Abstract
Purpose.
To evaluate the regulatory cross-talk of the vascular and neural networks in the cornea.
Methods.
b-FGF micropellets (80 ng) were implanted in the temporal side of the cornea of healthy C57Bl/6 mice. On day 7, blood vessels (hemangiogenesis) and nerves were observed by immunofluorescence staining of corneal flat mounts. The next group of mice underwent either trigeminal stereotactic electrolysis (TSE), or sham operation, to ablate the ophthalmic branch of the trigeminal nerve. Blood vessel growth was detected by immunohistochemistry for PECAM-1 (CD31) following surgery. In another set of mice following TSE or sham operation, corneas were harvested for ELISA (VEGFR3 and pigment epithelium-derived factor [PEDF]) and for quantitative RT-PCR (VEGFR3, PEDF, and CD45). PEDF, VEGFR3, beta-3 tubulin, CD45, CD11b, and F4/80 expression in the cornea were evaluated using immunostaining.
Results.
No nerves were detected in the areas subject to corneal neovascularization, whereas they persisted in the areas that were neovessel-free. Conversely, 7 days after denervation, significant angiogenesis was detected in the cornea, and this was associated with a significant decrease in VEGFR3 (57.5% reduction, P = 0.001) and PEDF protein expression (64% reduction, P < 0.001). Immunostaining also showed reduced expression of VEGFR3 in the corneal epithelial layer. Finally, an inflammatory cell infiltrate, including macrophages, was observed.
Conclusion.
Our data suggest that sensory nerves and neovessels inhibit each other in the cornea. When vessel growth is stimulated, nerves disappear and, conversely, denervation induces angiogenesis. This phenomenon, here described in the eye, may have far-reaching implications in understanding angiogenesis.
In this study, we use two different animal models. First, we induce corneal neovascularization and notice that the development of vessels is followed by the disappearance of corneal nerves. Second, we induce loss of corneal nerves, which is followed by the development of neovessels.
Introduction
There are considerable similarities between the development of the vascular and nervous systems. At the anatomical level, the nerves and the vascular network course parallel to one another, often with a similar branching pattern. At the cellular level, both systems require precise control over their guidance and growth,1–6 which is regulated by several common molecules with attractive and repulsive properties, including the semaphorins, slits, netrins, and their receptors.7–10 Despite these similarities, the interactions of these two networks are not well understood, in particular in the central nervous system, where an intricate “barrier” appears to modulate the neuronal–blood vessel interaction. This barrier, although well known as the blood-brain-barrier, is not limited to brain and includes blood-cerebral, blood–spinal cord, and blood-retinal barriers. Interestingly, angiogenesis has been shown to contribute to epileptogenesis in experimental and human epilepsy.11–14 These facts raise the question regarding the functional interactions of these two networks.
The normal cornea is the most densely innervated tissue in the human body,15 yet devoid of blood vessels. However, many corneal pathological conditions, such as inflammatory disorders, alkali burns, corneal graft rejection, infectious keratitis, and limbal stem cell deficiency, would disrupt the avascular microenvironment and lead to corneal angiogenesis.16 Thus, there is a delicate balance between angiogenic (e.g., angiogenin,17 FGF,18 hepatocyte growth factor,19 VEGFs,20 and so forth) and antiangiogenic factors (e.g., angiostatin,21 endostatin,22 VEGF receptors,23 pigment epithelium-derived factor [PEDF],24 thrombospondin 1 and 225) in the cornea. Because corneal avascularity is sharply demarcated at the limbus (the border between the vascularized conjunctiva and avascular cornea) the “limbal barrier” has been identified as a critical feature of corneal avascularity.22–25 In fact, the concept of a limbal barrier is corroborated by the increase in corneal neovascularization seen in pathological limbal stem cell deficiency and experimental limbal damage,26 where conjunctival (vascular) tissue grows into the cornea.
Similar to the vascular system, which is prone to perturbations of the ocular surface, corneal nerves are also prone to injury in many pathological conditions, such as ocular infection, topical anesthetic abuse, surgery, diabetes, stroke, and dry eye syndrome.27–29 While pathological corneal vascularization and nerve loss are correlated through inflammation, the direct relationship between nerve loss and corneal vascularization has not been studied per se. Here we aimed to examine whether the loss of the nerve in the cornea could result in disruption of the corneal vascular privilege. The avascularity of the cornea, despite its extensive innervation, provides a unique in vivo milieu to interrogate the interaction of neuronal and vascular networks in different settings.
In this article, we demonstrate for the first time that corneal nerves and vessels inhibit each other in a tight spatial and temporal fashion. We show that this effect is, in part, mediated by alterations in angiostatic molecules, PEDF, and VEGFR3, constitutively expressed by the normal cornea.
Methods
Animals
Male 6- to 8-week-old C57Bl/6 mice (Taconic Farms, Germantown, NY) were used in all experiments. Animals were anesthetized by intraperitoneal injection of ketamine (120 mg/kg) and xylazine (20 mg/kg) before any surgery and were treated in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and approved by the Schepens Eye Research Institute Animal Care and Use Committee.
Pellet Implantation
b-FGF micropellets (80 ng/pellet) were prepared as described previously.30 Briefly, the pellets were implanted through half-thickness linear incisions at the center of cornea using a disposable 30° microknife (F.S.T., Foster City, CA). Lamellar pocket incisions were then made parallel to the corneal plane using a Von Graefe knife (F.S.T.) and advanced to the temporal limbus at lateral canthal area. The pellets were positioned into the pocket 1.0 mm apart from the limbal vascular arcade, and tetracycline ophthalmic ointment was applied to the eye after pellet implantation.
Trigeminal Nerve Ablation
Trigeminal nerve ablation was performed as described previously.31 This technique leads to extensive disruption and vacuolization of the corneal nerves, followed by the development of neurotrophic keratitis.31 Briefly, animals were anesthetized and a stereotactic frame was fixed to the animal head. A median incision was made on the skull. The bregma (the point of conjunction of coronal and sagittal sutures) was identified and chosen as a point of reference. The skull was opened with a dental drill, a conductive unimodal electrode (FHC, Bowdoin, ME) was lowered (depth: 0.63 cm) at three different locations (relative to bregma: 0.15 cm anterior and 0.08 cm lateral; 0.09 anterior and 0.10 lateral; 0.09 anterior and 0.12 lateral) on the ophthalmic trigeminal nerve, and a 2-mA current was passed for 15 seconds. The electrode was removed and the skin sutured. A lateral tarsorrhaphy was performed to reduce the risk of infection. Finally, antibiotic ointment (bacitracin-neomycin-polymicin) was applied to the sutural area and the treated eye. Buprenorphine (0.1 mg/kg body weight every 8–12 hours for 72 hours) was injected subcutaneously. Corneal sensitivity using a cotton filament was recorded pre- and postoperatively. The eyelids were closed with a suture tarsorrhaphy after the procedure and opened 7 days later.
Immunohistochemistry and Morphometry
After taking photographs under the slit lamp, five mice per group were killed 7 days after b-FGF micropellet implantation or trigeminal nerve ablation, and freshly enucleated eyes were prepared into corneal flat mounts. The following primary antibodies were used for immunohistochemical staining: FITC-conjugated rat anti-mouse CD45 (pan-leukocyte marker; BD Biosciences; San Diego, CA), FITC-conjugated rat anti-mouse CD11b (BD Biosciences), FITC-conjugated anti-mouse F4/80 (eBioscience, San Diego, CA), polyclonal rabbit anti-mouse FLT-4, M-20 (Santa Cruz Biotechnology, Santa Cruz, CA), and goat polyclonal IgG anti-PEDF (Santa Cruz Biotechnology). Corneal neovessels and nerves were immunostained with FITC-conjugated CD31/PECAM-1 (rat anti- mouse antibody; Santa Cruz Biotechnology) and with anti-3 tubulin primary antibody (rabbit anti–beta-3 tubulin polyclonal antibody, Chemicon, Temecula, CA). For whole-mount corneal staining, freshly excised corneas were washed in PBS and fixed in acetone for 15 minutes. Nonspecific staining was blocked with anti-FcR CD16/CD32 antibody (BD Biosciences). Specimens were immunostained with primary for 2 hours, washed with PBS, incubated with secondary antibody, and mounted with Vector Shield mounting medium (Vector Laboratories, Burlingame, CA). Corneas were analyzed using confocal microscopy (Leica TCS 4D; Leica, Heidelberg, Germany) at ×40 magnification. At least three to five different corneas were examined per each double-staining experiment; representative data are presented below (Fig. 1).
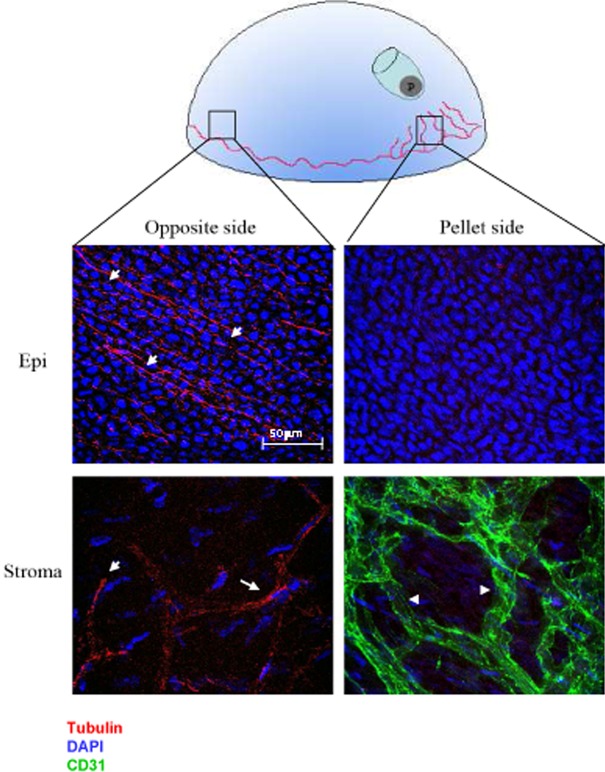
Figure 1. .
Growth of neovessels is associated with corneal nerve degeneration observed in both the epithelium and stroma. “P” = intracorneal pellet. Note that vessels are growing toward the pellet, whereas no vessels are visible opposite to the pellet. Beta-3 tubulin (red staining) reveals corneal nerves in the epithelium and stroma on the opposite side of the pellet (arrows). In contrast, adjacent to the pellet no nerves are detected, whereas PECAM staining (green) confirms the presence of neovessels (indicated by arrowheads).
Quantitative RT-PCR and ELISA
RNA was isolated from corneas by RNeasy Micro Kit (Qiagen, Valencia, CA) and reverse transcribed (Superscript III Kit; Invitrogen Life Technologies, Carlsbad, CA). Quantitative RT-PCR (qRT-PCR) was performed (TaqMan Universal PCR MasterMix; Applied Biosystems, Foster City, CA), and primers (Applied Biosystems) were preformulated for VEGFR-3 (assay ID: Mm00433337_ml), PEDF (assay ID: Mm 00441270_m1) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (assay ID: Mm99999915_gl), CD45 (assay ID: Mm00448490_m1). Results were derived from the comparative threshold cycle method and normalized by GAPDH as an internal control. Values obtained from normal corneas were then used to calculate relative mRNA expression for experimental corneas.
Levels of PEDF and ELISA in denervated and control corneas were analyzed using commercially available kits (MyBiosource, Inc., San Diego, CA) as per the manufacturer's instructions. Corneas were homogenized and diluted with RIPA buffer and Protease inhibition cocktail (Sigma, St. Louis, MO).
Statistical Analysis
Error bars displayed in the figures were calculated from the SEM (±SEM). Student's t-test was performed to compare the level of mRNA in qRT-PCR. P less than 0.05 was considered statistically significant. Results are presented as the mean ± SEM.
Results
Corneal Nerves Were Undetectable in the Area with Neovessel Formation
Corneas implanted with b-FGF pellets developed neovascularization toward the pellet site, as confirmed by slit lamp examination and immunostaining. Neovessels were evident histologically as a densely branched network (Fig. 1, right bottom panel). In the area where neovessels grew, both superficial intraepithelial and deeper stromal corneal nerves were entirely absent (right panels, Fig. 1). In contrast, in sectors of the cornea spared by neovascularization, abundant nerves could be detected, both in the epithelium and in the stroma, unchanged compared with the normal baseline (left panels, Fig. 1). Epithelial nerves appeared as a delicate and interconnected network, whereas stromal nerves were constituted by thicker trunks. These data suggest that corneal nerves are damaged and disappear following development of corneal neovascularization.
Corneal Vessels in the Denervation Model
As evaluated by immunohistochemistry, nerves could not be detected in the trigeminal stereotactic electrolysis (TSE)-treated (denervated) cornea, 7 days after TSE, in contrast to the normal contralateral eye and the sham-operated eye (Fig. 2A). Interestingly, 7 days following TSE-induced corneal denervation, neovessels were observed in the cornea by means of biomicroscopy (Fig. 2B). This was further confirmed by PECAM immunostaining (Fig. 2C), suggesting that corneal denervation is followed rapidly by corneal neovascularization.
Figure 2. .
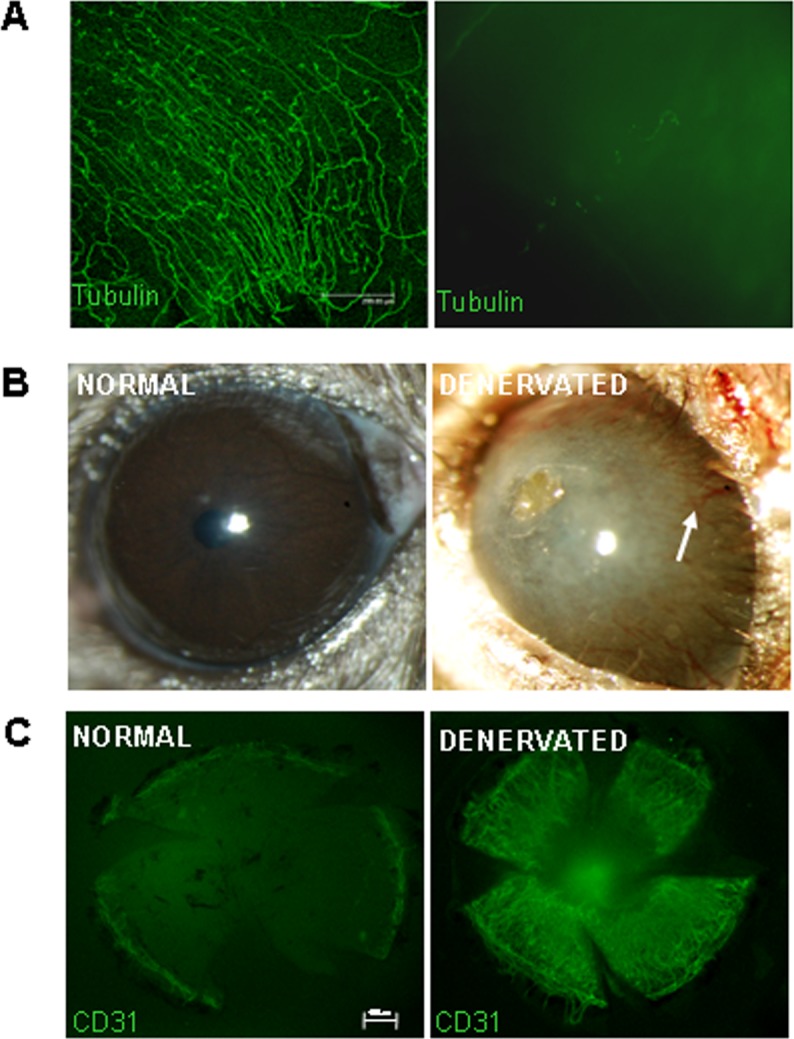
Corneal nerves and vessels inhibit each other in the cornea. (A) Corneal nerves are undetectable 48 hours following trigeminal stereotactic electrolysis (beta-3 tubulin staining), right: denervated eye; left: normal eye. (B) Neovascularization occurs 7 days after denervation in the cornea (right), in contrast to the normal cornea (left). (C) Immunostaining for PECAM showing growth of neovessels following denervation (right).
PEDF and VEGFR3 mRNA and Protein Expression Levels
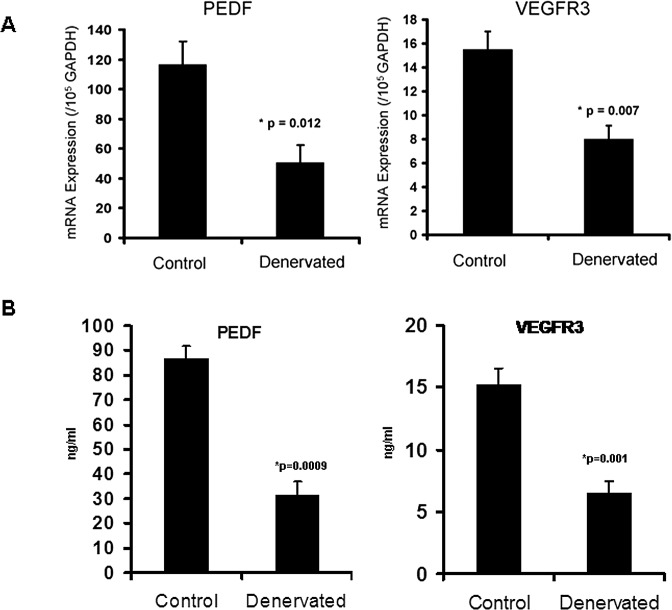
The expression of the angiostatic factors PEDF and VEGFR3, measured with real-time PCR, were both significantly reduced in the denervated compared with the normal corneas. PEDF expression was significantly reduced (56% reduction, P = 0.012). VEGFR3 expression was also significantly reduced after denervation (48% reduction, P = 0.007) (Fig. 3A). Protein levels of PEDF and VEGFR3 were also reduced after denervation (64%; P < 0.001 and 57.5%; P = 0.001) respectively (Fig. 3B).
Figure 3. .
Reduction of normally present angiostatic molecules following denervation of the cornea (A, mRNA expression; B, protein expression). PEDF and VEGFR3 are both significantly reduced 7 days after TSE. Bars indicate standard error.
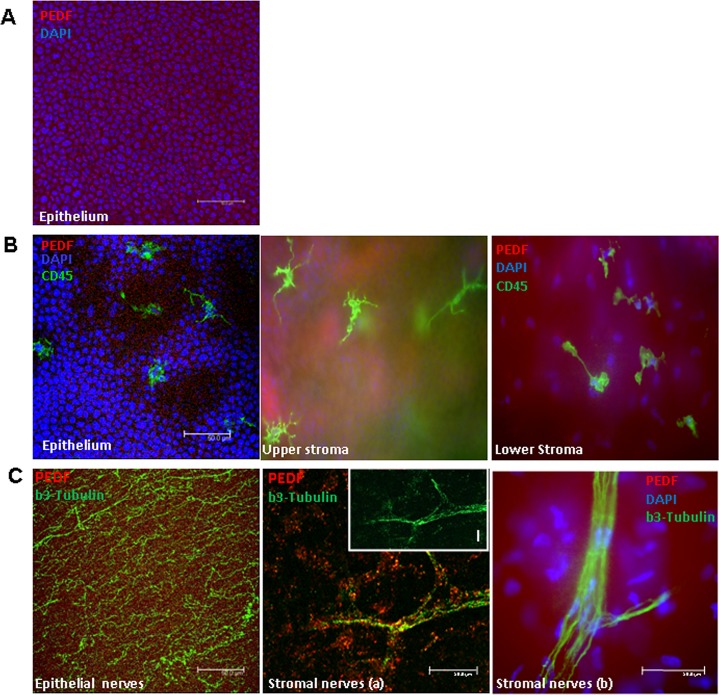
These data suggest that two major angiostatic mechanisms that are physiologically responsible for corneal avascularity are subverted following corneal denervation. The expression of CD45 receptor, a marker for inflammatory cell infiltration, was increased following corneal denervation when compared with normal controls (220 ± 110 vs. 111 ± 17; P < 0.05). Finally, PEDF immunostaining of corneal whole-mounts was robust in the corneal epithelium, sporadic in the stromal nerves, and absent in the epithelial nerves or CD45+ immune cells (Fig. 4), suggesting that epithelial cells and some of the stromal nerves are a source of PEDF.
Figure 4. .
Differential expression of PEDF, beta-3 tubulin, and CD45 in the corneal epithelium (A), stroma (B), and corneal nerves (C). (A) The normal corneal epithelium diffusely expresses PEDF (red). (B) Epithelial bone-marrow derived CD45+ cells (in green, arrows) do not double stain for PEDF (red). (C) Epithelial nerves, stained by anti-beta-3 tubulin (green), are not stained by anti-PEDF antibody. Stromal nerves co-stain for anti-PEDF antibody and anti–beta-3 tubulin in some areas (a, inset I), but not others (b).
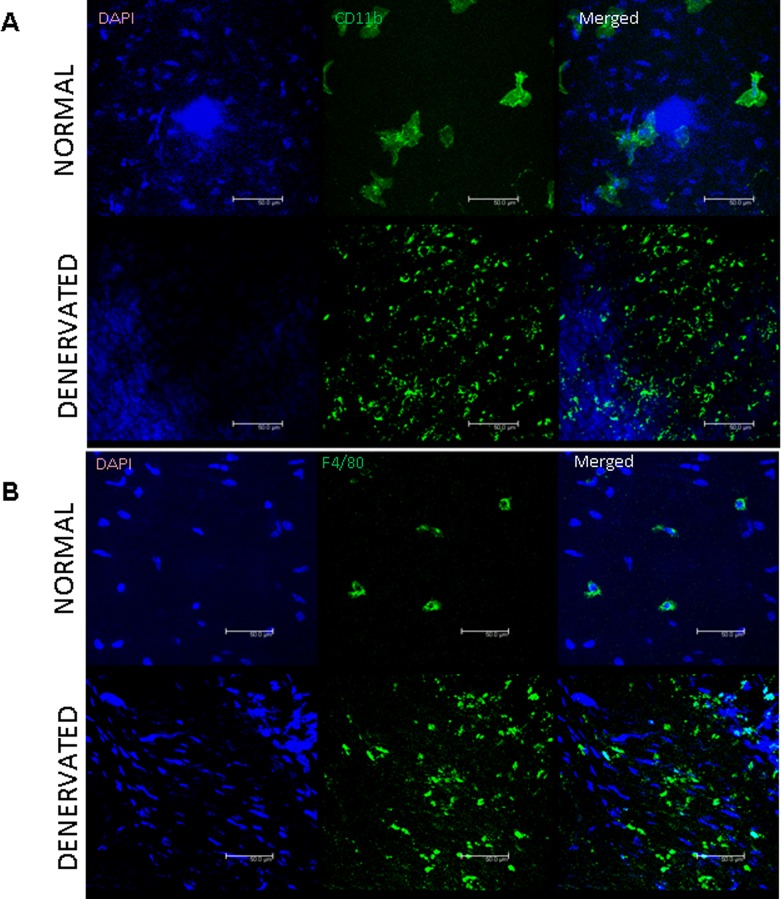
Immunostaining for VEGFR3, CD45, CD11b, and F4/80
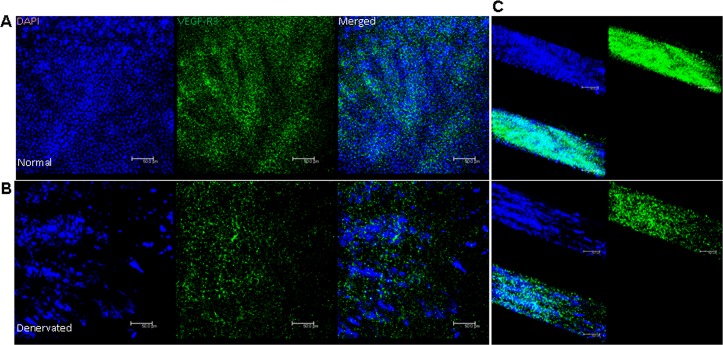
VEGFR3 expression was reduced in the epithelium following denervation (Figs. 5A, 5B; in X-Y plane). This was further confirmed by Z-plane stacking of all the scanned layers of epithelium, which revealed reduced signal intensity after TSE (Fig. 5C).
Figure 5. .
Confocal micrographs in X-Y plane showing that VEGFR3 expression is present in the normal corneal epithelium (A), and reduced following denervation (B). Z-plane stacking of all epithelial images in X-Y plane shows reduced expression in the denervated corneal epithelium (C).
Sporadic CD11b+ and F4/80+ cells were detected in normal corneas. Following denervation both markers appeared evidently increased in the corneal stroma (Fig. 6).
Figure 6. .
Characterization of inflammatory cell infiltrate following corneal denervation. Both CD11b (A, green) and F4/80 (B, green) expression is significantly increased in denervated corneas.
Discussion
The normal cornea is densely innervated (several hundred-fold higher than skin15), and completely avascular. The underlying reasons for corneal avascularity have been the subject of many studies and some controversy for decades. It has been shown that angiostatic molecules are constitutively expressed by the cornea, in particular the epithelium,23,32,33 and that limbal stem cells, together with the particular collagen ultrastructure of the cornea, may represent barriers to the ingrowth of neovessels.34,35 However, it has not been clear whether the dense innervation of the cornea is involved in the maintenance of avascularity. This question has been confounded by the fact that peripheral nerves are known to secrete neuropeptides, some of which promote angiogenesis (such as nerve growth factor36), while others are angiostatic (i.e., PEDF37).
Clinical observation provides some clues, in the form of corneal “neurotrophic keratopathy,” which is a disease characterized by disruption of corneal nerves. It is a frequent clinical observation that patients affected with this disease are more prone to developing corneal neovascularization. Moreover, patients affected by aniridia, a clinical condition caused by absence of the iris and other congenital ocular defects including reduced corneal nerve density,38,39 frequently present with corneal neovascularization.40
To test the hypothesis that vessels and nerves inhibit each other in the cornea, we used two different animal models. The first involved the use of proangiogenic pellets implanted in the cornea with the purpose of inducing sectoral corneal neovascularization so as to compare the effect of corneal neovascularization on distinct (vascularized and avascular) sections of the cornea. Interestingly, we found that both the superficial epithelial and deep stromal corneal nerves disappeared entirely in the sectors involved by neovessels, while persisting in the nonvascularized areas. b-FGF has been reported to have some neuroprotective effects under certain conditions of ischemia,41 despite our observation that high concentrations of b-FGF placed in the corneal micropocket led to complete corneal nerve depletion. One possibility to explain this is that infiltration of inflammatory cells induced by angiogenesis (in response to high local b-FGF levels) through the highly permeable “leaky” neovessel wall could directly induce local nerve loss. The relationship between inflammation and neural survival/death is complex, and is unlikely to be direct or linear. For example, interleukin-1 (IL-1) and b-FGF are increased significantly following tissue damage in the central nervous system,42,43 and although b-FGF has been implicated in neuroprotection in certain ischemia models, IL-1 has been shown to have detrimental effects in brain hypoxia.44 Another level of complexity is that different local concentrations of b-FGF and other cytokines that define the inflammatory tissue microenvironment may have very different effects on inducing nerve regeneration (versus death). In the aggregate, however, given the high density of nerves concurrent with the cornea's absolute avascularity in the normal state, and the vascularized cornea's association with near-total absence of nerves, suggests strongly that corneal innervation is a critical facet of its avascularity.
To formally test whether the ablation of corneal nerves could stimulate neovessel growth, we used a recently developed model of TSE, which we have demonstrated can induce both epithelial and stromal corneal nerve degeneration.15 As reduced blinking could lead to corneal infection and inflammation, potentially confounding factors, we performed a tarsorrhaphy (lid-closure procedure) and opened the eyes 7 days later. Just as we had found angiogenesis to lead to nerve loss, we found that denervation was associated with significant growth of neovessels in the cornea. Previous work has shown that removal of sympathetic nerves is associated with choroidal and retinal neovascularization.45 Similarly, ablation of peripheral dopaminergic nerves has been shown to stimulate angiogenesis in malignant tumors.46 However, to the best of our knowledge, there has been no report describing the influence of sensory innervation on ocular, including corneal, neovascularization thus far.
To better understand the mechanisms by which this regulation occurs, we hypothesized that the corneal epithelium, which is known for its angiostatic properties, such as constitutive expression of epithelial VEGFR3,3 may be altered after denervation. Ectopic expression of VEGFR3 by the corneal epithelium acts as a “sink” for VEGF ligands and hence contributes to preserve corneal avascularity.23 In this regard, we found that VEGFR3 protein expression was reduced by more than half following denervation. We also observed an evident reduction in the VEGFR3 with immunostaining. This supports the hypothesis that denervation induces a proangiogenic shift in the cornea. In addition, because inflammatory cells serve as key sources for proangiogenic cytokines, including VEGF, and thus serve as key stimulators of neovascularization,47,48 we measured the expression of CD45 (a receptor expressed broadly by bone marrow–derived leukocytes), and found it significantly increased following denervation. This is consistent with previous observations of CD45+ cell infiltration in the denervated corneas31 and was further confirmed by the increase of CD11b+ and F4/80+ cells following denervation. F4/80 is a marker for macrophages, which are known to be associated with corneal neovascularization.22
PEDF, a potent antiangiogenic molecule, has been shown to immunolocalize to the corneal epithelium and endothelium.49 It is expressed in a broad range of human fetal and adult tissues. Consistent with its angiostatic properties, PEDF-blocking antibodies implanted in the cornea facilitate corneal neovascularization.37 PEDF is the only molecule described so far in the cornea that presents both neurotrophic and angiostatic properties; hence, it is the ideal candidate for being involved in the “cross-talk” between corneal nerves and vessels. Accordingly, we hypothesized that denervation would cause a reduction in PEDF expression consistent with both nerve loss and resultant corneal angiogenesis. In accord with this hypothesis, we noted that after denervation, PEDF protein expression was significantly reduced (more than half), suggesting that suppressed PEDF expression could disinhibit angiogenesis and be (at least in part) responsible for the corneal neovascularization. To better delineate the cellular sources of PEDF in the cornea, we conducted experiments immunostaining for PEDF, CD45, and beta-3 tubulin. Our results demonstrate that corneal epithelium is the principal source of PEDF, supporting its angiostatic role for corneal stromal nerves through its constitutive PEDF expression. Stromal nerves were found to express PEDF sporadically. In contrast to the epithelium that contributes to corneal avascularity (its so-called “angiogenic privilege”), ample data suggest that CD45+ leukocytes that are mobilized in corneal inflammatory disorders, including the denervated cornea,31 stimulate angiogenesis through secretion of a broad array of proangiogenic cytokines, including VEGF-A.48
In summary, in this study, we provide direct evidence that corneal nerves and vessels inhibit one another, a cross-talk that our data suggest is mediated, at least in part, by the reduction of angiostatic molecules constitutively expressed in physiologic conditions by the cornea, including epithelial-derived PEDF and epithelial VEGFR3. It is conceivable that the inhibitory cross-regulation between the sensory nerves and angiogenesis, as described herein, may have implications beyond the cornea, such as inflammatory, and possibly neoplastic angiogenesis, in different tissues.
Footnotes
Supported by National Institutes of Health Grants EY-12963 and EY-20889, and the G.B. Bietti Eye Foundation, IRCCS, Rome, Italy.
These authors contributed equally to the work presented here and should therefore be regarded as equivalent authors.
Disclosure: G. Ferrari, None; A.R. Hajrasouliha, None; Z. Sadrai, None; H. Ueno, None; S.K. Chauhan, None; R. Dana, None
References
- 1. Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996; 274: 1123–1133 [DOI] [PubMed] [Google Scholar]
- 2. Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002; 298: 1959–1964 [DOI] [PubMed] [Google Scholar]
- 3. Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci. 2003; 26: 509–563 [DOI] [PubMed] [Google Scholar]
- 4. Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003; 161: 1163–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carmeliet P, Smet FD, Loges S, Mazzone M. Branching morphogenesis and antiangiogenesis candidates: tip cells lead the way. Na. Rev Clin Oncol. 2009; 6: 315–326 [DOI] [PubMed] [Google Scholar]
- 6. Dorrell MI, Aguilar E, Friedlander M. Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific R-cadherin adhesion. Invest Ophthalmol Vis Sci. 2002; 43: 3500–3510 [PubMed] [Google Scholar]
- 7. Suchting S, Bicknell R, Eichmann A. Neuronal clues to vascular guidance. Exp Cell Res. 2006; 312: 668–675 [DOI] [PubMed] [Google Scholar]
- 8. Larrivée B, Freitas C, Suchting S, Brunet I, Eichmann A. Guidance of vascular development: lessons from the nervous system. Circ Res. 2009; 104: 428–441 [DOI] [PubMed] [Google Scholar]
- 9. Jones CA, Li DY. Common cues regulate neural and vascular patterning. Curr Opin Genet Dev. 2007; 17: 332–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Desai BS, Monahan AJ, Carvey PM, Hendey B. Blood-brain barrier pathology in Alzheimer's and Parkinson's disease: implications for drug therapy. Cell Transplant. 2007; 16: 285–299 [DOI] [PubMed] [Google Scholar]
- 11. Torres-Vázquez J, Gitler AD, Fraser SD, et al. Semaphorin-plexin signaling guides patterning of the developing vasculature. Dev Cell. 2004; 7: 117–123 [DOI] [PubMed] [Google Scholar]
- 12. Friedman A, Kaufer D, Heinemann U. Blood-brain barrier breakdown-inducing astrocytic transformation: novel targets for the prevention of epilepsy. Epilepsy Res. 2009; 85: 142–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ndode-Ekane XE, Hayward N, Gröhn O, Pitkänen A. Vascular changes in epilepsy: functional consequences and association with network plasticity in pilocarpine-induced experimental epilepsy. Neuroscience. 2010; 166: 312–332 [DOI] [PubMed] [Google Scholar]
- 14. Tomkins O, Feintuch A, Benifla M, Cohen A, Friedman A, Shelef I. Blood-brain barrier breakdown following traumatic brain injury: a possible role in posttraumatic epilepsy. Cardiovasc Psychiatry Neurol. 2011; 2011: 765923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bonini S, Rama P, Olzi D, Lambiase A. Neurotrophic keratitis. Eye (Lond). 2003; 17: 989–995 [DOI] [PubMed] [Google Scholar]
- 16. Sellami D, Abid S, Bouaouaja G, Ben Amor S, Kammoun B, Masmoudi M, et al. Epidemiology and risk factors for corneal graft rejection. Transplant Proc. 2007; 38: 2609–2611 [DOI] [PubMed] [Google Scholar]
- 17. Hu G, Riordan JF, Vallee BL. Angiogenin promotes invasiveness of cultured endothelial cells by stimulation of cell-associated proteolytic activities. Proc Natl Acad Sci U S A. 1994; 91: 12096–12100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sunderkötter C, Roth J, Sorg C. Immunohistochemical detection of bFGF and TNF-alpha in the course of inflammatory angiogenesis in the mouse cornea. Am J Pathol. 1990; 137: 511–515 [PMC free article] [PubMed] [Google Scholar]
- 19. Xin X, Yang S, Ingle G, Zlot C, Rangell L, Kowalski J, et al. Hepatocyte growth factor enhances vascular endothelial growth factor-induced angiogenesis in vitro and in vivo. Am J Pathol. 2001; 158: 1111–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Amano S, Rohan R, Kuroki M, Tolentino M, Adamis AP. Requirement for vascular endothelial growth factor in wound- and inflammation-related corneal neovascularization. Invest Ophthalmol Vis Sci. 1998; 39: 18–22 [PubMed] [Google Scholar]
- 21. Ambati BK, Joussen AM, Ambati J, Moromizato Y, Guha C, Javaherian K, et al. Angiostatin inhibits and regresses corneal neovascularization. Arch Ophthalmol. 2002; 120: 1063–1068 [DOI] [PubMed] [Google Scholar]
- 22. Azar DT. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2006; 104: 264–302 [PMC free article] [PubMed] [Google Scholar]
- 23. Cursiefen C, Chen L, Saint-Geniez M, Hamrah P, Jin Y, Rashid S, et al. Nonvascular VEGF receptor 3 expression by corneal epithelium maintains avascularity and vision. Proc Natl Acad Sci U S A. 2006; 103: 11405–11410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abdiu O, Van Setten G. Antiangiogenic activity in tears: presence of pigment-epithelium-derived factor. New insights and preliminary results. Ophthalmic Res. 2008; 40: 16–18 [DOI] [PubMed] [Google Scholar]
- 25. Cursiefen C, Masli S, Ng TF, Dana MR, Bornstein P, Lawler J, et al. Roles of thrombospondin-1 and −2 in regulating corneal and iris angiogenesis. Invest Ophthalmol Vis Sci. 2004; 45: 1117–1124 [DOI] [PubMed] [Google Scholar]
- 26. Ashton N. Cornea vascularization. In: Duke-Elder S, Perkins ES. eds The Transparency of the Cornea. A Symposium. Oxford, UK: Blackwell Scientific Publications; 1960: 131–147 [Google Scholar]
- 27. Lim P, Fuchsluger TA, Jurkunas UV. Limbal stem cell deficiency and corneal neovascularization. Semin Ophthalmol. 2009; 24: 139–148 [DOI] [PubMed] [Google Scholar]
- 28. Epstein DL, Paton D. Keratitis from misuse of corneal anesthetics. N Engl J Med. 1968; 279: 396–399 [DOI] [PubMed] [Google Scholar]
- 29. Benitez del Castillo JM, Wasfy MA, Fernandez C, Garcia-Sanchez J. An in vivo confocal masked study on corneal epithelium and subbasal nerves in patients with dry eye. Invest Ophthal Vis Sci. 2004; 45: 3030–3035 [DOI] [PubMed] [Google Scholar]
- 30. Chung ES, Saban DR, Chauhan SK, Dana R. Regulation of blood vessel versus lymphatic vessel growth in the cornea. Invest Ophthalmol Vis Sci. 2009; 50: 1613–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ferrari G, Chauhan SK, Ueno H, Nallasamy N, Gandolfi S, Borges L, et al. A novel mouse model for neurotrophic keratopathy: trigeminal nerve stereotactic electrolysis through the brain. Invest Ophthalmol Vis Sci. 2011; 52: 2532–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Philipp W, Speicher L, Humpel C. Expression of vascular endothelial growth factor and its receptors in inflamed and vascularized human corneas. Invest Ophthalmol Vis Sci. 2000; 41: 2514–2522 [PubMed] [Google Scholar]
- 33. Singh N, Amin S, Richter E, Rashid S, Scoglietti V, Jani PD, et al. Flt-1 intraceptors inhibit hypoxia-induced VEGF expression in vitro and corneal neovascularization in vivo. Invest Ophthalmol Vis Sci. 2005; 46: 1647–1652 [DOI] [PubMed] [Google Scholar]
- 34. Huang AJ, Tseng SC. Corneal epithelial wound healing in the absence of limbal epithelium. Invest Ophthalmol Vis Sci. 1991; 32: 96–105 [PubMed] [Google Scholar]
- 35. Tseng SC. Concept and application of limbal stem cells. Eye (Lond). 1989; 3: 141–157 [DOI] [PubMed] [Google Scholar]
- 36. Lazarovici P, Gazit A, Staniszewska I, Marcinkiewicz C, Lelkes PI. Nerve growth factor (NGF) promotes angiogenesis in the quail chorioallantoic membrane. Endothelium. 2006; 13: 51–59 [DOI] [PubMed] [Google Scholar]
- 37. Meyer C, Notari L, Becerra SP. Mapping the type I collagen-binding site on pigment epithelium-derived factor. Implications for its antiangiogenic activity. J Biol Chem. 2002; 277: 45400–45407 [DOI] [PubMed] [Google Scholar]
- 38. Edén U, Riise R, Tornqvist K. Corneal involvement in congenital aniridia. Cornea. 2010; 29: 1096–1102 [DOI] [PubMed] [Google Scholar]
- 39. Leiper LJ, Ou J, Walczysko P. Control of patterns of corneal innervation by Pax6. Invest Ophthalmol Vis Sci. 2009; 50: 1122–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nishida K, Kinoshita S, Ohashi Y, Kuwayama Y, Yamamoto S. Ocular surface abnormalities in aniridia. Am J Ophthalmol. 1995; 120: 368–375 [DOI] [PubMed] [Google Scholar]
- 41. Alzheimer C, Werner S. Fibroblast growth factors and neuroprotection. Adv Exp Med Biol. 2002; 513: 335–351 [DOI] [PubMed] [Google Scholar]
- 42. Amantea D, Nappi G, Bernardi G, Bagetta G, Corasaniti MT. Post-ischemic brain damage: pathophysiology and role of inflammatory mediators. FEBS J. 2009; 276: 13–26 [DOI] [PubMed] [Google Scholar]
- 43. Albrecht PJ, Dahl JP, Stoltzfus OK, Levenson R, Levison SW. Ciliary neurotrophic factor activates spinal cord astrocytes, stimulating their production and release of FGF-2, to increase motor neuron survival. Exp Neurol. 2002; 173: 46–62 [DOI] [PubMed] [Google Scholar]
- 44. Basu A, Krady JK, Levison SW. Interleukin-1: a master regulator of neuroinflammation. J Neurosci Res. 2004; 78: 151–156 [DOI] [PubMed] [Google Scholar]
- 45. Steinle JJ, Pierce JD, Clancy RL, G Smith P. Increased ocular blood vessel numbers and sizes following chronic sympathectomy in rat. Exp Eye Res. 2002; 74: 761–768 [DOI] [PubMed] [Google Scholar]
- 46. Basu S, Sarkar C, Chakroborty D, Nagy J, Mitra RB, Dasgupta PS, et al. Ablation of peripheral dopaminergic nerves stimulates malignant tumor growth by inducing vascular permeability factor/vascular endothelial growth factor-mediated angiogenesis. Cancer Res. 2004; 64: 5551–5555 [DOI] [PubMed] [Google Scholar]
- 47. David Dong ZM, Aplin AC, Nicosia RF. Regulation of angiogenesis by macrophages, dendritic cells, and circulating myelomonocytic cells. Curr Pharm Des. 2009; 15: 365–379 [DOI] [PubMed] [Google Scholar]
- 48. Chung ES, Chauhan SK, Jin Y, Nakao S, Hafezi-Moghadam A, van Rooijen N, et al. Contribution of macrophages to angiogenesis induced by vascular endothelial growth factor receptor-3-specific ligands. Am J Pathol. 2009; 175: 1984–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Karakousis PC, John SK, Behling KC, Surace EM, Smith JE, Hendrickson A, et al. Localization of pigment epithelium derived factor (PEDF) in developing and adult human ocular tissues. Mol Vis. 2001; 7: 154–163 [PubMed] [Google Scholar]