Abstract
LcrV, the type III needle cap protein of pathogenic Yersinia, has been proposed to function as a tether between YscF, the needle protein, and YopB-YopD to constitute the injectisome, a conduit for the translocation of effector proteins into host cells. Further, insertion of LcrV-capped needles from a calcium-rich environment into host cells may trigger the low-calcium signal for effector translocation. Here, we used a genetic approach to test the hypothesis that the needle cap responds to the low-calcium signal by promoting injectisome assembly. Growth restriction of Yersinia pestis in the absence of calcium (low-calcium response [LCR+] phenotype) was exploited to isolate dominant negative lcrV alleles with missense mutations in its amber stop codon (lcrV*327). The addition of at least four amino acids or the eight-residue Strep tag to the C terminus was sufficient to generate an LCR− phenotype, with variant LcrV capping type III needles that cannot assemble the YopD injectisome component. The C-terminal Strep tag appears buried within the cap structure, blocking effector transport even in Y. pestis yscF variants that are otherwise calcium blind, a constitutive type III secretion phenotype. Thus, LcrV*327 mutants arrest the needle cap in a state in which it cannot respond to the low-calcium signal with either injectisome assembly or the activation of type III secretion. Insertion of the Strep tag at other positions of LcrV produced variants with wild-type LCR+, LCR−, or dominant negative LCR− phenotypes, thereby allowing us to identify discrete sites within LcrV as essential for its attributes as a secretion substrate, needle cap, and injectisome assembly factor.
INTRODUCTION
Three pathogenic Yersinia species—Yersinia enterocolitica, Yersinia pseudotuberculosis, and Yersinia pestis—employ a virulence plasmid-encoded type III secretion machine to establish disease (1–3). The key function of type III secretion machines during the pathogenesis of Yersinia infections is the transport of effectors [YopE, YopH, YopM, YopO, YopP (J), YopT, and YopQ] into host cells, thereby enabling bacterial escape from innate immune responses (4, 5). Assembly of the Yersinia type III secretion machine involves 23 different protein components encoded by ysc genes (Yop secretion), which catalyze the successive secretions of early, middle, and late (effector) substrates (6, 7). During the initial assembly stage of the Yersinia type III machine, a protein transport channel with a central conduit is formed across the bacterial inner and outer membranes (8). The type III machine promotes self-assembly of its basal membrane components and secretion of its inner rod (YscI) and needle (YscF) extensions (9, 10), followed by the transport of middle substrates to cap the needle (LcrV, YopD) (11). Contact of capped needles with host cells is thought to promote the assembly of the injectisome in host cell membranes (YopB, YopD) (12, 13). Establishment of a Yersinia type III conduit into host cells is associated with a decrease in calcium ions: the concentration of calcium ions is 1.2 mM in extracellular fluids and <10 μM within cells (14). Reception of this signal activates the Yersinia type III pathway for the translocation of effectors into host cells (14, 15).
Electron microscopy experiments with Y. enterocolitica needle complexes revealed the LcrV cap (11). Like Y. pestis yscF mutants, lcrV variants are unable to complete the type III conduit into host cells and cannot activate the pathway even when calcium ions are chelated (3, 16, 17). A different phenotype has been observed for yopB and yopD; Yersinia mutants lacking yopB or yopD respond to the low-calcium signal with type III secretion, yet these variants are unable to direct effectors into host cells (18, 19). When extracellular calcium ions are removed, wild-type Y. pestis cannot form colonies on laboratory media at 37°C (low-calcium response [LCR+] phenotype) (20); however, this growth restriction is abolished when yscF or lcrV is deleted (LCR−) (3, 21). In contrast, Y. pestis calcium-blind mutants secrete effectors even in the presence of extracellular calcium ions and cannot form colonies at 37°C (temperature sensitive for growth [ts] phenotype) (22). Torruellas and colleagues isolated yscF variants with calcium-blind ts phenotypes (21). These YscF mutants harbor single amino acid substitutions of negatively charged aspartic acid residues with neutral amino acids, suggesting that the association of aspartyl with calcium ions may enable the needle protein to act as a calcium sensor (21). The LCR pathway also involves factors in the Y. pestis cytoplasm. For example, LcrG and LcrE (YopN) are required for the Y. pestis blockade of effector secretion in the presence of calcium, as lcrG and lcrE mutants also display calcium-blind ts phenotypes (22–24). LcrG binds LcrV in the bacterial cytoplasm, and this association is required for LcrV secretion (17, 25).
LcrV, but not any other component of the type III pathway, is the plague-protective antigen; antibodies that bind LcrV prevent Y. pestis translocation of effectors into host cells (26, 27). Although wild-type LcrV could not be crystallized, the structure of a variant (K40A/D41A/K42A) was revealed by X-ray crystallography (28). Of note, the N and C termini of LcrV (residues 23 to 27 and 323 to 326), Y90, and two internal loops (residues 49 to 63 and 260 to 275) were not visible in the electron density map (28). LcrVK40A/D41A/K42A assumed an overall dumbbell-shaped structure with a central coiled coil connecting two globular folds (28). This structure was modeled into high-resolution electron microscopy images of the LcrV needle cap, which suggest an atomic model for a pentameric LcrV ring (13). The functional relevance of the proposed structure is, however, not clear. The LcrV residues involved in forming a central conduit, in associating with the needle protein (YscF), or in forming the injectisome (YopB and YopD) are not yet known. Furthermore, although the aforementioned hypothesis proposes a dynamic role for LcrV in capping type III needles and in promoting injectisome assembly, experimental proof for this conjecture is still missing. Here, we used genetic as well as molecular biology approaches to address these questions.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Y. pestis strain KIM D27 (wild type) (29) and the ΔlcrV mutant (KLD29) (27) were grown in heart infusion broth (HIB), M9-Casamino Acids (M9-Ca) minimal medium, or thoroughly modified Higuchi's (TMH) medium (27, 29, 30). Y. enterocolitica strain W22703 (wild type) (31) and the ΔlcrV mutant (CT1) (17) were grown in tryptic soy broth (TSB) or M9-Ca medium, as indicated. Chloramphenicol was added to Y. enterocolitica (30 μg/ml) or Y. pestis (10 μg/ml) cultures for plasmid retention. For PCR mutagenesis experiments, determination of the low-calcium response (LCR) was performed on solid medium by plating Y. pestis cultures onto tryptose blood agar (TBA) plates containing 20 mM MgCl2, 20 mM sodium oxalate, and 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). Duplicate plates were inoculated with transformants and incubated at 26°C or 37°C for 48 h and then scored for their LCR.
The construction of the plcrVW22703 (pNM142) and plcrVD27 (pNM77) mutants has been described previously (33). PCR mutagenesis of the plcrVD27 mutant (harboring Y. pestis KIM D27 lcrV) was performed with the GeneMorph II EZClone domain mutagenesis kit (Agilent) using primers complementary to the vector backbone at positions approximately 60 bp outside the lcrV coding region: 5′-TGCGCCGACATCATAACGGTTC-3′ (pHSG lcrV 5′) and 5′-TCTGCCTCCCAGAGCCTGATA-3′ (pHSG lcrV 3′). Plasmids carrying the LcrV-Strep alleles of Y. enterocolitica were generated with the QuikChange Lightning site-directed mutagenesis kit (Agilent) with reverse complementary primers containing the Strep tag sequence (5′-TGGTCTCATCCTCAATTTGAGAAG-3′), and plasmids harboring the sequential C-terminal additions of the Strep tag amino acid residues to LcrVD27 (plcrV+1, plcrV+2, plcrV+3, plcrV+4, plcrV+5, plcrV+6, plcrV+7) were assembled in a similar manner. Standard methods for transformation of the plasmids into Y. pestis were employed.
LCR growth assay.
To determine the bacterial growth phenotypes in the presence and absence of calcium, overnight cultures of Y. pestis were diluted 1:40 into 4 ml of TMH medium and incubated for 2 h at 26°C, at which time 200-μl aliquots of each sample were added in triplicate to a sterile 96-well flat-bottom growth plate (wells were supplemented in advance, where necessary, by the addition of CaCl2 and IPTG to final concentrations of 2.5 mM and 1 mM, respectively). Bacterial growth at 37°C was recorded by a plate reader over a period of 12 h by measuring the optical density at 600 nm for shaking cultures containing TMH medium alone or TMH medium supplemented with 2.5 mM CaCl2+.
Yersinia type III secretion.
To monitor secretion into the extracellular environment, Y. pestis and Y. enterocolitica overnight cultures were diluted 40-fold into 4 ml M9-Ca medium or TSB supplemented with 5 mM EGTA, respectively, incubated at 26°C for 2 h, and then shifted to 37°C for 3 h to induce type III secretion. When appropriate, IPTG was added to a final concentration of 1 mM to induce the expression of plasmid-borne lcrV alleles. Cultures were fractionated and analyzed for type III secretion as described previously (32). To screen for Yersinia type III translocation of effectors, overnight cultures of Y. pestis and Y. enterocolitica were diluted 1:40 into 4 ml HIB or TSB, respectively, and incubated for 2 h at 26°C. Bacteria were added at a multiplicity of infection (MOI) of 10 to HeLa cell monolayers of about 2 × 105 cells that had been seeded a day earlier. After 3 h of infection at 37°C, cells were fixed with 3.7% formaldehyde for 20 min, quenched with 0.1 M glycine for 5 min, washed with phosphate-buffered saline (PBS), permeabilized with 0.1% Triton X-100 for 30 min at 4°C, washed three times with PBS, and blocked with 5% nonfat dried milk in PBS for 20 min at room temperature. Filamentous actin was then labeled with 3 units of rhodamine-conjugated phalloidin (99 nM) for 20 min. After the labeling solution was removed, the cells were washed three times with PBS and visualized by phase-contrast imaging and fluorescence microscopy using a Nikon TE-2000 inverted microscope (33).
Purification of LcrV from Yersinia extracts.
To assay for cytosolic protein-protein interactions, 50 ml of overnight cultures of Y. pestis or Y. enterocolitica was inoculated into 1 liter of HIB supplemented with 20 mM MgCl2 and 20 mM sodium oxalate or TSB supplemented with 5 mM EGTA, respectively. Cultures were incubated at 26°C for 2 h and then grown at 37°C for another 3 h. IPTG, at a final concentration of 1 mM, was added prior to the temperature shift to induce expression of plasmid-borne LcrV. Bacteria were sedimented by centrifugation at 7,500 × g for 10 min, suspended in 20 ml of column buffer (100 mM Tris-HCl, 150 mM NaCl [pH 7.5]), and left at −20°C overnight. The cells were thawed the following day and French pressed twice at 15,000 lb/in2. The crude lysate was centrifuged twice at 8,000 × g for 15 min, and the supernatant (load) was subjected to chromatography on 1.5 ml of Strep-Tactin Sepharose (IBA BioTAGnology). The column was washed with 30 ml of column buffer, and the proteins were eluted with column buffer with 2.5 mM desthiobiotin. Samples were analyzed by 15% SDS-PAGE, Coomassie staining, or immunoblotting with rabbit antisera raised against purified recombinant Yersinia proteins or mouse monoclonal antibody directed against the Strep tag.
Purification and visualization of type III needles.
Needle purification was carried out by inoculation of a 50-ml overnight culture of Y. enterocolitica into 1 liter of M9-Ca medium, which was incubated at 26°C for 2 h and then grown at 37°C for 3 h. LcrV expression was induced by the addition of 1 mM IPTG prior to incubation at 37°C. Bacteria were sedimented by centrifugation at 2,700 × g for 10 min and suspended in 40 ml of 1 M Tris-HCl (pH 7.5). Needles on the bacterial surface were mechanically sheared by vortexing the samples for 5 min. Bacteria were again sedimented by centrifugation at 8,000 × g for 10 min, and the needle containing shearate was passed through a 0.45-μm cellulose acetate membrane filter (Whatman). Needles were sedimented by ultracentrifugation at 45,000 × g for 30 min. The needle sediment was suspended in 20 mM Tris-HCl (pH 7.5) and examined by either immunoblotting or electron microscopy. For electron microscopy, all samples were placed on a carbon-coated copper grid and stained with 1% uranyl acetate before viewing on a Tecnai F30 electron microscope at 300 kV.
RESULTS
Mutations in lcrV with a dominant negative LCR− phenotype in Y. pestis.
Y. pestis lcrVD27 was subjected to PCR mutagenesis, introducing mutations at frequencies ranging from 1 to 10 lesions per kilobase. Mutated plasmids were purified and transformed into wild-type Y. pestis strain KIM D27. Transformants were spread on agar with chelated calcium ions and incubated for 2 days at 37°C in the presence of 1 mM IPTG to induce the expression of plasmid-borne lcrV. Under these conditions, Y. pestis variants that lost the ability to secrete effectors formed colonies (20); these colonies were isolated, and the plasmids were purified and transformed again into Y. pestis KIM D27. Transformants that retained the LCR− phenotype on calcium-chelated agar supplemented with 1 mM IPTG were analyzed by DNA sequencing of plasmid-borne lcrV. Eighty-seven independent mutants were isolated, 18 of which harbored missense mutations in codon 327 (UGA amber stop), extending the lcrVD27 reading frame by 22 codons (Fig. 1A). Specifically, we identified 6 CGA (Arg), 2 GGA (Gly), 3 UGG (Trp), 2 UGU (Cys), 4 UUA (Lys), and 1 UCA (Ser) codon variants. The remaining mutants that answered the selection were stop or frameshift mutations at various locations throughout the lcrV gene.
Fig 1.
Yersinia pestis lcrV mutants with the dominant negative low-calcium response (LCR−) phenotype. (A) lcrVD27 was subjected to PCR mutagenesis, cloned under the control of the pHSG576 tac promoter, and electroporated into Y. pestis strain KIM D27. Transformants were quantified after colony formation on TBA supplemented with magnesium oxalate (MgOx) at 26°C or 37°C, at the latter temperature with selection for dominant negative LCR− mutants. Plasmids were isolated and again electroporated into Y. pestis strain KIM D27, growth of cells with the LCR− phenotype was verified, and plasmids were sequenced, which identified 18 mutations with missense mutations in codon 327 (UGA). These mutations extend the lcrV open reading frame by 22 codons (GSKQGGSVSPFFYQYCEYLRP*). (B) Y. pestis strains KIM D27 (wild-type [WT] lcrV) or KLD29 (ΔlcrV) harboring either no plasmid or plcrVD27, plcrV*327W, and plcrV*327R were cultured at 37°C in TMH medium supplemented with either 0 mM or 6.25 mM CaCl2. Growth was recorded as an increase in the optical density at 600 nm (OD600). pyopNF234S, which has been reported to cause a dominant negative LCR− phenotype (34), was used as a control.
Y. pestis KIM D27 strains harboring plasmids expressing wild-type lcrVD27 (plcrVD27) or the amber codon suppressors (plcrV*327R and plcrV*327W) were grown in liquid media at 37°C for 12 h under secretion-permissive (TMH medium) or secretion-nonpermissive conditions (TMH medium supplemented with 2.5 mM CaCl2) (Fig. 1B). In contrast to the plasmid expressing lcrVD27, plcrV*327R and plcrV*327W enabled IPTG-induced Y. pestis cultures to grow at 37°C in the absence of calcium ions (LCR−) (Fig. 1B). As controls, Y. pestis KIM D27 without plasmid was LCR+, whereas pyopNF234S resulted in the expected dominant negative LCR− phenotype (34) (Fig. 1B). Deletion of the lcrV gene on pCD1 from Y. pestis KIM D27 also caused an LCR− phenotype, as the ΔlcrV mutant strain (KLD29) continued to grow at 37°C in the absence of calcium; this defect was complemented by the transformation of KLD29 with plcrVD27 (Fig. 1B).
Y. pestis strains were grown in liquid media for 2 h at 26°C and then shifted to 37°C for 3 h to induce type III secretion. The cultures were centrifuged to separate the extracellular medium with the supernatant from the bacterial sediment. Proteins in both fractions were analyzed by immunoblotting with rabbit antisera raised against purified LcrV, YopE, or RNA polymerase subunit A (RpoA) (Table 1). Y. pestis strain KIM D27 (plcrVD27) secreted LcrV and YopE into the extracellular medium, whereas RpoA was found only in the bacterial sediment. As expected, the ΔlcrV mutant did not express LcrV and failed to secrete YopE, which was again complemented by plcrVD27 (Table 1). Y. pestis KIM D27 harboring plcrV*327R or plcrV*327W failed to secrete YopE yet retained the ability to secrete mutant LcrV*327R and LcrV*327W, albeit at reduced abundance (Table 1). Together, these data indicate that the IPTG-inducible expression of lcrV*327R and lcrV*327W causes a dominant negative LCR− phenotype in Y. pestis strain KIM D27. This is attributable to a type III secretion block of lcrV*327R and lcrV*327W mutants for effector Yops, whereas the secretion of LcrV was reduced but not blocked.
Table 1.
Missense mutations in lcrV codon 327 block Yersinia pestis type III secretion
| Y. pestis strain | Plasmid | % type III secretion ([S]/[S+P])a |
|
|---|---|---|---|
| YopE | LcrV | ||
| KIM D27 (wild type) | plcrVD27 | 36.6 | 28.3 |
| KIM D27 (wild type) | plcrV*327R | 1.3 | 12.6 |
| KIM D27 (wild type) | plcrV*327W | 1.2 | 10.3 |
| KLD29 (ΔlcrV) | None | 1.7 | NDb |
| KLD29 (ΔlcrV) | plcrVD27 | 33.2 | 36.2 |
Type III secretion of Y. pestis was measured after 3 h of growth in cultures that were grown in media with chelated calcium ions at 37°C. Following centrifugation, the extracellular medium was separated into the supernatant (S) and the bacterial pellet (P), and proteins in both fractions were precipitated with trichloroacetic acid and analyzed by immunoblotting with specific antibodies against the type III secretion substrates YopE and LcrV. Secretion was quantified by calculating the percent amount of secreted protein [S] derived from protein in both the medium and the pellet [S+P].
ND, no immunoreactive signal detected.
C-terminal extensions of LcrV block the Y. pestis type III pathway.
We wondered whether the LCR− phenotype of the lcrV*327R and lcrV*327W mutants critically depended on the 22 codons downstream of their stop codon suppressors. To test this, we extended the lcrVD27 open reading frame by insertions of random single amino acid codons immediately prior to codon 327 (UGA). The resulting variants were expressed from plasmids via IPTG induction of the lacUV5 promoter. Expression of lcrV alleles with one (plcrV+1), two (plcrV+2), or three codon insertions (plcrV+3) did not affect bacterial growth; however, lcrV alleles with four (plcrV+4), five (plcrV+5), six (plcrV+6), or seven (plcrV+7) codon insertions abolished the LCR of Y. pestis strain KIM D27 (Fig. 2). These data indicate that the mere extension of Y. pestis LcrV by four or more residues at its C-terminal end is sufficient to impose a dominant negative blockade on the type III pathway.
Fig 2.
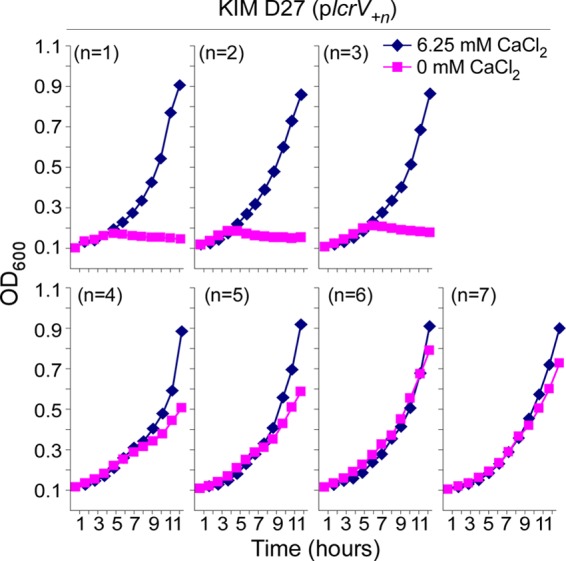
Short extensions at the C terminus of LcrV cause a dominant negative LCR− phenotype in Y. pestis. The open reading frame of lcrVD27 was extended by single-codon insertions at position 327, immediately prior to the UGA stop codon. Y. pestis strain KIM D27 harboring plcrV+1, plcrV+2, plcrV+3, plcrV+4, plcrV+5, plcrV+6, or plcrV+7 was cultured at 37°C in TMH medium supplemented with either 0 mM or 6.25 mM CaCl2. Growth was recorded as an increase in the OD600.
Strep tag insertions into Y. enterocolitica LcrV.
Earlier work revealed that LcrV caps the tip of Y. enterocolitica needles (11). As details regarding Y. pestis needles and their LcrV caps have not yet been revealed (35), we sought to examine the cap structure and other functional attributes of lcrV alleles in Y. enterocolitica W22703 (1, 31). LcrVW22703 is three residues shorter than LcrVD27; however, the two molecules are 96% identical (Fig. 3A). To generate a C-terminal extension of LcrVW22703, we appended the eight-residue Strep tag to the C-terminal end of the polypeptide chain by inserting the corresponding eight codons immediately upstream of the lcrVW22703 stop codon (plcrVS324). Furthermore, we wondered whether Strep tag insertions at other sites also cause a dominant negative type III secretion (LCR) phenotype, and we selected LcrVW22703 positions 1, 20, 55, 170, 228, and 270 for insertions. The relative positions of these insertions in the three-dimensional structure of LcrVK40A/D41A/K42A and their corresponding phenotypes are summarized in Fig. 3B. Plasmids expressing lcrVW22703 or its variants via the lacUV5 promoter were transformed into Y. enterocolitica CT1, the ΔlcrV variant of W22703. In contrast to Y. pestis, where the deletion of lcrV abolished the LCR and type III secretion, Y. enterocolitica CT1 (ΔlcrV) remains competent for both YopE expression and type III secretion (Fig. 3C) (17). Of note, LcrG, the cytoplasmic chaperone of LcrV, is expressed at very low abundance in the lcrV mutant strain (Fig. 3C) (17). Transformation of Y. enterocolitica CT1 with plcrVW22703 restored the expression of both LcrVW22703 and LcrG and enabled the secretion of LcrVW22703 (Fig. 3C). Similar phenotypes were observed with plcrVS1, plcrVS20, plcrVS55, plcrVS170, and plcrVS228. The plcrVS270 plasmid did not restore the expression of LcrG, and LcrVS270 was not secreted into the extracellular medium. Plasmid plcrVS324 restored the expression of LcrG, and only very small amounts of LcrVS324 were secreted into the extracellular medium (Fig. 3C).
Fig 3.
Strep tag insertions in LcrV. (A) Alignment of amino acid sequences derived from the lcrV gene of Y. enterocolitica strain W22703 (LcrVW22703) or Y. pestis strain KIM D27 (LcrVD27). LcrVs derived from Y. enterocolitica and Y. pestis were aligned. Arrowheads and S numbers identify the amino acids (codons) where the Strep tag (NH2-WSHPQFEK-COOH) was inserted into LcrVW22703. (B) Ribbon diagram illustrating the three-dimensional X-ray structure of LcrV (28) and the positions of the Strep tags. LcrVS1 and LcrVS228 (green) displayed wild-type LcrV phenotypes in both Y. enterocolitica and Y. pestis. LcrVS270 (blue) was nonfunctional, and LcrVS324 (red) caused a dominant negative blockade of the type III pathway in both Y. enterocolitica and Y. pestis. LcrVS20 and LcrVS55 (both orange) caused a dominant negative blockade of type III secretion only in Y. pestis and not in Y. enterocolitica. Finally, LcrVS170 (purple) did not affect type III secretion in vitro but failed to promote effector translocation for both Y. enterocolitica and Y. pestis. (C) Y. enterocolitica CT1 (ΔlcrV variant of W22703) without plasmid or harboring plcrVW22703, plcrVS1, plcrVS20, plcrVS55, plcrVS170, plcrVS228, plcrVS270, or plcrVS324 was grown for 3 h at 37°C in TSB supplemented with 5 mM EGTA to chelate calcium and 1 mM IPTG to induce the expression of plasmid-borne lcrV alleles. Yersinia cultures were centrifuged, and the extracellular medium was removed with the supernatant (S) and separated from the bacterial sediment (pellet [P]). Proteins in both fractions were precipitated with trichloroacetic acid and analyzed by immunoblotting with rabbit antisera raised against YopE (αYopE), LcrV (αLcrV), YopB (αYopB), YopD (αYopD), LcrG (αLcrG), or RpoA (αRpoA) or a monoclonal antibody specific for the Strep tag (αStrep).
To analyze the binding of LcrV mutants to LcrG, extracts of Y. enterocolitica CT1 harboring lcrV plasmids were subjected to affinity chromatography on Strep-Tactin Sepharose. Cleared lysate (L) and eluate (E) were analyzed by Coomassie blue-stained SDS-PAGE and immunoblotting (Fig. 4). LcrVS1, LcrVS20, LcrVS55, LcrVS228, and LcrVS324 were isolated by affinity chromatography and purified together with LcrG (Fig. 4). LcrVS170 did not bind Strep-Tactin resin, and the Strep tag was only poorly recognized by a specific monoclonal antibody. We therefore conclude that the Strep tag of LcrVS170 may be buried within the polypeptide and inaccessible to either antibody or Strep-Tactin (Fig. 4). LcrVS270 was purified from Yersinia lysate; however, this protein cannot copurify with LcrG, as the chaperone is not expressed by Y. enterocolitica CT1 harboring plcrVS270 (Fig. 4). Thus, with the exception of LcrVS270, all other variants do bind to their LcrG chaperone.
Fig 4.
Affinity chromatography of Strep-tagged LcrV. (A) Cleared lysates of Y. enterocolitica CT1 (ΔlcrV) harboring plcrVW22703 or Strep-tagged LcrV (plcrVS1, plcrVS20, plcrVS55, plcrVS170, plcrVS228, plcrVS270, and plcrVS324) were derived from cultures grown for 3 h at 37°C in TSB supplemented with 5 mM EGTA to chelate calcium and 1 mM IPTG to induce the expression of plasmid-borne lcrV alleles. Cleared lysates (L) were subjected to affinity chromatography on Strep-Tactin resin and eluted with desthiobiotin (E). Proteins in both samples were analyzed by Coomassie blue-stained SDS-PAGE. The migratory positions were proteins with known molecular mass (in kDa, indicated on the left). (B) Samples were subjected to immunoblotting with rabbit antisera raised against LcrV (αLcrV), LcrG (αLcrG), and RpoA (αRpoA) or with monoclonal antibody against the Strep tag (αStrep).
To assess the functions of lcrV alleles in needle cap assembly and injectisome functions, we measured Y. enterocolitica effector translocation into tissue culture cells. HeLa cells were infected with Y. enterocolitica CT1 expressing mutant lcrV. Phase-contrast microscopy and fluorescence microscopy images of phalloidin-stained tissue cultures were used to reveal actin filament rearrangements and cell rounding as measures for Yersinia type III translocation of effectors (5) (see Fig. S1 in the supplemental material). Of note, plcrVS1, plcrVS20, plcrVS55, and plcrVS228 enabled Y. enterocolitica CT1 (ΔlcrV) to inject effectors, whereas plcrVS170, plcrVS270, and plcrVS324 did not (see Fig. S1). When transformed into wild-type Y. enterocolitica W22703, only plcrVS324 caused a dominant negative effect on type III effector translocation, while plcrVS1, plcrVS20, plcrVS55, plcrVS170, plcrVS228, and plcrVS270 had no effect (see Fig. S1). These data indicate that the C-terminal extension of LcrVW22703 in Y. enterocolitica lcrVS324 blocked the type III secretion pathway similarly to lcrV*327W, lcrV*327R, and lcrVS324 in Y. pestis. Further, Strep tag insertions at positions 170 and 270 abolished the ability of LcrVS170 and LcrVS270 to assemble the type III injectisome and translocate effectors. For LcrVS270, this is attributable to a defect in binding and stabilizing LcrG, which results in a defect in the type III secretion of LcrVS270.
LcrVS228 and LcrVS324 cap Y. enterocolitica type III needles.
We sought to determine whether LcrVS324 can cap type III needle structures. Y. enterocolitica CT1 (ΔlcrV) without plasmid or with plcrVW22703, plcrVS228, or plcrVS324 was sheared to remove type III secretion needles, which were subsequently sedimented by ultracentrifugation. Electron microscopy experiments revealed the absence of a cap structure on Y. enterocolitica ΔlcrV needles (Fig. 5A). Plasmids plcrV and plcrVS228, as well as plcrVS324, restored the assembly of the LcrV cap on YscF needles (Fig. 5B). To examine whether capped needles also copurify with the injectisome component YopD, needle preparations were subjected to immunoblotting (11, 36). As expected, needles derived from Yersinia with plcrVW22703 or plcrVS228 were assembled from YscF, YopD, and LcrV, whereas the needle preparations from the ΔlcrV mutant harbored YscF, very little YopD, and no LcrV. In contrast, needles from Y. enterocolitica ΔlcrV (plcrVS324) were assembled from YscF and LcrVS324; however, the structures lacked YopD (Fig. 5B). Taken together, these data suggest that LcrVS324 can indeed cap the type III needle. In contrast to wild-type LcrV or LcrVS228, LcrVS324-capped needles lack YopD, which may explain the effector translocation defect of the lcrVS324 mutant strain. Electron microscopy experiments have not yet positioned YopD within the capped-needle complex. The finding that LcrVS228-capped needles, but not the nonfunctional LcrVS324 cap, associate with YopD provides evidence for a model whereby specific interactions between YscF, LcrV, and YopD promote the formation of capped needles that eventually progress toward injectisome assembly.
Fig 5.
LcrVS324 caps YscF needles that lack YopD. (A) Cultures of Y. enterocolitica CT1 (ΔlcrV) harboring plcrVW22703, plcrVS228, plcrVS324, or no plasmid were centrifuged, and the bacterial sediment was sheared to break off type III needle complexes. Bacteria were removed by slow-speed centrifugation, and the filtered supernatant was ultracentrifuged to sediment type III needle complexes, which were analyzed by transmission electron microscopy. The arrowheads identify LcrV caps of type III needle complexes. (B) Y. enterocolitica CT1 (ΔlcrV) and Y. enterocolitica ΔpYVe227 cultures were subjected to the isolation of type III needle complexes as described above. Samples were analyzed by immunoblotting with rabbit antisera raised against LcrV (αLcrV), YopD (αYopD), and YscF (αYscF).
Phenotypes of Strep-tagged LcrV expressed in Y. pestis.
Subtle differences between the type III pathways of Y. enterocolitica and Y. pestis have been reported (37). First, Y. pestis, but not Y. enterocolitica, displays an LCR phenotype (20, 38). Thus, low-calcium-induced activation of type III secretion in Y. enterocolitica does not lead to complete growth restriction even though large amounts of effectors are secreted into the extracellular medium (39). Second, even though lcrG and lcrV encode nearly identical products in pathogenic Yersinia, mutations in these genes cause different phenotypes. Deletion of Y. pestis lcrG causes a calcium-blind phenotype, whereas deletion of lcrV results in an LCR− phenotype, whereby the type III secretion of effectors is abolished even when bacteria are incubated in the absence of calcium (23, 24). In contrast, the deletion of Y. enterocolitica lcrV does not abrogate low-calcium-induced type III secretion of effectors (17). These differences cannot be explained by the amino acid polymorphisms of lcrV products, as the expression of lcrVW22703 in Y. pestis or lcrVD27 in Y. enterocolitica results in wild-type phenotypes (33). The deletion of Y. enterocolitica lcrG causes a class I secretion phenotype (40); although Y. enterocolitica lcrG mutants continue to grow, the variants secrete all effectors in both the presence and absence of calcium (17, 41).
To test whether lcrV alleles isolated here displayed different phenotypes in Y. enterocolitica and Y. pestis, plasmids plcrVS1, plcrVS20, plcrVS55, plcrVS170, plcrVS228, plcrVS270, and plcrVS324 were also transformed into Y. pestis strains KIM D27 and KLD29 (ΔlcrV). When analyzed for growth in the presence or absence of calcium, plasmids plcrVS1, plcrVS170, and plcrVS228 restored the LCR− phenotype of Y. pestis stain KLD29 (ΔlcrV) to LCR+, whereas plcrVS20, plcrVS55, plcrVS270, and plcrVS324 did not (Fig. 6). Further, plcrVS1, plcrVS170, and plcrVS228 complemented the type III secretion defect of the ΔlcrV mutant, whereas plcrVS270 and plcrVS324 blocked the secretion of both YopE and LcrVS270 or LcrVS324 (Table 2). Two lcrV plasmids produced different phenotypes in Y. pestis and Y. enterocolitica. Plasmids plcrVS20 and plcrVS55 caused a dominant negative LCR− and effector translocation phenotype in Y. pestis (Fig. 6) and reduced the type III secretion of YopE (Table 2). In contrast, plcrVS20 and plcrVS55 complemented the type III secretion and effector translocation defects of the Y. enterocolitica lcrV mutant. In comparison with plcrVS20 and plcrVS55, plcrVS324 caused a stronger LCR− phenotype in Y. pestis strain KIM D27 and abolished the type III secretion of YopE, similar to the effects on lcrV*327W and lcrV*327R (Tables 1 and 2).
Fig 6.
Strep-tagged LcrVs and their LCR phenotypes in Yersinia pestis. (A) Y. pestis KLD29 (ΔlcrV) expressing Strep-tagged LcrV (plcrVS1, plcrVS20, plcrVS55, plcrVS170, plcrVS228, plcrVS270, and plcrVS324) was cultured at 37°C in TMH medium supplemented with either 0 mM or 6.25 mM CaCl2. Growth was recorded as an increase in the OD600. (B) Y. pestis strain KIM D27 (wild-type lcrV) expressing Strep-tagged LcrV (plcrVS1, plcrVS20, plcrVS55, plcrVS170, plcrVS228, plcrVS270, and plcrVS324) was cultured at 37°C in TMH medium supplemented with either 0 mM or 6.25 mM CaCl2. Growth was recorded as an increase in the OD600.
Table 2.
Insertions of the eight-codon Strep tag at various positions into lcrV and its effect on Yersinia pestis type III secretion of YopE or LcrV
| Plasmid | % type III secretion ([S]/[S+P])a |
|||
|---|---|---|---|---|
| KIM D27 (wild type) |
KLD29 (ΔlcrV) |
|||
| YopE | LcrV | YopE | LcrV | |
| None | 47.1 | 21.3 | 3.7 | NDb |
| plcrVW22703 | 50.3 | 37.8 | 51.1 | 31.8 |
| plcrVS1 | 52.3 | 25.8 | 60.8 | 24.1 |
| plcrVS20 | 4.6 | 16.8 | 7.7 | 22.7 |
| plcrVS55 | 4.1 | 15.8 | 13.2 | 21.9 |
| plcrVS170 | 48.4 | 39.7 | 45.6 | 36.3 |
| plcrVS228 | 49.2 | 38.9 | 63.4 | 37.6 |
| plcrVS270 | 54.1 | 38.5 | 2.8 | 1.1 |
Type III secretion of Y. pestis strains without or with lcrV plasmids was measured after 3 h of growth in cultures that were grown in media with chelated calcium ions at 37°C. Following centrifugation, the extracellular medium was separated into the supernatant (S) and the bacterial pellet (P), and proteins in both fractions were precipitated with trichloroacetic acid and analyzed by immunoblotting with specific antibodies against the type III secretion substrates YopE and LcrV. Secretion was quantified by calculating the percent amount of secreted protein [S] compared to protein in both the medium and the pellet [S+P].
ND, no immunoreactive signal detected.
As with data obtained with Y. enterocolitica, affinity chromatography of Strep-tagged LcrV on Strep-Tactin resin revealed the copurification of LcrVS1, LcrVS20, LcrVS55, LcrVS228, and LcrVS324 with LcrG (see Fig. S2 in the supplemental material). As LcrG is not expressed in Y. pestis KLD29 (plcrVS270), LcrVS270 did not copurify with LcrG. GST-LcrG was purified from the cytoplasm of Escherichia coli using affinity chromatography on glutathione-Sepharose (17). GST-LcrG binding to LcrVS228, LcrVS170, or LcrVS270 was analyzed via chromatography of Yersinia lysates and immunoblotting; GST-LcrG retained LcrVS228 and LcrV170 but not LcrVS270 (see Fig. S2). As a control, YopB harboring a Strep tag at the N terminus was not retained during GST-LcrG chromatography (see Fig. S2). These data indicate that LcrV binding is required for LcrG expression. Presumably, LcrG that is not bound to LcrV is rendered unstable and may be degraded by Yersinia.
When analyzed for the type III injection of effectors into HeLa cells, Y. pestis strain KIM D27 with or without plcrVW22703 caused actin cable rearrangements and cell rounding (Fig. 7). Plasmids plcrVS1 and plcrVS228 did not affect the ability of Y. pestis KIM D27 type III machines to translocate effectors, whereas plcrVS170, plcrVS270, and plcrVS324 caused a dominant negative blockade of the Y. pestis type III pathway (Fig. 7). Of note, we observed an intermediate type III effector translocation phenotype for plcrVS20 and plcrVS55 (Fig. 7). When transformed into Y. pestis strain KLD29 (ΔlcrV), plcrVW22703, plcrVS1, and plcrVS228 complemented the type III injection phenotype, whereas plcrVS170, plcrVS270, and plcrVS324 did not. In contrast to Y. enterocolitica, expression of lcrVS20 or lcrVS55 in the Y. pestis ΔlcrV mutant did not restore function for the type III pathway (Fig. 7).
Fig 7.
Strep-tagged LcrV and Yersinia pestis effector translocation. (A) Y. pestis strains KIM D27 (wild-type lcrV) and KLD29 (ΔlcrV) were used to infect HeLa tissue culture cells for 3 h at an MOI of 10. Samples were fixed, stained with rhodamine-phalloidin, and imaged by fluorescence or phase-contrast microscopy to reveal actin cable rearrangements and cell rounding as a measure for effector translocation. (B) Y. pestis KIM D27 strains harboring lcrV plasmids (plcrVW22703, plcrVS1, plcrVS20, plcrVS55, plcrVS170, plcrVS228, plcrVS270, and plcrVS324) were subjected to the same assay as described for panel A. (C) Y. pestis KLD29 strains harboring lcrV plasmids (plcrVW22703, plcrVS1, plcrVS20, plcrVS55, plcrVS170, plcrVS228, plcrVS270, and plcrVS324) were subjected to the same assay as described for panel A.
LcrVS324 blocks the calcium-blind phenotype of Y. pestis yscF needle mutants.
Earlier work revealed that Y. pestis YP517 yscFD28A, yscFD46A, and yscFD28A,D46A mutants display a calcium-blind growth phenotype and secrete effectors constitutively when bacteria are grown at 37°C. IPTG-induced expression of lcrVS324 blocked the calcium-blind phenotype of yscFD28A, yscFD46A, and yscFD28A,D46A mutants, imposing an LCR− blockade on the type III pathway (Fig. 8 and Table 3). As a control, the expression of lcrVS324 did not affect the growth of yscFD77A LCR− mutants. When we analyzed for type III secretion, we found that the expression of lcrVS324 blocked the secretion of YopH in yscFD28A, yscFD46A, and yscFD28A,D46A mutants but did not restore the ability of the yscFD77A variant to secrete YopH (Table 3).
Fig 8.
LcrVS324 causes a dominant negative LCR− phenotype in Y. pestis harboring calcium-blind alleles yscFD28A and/or yscFD46A. (A) Y. pestis YP517 (ΔyopE/sycE) harboring wild-type yscF, the calcium-blind alleles yscFD28A and/or yscFD46A, or the LCR− yscFD77A allele were transformed with plcrVS324. Strains without (top panels) or with plcrVS324 (bottom panels) were cultured at 37°C in TMH medium supplemented with either 0 mM or 6.25 mM CaCl2. Growth was recorded as an increase in the OD600.
Table 3.
LcrVS324 blocks type III secretion of YopH in Y. pestis calcium-blind mutants with yscFD28A and yscFD46A mutations
| yscF allele |
Y. pestis (ΔyopE/sycE) % type III secretion ([S]/[S+P])a |
|||
|---|---|---|---|---|
| No plasmid |
plcrVS324 |
|||
| YopH | LcrV | YopH | LcrV | |
| yscF | 45.2 | 42.7 | 2.9 | 5.3 |
| yscFD28A | 44.9 | 39.8 | 4.5 | 18.2 |
| yscFD46A | 45.8 | 29.1 | 4.4 | 15.3 |
| yscFD28A,D46A | 47.3 | 45.8 | 2.2 | 9.3 |
| yscFD77A | 0.9 | 1.1 | 3.3 | 4.9 |
Type III secretion of Y. pestis strains with or without plcrVS324 was measured after 3 h of growth in cultures that were grown in media with chelated calcium ions at 37°C. Following centrifugation, the extracellular medium was separated into the supernatant (S) and the bacterial pellet (P), and proteins in both fractions were precipitated with trichloroacetic acid and analyzed by immunoblotting with specific antibodies against the type III secretion substrates YopH and LcrV. Secretion was quantified by calculating the percent amount of secreted protein [S] compared to protein in both the medium and the pellet [S+P].
DISCUSSION
LcrV is the protective antigen of Y. pestis, and subunit vaccines that raise high-titer antibodies against this polypeptide confer protection against bubonic and pneumonic plague in mice, rats, guinea pigs, rabbits, cynomolgus macaques, and African Green monkeys (26, 42). Both in vitro and in vivo studies have indicated that LcrV antibodies block Y. pestis type III translocation of effectors into immune cells (27, 33). Studies with Yersinia lcrV, yopB, and yopD mutants suggest that these type III secretion substrates are each required for the translocation of effectors into host cells (17–19). Electron microscopy and biochemistry experiments revealed that the YscF needle of Y. enterocolitica is capped by LcrV and perhaps by YopD (11). Contact of the needle with host cell membranes is thought to trigger the formation of the injectisome, a YopB-YopD complex that establishes a conduit across the plasma membrane of host cells (13, 43). Appreciation of the molecular details and the mechanistic features of this model is necessary in order to understand why some, but certainly not all, antibodies against LcrV block effector translocation by the Y. pestis type III machine (34, 44). We reported recently that the ability of LcrV antibodies to block Y. pestis effector translocation does not extend to Y. enterocolitica type III machines and that the observed differences are not based on the amino acid polymorphisms in LcrVW22703 and LcrVD27 (33). These observations suggest that type III machines in Y. pestis and Y. enterocolitica have evolved discrete differences in injectisome assembly. Here, we used a genetic approach to initiate the analysis of LcrV features essential for injectisome assembly and effector translocation.
Exploiting Y. pestis LCR growth restriction, we isolated dominant negative lcrV mutants and observed that these variants harbored missense mutations in the amber codon at position 327, which extended the lcrV open reading frame by 22 codons. Neither the identity of the amino acids in the C-terminal extension nor its length appears to be critical for the type III blockade, as four or more residues with seemingly random sequences caused the same dominant negative LCR− phenotype as the 22-residue extension. We achieved a similar effect by adding a C-terminal Strep tag to Y. enterocolitica strain W22703 and expressing lcrVS324 in Y. pestis. The LcrVS324 protein assembles as a cap on Y. enterocolitica YscF needles; however, the LcrVS324-capped needles are not competent for effector translocation into HeLa cells. Unlike wild-type LcrV, LcrVS324 needles lack YopD, suggesting that the C-terminal extension of LcrV may block the assembly of the injectisome. This assembly process appears to occur within the cap structure, as wild-type needles do not bind YopD antibodies and YopD antibodies cannot block effector translocation (45, 46). Further, needles capped by LcrVS324 do not bind Strep-Tactin resin or Strep tag-specific antibodies, and these needles lack the YopD component of the injectisome. We propose a model whereby the LcrV cap of the type III needle not only interacts with YscF but also contributes to the recruitment of YopD into the injectisome.
YscF, the needle protein, is thought to function as a calcium sensor and may bind calcium ions via aspartyls 28 and 46 (21). Y. pestis type III machines harboring YscFD28A,D46A are calcium blind and secrete effectors even in the presence of millimolar concentrations of calcium ions, presumably because the mutant needle complexes assume the conformation of a fully activated type III pathway (21). The experiments illustrated in Fig. 8 demonstrate that the expression of lcrVS324 in Y. pestis yscFD28A,D46A results in a dominant negative LCR− phenotype and in the complete blockage of type III secretion. We surmise that LcrVS324 is able to cap YscFD28A,D46A needles and lock the secretion machine in a conformation representing an inactive assembly intermediate. Such a model allows us to derive several predictions. First, lcrVS324 may exert a dominant negative phenotype for other calcium-blind mutants capable of LcrVS324 secretion, which may include the yopN, tyeA, sycN, and yscB mutants but not the lcrG mutant, which is required for LcrV secretion. Further, the contact sites between YscF and LcrV may be identified by the use of a suppressor screen for yscFD28A,D46A mutants, devised to restore the calcium-blind phenotype of strains also expressing wild-type lcrV. Lastly, the C-terminal end of LcrV may interact directly with YopD during the assemblies of the needle cap and injectisome. Of note, cap assembly does not require YopD (11); however, without YopD (or YopB), the cap cannot advance to generate an injectisome for effector translocation (19).
We noted phenotypic similarities but also differences for Strep-tagged LcrV mutants expressed in Y. enterocolitica or Y. pestis. LcrVS270 and LcrVS324 did not complement the ΔlcrV phenotype in Y. enterocolitica or in Y. pestis. Further, LcrVS324 displayed a dominant negative LCR− phenotype as well as a block in effector translocation in both Y. enterocolitica and Y. pestis. LcrVS270 did not bind to LcrG, and the mutant protein was not a substrate for type III secretion. The lcrV170 allele complemented the LCR− phenotype of the Y. pestis ΔlcrV mutant and enabled type III secretion of YopE and LcrV170. Nevertheless, lcrV170 failed to restore effector translocation in both Y. pestis and Y. enterocolitica ΔlcrV mutants. Although LcrVS170 did not bind to Strep-Tactin resin, the mutant copurified with GST-LcrG. Taken together, these data suggest that LcrVS170, similarly to LcrVS324, may assemble into a needle cap that is defective for injectisome assembly. In contrast, LcrVS270 cannot bind LcrG and appears not to be secreted by Yersinia. These results are in agreement with earlier work identifying residues 277, 289, and 292 as sites of 5-residue linker insertions that abolished the association between mutant LcrV and LcrG (47). Finally, LcrVS20 and LcrVS55 are secreted by ΔlcrV mutant Y. pestis and Y. enterocolitica. In Y. enterocolitica, lcrVS20 and lcrVS55 restore YopE secretion and effector translocation, whereas in Y. pestis, lcrVS20 and lcrVS55 cause a dominant negative LCR− phenotype and a block in effector translocation. These data suggest that LcrVS20 and LcrVS55 form a functional needle cap in Y. enterocolitica. In contrast, LcrVS20 and LcrVS55 needle cap assembly may occur in Y. pestis; however, if formed, these caps cannot respond to the low-calcium signal or activate effector translocation. Of note, the alleles lcrVS20 and lcrVS55 enabled us to identify for the first time LcrV mutants that exert different phenotypes when expressed in Y. enterocolitica and Y. pestis. Thus, these mutants may be useful in elucidating the subtle differences between Y. enterocolitica and Y. pestis type III machines.
Sato and colleagues performed linker-scanning mutagenesis of pcrV, encoding the cap protein of Pseudomonas aeruginosa type III needles (48). Of note, PcrV and LcrV are 37% identical and 67% similar at the amino acid level, and the two proteins are presumed to assemble into structures with similar functions (43, 49). The insertion of a 19-residue linker at position 119 (D151), 120 (S152), 131 (L153), 134 (E156), 138 (L160), or 279 (F308) of PcrV abolished effector translocation in P. aeruginosa (in parentheses are the corresponding residues of the LcrVW22703 orthologue) (48). The linker insertions map to helices α7 and α12 of LcrV, and the insertions likely interfere with the binding of mutant PcrV to PcrG, the LcrG orthologue in P. aeruginosa (48, 50).
Supplementary Material
ACKNOWLEDGMENTS
We thank members of our laboratory for critical comments and discussion. We also thank Antoni Hendrickx for guidance with transmission electron microscopy experiments.
This work was supported by a grant (AI42797) from the National Institute of Allergy and Infectious Diseases (NIAID), Infectious Diseases Branch (to O.S.). K.G.L. was a trainee of the Graduate Training in Growth and Development program at the University of Chicago (grant HD009007). The authors acknowledge membership within and support from the Region V “Great Lakes” Regional Center of Excellence in Biodefense and Emerging Infectious Diseases Consortium (NIH award 1-U54-AI-057153).
Footnotes
Published ahead of print 7 December 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.02021-12.
REFERENCES
- 1. Cornelis GR, Boland A, Boyd AP, Geuijen C, Iriarte M, Neyt C, Sory MP, Stainier I. 1998. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 62: 1315–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosqvist R, Bolin I, Wolf-Watz H. 1988. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involved in the Yop2b protein. Infect. Immun. 56: 2139–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Perry RD, Harmon PA, Bowmer WS, Straley SC. 1986. A low-Ca2+ response operon encodes the V antigen of Yersinia pestis. Infect. Immun. 54: 428–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cornelis GR. 2002. Yersinia type III secretion: send in the effectors. J. Cell Biol. 158: 401–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rosqvist R, Magnusson KE, Wolf-Watz H. 1994. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 13: 964–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cornelis GR. 2006. The type III injectisome. Nat. Rev. Microbiol. 4: 811–825 [DOI] [PubMed] [Google Scholar]
- 7. Sorg JA, Blaylock B, Schneewind O. 2006. Secretion signal recognition by YscN, the Yersinia type III secretion ATPase. Proc. Natl. Acad. Sci. U. S. A. 103: 16490–16495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Galan JE, Wolf-Watz H. 2006. Protein delivery into eukaryotic cells by type III secretion machines. Nature 444: 567–573 [DOI] [PubMed] [Google Scholar]
- 9. Hoiczyk E, Blobel G. 2001. Polymerization of a single protein of the pathogen Yersinia enterocolitica into needles punctures eukaryotic cells. Proc. Natl. Acad. Sci. U. S. A. 98: 4669–4674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diepold A, Wiesand U, Amstutz M, Cornelis GR. 2012. Assembly of the Yersinia injectisome: the missing pieces. Mol. Microbiol. 85: 878–892 [DOI] [PubMed] [Google Scholar]
- 11. Mueller CA, Broz P, Muller SA, Ringler P, Erne-Brand F, Sorg I, Kuhn M, Engel A, Cornelis GR. 2005. The V-antigen of Yersinia forms a distinct structure at the tip of injectisome needles. Science 310: 674–676 [DOI] [PubMed] [Google Scholar]
- 12. Sorg JA, Miller NC, Schneewind O. 2005. Substrate recognition of type III secretion machines: testing the RNA signal hypothesis. Cell. Microbiol. 7: 1217–1225 [DOI] [PubMed] [Google Scholar]
- 13. Mueller CA, Broz P, Cornelis GR. 2008. The type III secretion system tip complex and translocon. Mol. Microbiol. 68: 1085–1095 [DOI] [PubMed] [Google Scholar]
- 14. Pollack C, Straley SC, Klempner MS. 1986. Probing the phagolysosomal environment of human macrophages with a Ca2+-responsive operon fusion in Yersinia pestis. Nature 322: 834–836 [DOI] [PubMed] [Google Scholar]
- 15. Lee VT, Mazmanian SK, Schneewind O. 2001. A program of Yersinia enterocolitica type III secretion reactions is triggered by specific host signals. J. Bacteriol. 183: 4970–4978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nilles ML, Fields KA, Straley SC. 1998. The V antigen of Yersinia pestis regulates Yop vectorial targeting as well as Yop secretion through effects on YopB and LcrG. J. Bacteriol. 180: 3410–3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee VT, Tam C, Schneewind O. 2000. LcrV, a substrate for Yersinia enterocolitica type III secretion, is required for toxin targeting into the cytosol of HeLa cells. J. Biol. Chem. 275: 36869–36875 [DOI] [PubMed] [Google Scholar]
- 18. Hakansson S, Schesser K, Persson C, Galyov EE, Rosqvist R, Homble F, Wolf-Watz H. 1996. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact-dependent membrane disrupting activity. EMBO J. 15: 5812–5823 [PMC free article] [PubMed] [Google Scholar]
- 19. Hakansson S, Bergman T, Vanooteghem JC, Cornelis G, Wolf-Watz H. 1993. YopB and YopD constitute a novel class of Yersinia Yop proteins. Infect. Immun. 61: 71–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goguen JD, Yother J, Straley SC. 1984. Genetic analysis of the low calcium response in Yersinia pestis Mud1(Ap lac) insertion mutants. J. Bacteriol. 160: 842–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Torruellas J, Jackson MW, Pennock JW, Plano GV. 2005. The Yersinia pestis type III secretion needle plays a role in the regulation of Yop secretion. Mol. Microbiol. 57: 1719–1733 [DOI] [PubMed] [Google Scholar]
- 22. Yother J, Goguen JD. 1985. Isolation and characterization of Ca2+-blind mutants of Yersinia pestis. J. Bacteriol. 164: 704–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Skrzypek E, Straley SC. 1995. Differential effects of deletions in lcrV on secretion of V antigen, regulation of the low-Ca2+ response, and virulence of Yersinia pestis. J. Bacteriol. 177: 2530–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Skrzypek E, Straley SC. 1993. LcrG, a secreted protein involved in negative regulation of the low-calcium response in Yersinia pestis. J. Bacteriol. 175: 3520–3528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nilles ML, Williams AW, Skrzypek E, Straley SC. 1997. Yersinia pestis LcrV forms a stable complex with LcrG and may have a secretion-related regulatory role in the low-Ca2+ response. J. Bacteriol. 179: 1307–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burrows TW. 1956. An antigen determining virulence in Pasteurella pestis. Nature 177: 426–427 [DOI] [PubMed] [Google Scholar]
- 27. DeBord KL, Anderson DM, Marketon MM, Overheim KA, DePaolo RW, Ciletti NA, Jabri B, Schneewind O. 2006. Immunogenicity and protective immunity against bubonic plague and pneumonic plague by immunization of mice with the recombinant V10 antigen, a variant of LcrV. Infect. Immun. 74: 4910–4914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Derewenda U, Mateja A, Devedjiev Y, Routzahn KM, Evdokimov AG, Derewenda ZS, Waugh DS. 2004. The structure of Yersinia pestis V-antigen, an essential virulence factor and mediator of immunity against plague. Structure 12: 301–306 [DOI] [PubMed] [Google Scholar]
- 29. Brubaker RR. 1969. Mutation rate to non-pigmentation in Pasteurella pestis. J. Bacteriol. 98: 1404–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Higuchi K. 1970. An improved chemically defined culture medium for strain L mouse cells based on growth responses to graded levels of nutrients including iron and zinc ions. J. Cell. Physiol. 75: 65–72 [DOI] [PubMed] [Google Scholar]
- 31. Cornelis GR, Colson C. 1975. Restriction of DNA in Yersinia enterocolitica detected by the recipient ability for a derepressed R factor from Escherichia coli. J. Gen. Microbiol. 87: 285–291 [DOI] [PubMed] [Google Scholar]
- 32. Cheng LW, Anderson DM, Schneewind O. 1997. Two independent type III secretion mechanisms for YopE in Yersinia enterocolitica. Mol. Microbiol. 24: 757–765 [DOI] [PubMed] [Google Scholar]
- 33. Miller NC, Quenee LE, Elli D, Ciletti N, Schneewind O. 2012. Polymorphisms in the lcrV gene of Yersinia enterocolitica do not provide for escape from plague protective immunity. Infect. Immun. 80: 1572–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ferracci F, Schubot FD, Waugh DS, Plano GV. 2005. Selection and characterization of Yersinia pestis YopN mutants that constitutively block Yop secretion. Mol. Microbiol. 57: 970–987 [DOI] [PubMed] [Google Scholar]
- 35. Blaylock B, Berube BJ, Schneewind O. 2010. YopR impacts type III needle polymerization in Yersinia species. Mol. Microbiol. 75: 221–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Olsson J, Edqvist PJ, Bröms JE, Forsberg A, Wolf-Watz H, Francis MS. 2004. The YopD translocator of Yersinia pseudotuberculosis is a multifunctional protein comprised of discrete domains. J. Bacteriol. 186: 4110–4123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ramamurthi KS, Schneewind O. 2002. Type III protein secretion in Yersinia species. Annu. Rev. Cell Dev. Biol. 18: 107–133 [DOI] [PubMed] [Google Scholar]
- 38. Cornelis G, Vanootegem JC, Sluiters C. 1987. Transcription of the yop regulon from Y. enterocolitica requires trans acting pYV and chromosomal genes. Microb. Pathog. 2: 367–379 [DOI] [PubMed] [Google Scholar]
- 39. Michiels T, Wattiau P, Brasseur R, Ruysschaert JM, Cornelis G. 1990. Secretion of Yop proteins by yersiniae. Infect. Immun. 58: 2840–2849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Anderson DM, Ramamurthi KS, Tam C, Schneewind O. 2002. YopD and LcrH regulate the expression of Yersinia enterocolitica YopQ at a post-transcriptional step and bind to yopQ mRNA. J. Bacteriol. 184: 1287–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. DeBord K, Lee VT, Schneewind O. 2001. Roles of LcrG and LcrV during the type III targeting of effector Yops by Yersinia enterocolitica. J. Bacteriol. 183: 4588–4598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Quenee LE, Ciletti NA, Elli D, Hermanas T, Schneewind O. 2011. Prevention of pneumonic plague in mice, rats, guinea pigs and non-human primates with clinical grade rV10, rV10-2 or F1-V vaccines. Vaccine 29: 6572–6583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Broz P, Mueller CA, Muller SA, Phillipsen A, Sorg I, Engel A, Cornelis GR. 2007. Function and molecular architecture of the Yersinia injectisome tip complex. Mol. Microbiol. 65: 1311–1320 [DOI] [PubMed] [Google Scholar]
- 44. Quenee LE, Berube B, Segal J, Elli D, Ciletti NA, Anderson DM, Schneewind O. 2010. Amino acid residues 196–225 of LcrV represent a plague protective epitope. Vaccine 28: 1870–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Benner GE, Andrews GP, Byrne WR, Strachan SD, Sample AK, Heath DG, Friedlander AM. 1999. Immune response to Yersinia outer proteins and other Yersinia pestis antigens after experimental infection in mice. Infect. Immun. 67: 1922–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ivanov MI, Noel BL, Rampersaud R, Mena P, Benach JL, Bliska JB. 2008. Vaccination of mice with a Yop translocon complex elicits antibodies that are protective against infection with F1− Yersinia pestis. Infect. Immun. 76: 5181–5190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hamad MA, Nilles ML. 2007. Structure-function analysis of the C-terminal domain of LcrV from Yersinia pestis. J. Bacteriol. 189: 6734–6739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sato H, Hunt ML, Weiner JJ, Hansen AT, Frank DW. 2011. Modified needle-tip PcrV proteins reveal distinct phenotypes relevant to the control of type III secretion and intoxication by Pseudomonas aeruginosa. PLoS One 6: e18356 doi:10.1371/journal.pone.0018356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sato H, Frank DW. 2011. Multi-functional characteristics of the Pseudomonas aeruginosa type III needle-tip protein, PcrV; comparison to orthologs in other Gram-negative bacteria. Front. Microbiol. 2: 142 doi:10.3389/fmicb.2011.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee PC, Stopford CM, Svenson AG, Rietsch A. 2010. Control of effector export by the Pseudomonas aeruginosa type III secretion proteins PcrG and PcrV. Mol. Microbiol. 75: 924–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.