Abstract
Sucrose is perhaps the most efficient carbohydrate for the promotion of dental caries in humans, and the primary caries pathogen Streptococcus mutans encodes multiple enzymes involved in the metabolism of this disaccharide. Here, we engineered a series of mutants lacking individual or combinations of sucrolytic pathways to understand the control of sucrose catabolism and to determine whether as-yet-undisclosed pathways for sucrose utilization were present in S. mutans. Growth phenotypes indicated that gtfBCD (encoding glucan exopolysaccharide synthases), ftf (encoding the fructan exopolysaccharide synthase), and the scrAB pathway (sugar-phosphotransferase system [PTS] permease and sucrose-6-PO4 hydrolase) constitute the majority of the sucrose-catabolizing activity; however, mutations in any one of these genes alone did not affect planktonic growth on sucrose. The multiple-sugar metabolism pathway (msm) contributed minimally to growth on sucrose. Notably, a mutant lacking gtfBC, which cannot produce water-insoluble glucan, displayed improved planktonic growth on sucrose. Meanwhile, loss of scrA led to growth stimulation on fructooligosaccharides, due in large part to increased expression of the fruAB (fructanase) operon. Using the LevQRST four-component signal transduction system as a model for carbohydrate-dependent gene expression in strains lacking extracellular sucrases, a PlevD-cat (EIIALev) reporter was activated by pulsing with sucrose. Interestingly, ScrA was required for activation of levD expression by sucrose through components of the LevQRST complex, but not for activation by the cognate LevQRST sugars fructose or mannose. Sucrose-dependent catabolite repression was also evident in strains containing an intact sucrose PTS. Collectively, these results reveal a novel regulatory circuitry for the control of sucrose catabolism, with a central role for ScrA.
INTRODUCTION
Sucrose is among the most cariogenic carbohydrates (1, 2), and this disaccharide, composed of β2,1-linked fructose and glucose, influences the development of caries in multiple ways. First, sucrose can serve as a readily metabolizable carbon and energy source for many members of the oral microbiome and is a particularly effective substrate for generation of organic acids via glycolysis by the abundant oral streptococci and Actinomyces spp. that comprise a large proportion of the oral microbiome. Many oral bacteria possess transport systems for sucrose but can also hydrolyze sucrose outside the cell. Sucrose is a substrate for a variety of glucosyltransferase enzymes (GTFs) that are secreted mainly by oral streptococci. For example, the GTF enzymes of Streptococcus mutans, the primary etiologic agent of human dental caries, release free fructose and form high-molecular-mass α1,3- and α1,6-linked homopolymers of glucose, commonly called glucans or mutan, which act as an adhesive scaffolding to promote the formation of oral biofilms, particularly on the smooth surfaces of the teeth (3, 4). Many oral streptococci and certain Actinomyces spp. also produce fructosyltransferase (FTF) enzymes that convert sucrose into homopolymers of fructose (fructans) and free glucose (5, 6). Fructan polymers appear to serve mainly as extracellular energy storage compounds that can be hydrolyzed by fructanase enzymes to extend the depth and duration of oral biofilm acidification (7–9).
In S. mutans, sucrose can be internalized by the sugar:phosphotransferase system (PTS) via a high-affinity permease, EIIScr, encoded by the scrA gene (10, 11). Intracellular metabolism of sucrose-6-PO4 is then initiated by ScrB, a sucrose-6-PO4 hydrolase, to produce glucose-6-PO4 and fructose. A fructokinase (ScrK) encoded by a gene immediately downstream of scrA can channel the fructose into the glycolytic pathway (for a diagram, see Fig. S1 in the supplemental material) (12, 13). The expression of both scrA and scrB is under the control of a negative regulator, ScrR, encoded downstream of scrB, which binds to the promoter regions of scrA and scrB, and sucrose-grown cells showed the highest expression levels of scrAB (12). Another PTS permease, EIITre, which appears to be the primary transporter for trehalose, has been suggested to internalize sucrose in S. mutans GS-5 (14). Besides the PTS, two binding-protein-dependent carbohydrate transport systems, the multiple-sugar metabolism system (Msm) (15, 16) and the maltose/maltodextrin ABC transporter (17), have been implicated in sucrose uptake by S. mutans. The metabolism of sucrose after internalization via ABC transporters likely involves the sucrose phosphorylase GtfA, which converts sucrose into glucose-1-PO4 and fructose (18).
Three major GTFs are encoded by S. mutans: GtfB and GtfC produce predominantly α1,3-linked water-insoluble glucans, and GtfD produces an α1,6-rich, water-soluble glucan (4). The fructosyltransferase (FTF) (6) of S. mutans converts sucrose into a predominantly β2,1-linked inulin-type fructan polymer, although many oral bacteria make a levan-type β2,6-linked fructan. Both types of polymers can be hydrolyzed by S. mutans by a single secreted exo-β-d-fructosidase (FruA) (7, 19, 20). Expression of fruA is governed by an unusual four-component regulatory system that is composed of a conventional histidine kinase (LevS) and response regulator (LevR) and two membrane-associated carbohydrate-binding proteins (LevQ and LevT) that work in concert with LevS and LevR (21–24). The same regulatory system also controls expression of the levDEFG operon that encodes the A, B, C, and D domains of a fructose/mannose-PTS EII permease (24).
Prioritization of carbohydrate utilization in the human oral cavity is a critical function and is achieved in large part through carbon catabolite repression (CCR), the selective expression of genes involved in the uptake and metabolism of preferred carbohydrates coupled with repression of alternative catabolic pathways (25). In most low-G+C Gram-positive bacteria, CCR is effected primarily by catabolite control protein A, CcpA, which regulates gene expression by binding to conserved catabolite response elements (CRE) in the promoter regions of CCR-sensitive genes, although many alternative mechanisms to selectively regulate carbohydrate uptake and catabolic pathways exist. In S. mutans, CcpA does play an important role in the global control of gene expression (26), but CcpA-independent mechanisms involving the PTS phosphocarrier protein HPr and various EII permeases play dominant roles in CCR, regulating catabolic pathways such as the fruAB operon, the fructose/mannose-PTS encoded by levDEFG (22, 27), and transport and catabolism of cellobiose (28) and lactose (29). There are data to support that sucrose may be a preferred carbohydrate source that can elicit CCR in S. mutans (30–33). For example, a microarray-based transcriptomic analysis (30) indicated that growth in sucrose resulted in 2- to 3-fold-lower expression of the maltose/maltodextrin ABC transporter (SMU1568 to SMU1571) and the msm operon. In contrast, expression of the scrAB cluster was found to be relatively constant in cells growing on various carbohydrates (30).
The inherent complexity of multiple overlapping systems for sucrose dissimilation has been the primary impediment to the development of a clear understanding of the control of sucrose-metabolism and how the presence of sucrose exerts its effects on gene expression and virulence of S. mutans. In particular, there have not yet been any mutants of S. mutans constructed or identified that completely lack the ability to grow on sucrose. In addition, GTFs, FTF, and FruA are capable of releasing free fructose and glucose in the extracellular environment, which can affect the expression of PTS porters and trigger CCR. Further, production of glucan leads to clumping of bacterial cells, which makes it difficult to monitor planktonic growth and to be confident that factors other than the growth carbohydrate, for example, cell density or quorum-sensing pathways, are inducing the observed changes in gene expression and cell behavior. In this study, we overcame many of these challenges by engineering a series of mutants deficient in various combinations of sucrose-utilizing enzymes. The results provide some definitive answers for long-standing questions about sucrose metabolism and its role in gene expression and provide new insights into the ability of S. mutans to regulate gene expression in response to sucrose availability.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
S. mutans strain UA159 and various mutant derivatives of this strain were maintained on brain heart infusion (BHI; Difco Laboratories, Detroit, MI) agar plates and routinely cultured in liquid BHI medium at 37°C in a 5% CO2–95% air incubator. Escherichia coli strain DH10B was maintained in Luria-Bertani medium at 37°C in air. BHI was supplemented with antibiotics when needed at the following concentrations: kanamycin (Km), 1 mg/ml; erythromycin (Em), 10 μg/ml; spectinomycin (Sp), 1 mg/ml; and tetracycline (Tc), 10 μg/ml.
When preparing cells for chloramphenicol acetyltransferase (CAT) assays (34) and RNA extraction, S. mutans strains were first cultured overnight in tryptone-vitamin (TV) base medium (21) supplemented with various carbohydrates, subcultured in the same fresh medium by diluting 50-fold, and then incubated until the optical density at 600 nm (OD600) reached ∼0.5. Two different types of fructooligosaccharides (FOS) were purchased: an FOS powder (NutraFlora FOS) from The Vitamin Shoppe (North Bergen, NJ) that was enzymatically prepared from sucrose (hence, the major component of this FOS was of the glucose-fructosen [GFn] type) (35); and an inulin-based FOS (Fn-FOS) from Jarrow Formulas (Los Angeles, CA) that was derived from partial hydrolysis of long-chain inulins. To eliminate sucrose contamination, a 10% (wt/vol) GFn-FOS solution was treated with 1 mg/ml of invertase (from yeast) (Sigma, St. Louis, MO) at 37°C for 1 h, passed through a sterile 0.2-μm filter, and added to TV base medium at a concentration of 0.5% (wt/vol). For monitoring of growth, S. mutans strains were precultured overnight in BHI, subcultured in BHI until the OD600 reached 0.4 to 0.5, and inoculated at a dilution of 1:300 into fresh TV medium containing the desired carbohydrates. The optical density of the cultures was monitored over a period of 24 to 48 h using a Bioscreen C (OyGrowth Curves AB, Helsinki, Finland) with individual wells covered with sterile mineral oil and maintained at 37°C.
To explore the effects of carbohydrates on catabolite repression and gene expression in strains carrying reporter gene fusions, cells were cultivated overnight in a 5% CO2 aerobic atmosphere at 37°C with TV base medium supplemented with 0.5% of galactose. Subsequently, the cultures were diluted 1:12 into fresh TV-galactose medium and incubated for 2 to 2.5 h to allow the cultures to reach an OD600 of 0.2. Various concentrations of sucrose, fructose, mannose, or glucose were then added to the culture medium, and cells were incubated for an additional 3.5 h before harvesting and immediately freezing at −80°C.
DNA manipulation and mutant strain construction.
Standard techniques were applied for preparation and manipulation of DNA (36). All DNA restriction and modifying enzymes were purchased from New England BioLabs (Beverly, MA) and used as recommended by the supplier. Oligonucleotides for PCR amplifications were custom synthesized by Integrated DNA Technologies, Inc. (Coralville, IA).
Multiple mutagenesis steps were carried out to construct various recombinant S. mutans strains, each achieved by transformation using competent cells prepared by growth in the presence of 10% horse serum and 100 nM competence-stimulating peptide (CSP) in BHI medium (37). First, an allelic-exchange mutagenesis was performed in the wild-type strain UA159, using ligation products of DNA fragments containing the flanking sequences of gtfBC and a Tc-resistant marker inserted in between. Briefly, DNA oligonucleotide primers (for the upper flanking fragment, gtfBC-1, 5′-GCT AAA GTT GGA GTT TGT AAT CTC C-3′, and gtfBC-2, 5′-GAC GAT ACC AAC TTT CGG CTG TCA A-3′; for the lower fragment, gtfBC-3, 5′-GGG ACT CCT GTT GCA GGA AGT CA-3′, and gtfBC-4, 5′-CTT AGA TGT CCA TCT GCA TCT CTG ATA-3′) were used to PCR amplify two DNA fragments. After digestion of the upper and lower fragments with BclI and BamHI, respectively, the DNA fragments were purified and put into a ligation mixture together with a Tc gene cassette released from plasmid pLN2 (9) with BamHI digestion. After transformation, Tc-resistant colonies were confirmed by PCR coupled with DNA sequencing to rule out the introduction of any unintended mutations into the regions flanking gtfBC. Mutants with similar deletions of gtfA (em), scrA (em, km, or sp), scrB (km), treB (sp), fruA (km), and fruB (sp) were constructed. All three markers, em, km, and sp, have been used commonly in our genetic study of S. mutans, and none have displayed any apparent polar effects (23). On the other hand, a polar km marker (Ωkm) was also used in certain cases when specified.
In addition, a PCR-based site-directed mutagenesis technique was applied to UA159 to convert the Met9 codon (ATG) of the gtfD coding sequence into a stop codon (TAG). As detailed elsewhere (23), a recombinant PCR was conducted to produce an approximately 2-kbp DNA fragment containing a single gtfD(M9stop) mutation located near the middle of the fragment. This DNA product (mutator DNA) was used to transform UA159 along with a suicide plasmid (pLacG-em) that inactivates the lacG gene encoding a phospho-β-galactosidase that is required for growth on lactose (29) via a single crossover recombination. After screening the Em-resistant transformants for gtfD(M9stop) mutations using MAMA-PCR (mismatch amplification mutation analysis) and an allele-specific primer (gtfDM9stop-3′MAMA, 5′-ACC CAG TGC TTT TTA ACC TTG TAG AT-3′) (38), positive clones were further confirmed by DNA sequencing.
A confirmed clone of the gtfD(M9stop) lacG::Em mutant was then subjected to a second round of site-directed mutagenesis, this time targeting the Met9 codon of the ftf sequence. Similarly, a homologous mutator DNA fragment was created by recombinant PCR to include a ftf(M9stop) mutation (to TAG); another PCR product was generated using the chromosomal DNA of the gtfBC::Tc mutant (see above) as the template to include the gtfBC::Tc marker. After cotransformation of gtfD(M9stop) lacG::Em with the mutator DNA and gtfBC::Tc marker, Tc-resistant colonies were then screened by MAMA-PCR to identify ftf(M9stop) mutants. After confirmation by sequencing, gtfBC gtfD(M9stop) ftf(M9stop) lacG::Em mutants were patched onto TV-lactose agar plates to select for Em-sensitive revertants that had lost the suicide plasmid pLacG-em, resulting in strain MMZ950 [gtfBC gtfD(M9stop) ftf(M9stop)]. Similarly constructed was a fruA(M12stop) point mutation (in strains MMZ952 and MMZ966) that had its Met12 codon replaced with a stop codon (TAG). Also, a gtfD(M9stop) strain (MMZ948) was created by curing pLacG-em from gtfD(M9stop) lacG:Em. All antibiotic replacement and point mutations maintained in various mutant strains in this report have been confirmed by PCR and sequencing.
RNA preparation and quantitative RT-PCR.
Total RNA was extracted from exponentially growing S. mutans cells using an RNeasy minikit (Qiagen, Germantown, MD), according to procedures detailed elsewhere (39). For preparation of first-strand cDNA, random hexamers were used along with 1 μg total RNA in each 10-μl reverse transcription reactions, following instructions from the supplier (Invitrogen by Life Technologies, Carlsbad, CA). The levels of various mRNA transcripts were quantified by real-time PCR (RT-PCR) using specific primers: for levD, 5′-GGA AGC CCT TTG ACA ACA GC-3′ (forward) and 5′-CTG CCA TTG GTA AGT TCA TCC C-3′ (reverse); for gtfD, 5′-CAC AGG CAA AAG CTG AAT TAA CA-3′ (forward) and 5′-GAA TGG CCG CTA AGT CAA CAG-3′ (reverse); for ftf, 5′-AAA TAT GAA GGC GGC TAC AAC G-3′ (forward) and 5′-CTT CAC CAG TCT TAG CAT CCT GAA-3′ (reverse); and for 16S rRNA as a control, 5′-CAC ACC GCC CGT CAC ACC-3′ (forward) and 5′-CAG CCG CAC CTT CCG ATA CG-3′ (reverse).
Biochemical assays.
When preparing cells for PTS assays, bacterial strains were cultured overnight in BHI medium, diluted 1:50 into fresh BHI, and grown to mid-exponential phase (OD600, ∼0.5). Cells were harvested by centrifugation and immediately frozen at −80°C. CAT (34) and PTS (40) assays were carried out according to previously published protocols, and each experiment was repeated with three independent cultures. For the measurement of glucose and fructose in culture supernatant fluids, supernates were first passed through a 0.2-μm filter, followed by incubation in boiling water for 15 min to inactivate enzymes, and then processed using obtained glucose and fructose assay kits (Sigma).
RESULTS
Growth phenotypes of mutants lacking various sucrose-metabolizing enzymes.
To begin to investigate the impact of the complement of sucrose-metabolizing enzymes of S. mutans on growth phenotypes, a series of mutants were created, employing both marker-dependent and markerless approaches. The panel of mutant strains utilized in this study are described in Table 1 and were first tested for their ability to grow in TV medium supplemented with 0.2% sucrose.
Table 1.
Bacterial strains used in this study
| Strain | Description | Source or reference |
|---|---|---|
| UA159 | Wild type | University of Alabama |
| MMZ108 | UA159 PfruA-cat | 24 |
| ΔEI | ptsI::Em | 27 |
| MMZ931 | scrB::Km | UA159 |
| MMZ932 | scr::Em | UA159 |
| MMZ934 | scrAB::Km | UA159 |
| MMZ957 | scrAB::ΩKm | UA159 |
| MMZ945 | gtfBC::Tc | UA159 |
| MMZ948 | gtfD(M9stop) | UA159 |
| MMZ950 | gtfBC gtfD(M9stop) ftf(M9stop) | MMZ945 |
| MMZ952 | gtfA::Em gtfBCD ftf fruA(M12stop) | MMZ950 |
| MMZ966 | gtfBCD ftf scrAB::ΩKm fruA(M12stop) | MMZ950 |
| MMZ967 | gtfBCD ftf scrAB fruA msmE::Em | MMZ966 |
| MMZ968 | gtfBCD ftf scrAB fruA fruB::Sp msmE | MMZ967 |
| MMZ974 | scrA PfruA-cat | MMZ932 |
| MMZ983 | gtfBCD ftf scrAB::ΩKm | MMZ950 |
| MMZ984 | gtfBCD ftf fruA::Km | MMZ950 |
| MMZ993 | gtfABCD ftf fruA scrA::Km | MMZ952 |
| MMZ996 | gtfABCD ftf fruA scrA::Sp | MMZ952 |
| MMZ997 | gtfABCD ftf fruA scrA treB::Sp | MMZ993 |
| MMZ998 | gtfABCD ftf fruA PlevD-cat | MMZ952 |
| MMZ1002 | gtfABCD ftf fruA scrA PlevD-cat | MMZ996 |
| MMZ998Q | gtfABCD ftf fruA PlevD-cat levQ::Sp | MMZ998 |
| MMZ998T | gtfABCD ftf fruA PlevD-cat levT::Sp | MMZ998 |
| MMZ998R | gtfABCD ftf fruA PlevD-cat levR::Sp | MMZ998 |
| MMZ1025 | gtfBC scrAB::ΩKm | MMZ945 |
| SP8 | fruA::Km | UA159 |
gtfBCD and ftf.
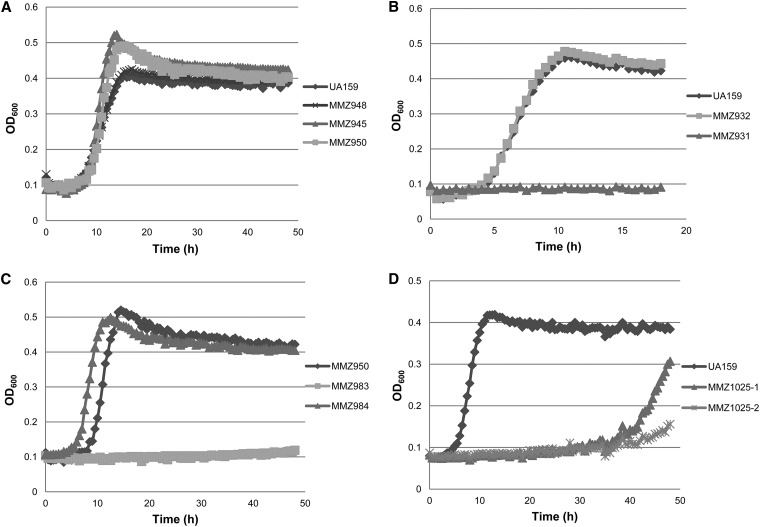
Three extracellular glucosyltransferase enzymes produced by S. mutans, GtfB, GtfC, and GtfD, convert sucrose into glucans while releasing free fructose. The fructosyltransferase (FTF) of S. mutans is able to convert exogenous sucrose into fructans and free glucose. Thus, when strains possess these enzymes, extracellular fructose and glucose are produced for consumption by S. mutans. As shown in Fig. 1A, loss of gtfD (MMZ948) had no significant impact on the growth of S. mutans in TV containing 0.2% sucrose, whereas a mutant (MMZ945) that was deficient in both gtfB and gtfC showed improved growth on sucrose, compared to the otherwise-isogenic wild-type strain UA159. As production of extracellular glucan, especially water-insoluble glucans synthesized by GtfBC, greatly enhances bacterial adherence and clumping, this phenotype of MMZ945 was likely a combination of less aggregation by the cells and less inhibition of growth from accumulation of inhibitory end products within cell aggregates. S. mutans MMZ950, which is deficient in all four exopolysaccharide-producing enzymes (GtfBCD and Ftf), displayed a growth phenotype similar to that of MMZ945 (Fig. 1A).
Fig 1.
Growth curves of the mutants of major sucrose-metabolizing pathways in S. mutans. All strains were grown in BHI medium overnight, subcultured in BHI to mid-exponential phase, and then diluted 1:300 into TV medium containing 0.2% sucrose. Growth was monitored using a Bioscreen C reader set at 37°C with readings (OD600) taken every 30 min. (A) UA159 (wild type), MMZ948 [gtfD(M9stop)], MMZ945 (gtfBC), and MMZ950 (gtfBCD ftf); (B) UA159, MMZ932 (scrA), and MMZ931 (scrB); (C) MMZ950, MMZ983 (gtfBCD ftf scrAB), and MMZ984 (gtfBCD ftf fruA); (D) UA159 and MMZ1025 (gtfBC scrAB). Two isolates were included for MMZ1025, as variability in lag phase was noted.
EIIScr.
S. mutans also possesses a specific sucrose-PTS permease, EIIScr, encoded by a single gene, scrA (10). The sucrose-6-PO4 produced by PTS-mediated uptake is cleaved by a sucrose-6-PO4 hydrolase (ScrB) that releases intracellular glucose-6-PO4 and fructose. A fructose kinase gene is carried immediately downstream of scrA. It has been suggested that the majority of the sucrose that is presented to S. mutans is directly internalized and then catabolized (13, 41). When the entire coding region of scrA was replaced with a nonpolar antibiotic marker (em) in the parental strain to create strain MMZ932, there was little change in the ability of the mutant to grow on sucrose (Fig. 1B). The same was true when the coding sequences of both scrA and scrB were replaced by a nonpolar km or a polar Ωkm cassette (MMZ934 and MMZ957, respectively; data not shown). These results were not surprising, given the presence of multiple GTF and FTF enzymes as well as FruA and GtfA (sucrose phosphorylase), all of which have been shown to be capable of hydrolyzing sucrose. However, a deletion mutant of scrB alone (MMZ931) presented a sucrose-sensitive phenotype (Fig. 1B), consistent with a previous report that suggested that accumulation of sucrose-6-PO4 may be toxic to the cells (42).
An interesting phenotype of the scrA mutant MMZ932 was observed when it was growing in TV base medium supplemented with glucose, fructose, mannose, or galactose. In particular, the cells showed excessive clumping in the absence of sucrose (see Fig. S2 in the supplemental material), apparently indicative of pleiotropic effects on the cell that may have influenced envelope composition or cell division in ScrA-deficient strains.
An S. mutans strain containing mutations in the gtfBCD, ftf, and scrAB genes (MMZ983) was also constructed by introducing a PCR product that contained the replacement of scrAB by the Ωkm cassette into strain MMZ950. Consistent with the current view of the functions of the GTF and FTF enzymes and the ScrAB cluster, very little growth of MMZ983 was noted in TV-sucrose (Fig. 1C), indicating that GtfBCD, Ftf, and ScrAB are the main contributors to sucrose metabolism by S. mutans. Surprisingly, a mutant strain that lacked only gtfBC and scrAB (MMZ1025) displayed an extended lag phase, ranging from 35 to 45 h, when growing on sucrose (Fig. 1D). A previous study indicated that the expression of gtfD is relatively constant whether under glucose or sucrose conditions and that the ftf mRNA level is induced only about 2- to 3-fold by sucrose (32). We confirmed that gtfD and ftf transcript levels were not greatly affected by growth in sucrose (data not shown). Therefore, the behavior of strain MMZ1025 may indicate that, in contrast to GtfB and GtfC, GtfD and Ftf contribute in only minor ways to growth of S. mutans on sucrose under the conditions tested.
FruA.
When a fruA::Km replacement mutation was introduced into S. mutans MMZ950, resulting in strain MMZ984, little change in growth rate on sucrose was noted, although MMZ984 did appear to have a shorter lag phase than MMZ950 (Fig. 1C). FruA is an exo-β-d-fructosidase that has been shown to contribute to the virulence of S. mutans in a rat model (9). The preferred substrates of FruA are β2,6-linked levans, but it is also active on other β-fructosides, including inulins, fructooligosaccharides (FOS), sucrose, and raffinose (7). Since expression of the fruAB operon is under tight regulation by CCR (21), it is not surprising that loss of fruA resulted in little change in growth on sucrose.
MSM.
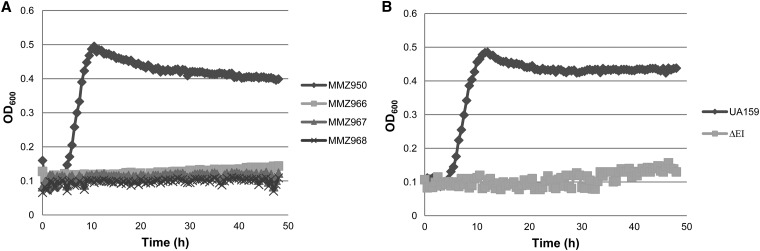
The multiple-sugar metabolism (msm) pathway in S. mutans has been implicated in the transport and metabolism of sucrose, although this hypothesis has not been directly tested (15, 16). Thus, a new strain (MMZ966) was created in the background of MMZ950 (gtfBCD ftf) by introducing both the scrAB::ΩKm replacement and a new fruA point mutation that replaced the translational start codon with a stop codon. Subsequently, an msmE::Em replacement mutation was introduced into strain MMZ966, resulting in MMZ967. As shown in Fig. 2A, similar to strain MMZ983, which produced low levels of growth on sucrose, MMZ966 managed to increase the optical density (OD600) of the culture by only 0.05 units after 48 h of incubation in the presence of 0.2% sucrose. However, strain MMZ967, which further lacks msmE, produced no detectable growth during the same period. Therefore, under conditions used in these growth assays, a minor role was established for the msm system in sucrose catabolism by S. mutans. Furthermore, a mutant lacking the ptsI gene that encodes Enzyme I of the PTS (designated ΔEI) (27) that was constructed in strain UA159 failed to produce substantial growth on sucrose (Fig. 2B). Given the fact that glucose but not fructose has been shown to be able to sustain the growth of an EI mutant of S. mutans (43), the largely abolished growth of the ΔEI strain on sucrose is consistent with our view that both FTF, which releases free glucose from sucrose, and the msm pathway contribute very little to sucrose catabolism by S. mutans.
Fig 2.
Minor contributions from the Msm pathway to growth on sucrose. Growth tests were performed as described for Fig. 1. (A) Strains MMZ950 (gtfBCD ftf), MMZ966 (gtfBCD ftf scrAB fruA), MMZ967 (gtfBCD ftf scrAB fruA msmE), and MMZ968 (gtfBCD ftf scrAB fruAB msmE); (B) strains UA159 and ΔEI (ptsI).
FruB.
The fruA gene of S. mutans is borne in an operon with the fruB gene, encoding a predicted β-fructosidase. To date, an activity has not been associated with this open reading frame (ORF). Since deficiency of gtfBCD ftf scrAB fruA msmE (strain MMZ967) resulted in complete loss of growth on sucrose, we concluded that FruB, similar to FruA, does not contribute in any major way to sucrose catabolism under the conditions tested. When we further deleted fruB in strain MMZ967 by introducing a fruB::Sp replacement, the resultant mutant MMZ968 showed no growth after 48 h of incubation in TV-sucrose (Fig. 2A).
EIITre.
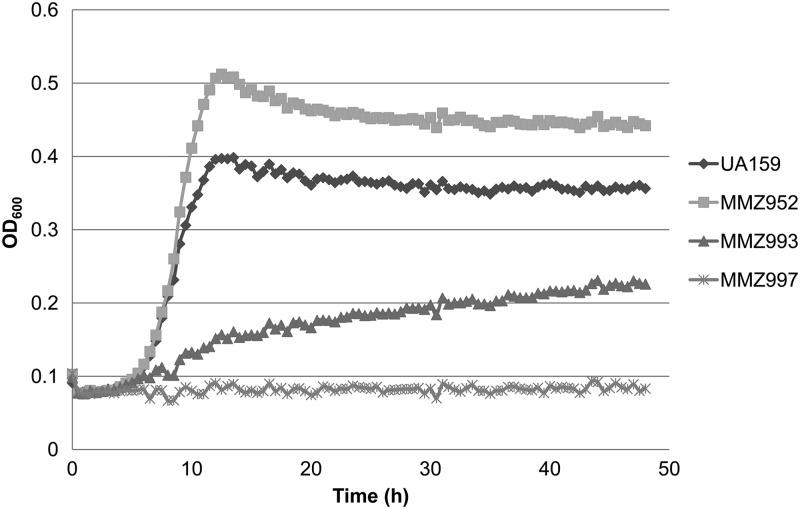
We also tested the hypothesis that the trehalose-PTS pathway can contribute to the catabolism of sucrose by creating strain MMZ993, which was constructed by introducing a scrA deletion into the background of strain MMZ952 (gtfABCD ftf fruA), in which gtfA had been inactivated to eliminate any contribution of the msm pathway. In comparison with the parental strain MMZ952, loss of scrA led to markedly reduced growth on sucrose, but a significant growth rate and yield were still observed (Fig. 3). Also similar to the strain carrying the scrA mutation (MMZ932), strain MMZ993 clumped in TV medium supplemented with sucrose, glucose, or galactose, whereas MMZ952 showed no clumping, even in the presence of sucrose. Another strain, MMZ996, which had a genetic makeup nearly identical to that of MMZ993, except that a different antibiotic marker was used to replace the scrA gene (Table 1), also behaved similarly to MMZ993 (data not shown). Importantly, as we demonstrated that a scrAB double mutation in a similar genetic background (gtfBCD ftf) led to near complete loss of growth on sucrose (Fig. 1C), these results suggested that additional sucrose-PTS permeases are likely contributing to the residual growth by MMZ993 in the absence of ScrA. A previous study in S. mutans has suggested that EIITre, the PTS permease for trehalose, might function as a low-affinity sucrose permease (14). To test this hypothesis, a treB::Sp replacement deletion was constructed using MMZ993 as the base strain, and the resultant mutant (MMZ997) failed to show growth in TV sucrose (Fig. 3). Therefore, the results confirmed that the trehalose-PTS may internalize sucrose as sucrose-6-PO4, which can then be catabolized by ScrB.
Fig 3.
Role of EIITre in sucrose metabolism by S. mutans. Strains UA159, MMZ952 (gtfABCD ftf fruA), MMZ993 (gtfABCD ftf fruA scrA), and MMZ997 (gtfABCD ftf fruA scrA treB) were grown in TV medium containing 0.2% sucrose.
Transport of sucrose via the PTS.
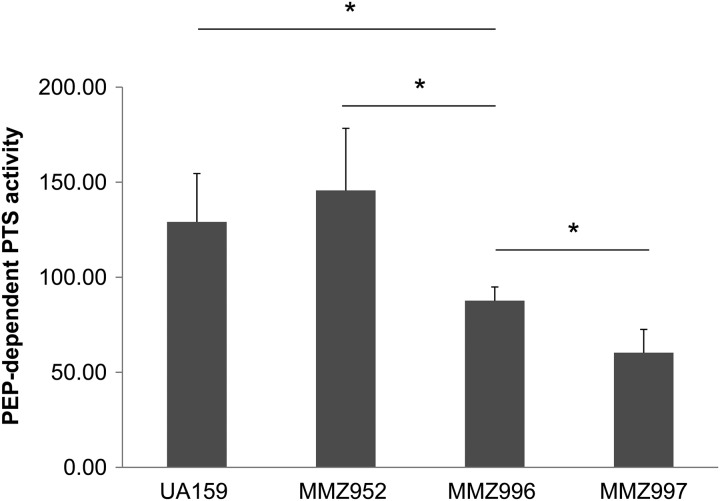
Despite extensive genetic studies of sucrose uptake and metabolism by S. mutans, obtaining a definitive and accurate assessment of PTS-dependent sucrose transport activity has been confounded in part by the presence of the secreted GTF, Ftf, and FruA enzymes. Taking advantage of the mutants created for this study, an in vitro PTS assay was carried out using strains UA159, MMZ952, MMZ996, and MMZ997. To circumvent the issues of clumping in TV-sucrose by strains UA159 and MMZ996 and of the inability to grow strain MMZ997 in sucrose, cells were cultivated in BHI medium for PTS assays. As shown in Fig. 4, strain MMZ952, which lacks the GTFs, Ftf, and FruA, showed levels of PTS activities similar to those of the wild-type strain (P = 0.456). However, in the absence of scrA, as in strain MMZ996, significant reductions in sucrose PTS activity were noted (P = 0.034) compared with strain MMZ952, confirming the role of EIIScr in sucrose transport. Additionally, in strain MMZ997, which lacked ScrA and EIITre, a further reduction in PTS activity relative to MMZ996 was observed (P = 0.040). It was surprising that MMZ997 still had detectable levels of sucrose-PTS activity. The two most plausible explanations are that other PTS permeases are able to internalize sucrose under our test conditions (e.g., cellobiose PTS) or that there are other enzymes produced intracellularly that have low levels of sucrolytic activity, such that they release glucose and/or fructose into the reaction mixture, which can then serve as substrates for PTS permeases that act on the monosaccharides released from sucrose.
Fig 4.
Sucrose-transporting activities measured in strains UA159, MMZ952, MMZ996, and MMZ997. Cells were harvested from exponentially growing cultures in BHI, washed in Na/K-phosphate buffer, permeabilized with toluene:acetone, and tested in PTS assays. The results are the average activities measured using three independent cultures. Units are expressed as nmol NADH oxidized (mg of protein)−1 (min)−1 in a PEP-dependent manner. The error bars represent standard deviations, and the asterisks indicate P values of <0.05 (Student's t test).
Sucrose transport affects fructan catabolism.
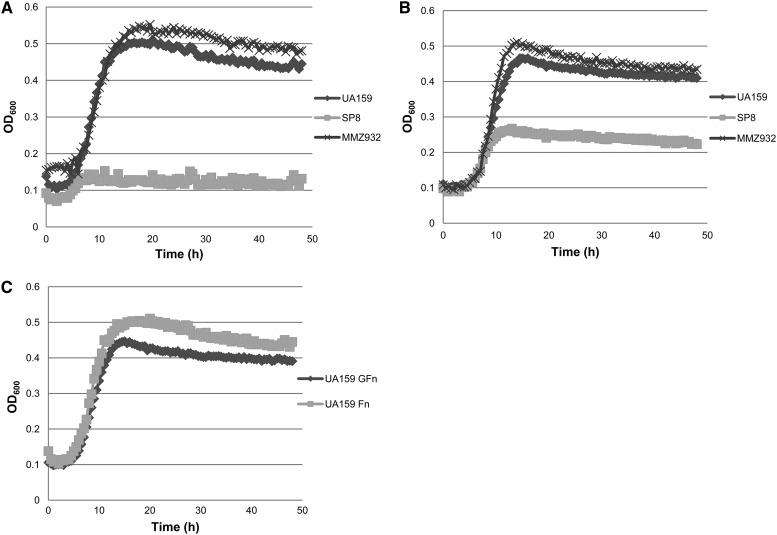
The production of fructan polymers from sucrose enhances the cariogenic potential of S. mutans by extending the depth and duration of acidification of oral biofilms (9). As part of our ongoing effort to explore the relationship between fructan metabolism and cariogenicity, various S. mutans strains were grown in TV medium containing 0.2% of commercially obtained fructooligosaccharide (FOS). Two different sources of FOS were tested: one was derived from inulin via limited acid hydrolysis and contained mostly the fructosen (Fn) form of FOS, whereas the other was enzymatically synthesized from sucrose and would be composed primarily of the glucose-Fn (GFn) type and also would contain significant quantities of sucrose (35).
When Fn-FOS was supplied as the sole carbohydrate in TV medium, a nonpolar fruA mutant (strain SP8) showed largely abolished growth, consistent with the proven function of FruA to metabolize fructan polymers (Fig. 5A). Likewise, when GFn-FOS was used as the carbon source, the fruA mutant showed reduced growth, albeit significantly better growth than when Fn-FOS was the carbon source (Fig. 5B), due to the presence of significant quantities of contaminating sucrose.
Fig 5.
Growth of UA159 and fruA and scrA mutants on fructooligosaccharides (FOS). Strains UA159, SP8 (fruA), and MMZ932 (scrA) were cultured to mid-exponential phase in BHI medium and used to inoculate TV base medium containing 0.2% of Fn-FOS (A) or GFn-FOS (B). Also compared were the growths of UA159 in the two FOS preparations (C).
Interestingly, when the scrA mutant strain MMZ932 was tested for its ability to grow in TV-GFn, improved growth and final yield were noted in comparison to the wild-type strain (Fig. 5B). Similar results were obtained with the scrAB mutants (data not shown). However, this was not the case in TV-Fn, where little difference was seen between a scrA mutant and UA159, except for a slightly higher yield by the mutant (Fig. 5A). Therefore, it appears that loss of ScrA in S. mutans resulted in altered expression or activities of the enzymes responsible for catabolizing certain types of FOS.
Loss of scrA results in increased activity of the fruA promoter.
In order to understand the growth behavior of the scrA mutant on FOS, we examined the expression of the fruA gene using a fruA promoter::cat fusion (24), which was established in the wild-type and scrA mutant backgrounds. The strains were cultivated in TV medium containing 0.5% of FOS. As indicated by the CAT assays (Table 2), fruA promoter activity in the wild-type genetic background was approximately 30-fold higher when cells were growing on the Fn-FOS than on the GFn-FOS, consistent with the fact that the wild-type strain grew better on Fn-FOS than on GFn-FOS (Fig. 5C). Introduction of the scrA mutation resulted in a large increase in fruA promoter activity on both growth substrates, such that equivalent levels of expression were noted in cells growing on Fn-FOS and GFn-FOS (Table 2). Apparently, the baseline level of fruA expression in the wild-type cells growing on Fn-FOS yields sufficient FruA enzyme so that there is no further improvement in the growth of cells when the scrA mutation is introduced. In contrast, there is a notable improvement in the growth of the scrA mutant on GFn-FOS, presumably because the baseline levels of fruA expression in the wild-type background are so low in GFn-FOS medium.
Table 2.
CAT activities of PfruA-cat in the background of wild-type UA159 and its scrA mutant
| Background | Avg (SD) CAT sp acta [nmol (mg protein)−1 min−1] |
|||
|---|---|---|---|---|
| GFn |
Fn |
|||
| Untreated | + Invertase | Untreated | + Invertase | |
| UA159 | 0.14 (0.25) | 0.47 (0.31) | 4.40b (2.02) | 2.58b (0.43) |
| scrA | 63.96c (4.52) | 0.74 (0.30) | 62.65c (14.2) | 2.88b (0.20) |
Cells were growing exponentially in TV medium containing 0.5% of fructooligosaccharide (GFn or Fn), with or without prior treatment of the FOS with invertase. Values are from three separate experiments.
The data are statistically different from those obtained under corresponding GFn conditions as verified by Student's t test (P < 0.05).
The data are statistically different from those obtained for UA159 under corresponding conditions as verified by Student's t test (P < 0.05).
As mentioned above, GFn is likely contaminated by sucrose. To test the hypothesis that increased fruA promoter activity in the scrA mutant background is associated with loss of sucrose transport by EIIScr, the stock solutions of GFn- and Fn-FOS were treated with invertase as detailed in Materials and Methods. When the CAT assays were repeated using cells growing on invertase-treated FOS, the PfruA-cat activities in the scrA mutant background were now reduced to the wild-type levels (Table 2), supporting the notion that ScrA-dependent sucrose transport is essential for the observed repressive effect of ScrA on fruA expression. The simplest interpretation of these data is that ScrA is able to effect CCR of the fruA operon through some or all of the mechanisms previously detailed by our laboratory (27). However, further investigation has revealed a novel mechanism whereby sucrose and ScrA can affect gene expression in S. mutans.
ScrA can affect fruA expression through the LevQRST system.
The studies with FOS led us to investigate in more detail how the presence of sucrose and ScrA may influence fruA expression by studying the induction of fruA by the levQRST circuit in the context of sucrose metabolism by S. mutans. We previously established the levD promoter as a useful model system for LevR-dependent activation of gene expression, particularly because its promoter region has the binding site for LevR; levD promoter activity is minimal in the absence of LevR-dependent activation; and, unlike the fruA promoter, there is no CRE for CcpA binding in the levD promoter, and therefore the results would not be confounded by the activity of an additional regulatory pathway (23, 24).
Since fructose is a potent signal for activation of the LevQRST pathway, a PlevD-cat fusion was introduced into the background of MMZ952 (gtfABCD ftf fruA), resulting in strain MMZ998. Similarly created was another PlevD-cat reporter strain, MMZ1002, which also lacks scrA in addition to the mutations in MMZ952. When these two strains were cultured in TV with 0.5% sucrose, no free glucose was detectable in the culture supernatant fluids (data not shown). Analysis of the CAT activity revealed that expression from the levD promoter was significantly higher in the strain lacking ScrA, MMZ1002 (728 ± 17 CAT units), than in the ScrA-proficient strain MMZ998 (263 ± 4 CAT units). Further tests using two additional strains bearing a PfruA-cat fusion in the same genetic backgrounds showed similarly higher activities in the scrA mutant (data not shown). However, while these results confirmed that sucrose, and not some other compound(s) in FOS preparations, was required for the effects on gene expression, these experiments still did not allow us to distinguish whether CCR alone, or effects mediated through LevQRST, could account for these observations.
We previously employed a methodology to study the regulation of the LevQRST regulon by first cultivating cells in medium that did not efficiently induce CCR, i.e., TV-galactose, followed by pulsing with inducing substrates to activate expression of the reporter fusion (24). This technique allowed us to successfully discriminate the activation of gene expression by LevQRST from the effects of catabolite repression by the inducing substrates, which include fructose, fructans, and mannose (22, 24, 26). Using this approach, strain MMZ998 was first cultured in TV-galactose to early exponential phase, and then various concentrations of sucrose were added. The cells were incubated for 3.5 h and collected for CAT assays. As shown in Table 3, when strain MMZ998 was pulsed with sucrose, a concentration-dependent activation of the levD promoter was evident. As no extracellular release of free fructose could occur in this particular strain, it appears that sucrose itself has the capacity to contribute to activation of the LevQRST pathway. This conclusion was further supported by the transcript levels of levD in strain MMZ952 measured using quantitative real-time RT-PCR, which showed a 12-fold increase in cells growing in TV containing 0.5% sucrose compared with cells in TV-glucose medium. Also clear was that when sucrose concentrations reached 5 mM or higher, expression of levD was significantly reduced in comparison to that measured at 1 mM, providing evidence of CCR being effected by sucrose (Table 3). Interestingly, when a scrA mutant strain, MMZ1002, was subjected to the same conditions, there was no evidence of activation of levD expression by sucrose at concentrations of 0.05 to 10 mM (Table 3). Thus, the EIIScr permease appears to be required for activation of the LevQRST pathway by sucrose under our pulsing conditions.
Table 3.
PlevD-cat activities measured in various strains under carbohydrate pulsinga
| Pulsing sugar(s) and strain | Avg CAT sp act (SD) when pulsed with indicated concn of sugar |
|||||
|---|---|---|---|---|---|---|
| 0 mM | 0.05 mM | 0.2 mM | 1 mM | 5 mM | 10 mM | |
| Sucrose | ||||||
| MMZ998 | 15.8 (5.9) | 22.4 (4.9) | 117.3 (12) | 308.7 (37) | 169.4 (18) | 185.1 (12) |
| MMZ1002 | 17.3 (6.2) | 14.2 (5.7) | 12.5b (4.4) | 16.4b (5.0) | 24.0b (7.1) | 31.0b (7.3) |
| MMZ998Q | 16.3 (1.3) | ND | 9.3b (0.5) | 9.6b (1.1) | 2.2b (0.3) | ND |
| MMZ998T | 242b (6.4) | ND | 247b (8.5) | 242 (43) | 37.1b (1.1) | ND |
| MMZ998R | 0b (0) | ND | 0.23b (0.2) | 0.25b (0.2) | 0.18b (0.04) | ND |
| Fructose | ||||||
| MMZ998 | ND | 190 (6.8) | 387 (7.5) | 511 (12) | ND | |
| MMZ1002 | ND | 180 (15) | 388 (38) | 486 (93) | ND | |
| Mannose | ||||||
| MMZ998 | ND | 251 (6.5) | 564 (30) | 359 (6.3) | ND | |
| MMZ1002 | ND | 241 (29) | 557 (97) | 471 (81) | ND | |
| Glucose and fructose | ||||||
| MMZ998 | ND | 258 (11) | 556 (2.1) | 677 (33) | ND | |
| MMZ1002 | ND | 281 (46) | 651 (114) | 692 (179) | ND | |
The cells were cultured in TV containing 0.5% galactose to an OD600 of 0.2, and then the indicated concentrations of carbohydrates were added. Cells were then incubated for 3.5 h before CAT assays. CAT activity is expressed as nmol of chloramphenicol acetylated (mg of protein)−1 (min)−1. The data are averages from three independent cultures. ND, not determined.
The data are statistically different from those obtained for MMZ998 under corresponding conditions as verified by Student's t test (P < 0.05).
As reported previously, fructose and mannose are very effective at activating the expression of fruA and levD through the LevQRST complex (22, 24). LevS and LevR are absolutely required for the function of this circuit, while LevQ and LevT are also required for optimal activation and affect the efficiency of induction by specific carbohydrates (23, 24). Thus, it was of interest to determine whether the effects of EIIScr on the LevQRST system could modify responses by the complex to the cognate signals, fructose and mannose. As shown in Table 3, when both MMZ998 and MMZ1002 were pulsed with fructose, the levD promoter was activated to essentially the same levels regardless of whether the scrA gene was intact. The same was true with mannose (Table 3). Thus, EIIScr is not required for activation of LevQRST by fructose or mannose.
To further prove that sucrose was the actual inducing reagent and to rule out any effects from the unexpected extracellular hydrolysis of sucrose, we also repeated this pulsing experiment with equivalent concentrations of an equimolar mixture of glucose and fructose. Similar to what was seen with fructose alone (Table 3), the combination of glucose and fructose induced the PlevD-cat fusion in a concentration-dependent fashion regardless of the presence of ScrA.
Finally, we investigated the involvement of individual components of the LevQRST circuit in sucrose-dependent activation of the levD promoter. Additional mutations were introduced into the background of MMZ998, using DNA fragments carrying antibiotic insertions (sp marker) into the levQ, levT, or levR genes, yielding strains MMZ998Q, MMZ998T, and MMZ998R, respectively. These three strains were grown in TV-galactose and pulsed with sucrose as above (Table 3). As expected, loss of the response regulator LevR led to nearly complete loss of expression by the levD promoter fusion. Interestingly, loss of LevQ largely abolished promoter activity, reinforcing our previous conclusion that LevQ could be playing a role in maintaining the structural integrity of the LevQRST four-component system (23). When LevT was deleted, levD promoter activity was shown to be higher without sucrose or when lower concentrations of sucrose were added; however, activity again declined when 5 mM sucrose was utilized. Thus, mutations in the carbohydrate binding proteins of the four-component system differentially affect how sucrose and ScrA modulate activation of the LevQRST circuit, and a potential interaction between LevT and ScrA could impact activation of the complex. Clearly, though, the results indicate that ScrA cannot bypass the LevQRST circuit to activate levD through some alternative pathway.
DISCUSSION
We set out to determine the relative contributions of various sucrose-metabolizing enzymes found in S. mutans to growth and to explore the capacity of the sucrose-PTS permease to affect gene expression in a way that the liberation of glucose or fructose from sucrose would not confound the analysis. We have shown that there was no apparent growth defect in various mutants that lack GTFs and FTF (MMZ950 and MMZ952) or ScrA and ScrB (MMZ932, MMZ934, and MMZ957, but not MMZ931), yet a deficiency in all three systems (MMZ983) largely abolished the ability to grow on sucrose. We were a bit surprised to find that a mutant deficient in GtfBC and ScrAB (MMZ1025) also failed to yield significant growth on sucrose, since this strain should be able to liberate fermentable carbohydrates from sucrose via GtfD, FTF, and FruA and still had an active Msm pathway. Further analysis of the transcript levels of both gtfD and ftf in strain MMZ1025 showed little change in comparison to that of UA159. In fact, we found that the levels of expression of these genes in strain UA159 did not significantly differ in cells cultured with glucose or sucrose (data not shown). Thus, we conclude that while EIIScr appears to be the main sucrose transporter, extracellular sucrolytic enzymes, especially GtfBC, can release free hexoses at a sufficiently high rate to facilitate wild-type levels of planktonic growth. In fact, when strain UA159 was cultured to an OD600 of 0.25 to 0.3 in TV containing 0.5% sucrose (14.6 mM), glucose concentrations in the supernatant were measured at 0.05 ± 0.003 mg/ml (0.28 mM) and fructose at 0.27 ± 0.05 mg/ml (1.5 mM). Conversely, but in agreement with previous research indicating that the majority of sucrose encountered by S. mutans is metabolized intracellularly (14), our findings further reveal that the levels of free hexoses in sucrose-grown cultures are probably not high enough to trigger catabolite repression (22, 26).
By creating strains lacking multiple sucrolytic enzymes, especially the GTFs and FTF (MMZ952, MMZ998, and MMZ1002), to eliminate the ability for free hexose to be produced from sucrose in the supernatant fluid, we were able to detect the ability of sucrose both to activate gene expression (levD and fruA) and to trigger CCR when present at relatively high, albeit physiologically relevant concentrations (>5 mM). Given that fructose is a potent inducer of expression of levD and fruA and that high concentrations of fructose and glucose can repress expression of these genes, the use of strains lacking exported sucrases was critical for reliable quantitation of the effects of sucrose on gene expression. It is also only by using these mutants that we were able to observe the dependence on ScrA for activation of the LevQRST-controlled genes by sucrose, an unusual and novel observation. Obviously, the control of fruA and levD in this manner is of interest, as it adds a new dimension to the regulation of exopolysaccharide metabolism and may influence how S. mutans responds to the intermittent introduction of sucrose in the diet. From a mechanistic perspective, we postulate that interactions between ScrA, most likely in its dephosphorylated form, and components of the LevQRST system, in particular LevQ and LevT, influence the signal transduction events that lead to activation of the lev and fru operons. We were not able to include a strain lacking the sensor kinase LevS in our test system for reasons that remain unclear. Specifically, all attempts to create a levS::Sp allelic exchange mutation, either in the backgrounds of UA159 or MMZ998, were not successful. This is odd, particularly in light of the fact that we had successfully constructed levS deletion mutants using other antibiotic resistance markers. However, since we have already demonstrated that loss of the sensor kinase LevS results in an inability to activate LevR (22), we anticipate that the effects of ScrA in a levS mutant would be minimal. It should also be noted that we examined the ability of three sugars with some similarities to sucrose, i.e., isomaltulose (also called palatinose; 6-O-α-d-glucopyranosyl-d-fructose), turanose (3-O-α-d-glucopyranosyl-d-fructose), and sucralose (trichlorogalactosucrose) (Sigma) to activate expression of the levD promoter. When used at levels comparable to sucrose to pulse galactose-grown MMZ998 cultures, the results showed no induction of the levD promoter by any of these compounds. Further, none of these sucrose analogs were able to interfere with induction by sucrose (data not shown).
Findings made using these various mutants lacking sucrolytic activities also allowed us to conclude that sucrose efficiently induces CCR without needing to be processed into free hexoses. Interestingly, while both ScrA and the LevQRST system are needed to fully activate the levD promoter, the ability of sucrose to negatively regulate the CCR-sensitive targets of LevR remains even in the absence of LevQ and LevT (Table 3). Notably, these observations mirror some of the phenotypes of CCR of the LevQRST system that are elicited by the major glucose-PTS permease, EIIMan (22). Prior studies have indicated that scrA is expressed at high levels in cells growing in a variety of different carbohydrate sources (30), suggesting that ScrA may be constitutively produced in S. mutans and could therefore rapidly detect the presence of sucrose as it is introduced intermittently in the diet of the host. Notably, there are many cases in which a bacterial membrane transporter plays a major role as a modulator of gene transcription, imparting regulatory information in response to the very substrate it is transporting (44, 45). For example, a recent report described how a maltose-specific ABC transporter, MalFGK2, can regulate the expression of the maltose regulon in E. coli by sequestering the major activator MalT only when not actively engaged in sugar transport (46). Since sucrose can activate levD in a ScrA-dependent fashion at concentrations that are below that which triggers catabolite repression, we hypothesize that ScrA works as a sucrose sensor that transduces regulatory information to other circuits, such as LevQRST, that govern secondary catabolic pathways. Since a clear and significant phenotype of the scrA mutant is its marked clumping in various carbohydrates besides sucrose, the regulation of gene expression by ScrA likely extends beyond the LevQRST regulon. Further transcriptomic studies are planned to investigate the scope of the EIIScr regulon.
It should be noted that while ScrA was required for the activation of the LevQRST system by sucrose in our pulsing experiment, the same strains (MMZ998 and MMZ1002, both deficient in gtfABCD, ftf, and fruA) when grown for extended periods in TV-sucrose showed higher fru and lev promoter activities in the scrA mutant than the scrA+ background. Since for the latter experiment, the strains were first cultured in TV-sucrose overnight before subculturing again for CAT assays, we tested the fructose concentrations in the supernates of the overnight cultures of MMZ998. Fructose could be detected at levels that were low (0.65 ± 0.16 mM) yet sufficient to induce the LevQRST system (24). The presence of fructose in these overnight cultures is probably the result of cell lysis. However, we cannot exclude that there are other unknown very-low-activity extracellular sucrases or that inducer expulsion may result in the transport of fructose into the extracellular environment after it is released from sucrose-6-PO4 (47). In fact, preliminary measurement of fructose in cultures of strain MMZ998 pulsed with various amounts of sucrose, similar to those described above for the CAT assays, has indicated that there may be sufficient fructose present in the extracellular environment to induce gene expression through the LevQRST complex (data not shown).
Our previous studies utilizing the LevQRST model system have revealed a dominant role of CcpA-independent CCR mechanisms in regulating carbohydrate metabolism by S. mutans, a process effected primarily by the HPr protein and a number of glucose/fructose-PTS permeases of the PTS (22, 27). With evidence for what appears to be a direct role for the major sucrose-PTS ScrA in transcriptional regulation of exopolysaccharide metabolism, the findings described in this report add to the theme that prioritization by S. mutans of carbohydrate catabolism takes place primarily at the level of substrate sensing and transport rather than centrally through CcpA, as occurs in many related Gram-positive bacteria. The present study significantly extends our general knowledge of sucrose metabolism by S. mutans and enhances our understanding of the role of sucrose and S. mutans in the ecology of the oral microbiome and dental caries.
Supplementary Material
ACKNOWLEDGMENT
This study was supported by funding from the National Institute of Dental and Craniofacial Research (DE12236).
Footnotes
Published ahead of print 7 December 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.02042-12.
REFERENCES
- 1. Woodward M, Walker AR. 1994. Sugar consumption and dental caries: evidence from 90 countries. Br. Dent. J. 176:297–302 [DOI] [PubMed] [Google Scholar]
- 2. Burt BA. 1993. Relative consumption of sucrose and other sugars: has it been a factor in reduced caries experience? Caries Res. 27(Suppl 1):56–63 [DOI] [PubMed] [Google Scholar]
- 3. Tanzer JM. 1979. Essential dependence of smooth surface caries on, and augmentation of fissure caries by, sucrose and Streptococcus mutans infection. Infect. Immun. 25:526–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bowen WH, Koo H. 2011. Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. 45:69–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Birkhed D, Rosell KG, Granath K. 1979. Structure of extracellular water-soluble polysaccharides synthesized from sucrose by oral strains of Streptococcus mutans, Streptococcus salivarius, Streptococcus sanguis and Actinomyces viscosus. Arch. Oral Biol. 24:53–61 [DOI] [PubMed] [Google Scholar]
- 6. Shiroza T, Kuramitsu HK. 1988. Sequence analysis of the Streptococcus mutans fructosyltransferase gene and flanking regions. J. Bacteriol. 170:810–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burne RA, Schilling K, Bowen WH, Yasbin RE. 1987. Expression, purification, and characterization of an exo-β-D-fructosidase of Streptococcus mutans. J. Bacteriol. 169:4507–4517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miller CH, Somers PJ. 1978. Degradation of levan by Actinomyces viscosus. Infect. Immun. 22:266–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burne RA, Chen YY, Wexler DL, Kuramitsu H, Bowen WH. 1996. Cariogenicity of Streptococcus mutans strains with defects in fructan metabolism assessed in a program-fed specific-pathogen-free rat model. J. Dent. Res. 75:1572–1577 [DOI] [PubMed] [Google Scholar]
- 10. Sato Y, Poy F, Jacobson GR, Kuramitsu HK. 1989. Characterization and sequence analysis of the scrA gene encoding enzyme IIScr of the Streptococcus mutans phosphoenolpyruvate-dependent sucrose phosphotransferase system. J. Bacteriol. 171:263–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ajdic D, McShan WM, McLaughlin RE, Savic G, Chang J, Carson MB, Primeaux C, Tian R, Kenton S, Jia H, Lin S, Qian Y, Li S, Zhu H, Najar F, Lai H, White J, Roe BA, Ferretti JJ. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. U. S. A. 99:14434–14439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hiratsuka K, Wang B, Sato Y, Kuramitsu H. 1998. Regulation of sucrose-6-phosphate hydrolase activity in Streptococcus mutans: characterization of the scrR gene. Infect. Immun. 66:3736–3743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chassy BM, Porter EV. 1979. Initial characterization of sucrose-6-phosphate hydrolase from Streptococcus mutans and its apparent identity with intracellular invertase. Biochem. Biophys. Res. Commun. 89:307–314 [DOI] [PubMed] [Google Scholar]
- 14. Poy F, Jacobson GR. 1990. Evidence that a low-affinity sucrose phosphotransferase activity in Streptococcus mutans GS-5 is a high-affinity trehalose uptake system. Infect. Immun. 58:1479–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Russell RR, Aduse-Opoku J, Sutcliffe IC, Tao L, Ferretti JJ. 1992. A binding protein-dependent transport system in Streptococcus mutans responsible for multiple sugar metabolism. J. Biol. Chem. 267:4631–4637 [PubMed] [Google Scholar]
- 16. Tao L, Sutcliffe IC, Russell RR, Ferretti JJ. 1993. Transport of sugars, including sucrose, by the msm transport system of Streptococcus mutans. J. Dent. Res. 72:1386–1390 [DOI] [PubMed] [Google Scholar]
- 17. Kilic AO, Honeyman AL, Tao L. 2007. Overlapping substrate specificity for sucrose and maltose of two binding protein-dependent sugar uptake systems in Streptococcus mutans. FEMS Microbiol. Lett. 266:218–223 [DOI] [PubMed] [Google Scholar]
- 18. Russell RR, Mukasa H, Shimamura A, Ferretti JJ. 1988. Streptococcus mutans gtfA gene specifies sucrose phosphorylase. Infect. Immun. 56:2763–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burne RA, Penders JE. 1992. Characterization of the Streptococcus mutans GS-5 fruA gene encoding exo-b-D-fructosidase. Infect. Immun. 60:4621–4632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burne RA, Penders JE, Wexler DL, Jayaraman GC, Clancy KA. 1995. Regulation of fructan degradation by Streptococcus mutans. Dev. Biol. Stand. 85:323–331 [PubMed] [Google Scholar]
- 21. Burne RA, Wen ZT, Chen YY, Penders JE. 1999. Regulation of expression of the fructan hydrolase gene of Streptococcus mutans GS-5 by induction and carbon catabolite repression. J. Bacteriol. 181:2863–2871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zeng L, Burne RA. 2008. Multiple sugar: phosphotransferase system permeases participate in catabolite modification of gene expression in Streptococcus mutans. Mol. Microbiol. 70:197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zeng L, Das S, Burne RA. 2011. Genetic analysis of the functions and interactions of components of the LevQRST signal transduction complex of Streptococcus mutans. PLoS One 6:e17335 doi:10.1371/journal.pone.0017335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zeng L, Wen ZT, Burne RA. 2006. A novel signal transduction system and feedback loop regulate fructan hydrolase gene expression in Streptococcus mutans. Mol. Microbiol. 62:187–200 [DOI] [PubMed] [Google Scholar]
- 25. Deutscher J. 2008. The mechanisms of carbon catabolite repression in bacteria. Curr. Opin. Microbiol. 11:87–93 [DOI] [PubMed] [Google Scholar]
- 26. Abranches J, Nascimento MM, Zeng L, Browngardt CM, Wen ZT, Rivera MF, Burne RA. 2008. CcpA regulates central metabolism and virulence gene expression in Streptococcus mutans. J. Bacteriol. 190:2340–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zeng L, Burne RA. 2010. Seryl-phosphorylated HPr regulates CcpA-independent carbon catabolite repression in conjunction with PTS permeases in Streptococcus mutans. Mol. Microbiol. 75:1145–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zeng L, Burne RA. 2009. Transcriptional regulation of the cellobiose operon of Streptococcus mutans. J. Bacteriol. 191:2153–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zeng L, Das S, Burne RA. 2010. Utilization of lactose and galactose by Streptococcus mutans: transport, toxicity, and carbon catabolite repression. J. Bacteriol. 192:2434–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ajdic D, Pham VT. 2007. Global transcriptional analysis of Streptococcus mutans sugar transporters using microarrays. J. Bacteriol. 189:5049–5059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Klein MI, DeBaz L, Agidi S, Lee H, Xie G, Lin AH, Hamaker BR, Lemos JA, Koo H. 2010. Dynamics of Streptococcus mutans transcriptome in response to starch and sucrose during biofilm development. PLoS One 5:e13478 doi:10.1371/journal.pone.0013478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shemesh M, Tam A, Feldman M, Steinberg D. 2006. Differential expression profiles of Streptococcus mutans ftf, gtf and vicR genes in the presence of dietary carbohydrates at early and late exponential growth phases. Carbohydr. Res. 341:2090–2097 [DOI] [PubMed] [Google Scholar]
- 33. Shemesh M, Tam A, Steinberg D. 2007. Expression of biofilm-associated genes of Streptococcus mutans in response to glucose and sucrose. J. Med. Microbiol. 56:1528–1535 [DOI] [PubMed] [Google Scholar]
- 34. Shaw WV. 1975. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol. 43:737–755 [DOI] [PubMed] [Google Scholar]
- 35. Kaplan H, Hutkins RW. 2000. Fermentation of fructooligosaccharides by lactic acid bacteria and bifidobacteria. Appl. Environ. Microbiol. 66:2682–2684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 37. Petersen FC, Scheie AA. 2010. Natural transformation of oral streptococci. Methods Mol. Biol. 666:167–180 [DOI] [PubMed] [Google Scholar]
- 38. Cha RS, Zarbl H, Keohavong P, Thilly WG. 1992. Mismatch amplification mutation assay (MAMA): application to the c-H-ras gene. PCR Methods Appl. 2:14–20 [DOI] [PubMed] [Google Scholar]
- 39. Ahn SJ, Lemos JA, Burne RA. 2005. Role of HtrA in growth and competence of Streptococcus mutans UA159. J. Bacteriol. 187:3028–3038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. LeBlanc DJ, Crow VL, Lee LN, Garon CF. 1979. Influence of the lactose plasmid on the metabolism of galactose by Streptococcus lactis. J. Bacteriol. 137:878–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tanzer JM, Chassy BM, Krichevsky MI. 1971. Sucrose metabolism by Streptococcus mutans, SL-I. Biochim. Biophys. Acta 261:379–387 [DOI] [PubMed] [Google Scholar]
- 42. Macrina FL, Jones KR, Alpert CA, Chassy BM, Michalek SM. 1991. Repeated DNA sequence involved in mutations affecting transport of sucrose into Streptococcus mutans V403 via the phosphoenolpyruvate phosphotransferase system. Infect. Immun. 59:1535–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cvitkovitch DG, Boyd DA, Thevenot T, Hamilton IR. 1995. Glucose transport by a mutant of Streptococcus mutans unable to accumulate sugars via the phosphoenolpyruvate phosphotransferase system. J. Bacteriol. 177:2251–2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu Y, Zeng L, Burne RA. 2009. AguR is required for induction of the Streptococcus mutans agmatine deiminase system by low pH and agmatine. Appl. Environ. Microbiol. 75:2629–2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kuper C, Jung K. 2005. CadC-mediated activation of the cadBA promoter in Escherichia coli. J. Mol. Microbiol. Biotechnol. 10:26–39 [DOI] [PubMed] [Google Scholar]
- 46. Richet E, Davidson AL, Joly N. 2012. The ABC transporter MalFGK(2) sequesters the MalT transcription factor at the membrane in the absence of cognate substrate. Mol. Microbiol. 85:632–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Egan JB, Morse ML. 1966. Carbohydrate transport in Staphylococcus aureus. 3. Studies of the transport process. Biochim. Biophys. Acta 112:63–73 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.