | 0 | 0 | 0 |
| Neurological phenotype | ||||||||
| XP-type neurodegeneration presentb | 17 | 7 | 10 | 15 | 2 | 0 | 0 | 0 |
| No neurodegeneration | 55 | 24 | 31 | 39 | 8 | 4 | 3 | 1 |
| Non-XP neurological involvementc | 7 | 3 | 4 | 3 | 3 | 0 | 0 | 1 |
| Burning phenotype | ||||||||
| Acute burning on minimal sun exposure | 32 | 19 | 13 | 25 | 5 | 0 | 1 | 1 |
| Does not burn on minimal sun exposure | 47 | 15 | 32 | 32 | 8 | 4 | 2 | 1 |
| Complementation group | ||||||||
| XP-A | 9 | 3 | 6 | 6 | 2 | 0 | 1 | 0 |
| XP-Bd | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| XP-C | 38 | 20 | 18 | 24 | 7 | 4 | 2 | 1 |
| XP-D | 17 | 7 | 10 | 15 | 1 | 0 | 0 | 1 |
| XP-E | 2 | 0 | 2 | 2 | 0 | 0 | 0 | 0 |
| XP-Ge | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 0 |
| Variant | 5 | 2 | 3 | 5 | 0 | 0 | 0 | 0 |
| Unknownf | 5 | 1 | 4 | 3 | 2 | 0 | 0 | 0 |
| Skin cancer? | ||||||||
| Yes | 58 | 24 | 34 | 42 | 6 | 3 | 3 | 1 |
| No | 21 | 10 | 11 | 14 | 5 | 1 | 0 | 1 |
a Clinically significant hearing loss defined as 4F-PTA (0.5/1/2/4 kHz) >20 dB sensorineural hearing loss.
d Xeroderma pigmentosum/Cockayne syndrome (Patient XP11BE) Robbins et al. (1974).
e One patient is an African American male with a mutation in the XP-G gene and is phenotypically xeroderma pigmentosum/Cockayne syndrome (Patient XP218BE).
f Five patients were tested for complementation groups, and they did not have A, B, C, D, E, F, G, ERCC1 or variant defects.
AA = African American; HW = Hispanic white; NHW = non-Hispanic white; XP = xeroderma pigmentosum.
DNA repair complementation group was determined for 74 of the patients. The most common complementation group was XP-C (48%, n = 38), followed by XP-D (22%, n = 17) (Table 1). No patients with mutations in XP-F (Imoto et al., 2005) were examined at the National Institutes of Health, but previous studies have shown defects in these genes in patients with xeroderma pigmentosum and late onset neurological degeneration. Cells from five patients could not be assigned to a known complementation group and may have defects in genes not yet identified (Bradford et al., 2011).
Neurodegeneration and hearing status analysis
Although most patients (70%, n = 55) had no neurological symptoms, 21% (n = 17) had xeroderma pigmentosum-type neurological degeneration (Table 1). Non-xeroderma pigmentosum type neurological involvement was present in seven patients (9%). Neurological involvement in this group included xeroderma pigmentosum/Cockayne syndrome complex, foetal alcohol syndrome, chromosomal abnormalities, neonatal streptococcal meningitis, enlarged ventricles and one patient of unknown cause.
Overall, 71% (n = 56) of patients had normal hearing, 23% (n = 18) had sensorineural hearing loss, 1% (n = 1) had conductive hearing loss and 5% (n = 4) had hearing loss of unknown type (Table 2). Of those patients with xeroderma pigmentosum without neurological involvement, 89% (n = 49) had normal hearing, whereas only 11% (n = 6) had hearing loss. In contrast, 76% (n = 13) of patients in the xeroderma pigmentosum-type neurological degeneration group had hearing loss, including nine with sensorineural hearing loss and four with an unknown type of hearing loss.
Table 2.
Hearing loss in patients with xeroderma pigmentosum by neurological status
| Clinically significant hearing loss typea | No neurological involvement (n = 55) (%) | XP-type neurological involvement (n = 17) (%) | Non-XP type neurological involvement (n = 7) (%) | Total patient population (n = 79) (%) |
|---|---|---|---|---|
| Sensorineural hearing loss | 5 (9) | 9 (53) | 4 (57) | 18 (23) |
| Conductive | 1 (2) | 0 (0) | 0 (0) | 1 (1) |
| Hearing loss, type unknown | 0 (0) | 4 (23.5) | 0 (0) | 4 (5) |
| Normal | 49 (89) | 4 (23.5) | 3 (43) | 56 (71) |
a Clinically significant hearing loss defined as 4F-PTA (0.5/1/2/4 kHz) >20 dBHL.
Neurological status was determined in accordance with Nishigori et al. (1994).
XP = xeroderma pigmentosum.
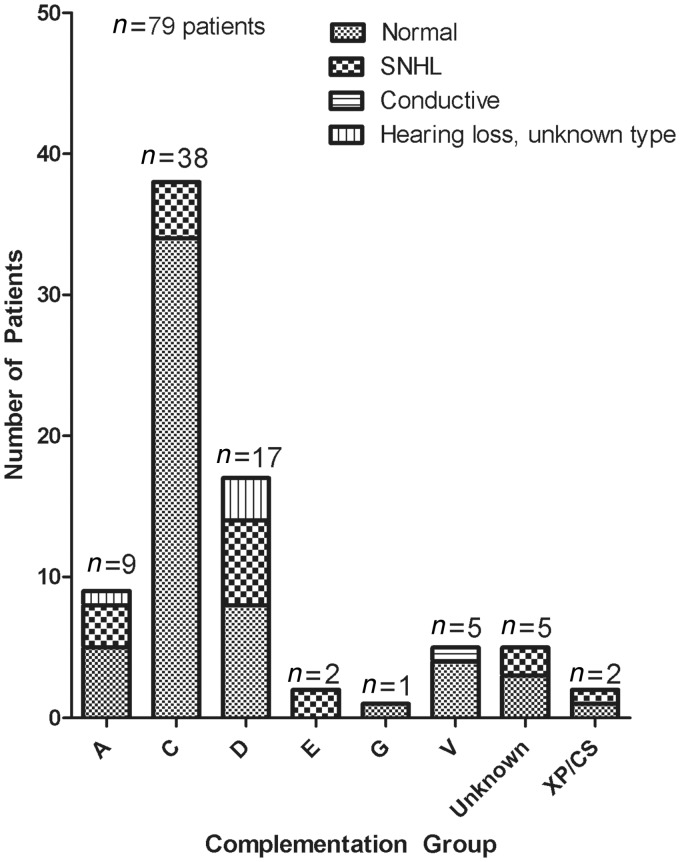
Patients with complementation group XP-C had the greatest proportion of normal hearing (89%, n = 34), consistent with their predominance in the no neurological involvement group (Fig. 1). All of the patients with xeroderma pigmentosum-type neurological degeneration were in complementation groups XP-A or XP-D. These complementation groups had the highest proportion of patients with hearing loss at 44% (n = 4) and 53% (n = 9), respectively (Fig. 1).
Figure 1.
Type of hearing loss by complementation group in patients with xeroderma pigmentosum seen at the National Institutes of Health from 1971 to 2012. Type of hearing loss is based on thresholds (dBHL) in the worse hearing ear at reference audiogram. XP/CS = xeroderma pigmentosum/Cockayne syndrome; SNHL = sensorineural hearing loss.
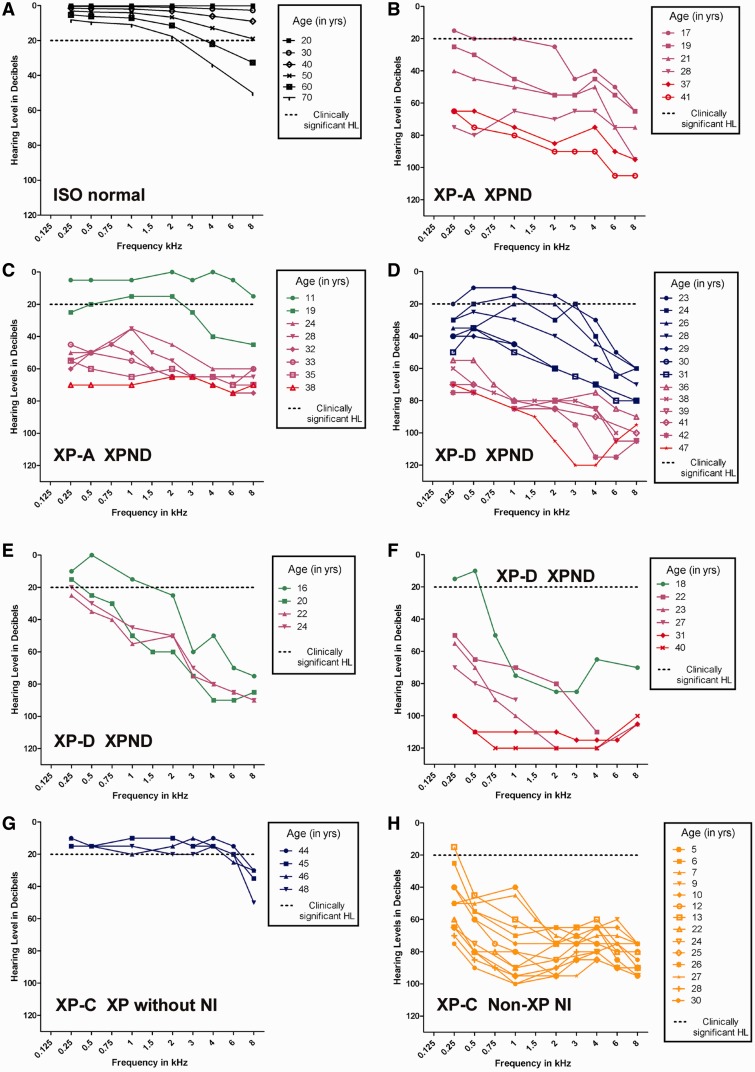
Longitudinal audiograms for five patients with xeroderma pigmentosum-type neurological degeneration are shown in Fig. 2B–F. Compared with the ISO normative data (Fig. 2A), these patients showed substantially poorer hearing thresholds with a significantly greater decline in hearing associated with a concomitant decline in neurological status. Patient XP1BE did not have neurological involvement, and her hearing thresholds were within clinical and/or age-based normal limits (Robbins et al., 2002) (Fig. 2G). Patient XP21BE (XP-C) was the child of a close consanguineous mating. She had a mild-to-severe sensorineural hearing loss as early as age 5 years, with progression to a severe to profound hearing loss by age 30 years, thought to be the result of a separate and distinct genetic abnormality (Khan et al., 2009) (Fig. 2H). She had non-xeroderma pigmentosum-type neurological involvement with hearing loss at a younger age, and less rapid progression than that observed in patients with xeroderma pigmentosum-type neurological degeneration. In the 17 patients with xeroderma pigmentosum-type neurological degeneration (Table 3), ANOVA testing showed that 4F-PTA was different based on the degree of neurodegeneration (P < 0.001). The results showed that more severe neurodegeneration was associated with higher 4F-PTA.
Figure 2.
Longitudinal pure-tone air-conduction audiograms and degree of neurological involvement for individual patients with xeroderma pigmentosum. Individual patient thresholds for the worse hearing ear. Neurological status is colour coded: blue = no neurological involvement; green = mild mental retardation (IQ > 50), only hyporeflexia; purple = gait disturbance because of spasticity or ataxia, moderate mental retardation (IQ < 50); red = severe mental retardation, cannot walk, cannot speak and/or bed resting; orange = non-xeroderma pigmentosum-type neurological involvement [modified from Nishigori et al. (1994)]. (A) Age-based normative thresholds derived from combined male and female subject data from ISO 7029. (B) Patient XP12BE, XP-A with xeroderma pigmentosum-type neurological degeneration. (C) Patient XP19BE, XP-A with xeroderma pigmentosum-type neurological degeneration. (D) Patient XP33BE, XP-D with xeroderma pigmentosum-type neurological degeneration. (E) Patient XP29BE, XP-D with xeroderma pigmentosum-type neurological degeneration. (F) Patient XP18BE, XP-D with xeroderma pigmentosum-type neurological degeneration. (G) Patient XP-1BE, XP-C, xeroderma pigmentosum without neurological involvement (NI). (H) Patient XP21BE, XP-C with non-xeroderma pigmentosum type neurological involvement. HL = hearing loss; XP = xeroderma pigmentosum; XPND = xeroderma pigmentosum-type neurological degeneration.
Table 3.
Neurological status of patients with xeroderma pigmentosum
| Complementation group | No neurological involvement | Xeroderma pigmentosum neurological involvementa | Non-XP neurological involvement | Total | ||
|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | ||||
| A | 2 | 2 | 1 | 3 | 1 | 9 |
| B | 0 | 0 | 0 | 0 | 1 | 1 |
| C | 36 | 0 | 0 | 0 | 2 | 38 |
| D | 5 | 4 | 2 | 5 | 1 | 17 |
| E | 2 | 0 | 0 | 0 | 0 | 2 |
| G | 1 | 0 | 0 | 0 | 1 | 2 |
| XP-variant | 5 | 0 | 0 | 0 | 0 | 5 |
| Unknown | 4 | 0 | 0 | 0 | 1 | 5 |
| Total | 55 | 6 | 3 | 8 | 7 | 79 |
a Modified from Nishigori et al. (1994) (Fig. 2).
XP = xeroderma pigmentosum.
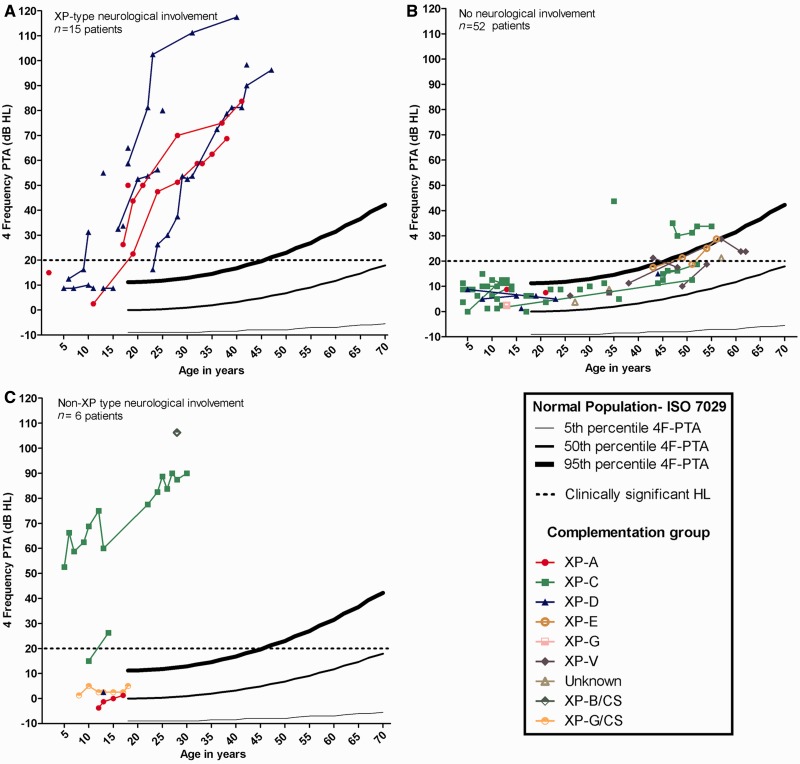
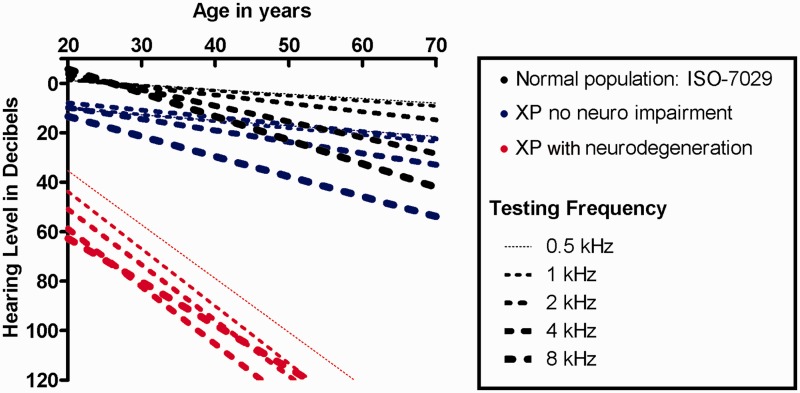
Similar hearing patterns existed between better and worse hearing ears for all participants, with no gross asymmetries between type and degree (data not shown). Figure 3 shows the 4F-PTA in the worse ear for patients with xeroderma pigmentosum-type neurological degeneration, xeroderma pigmentosum without neurological involvement and non-xeroderma pigmentosum-type neurological involvement. Most patients with xeroderma pigmentosum-type neurological degeneration demonstrate a significant hearing impairment as compared with the normal population (Fig. 3A). All patients with xeroderma pigmentosum-type neurological degeneration developed clinically significant hearing loss by age 24 years, except for four patients who were aged <16 years, and one profoundly hearing impaired patient who was not tested at the National Institutes of Health until age 42 years. The mean age of documented onset of clinically significant hearing loss for patients with xeroderma pigmentosum-type neurological degeneration was 19 years. This onset was ∼54 years younger than the predicted 73 years for the general population (Fig. 3A).
Figure 3.
Worse-hearing ear 4F-PTA plotted against the 95th, 50th and 5th percentiles obtained from age-matched normative data (ISO 7029) and the criterion for clinically normal hearing (20 dBHL). Data are plotted for all patients with xeroderma pigmentosum (XP) grouped by neurological status and colour-coded for xeroderma pigmentosum complementation group. (A) Patients with xeroderma pigmentosum-type neurological degeneration. (B) Patients with xeroderma pigmentosum without neurological involvement. The two XP-C patients above the 95th percentile range have documented noise exposure. (C) Patients with xeroderma pigmentosum with non-xeroderma pigmentosum-type neurological involvement. HL = hearing loss.
Eighty-nine per cent (49/55) of patients with xeroderma pigmentosum without neurological involvement had normal hearing (Fig. 3B), with 4F-PTAs falling between the 50th and 95th percentiles of the normal distribution. Two XP-C patients, who are cousins, had documented noise exposure as a result of farm machinery and had exhibited sensorineural hearing loss above the 95th percentile. In the remaining patients with sensorineural hearing loss and no neurological involvement, the hearing loss developed after 49 years of age. Sixty-five per cent (36/55) of patients with xeroderma pigmentosum without neurological involvement were in complementation group XP-C (Fig. 3B). For the six patients without neurological involvement who demonstrated clinically significant hearing loss, the average age at reference audiogram was 52 years, whereas the average age of the 13 patients with xeroderma pigmentosum-type neurological degeneration and clinically significant hearing loss was 26 years. These are both substantially lower than the average age (73 years) of developing clinically significant hearing loss calculated from the ISO-7029 normal data. Unlike the other two groups, the group with non-xeroderma pigmentosum-type neurological involvement had no distinct pattern of hearing loss (Fig. 3C).
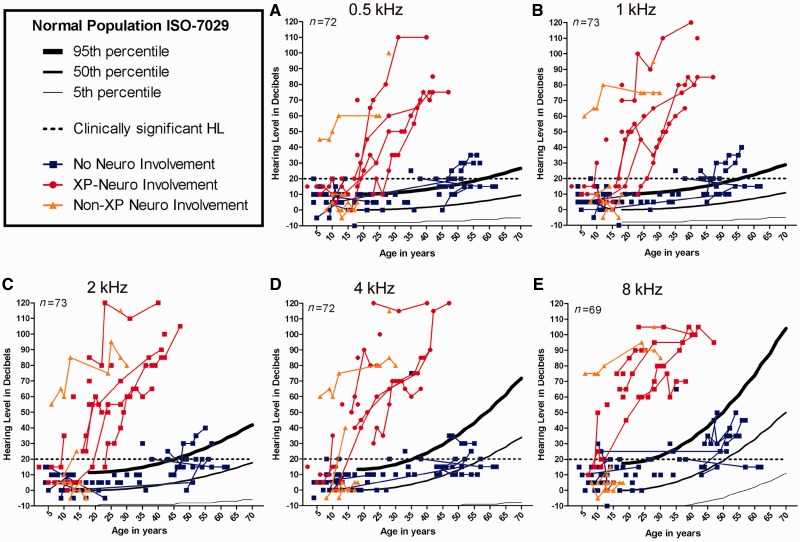
Pure-tone hearing thresholds were similar within each xeroderma pigmentosum group (xeroderma pigmentosum-type neurological degeneration, no neurological involvement and non-xeroderma pigmentosum-type neurological involvement); however, a distinct difference in the degree of hearing thresholds between the neurological versus the non-neurological xeroderma pigmentosum groups (Fig. 4A–E) was observed. This finding is independent of the frequency tested with the largest disparity in pure-tone hearing between xeroderma pigmentosum neurological groups occurring at 8 kHz for the younger age groups. Regression analysis (Fig. 5) demonstrates both a greater degree and more rapid progression of hearing loss in the xeroderma pigmentosum-type neurological degeneration group as compared with otologically normal persons (ISO 7029) and the xeroderma pigmentosum with no neurological involvement group. Although the patients with xeroderma pigmentosum without neurological involvement and the ISO normal hearing population had similar rates of hearing decline for all frequencies, the patients with xeroderma pigmentosum without neurological involvement had a higher y-intercept for hearing thresholds that accounts for the difference in age-based hearing (Fig. 5).
Figure 4.
Worse-hearing ear pure-tone thresholds plotted against the percentiles obtained from age-matched normative data (ISO 7029) and the criterion for clinically normal hearing (20 dB hearing loss) for 0.5, 1, 2, 4 and 8 kHz. Neurological status, xeroderma pigmentosum-type neurological degeneration, patients with xeroderma pigmentosum (XP) without neurological involvement, and non-xeroderma pigmentosum type neurological involvement, is indicated by colour-coding. HL = hearing loss.
Figure 5.
Linear regression analysis for age-based hearing decline at 0.5, 1, 2, 4 and 8 kHz. Regression is based on combined gender normative data (ISO 7029) and the worse hearing ear at the reference audiogram for patients with xeroderma pigmentosum-type neurological degeneration and patients with xeroderma pigmentosum without neurological involvement.
Acute burning on minimal sun exposure
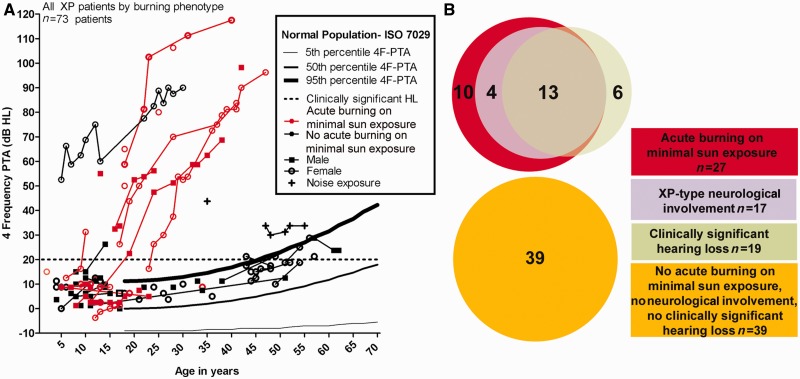
The 4F-PTA showed a strong association between a history of acute burning on minimal sun exposure and degree of hearing loss (Fig. 6A). The hearing loss pattern in Fig. 6A is similar to that observed in Fig. 3A. This is predominantly because all patients with xeroderma pigmentosum with xeroderma pigmentosum-type neurological degeneration also experienced acute burning on minimal sun exposure (Fig. 6B). There was no difference in hearing patterns between male and female subjects. After excluding patients with non-xeroderma pigmentosum-type neurological involvement, 27 patients had acute burning on minimal sun exposure; 17 with xeroderma pigmentosum-type neurological degeneration and 10 without neurological involvement. Of the 10 patients with xeroderma pigmentosum without neurological involvement who had acute burning on minimal sun exposure, all had normal hearing. The combination of acute burning on minimal sun exposure and 4F-PTA ≥10 dBHL was associated with a 39-fold increased odds ratio (P = 0.002) of having xeroderma pigmentosum neurological degeneration in patients between the ages of 4–30 years. None of the six patients with xeroderma pigmentosum with clinically significant hearing loss without neurological involvement experienced acute burning on minimal sun exposure (Fig. 6B).
Figure 6.
Acute burning on minimal sun exposure as an indicator of hearing loss (HL) and neurological involvement in patients with xeroderma pigmentosum. (A) Worse ear 4F-PTA in all patients with xeroderma pigmentosum grouped by acute burning on minimal sun exposure and plotted against the percentiles obtained from age-matched normative data (ISO 7029) and the criterion for clinically normal hearing (20 dB hearing loss). (B) Venn diagram showing acute burning on minimal sun exposure, hearing status and neurological involvement for patients with xeroderma pigmentosum with xeroderma pigmentosum-type neurological degeneration and no neurological involvement.
Cochlear histopathology in patients with xeroderma pigmentosum-type neurological degeneration
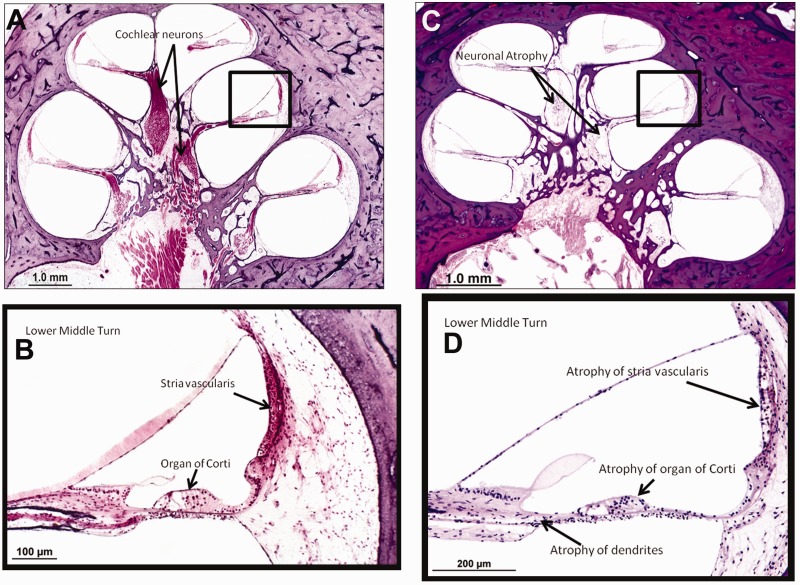
Temporal bone histopathology was analysed for Patient XP12BE with xeroderma pigmentosum-type neurological degeneration, who was 44 years old at autopsy (Ramkumar et al., 2011) and was compared with a normal cochlea (Fig. 7A–D). This patient had a defect in the XP-A gene and severe, bilateral progressive sensorineural hearing loss first documented in her second decade of life (Robbins et al., 1991) (Fig. 2B). Cochlear pathology shows diffuse and severe atrophy of the organ of Corti, moderate to severe atrophy of the stria vascularis and severe diffuse atrophy of cochlear neurons (Fig. 7C and D) when compared with a normal hearing cochlea (Fig. 7A and B). There was also mild basal atrophy of the spiral limbus. Both cochleae in this patient demonstrated similar pathology.
Figure 7.
Cochlear histopathology in a patient with xeroderma pigmentosum-type neurodegeneration. (A) Cochlear histopathology of a normal-hearing individual at 63 years of age shown for comparison. (B) Higher power magnification of boxed area in (A). (C) Left ear cochlear pathology of Patient XP12BE (age 44 years) who developed xeroderma pigmentosum-type neurological degeneration. (D) Higher power magnification of boxed area in (C). Pathology shows diffuse atrophy of the organ of Corti, moderate to patchy atrophy of the stria vascularis and severe atrophy of cochlear neurons as compared with the control cochlea with normal hearing.
Prediction of neurodegeneration
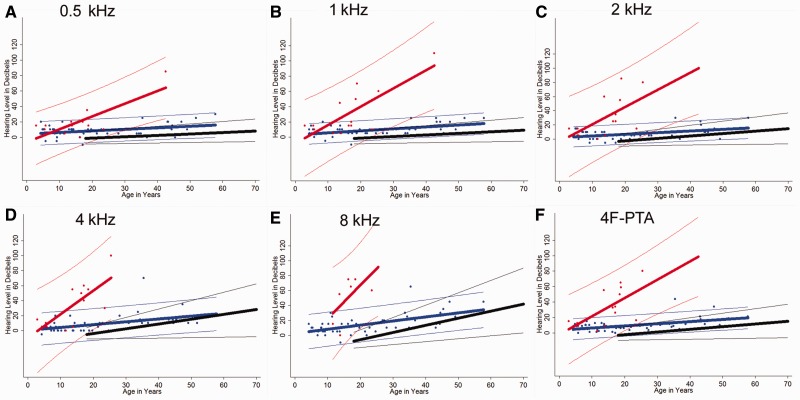
Figure 8 provides a model for charting hearing levels over time for patients with xeroderma pigmentosum to determine which curve their threshold values follow most closely to predict whether they will develop neurodegeneration. The earliest audiogram was used for each patient so that this regression could more accurately predict threshold values for young patients with xeroderma pigmentosum for whom prediction of xeroderma pigmentosum-type neurological degeneration is most critical and difficult to accomplish. After Bonferroni correction, the Chow test showed that each group was different from the others (P < 0.001) at all frequencies tested (0.5/1/2/4/8 kHz) with respect to the combined slope and intercept, except when comparing the xeroderma pigmentosum no neurological involvement group to normative data for a 4 kHz stimulus. Ranges of hearing level values overlapped most closely at the younger age groups for all frequencies, but the greatest differences in range between xeroderma pigmentosum-type neurological degeneration and xeroderma pigmentosum with no neurological involvement groups occurred at 8 kHz.
Figure 8.
Regression curve analysis by Chow test. (A–F) We compared combined gender ISO 7029 normative data (black), patients with xeroderma pigmentosum without neurological involvement (blue) and patients with xeroderma pigmentosum-type neurodegeneration (red) for 0.5, 1, 2, 4 and 8 kHz and the 4F-PTA. Regression curves for the patients with xeroderma pigmentosum are derived from the worse hearing ear at the earliest complete audiogram for each patient. The thicker line in the middle of each group of coloured data points represents a linear regression line corresponding to decibel level as predicted by age for that particular group. The thinner outer lines of each group mark the boundaries within which you would expect to find 95% of the sample’s values for a given age based on calculated prediction intervals. For the normal group, the recorded intervals represent those within which 95% of the population lies. P-values are <0.001 for all frequencies (including 4F-PTA) when comparing the combination of intercepts and slope for regression curves between xeroderma pigmentosum-type neurological degeneration, patients with xeroderma pigmentosum without neurological involvement and ISO normal populations, with the exception of ISO normal versus xeroderma pigmentosum no neurological involvement at 4 kHz (P = 0.006). All values are significant at Bonferroni-adjusted value of P < 0.002.
Discussion
Prediction of patients with xeroderma pigmentosum who will develop xeroderma pigmentosum-type neurological degeneration is clinically challenging. Here, we report that audiometric status, xeroderma pigmentosum complementation group and acute burning on minimal sun exposure are important indicators of xeroderma pigmentosum-type neurological degeneration, and we present a model for predicting xeroderma pigmentosum-type neurological degeneration in patients with xeroderma pigmentosum. In this 41-year retrospective follow-up study of hearing loss in patients with xeroderma pigmentosum, we have shown that degree of hearing loss is directly correlated with neurological involvement. Although hearing loss in patients with xeroderma pigmentosum with xeroderma pigmentosum-type neurological degeneration has been implicated in the past (Robbins et al., 1974, 1991; Kenyon et al., 1985; Mamada et al., 1988; Rapin et al., 2000; Lindenbaum et al., 2001; Kraemer et al., 2007; Anttinen et al., 2008) mainly through case reports, this study analyses audiometric data in a large cohort of patients with xeroderma pigmentosum. Xeroderma pigmentosum-type neurological degeneration has been documented in patients with XP-A, XP-B, XP-D, XP-F and XP-G. Patients with xeroderma pigmentosum seen at the National Institutes of Health with xeroderma pigmentosum-type neurological degeneration were either XP-A or XP-D, the more common xeroderma pigmentosum complementation groups in the USA (Moriwaki and Kraemer, 2001). No patients with XP-C reported acute burning on minimal sun exposure or developed xeroderma pigmentosum-type neurological degeneration, whereas all patients with xeroderma pigmentosum-type neurological degeneration had acute burning on minimal sun exposure and were all XP-A or XP-D. Fibroblasts from patients with acute burning on minimal sun exposure of complementation groups XP-A and XP-D have been shown to be more susceptible to ultraviolet radiation damage compared with patients with XP-C who do not burn easily (Robbins and Moshell, 1979). This may indicate that transcription-coupled DNA repair (which is defective in XP-A and XP-D but normal in XP-C) (DiGiovanna and Kraemer, 2012) plays an important role in the pathogenesis of the sunburn response and in the development of DNA damage that affects neuronal cell death.
In studying rare diseases, sample size always presents a limitation. Our report of 79 patients with xeroderma pigmentosum with audiometric data measured during the past four decades is one of the largest of its kind. We identified a 54-year reduction in age of detected onset of clinically significant hearing loss in patients with xeroderma pigmentosum-type neurological degeneration as compared with the ISO normal hearing population. This is similar to the 58-year reduction in age at first non-melanoma skin cancer reported by Bradford et al. (2011) in patients with xeroderma pigmentosum compared with the general population, suggesting that xeroderma pigmentosum is an important model of premature ageing through the accelerated accumulation of DNA damage. The 19-year mean age of detection of onset of clinically significant hearing loss in the patients with xeroderma pigmentosum-type neurological degeneration was based on data in the few patients who were tested before and after clinically significant hearing loss developed. However, it was a conservative estimate of the mean age of clinically significant hearing loss given that the majority of patients with xeroderma pigmentosum-type neurological degeneration had a 4F-PTA well above the 95th percentile of normal hearing at initial testing.
Patients with xeroderma pigmentosum with xeroderma pigmentosum-type neurological degeneration and those without neurological involvement had different rates of hearing decline. One possible explanation for this is that patients with xeroderma pigmentosum without neurological involvement may experience a slower progression in accumulation of DNA damage to their auditory system when compared with patients with xeroderma pigmentosum-type neurological degeneration, thus accounting for the normal histological appearance of the temporal bone in an XP-C patient (Robbins et al., 2002). In addition, those with severe neurological involvement may have greater test–retest variability.
Our data show a definitive difference between patients with xeroderma pigmentosum with xeroderma pigmentosum-type neurological degeneration and the ISO normal hearing population; however, the relationship between the normal population and the patients with xeroderma pigmentosum without neurological involvement is more difficult to interpret (Robbins et al., 2002). The Chow test showed that the ISO normative data were different from those for the patients with xeroderma pigmentosum without neurological involvement (P < 0.001) at all frequencies tested except for 4 kHz, suggesting that patients with xeroderma pigmentosum without neurological involvement could have slightly worse hearing than expected for their age. Audio profile regression analysis also shows a difference between the ISO normal population and the xeroderma pigmentosum without neurological involvement group, yet the slopes for hearing loss over time are similar. The difference lies in the y-intercept, with the patients with xeroderma pigmentosum starting out at higher hearing thresholds. This may be interpreted as a true difference in hearing between the two populations, with patients with xeroderma pigmentosum without neurological involvement developing subclinical hearing loss. However, this difference may also be accounted for by variations in hearing test methodologies or subject test-taking abilities between the otologically normal ISO population and our cohort with xeroderma pigmentosum. Further studies will be necessary to more accurately describe this relationship, and to determine whether DNA repair defects in patients with xeroderma pigmentosum without neurological disease could be affecting the hearing apparatus over time.
Here, we report xeroderma pigmentosum temporal bone histopathology that correlates with audiometric findings and neurological decline. Our findings in Patient XP12BE with xeroderma pigmentosum-type neurological degeneration contrast with those of Patient XP1BE, who had xeroderma pigmentosum but no neurological involvement. This patient was previously reported by Robbins et al. (2002) to have normal cochlear neuron cell loss for her age by temporal bone histopathology. Patient XP1BE had essentially normal hearing with some hearing loss limited to the higher frequencies consistent with age-matched normative data (Fig. 2G). The cochlear neuronal atrophy in 44-year-old Patient XP12BE with xeroderma pigmentosum-type neurological degeneration was much greater than expected for an individual of her age (Fig. 7). Temporal bone histopathology for patient XP12BE indicates that her hearing loss has a cochlear component. Extended audiometric evaluation, including word recognition, acoustic reflexes and auditory brainstem responses, provided no evidence of a neural component to this patient’s hearing loss through 21 years of age (data not shown). At age 44 years, the histopathological correlate of the sensorineural hearing loss demonstrated in this patient was severe degeneration of the hair cells of the organ of Corti and also of the primary cochlear neurons. It is impossible to state with certainty that the neural degeneration is primary rather than secondary to hair cell degeneration. However, the fact that in the apical half of the cochlea there were remaining hair cells despite severe degeneration of cochlear neurons, suggests that at least in part, the neural degeneration is primary. In addition, the fact that the vestibular hair cells were either normal for age or only mildly decreased in numbers despite severe atrophy of Scarpa’s (vestibular) ganglion also suggests primary neuronal degeneration. Further temporal bone analyses in patients with xeroderma pigmentosum-type neurological degeneration will be necessary to determine whether these histopathological findings are similar in the entire xeroderma pigmentosum patient population. Assessment of auditory neural function throughout the lifespan with both behavioural and physiological measures may contribute to a more complete understanding of the pathophysiology of the auditory dysfunction in patients with xeroderma pigmentosum.
Although yet to be definitively proven, a mechanism underlying age-related hearing decline, or presbycusis, is thought to involve the progressive accumulation of DNA damage caused by reactive oxygen species (McFadden et al., 1999; Hanawalt, 2001; Willott et al., 2001; Seidman et al., 2002; Pickles, 2004; Darrat et al., 2007; Jiang et al., 2007). Our data support the hypothesis that DNA damage may contribute to presbycusis by showing greatly accelerated rates of hearing loss in patients with xeroderma pigmentosum-related neurological disease and defects in DNA repair genes XP-A and XP-D. A recent study using ERCC1 δ/− mutant mice deficient in nucleotide excision repair, inter-strand cross-link repair and double-strand break repair showed progressive and accelerated increases in hearing level thresholds thought to arise from diminishing cochlear function (Spoor et al., 2012). Although the molecular mechanism remains unknown, this provides evidence that the pathogenesis underlying age-related hearing loss may relate to reactive oxygen species-induced DNA damage over time.
Hearing loss has also been reported in patients with other defects in DNA repair, namely Cockayne syndrome, cerebro-oculo-facial-skeletal syndrome and trichothiodystrophy. Patients with Cockayne syndrome and cerebro-oculo-facial-skeletal syndrome have progressive sensorineural hearing loss similar to our group of patients with xeroderma pigmentosum with neurodegeneration (Iwasaki and Kaga, 1994; Iwasaki et al., 1996; Del Bigio et al., 1997; Rapin et al., 2000, 2006; Fish et al., 2001; Lindenbaum et al., 2001; Weidenheim et al., 2009). A few case reports have described sensorineural hearing loss in trichothiodystrophy (Toelle et al., 2001; Yoon et al., 2005) and preliminary data from our National Institutes of Health cohort of 23 patients with trichothiodystrophy (Brooks et al., 2011; Tamura et al., 2011) provide further evidence that this may be the case in a subset. Interestingly, patients with Cockayne syndrome and cerebro-oculo-facial-skeletal syndrome demonstrate neurological degeneration in addition to dysmyelination of the brain, whereas patients with trichothiodystrophy only demonstrate dysmyelination (Rapin et al., 2000; Lindenbaum et al., 2001; Seidman et al., 2002; Yoon et al., 2005; Kraemer et al., 2007; Weidenheim et al., 2009). These neurological findings suggest different mechanisms that may underlie the neurological involvement and hearing loss in these patients (Brooks et al., 2008). When available, results of acoustic reflex assessment, otoacoustic emissions, word recognition scores and auditory brainstem responses were examined for evidence of a neural or other retrocochlear contribution to the hearing loss. We observed a small number of cases with a single retrocochlear finding. Although our data support a predominant cochlear hearing loss in xeroderma pigmentosum, prospective assessment with tests sensitive to sensory versus neural function should be conducted to further elucidate site of lesion in the auditory system. Further studies on disease mechanisms will be critical in understanding differences present among patients with xeroderma pigmentosum in addition to the other disorders of DNA repair, so that targeted therapeutics may be developed.
This study implicates DNA repair as an essential part of the auditory system to maintain normal hearing function as we age. Here, we report a 39-fold increased odds of developing neurodegeneration in patients with xeroderma pigmentosum between the ages of 4–30 years with acute burning on minimal sun exposure, and who have a 4F-PTA ≥10 dBHL. Graphical analysis of hearing levels in patients with xeroderma pigmentosum (Figs 6 and 8) provides a useful clinical method for predicting xeroderma pigmentosum-type neurological degeneration and for determining progression of neurological disease in those who develop xeroderma pigmentosum-type neurological degeneration. Although all audiograms were carefully reviewed for internal consistency, a limitation of this study is the use of retrospective data in which testing took place in multiple sites. Future studies will help in determining when and how often audiometric testing should be performed in patients with xeroderma pigmentosum for predicting xeroderma pigmentosum-type neurological degeneration and for monitoring neurological decline. These factors taken together provide an important tool to direct clinical care in patients with xeroderma pigmentosum and for guiding current and potential future treatments. Avoidance of high levels of noise/music exposure should be a routine part of counselling for these patients who are already at risk for xeroderma pigmentosum-related hearing loss. Annual hearing exams may be an important part of management for all patients with xeroderma pigmentosum not only to monitor their hearing status and identify the need for auditory habilitative interventions such as hearing aids, but also to assist in prediction and surveillance of neurological symptoms.
Funding
Intramural Research Programs of the Center for Cancer Research, National Cancer Institute (M.B.T., D.T., S.G.K., J.J.D. and K.H.K.), of the Division of Cancer Epidemiology and Genetics, National Cancer Institute (P.T.B.), of the National Institute on Deafness and Other Communication Disorders (NIDCD) (C.Z., A.J.G. and C.C.B.) and of the National Institute of Aging (M.S.P.) of the National Institutes of Health and from NIDCD (1U24DC011943-01 to S.M. and J.N.). Clinical Research Training Program (to M.B.T. and M.S.P.), a public–private partnership supported jointly by the National Institutes of Health and Pfizer Inc (via a grant to the Foundation for National Institutes of Health from Pfizer Inc).
Acknowledgements
The authors thank Ms. Talah Wafa, Pamela Buethe, Ruth Marin and Elizabeth Hellmuth, doctoral students at Gallaudet University, for audiology data entry and initial analysis of a subset of these data. They also thank Barbara Burgess, Diane Jones and Jon Pack from the Otopathology Laboratory at Massachusetts Eye and Ear Infirmary for assistance with the temporal bone pathology examinations. They want to recognize the contributions of Dr Jay Robbins who initiated these studies of xeroderma pigmentosum at the National Institutes of Health.
Glossary
Abbreviations
- XP-A–G
xeroderma pigmentosum complementation groups A–G
- 3F-PTA
three-frequency (0.5/1/2 kHz) pure-tone average
- 4F-PTA
four-frequency (0.5/1/2/4-kHz) pure-tone average
- dBHL
decibels hearing level
- HL
hearing loss
References
- American National Standard Institute. New York: American National Standards Institute; 2003. Maximum permissible ambient noise levels for audiometric test rooms (Standard S3.1) (ANSI. S3.1-1999) [Google Scholar]
- American National Standards Institute. New York: American National Standards Institute; 2004. American national standard specification for audiometers (Standard S3.6) (ANSI. S3.6-1996) [Google Scholar]
- Anttinen A, Koulu L, Nikoskelainen E, Portin R, Kurki T, Erkinjuntti M, et al. Neurological symptoms and natural course of xeroderma pigmentosum. Brain. 2008;131:1979–89. doi: 10.1093/brain/awn126. [DOI] [PubMed] [Google Scholar]
- Bradford PT, Goldstein AM, Tamura D, Khan SG, Ueda T, Boyle J, et al. Cancer and neurologic degeneration in xeroderma pigmentosum: long term follow-up characterises the role of DNA repair. J Med Genet. 2011;48:168–76. doi: 10.1136/jmg.2010.083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks BP, Thompson AH, Clayton JA, Chan CC, Tamura D, Zein WM, et al. Ocular manifestations of trichothiodystrophy. Ophthalmology. 2011;118:2335–42. doi: 10.1016/j.ophtha.2011.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks PJ, Cheng TF, Cooper L. Do all of the neurologic diseases in patients with DNA repair gene mutations result from the accumulation of DNA damage? DNA Repair (Amst) 2008;7:834–48. doi: 10.1016/j.dnarep.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrat I, Ahmad N, Seidman K, Seidman MD. Auditory research involving antioxidants. Curr Opin Otolaryngol Head Neck Surg. 2007;15:358–63. doi: 10.1097/MOO.0b013e3282efa641. [DOI] [PubMed] [Google Scholar]
- Del Bigio MR, Greenberg CR, Rorke LB, Schnur R, McDonald-McGinn DM, Zackai EH. Neuropathological findings in eight children with cerebro-oculo-facio-skeletal (COFS) syndrome. J Neuropathol Exp Neurol. 1997;56:1147–57. doi: 10.1097/00005072-199710000-00009. [DOI] [PubMed] [Google Scholar]
- DiGiovanna JJ, Kraemer KH. Shining a light on xeroderma pigmentosum. J Invest Dermatol. 2012;132:785–96. doi: 10.1038/jid.2011.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish JH, III, Scholtz AW, Hussl B, Kreczy A, Schrott-Fischer A. Cerebro-oculo-facio-skeletal syndrome as a human example for accelerated cochlear nerve degeneration. Otol Neurotol. 2001;22:170–7. doi: 10.1097/00129492-200103000-00009. [DOI] [PubMed] [Google Scholar]
- Gelfand SA, Schwander T, Silman S. Acoustic reflex thresholds in normal and cochlear-impaired ears: effects of no-response rates on 90th percentiles in a large sample. J Speech Hear Disord. 1990;55:198–205. doi: 10.1044/jshd.5502.198. [DOI] [PubMed] [Google Scholar]
- Hanawalt PC. Controlling the efficiency of excision repair. Mutat Res. 2001;485:3–13. doi: 10.1016/s0921-8777(00)00071-9. [DOI] [PubMed] [Google Scholar]
- Imoto K, Slor H, Orgal S, Khan SH, Oh KS, Busch DB, et al. Xeroderma pigmentosum group F patients with late onset neurological disease. J Invest Dermatol. 2005;124:A78. [Google Scholar]
- International Standard Organization. Geneva: International Organization for Standardization; 2000. ISO 7029-1, Acoustics—statistical distribution of hearing thresholds as a function of age. [Google Scholar]
- Iwasaki S, Kaga K. Chronological changes of auditory brainstem responses in Cockayne's syndrome. Int J Pediatr Otorhinolaryngol. 1994;30:211–21. doi: 10.1016/0165-5876(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Kaga K, Yagi M, Kuroda M. Vestibular findings and brainstem pathology in two siblings with Cockayne's syndrome. ORL J Otorhinolaryngol Relat Spec. 1996;58:343–6. doi: 10.1159/000276867. [DOI] [PubMed] [Google Scholar]
- Jiang H, Talaska AE, Schacht J, Sha SH. Oxidative imbalance in the aging inner ear. Neurobiol Aging. 2007;28:1605–12. doi: 10.1016/j.neurobiolaging.2006.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon GS, Booth JB, Prasher DK, Rudge P. Neuro-otological abnormalities in xeroderma pigmentosum with particular reference to deafness. Brain. 1985;108 (Pt 3):771–84. doi: 10.1093/brain/108.3.771. [DOI] [PubMed] [Google Scholar]
- Khan SG, Oh KS, Emmert S, Imoto K, Tamura D, Digiovanna JJ, et al. XPC initiation codon mutation in xeroderma pigmentosum patients with and without neurological symptoms. DNA Repair (Amst) 2009;8:114–25. doi: 10.1016/j.dnarep.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KA, Makishima T, Zalewski CK, Bakalov VK, Griffith AJ, Bondy CA, et al. Analysis of auditory phenotype and karyotype in 200 females with Turner syndrome. Ear Hear. 2007;28:831–41. doi: 10.1097/AUD.0b013e318157677f. [DOI] [PubMed] [Google Scholar]
- Kraemer KH, Lee MM, Andrews AD, Lambert WC. The role of sunlight and DNA repair in melanoma and nonmelanoma skin cancer. The xeroderma pigmentosum paradigm. Arch Dermatol. 1994;130:1018–21. [PubMed] [Google Scholar]
- Kraemer KH, Lee MM, Scotto J. Xeroderma pigmentosum. Cutaneous, ocular, and neurologic abnormalities in 830 published cases. Arch Dermatol. 1987;123:241–50. doi: 10.1001/archderm.123.2.241. [DOI] [PubMed] [Google Scholar]
- Kraemer KH, Patronas NJ, Schiffmann R, Brooks BP, Tamura D, DiGiovanna JJ. Xeroderma pigmentosum, trichothiodystrophy and Cockayne syndrome: a complex genotype-phenotype relationship. Neuroscience. 2007;145:1388–96. doi: 10.1016/j.neuroscience.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbaum Y, Dickson D, Rosenbaum P, Kraemer K, Robbins I, Rapin I. Xeroderma pigmentosum/cockayne syndrome complex: first neuropathological study and review of eight other cases. Eur J Paediatr Neurol. 2001;5:225–42. doi: 10.1053/ejpn.2001.0523. [DOI] [PubMed] [Google Scholar]
- Mamada A, Kondo S, Kawada A, Satoh Y, Fujiwara Y. Delayed sensorineural deafness and skin carcinogenesis in a Japanese xeroderma pigmentosum group D patient. Photodermatol. 1988;5:83–91. [PubMed] [Google Scholar]
- Margolis RH, Heller JW. Screening tympanometry: criteria for medical referral. Audiology. 1987;26:197–208. doi: 10.3109/00206098709081549. [DOI] [PubMed] [Google Scholar]
- Mazzoli M, van Camp G, Newton V, Giarbini N, Declau F, Parving A. Recommendations for the description of genetic and audiological data for families with nonsyndromic hereditary hearing impairment. Audiol Med. 2003;1:148–50. [Google Scholar]
- McFadden SL, Ding D, Reaume AG, Flood DG, Salvi RJ. Age-related cochlear hair cell loss is enhanced in mice lacking copper/zinc superoxide dismutase. Neurobiol Aging. 1999;20:1–8. doi: 10.1016/s0197-4580(99)00018-4. [DOI] [PubMed] [Google Scholar]
- Moriwaki S, Kraemer KH. Xeroderma pigmentosum—bridging a gap between clinic and laboratory. Photodermatol Photoimmunol Photomed. 2001;17:47–54. doi: 10.1034/j.1600-0781.2001.017002047.x. [DOI] [PubMed] [Google Scholar]
- Nishigori C, Moriwaki S, Takebe H, Tanaka T, Imamura S. Gene alterations and clinical characteristics of xeroderma pigmentosum group A patients in Japan. Arch Dermatol. 1994;130:191–7. [PubMed] [Google Scholar]
- Pickles JO. Mutation in mitochondrial DNA as a cause of presbyacusis. Audiol Neurootol. 2004;9:23–33. doi: 10.1159/000074184. [DOI] [PubMed] [Google Scholar]
- Ramkumar HL, Brooks BP, Cao X, Tamura D, Digiovanna JJ, Kraemer KH, et al. Ophthalmic manifestations and histopathology of xeroderma pigmentosum: two clinicopathological cases and a review of the literature. Surv Ophthalmol. 2011;56:348–61. doi: 10.1016/j.survophthal.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapin I, Lindenbaum Y, Dickson DW, Kraemer KH, Robbins JH. Cockayne syndrome and xeroderma pigmentosum. Neurology. 2000;55:1442–9. doi: 10.1212/wnl.55.10.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapin I, Weidenheim K, Lindenbaum Y, Rosenbaum P, Merchant SN, Krishna S, et al. Cockayne syndrome in adults: review with clinical and pathologic study of a new case. J Child Neurol. 2006;21:991–1006. doi: 10.1177/08830738060210110101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins JH, Brumback RA, Mendiones M, Barrett SF, Carl JR, Cho S, et al. Neurological disease in xeroderma pigmentosum. Documentation of a late onset type of the juvenile onset form. Brain. 1991;114 (Pt 3):1335–61. doi: 10.1093/brain/114.3.1335. [DOI] [PubMed] [Google Scholar]
- Robbins JH, Kraemer KH, Lutzner MA, Festoff BW, Coon HG. Xeroderma pigmentosum. An inherited disease with sun sensitivity, multiple cutaneous neoplasms, and abnormal DNA repair. Ann Intern Med. 1974;80:221–48. doi: 10.7326/0003-4819-80-2-221. [DOI] [PubMed] [Google Scholar]
- Robbins JH, Kraemer KH, Merchant SN, Brumback RA. Adult-onset xeroderma pigmentosum neurological disease–observations in an autopsy case. Clin Neuropathol. 2002;21:18–23. [PubMed] [Google Scholar]
- Robbins JH, Moshell AN. DNA repair processes protect human beings from premature solar skin damage: evidence from studies on xeroderma pigmentosum. J Invest Dermatol. 1979;73:102–7. doi: 10.1111/1523-1747.ep12532789. [DOI] [PubMed] [Google Scholar]
- Seidman MD, Ahmad N, Bai U. Molecular mechanisms of age-related hearing loss. Ageing Res Rev. 2002;1:331–43. doi: 10.1016/s1568-1637(02)00004-1. [DOI] [PubMed] [Google Scholar]
- Spoor M, Nagtegaal AP, Ridwan Y, Borgesius NZ, van Alphen B, van der Pluijm I, et al. Accelerated loss of hearing and vision in the DNA-repair deficient Ercc1(delta/-) mouse. Mech Ageing Dev. 2012;133:59–67. doi: 10.1016/j.mad.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Tamura D, Merideth M, DiGiovanna JJ, Zhou X, Tucker MA, Goldstein AM, et al. High-risk pregnancy and neonatal complications in the DNA repair and transcription disorder trichothiodystrophy: report of 27 affected pregnancies. Prenat Diagn. 2011;31:1046–53. doi: 10.1002/pd.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toelle SP, Valsangiacomo E, Boltshauser E. Trichothiodystrophy with severe cardiac and neurological involvement in two sisters. Eur J Pediatr. 2001;160:728–31. doi: 10.1007/s004310100845. [DOI] [PubMed] [Google Scholar]
- Weidenheim KM, Dickson DW, Rapin I. Neuropathology of Cockayne syndrome: Evidence for impaired development, premature aging, and neurodegeneration. Mech Ageing Dev. 2009;130:619–36. doi: 10.1016/j.mad.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Willott JF, Hnath CT, Lister JJ. Modulation of presbycusis: current status and future directions. Audiol Neurootol. 2001;6:231–49. doi: 10.1159/000046129. [DOI] [PubMed] [Google Scholar]
- Yoon HK, Sargent MA, Prendiville JS, Poskitt KJ. Cerebellar and cerebral atrophy in trichothiodystrophy. Pediatr Radiol. 2005;35:1019–23. doi: 10.1007/s00247-005-1495-6. [DOI] [PubMed] [Google Scholar]