Abstract
Purpose.
To investigate the association between myopia and the prevalence of glaucoma.
Methods.
This cross-sectional study included 5277 participants from the 2005 to 2008 National Health and Nutrition Examination Survey, greater than or equal to 40 years old, without history of cataract or refractive surgery, who underwent auto-refraction measurement. The predictor was refractive status; emmetropia (−0.99 to +0.99 diopters [D]), mild myopia (−1.00 to −2.99 D), moderate myopia (−3.00 to −5.99 D), severe myopia (> −6.00 D), and hyperopia (> 1.00 D). The outcomes were self-reported glaucoma, vertical cup-to-disc ratio and visual field defects as found on frequency doubling technology (FDT) testing
Results.
Odds of self-reported glaucoma were not significantly increased in mild (odds ratio [OR] 0.90, confidence interval [CI] 0.56–1.45), moderate (OR 1.40, CI 0.62–3.16), or severe (OR 0.26, CI 0.08–0.80) myopes compared with emmetropes. Odds of vertical cup-to-disc ratio greater than or equal to 0.7 were not significantly increased in mild (OR 0.84, CI 0.31–2.25), moderate (OR 0.37, CI 0.04–3.57), or severe (OR 0.85, CI 0.09–8.42) myopes compared with emmetropes. Odds of any visual field defects were significantly increased in mild (OR 2.02, CI 1.28–3.19), moderate (OR 3.09, CI 1.42–6.72), and severe (OR 14.43, CI 5.13–40.61) myopes compared with emmetropes. The χ2 test indicated a significant difference (P = 0.001) in the distribution of subjects with each category of visual field status across subjects with each refractive status; the proportion of subjects with worse visual field defects increased with worsening myopia severity.
Conclusions.
The association between myopia and visual field defects may represent an increased risk of glaucoma among myopes, and the lack of association with self-reported glaucoma may suggest a need for greater glaucoma surveillance in this population.
This study found an association between myopia and visual field defects, which may represent an increased risk of glaucoma among myopes, and the lack of association between myopia and self-reported glaucoma may suggest a need for greater glaucoma surveillance in this population.
Introduction
Glaucoma, the leading cause of irreversible blindness worldwide,1 can adversely impact quality of life for patients with visual field defects even if they are unaware of their diagnosis.2–4 In many populations, POAG is the most common form of the disease.5–9 The only known modifiable risk factor for POAG is IOP, and the goal of treatment is to lower this parameter in afflicted individuals. To improve screening in individuals at high risk for glaucomatous disease, the Centers for Medicare and Medicaid initiated glaucoma screening coverage for beneficiaries with diabetes mellitus, those with a family history of glaucoma, African Americans aged 50 years or older, and Hispanic Americans aged 65 years or older.10 The underlying pathogenesis of glaucoma is not completely understood and is likely influenced by both genetic and environmental factors,11–13 so the identification of other risk factors may allow for earlier and more aggressive screening targeted toward susceptible populations as well as shed light on the pathophysiology of this disease.
Myopia has become increasingly prevalent worldwide over the past century.14 The association between myopia and POAG has been recognized for decades15–17 and has been documented in many case series18–23 and case-control studies.24,25 The underlying hypothesis explaining this association is that individuals with axial myopia have weaker scleral support at the optic nerve, and this contributes to a greater susceptibility of the optic nerve to glaucomatous damage.10 More recently, large cross-sectional epidemiologic studies in Australia, China, Japan, India, and the Netherlands also suggest that individuals with myopia have a higher prevalence of open-angle glaucoma than those without myopia.26–30
In the United States (US), there have been similar studies about the association between myopia and glaucoma in certain ethnic or geographic groups31,32 However, to date, there are no large cross-sectional epidemiologic studies of a representative sample of the entire US population investigating the importance of myopia as a risk factor for glaucoma, the results of which might have potential implications for glaucoma screening guidelines. The purpose of this study is to characterize the rates of self-reported glaucoma, glaucomatous optic nerve damage, and visual field defects in individuals with mild myopia, moderate myopia, severe myopia, and hyperopia, compared with those with emmetropia, using data from the National Health and Nutrition Examination Survey (NHANES),32 a large population-based survey conducted annually in the US.
Methods
Sample and Population
We used data from the 2005 to 2008 NHANES,29 a cross-sectional series of interviews and examinations of the civilian, noninstitutionalized US population administered by the Centers for Disease Control. The purpose of NHANES is to provide US health statistics, and the de-identified data are made publically available. NHANES uses a stratified multistage sampling design with a weighting scheme to most accurately estimate disease prevalence in the US population.
Our analysis included all NHANES participants between the years 2005 to 2008 aged 40 years and older who had an autorefraction reading in the right eye and had not previously undergone cataract or refractive surgery in that eye (n = 5277). Two variables that we did not include in the presented analysis were a history of retinal detachment and a self-reported diagnosis of macular degeneration, since information pertaining to the former was not available in NHANES and correction for the latter did not alter our findings. For the purpose of internal validation, we also repeated all of our analyses for subjects meeting the same inclusion criteria for the left eye (n = 5247), and since the results for the left eye were not substantially different from those found for the right eye, only the results of the right eye are reported here.
Measures
Predictor.
The primary predictor variable was refractive error as measured by the Nidek Auto Refractor Model ARK-760 instrument (Nidek Co., Ltd., Gamagori, Japan). Trained technicians took three measurements from each eye and the final result was the median of the three measurements. The spherical equivalent of each eye was calculated by taking the sum of the spherical power and half of the cylindrical power.
While there is no universal consensus for defining myopia severity, we opted, with some modification, to base our myopia categories on those employed in the Blue Mountains Eye Study (BMES), a large population-based study that assessed the association between myopia and glaucoma in Australia.26 The BMES criteria for refractive error were selected because the subjects in the BMES were mostly Caucasian, a feature shared with the NHANES study population.
We defined emmetropia (−0.99 to +0.99 diopters [D]), mild myopia (−1.00 to −2.99 D), and hyperopia (> +1.00 D) based upon the BMES criteria. We modified the BMES category of moderate to severe myopia (> −3.00 D) by further subdividing it into moderate myopia (−3.00 to −5.99 D) and severe myopia (>−6.00D), using cutoffs established in the Beijing Eye Study (BES).27
As a sensitivity analysis, we also repeated all of our analyses using different refractive error criteria modified from the BES. We used the BES criteria for emmetropia (−0.49 to +1.99 D), hyperopia (> +2.00 D), mild myopia (−0.50 to −2.99 D), and moderate myopia (−3.00 to −5.99 D), but combined their marked myopia and high myopia categories into a severe myopia (> −6.00 D) category, because the BES did not find differences between marked and high myopes with regard to glaucoma risk. Since our results using the modified BES criteria were not substantially different from those found using the modified BMES criteria, only the results from the modified BMES criteria are reported here.
Potential confounders in our analysis included age, sex, ethnicity, annual household income, and education level.
Outcomes.
The primary outcome variable was self-reported glaucoma, as determined by the answer to the survey question: “Have you ever been told by an eye doctor that you have glaucoma, sometimes called high pressure in your eyes?” Secondary outcomes included vertical cup-to-disc ratio and visual field defects. The vertical cup-to-disc ratio was graded from fundus photographs taken with a Canon Non-Mydriatic Retinal Camera CR6-45NM (Canon, Tokyo, Japan). The vertical cup-to-disc ratio was used as a continuous variable and also as a categorical variable in which a value of greater or equal to 0.7 was considered abnormal. The presence of any visual field defects was determined with a 19-point suprathreshold screening test using N-30-5 frequency doubling technology (FDT). Each subject underwent two FDT visual field tests per eye. In NHANES, abnormal FDT status was defined by a 2-2-1 algorithm: at least 2 test points in the first test below the 1% threshold level, and at least 2 test points in the second test below the 1% threshold level, and at least 1 failed test point in the same location on both tests. Examinations were considered unreliable if either of the 2 tests on each eye had at least 2 out of 3 false-positive or fixation errors, or the technician supervising the test noted lack of fixation.33 The NHANES 2-2-1 algorithm for FDT N-30-5 had a previously demonstrated sensitivity of 54.8% and specificity of 91.9% in detecting subjects with glaucoma.34 In addition, we further stratified results of the first visual field test administered for each eye into normal, early, moderate, or severe visual field defects based on the clinical classification scheme previously published and validated against the Glaucoma Staging System, which showed a Cohen Kappa agreement of 0.679 and specificity of 95%.14 The classification of severe glaucoma was slightly modified for our study and defined as more than nine P values less than 1% defects (same as the original criteria), or more than 12 abnormal points with more than six P values less than 1% defects (modified from the original criteria where the cutoff was 0.5% rather than our 1%). This slight modification was necessary due to lack of P values less than 0.5% threshold data in the NHANES dataset.
Data Analysis
We compared the distribution of possible confounding variables between subjects with emmetropia, mild myopia, moderate myopia, severe myopia, and hyperopia using design-adjusted Rao-Scott Pearson-type χ2 and Wald tests for categorical and continuous variables, respectively. Multivariate logistic regression models were created to examine the independent association between refractive status and the binary outcome variables self-reported glaucoma, any visual field defects, and optic nerve vertical cup-to-disc ratio greater than or equal to 0.7, while adjusting for potential confounders. Odds ratios (OR) and 95% confidence intervals (CI) were calculated. A multivariate linear regression model was created to examine the independent association between refractive error and vertical cup-to-disc ratio as continuous numerical variables, while adjusting for potential confounders; subjects with hyperopia were excluded. We also performed a design-adjusted Rao-Scott Pearson-type χ2 test to determine if subjects with normal visual fields, early visual field defects, moderate visual field defects, and severe visual defects were distributed differently across subjects with emmetropia, mild myopia, moderate myopia, severe myopia, and hyperopia. To most accurately calculate CIs around estimates for the US national population, we performed all data analyses (Stata 12.0; Stata Statistical Software, College Station, TX) using weighted data, and SEs of population estimates were calculated by Taylor linearization methods.
Results
Population Characteristics
The 2005 to 2008 NHANES data yielded 5277 participants aged 40 years and older who had an autorefraction measurement in the right eye and had not previously undergone cataract or refractive surgery in that eye. Of these participants, 2638 (50.0%) had emmetropia, 782 (14.8%) had mild myopia, 341 (6.5%) had moderate myopia, 118 (2.2%) had severe myopia, and 1398 (26.5%) had hyperopia.
Table 1 presents demographic characteristics regarding those with emmetropia, mild myopia, moderate myopia, severe myopia, and hyperopia. There were substantial differences in the frequencies of all of the listed demographic parameters between these five subgroups. The mean age of subjects was lower amongst those with greater myopia, and the mean age was higher in subjects with hyperopia, compared with those with emmetropia. Worse myopia was also associated with female sex, non-Hispanic, white race, college graduation and an annual household income greater than $75,000.
Table 1. .
Demographic Characteristics of Subjects with Emmetropia, Mild Myopia, Moderate Myopia, Severe Myopia, or Hyperopia
|
|
Emmetropia, Mean† or % (SE)* |
Mild Myopia* % Mean† or % (SE)* |
Moderate Myopia* Mean† or % (SE)* |
Severe Myopia* Mean† or % (SE)* |
Hyperopia* Mean† or % (SE)* |
P
Value‡ |
| Number of subjects, N (%) | 2638 (50.0%) | 782 (14.8%) | 341 (6.5%) | 118 (2.2%) | 1398 (26.5%) | |
| Age, y | 53.1 (0.33) | 53.5 (0.52) | 52.2 (0.50) | 50.3 (0.86) | 62.4 (0.61) | <0.0001 |
| Female | 47.4 (1.2) | 49.7 (2.0) | 60.9 (3.5) | 61.5 (5.8) | 51.6 (0.7) | 0.0001 |
| Race | 0.0047 | |||||
| Mexican | 7.3 (0.9) | 4.5 (0.9) | 2.5 (0.4) | 3.2 (0.9) | 5.4 (1.1) | |
| Other Hispanic | 3.5 (0.8) | 2.8 (0.7) | 2.1 (0.9) | 1.5 (0.7) | 4.3 (1.0) | |
| Non-Hispanic white | 71.8 (2.3) | 76.9 (2.5) | 85.2 (2.3) | 82.9 (4.0) | 75.3 (3.2) | |
| Non-Hispanic black | 12.0 (1.6) | 11.3 (1.5) | 7.8 (1.5) | 7.6 (2.2) | 9.0 (1.5) | |
| Other and multiracial | 5.4 (0.7) | 4.5 (1.5) | 2.4 (1.1) | 4.9 (2.5) | 6.0 (1.5) | |
| Education | <0.0001 | |||||
| < Ninth grade | 6.9 (0.6) | 4.3 (0.8) | 2.7 (0.8) | 0.4 (0.3) | 11.7 (1.6) | |
| Ninth grade to less than HS graduate | 12.4 (1.2) | 9.8 (1.3) | 6.6 (2.1) | 3.5 (1.9) | 12.5 (1.1) | |
| HS graduate or GED equivalent | 27.2 (1.2) | 25.2 (2.2) | 19.0 (2.5) | 13.3 (4.6) | 28.7 (1.7) | |
| Some college | 29.5 (1.5) | 28.3 (2.5) | 30.5 (3.2) | 27.0 (4.7) | 26.8 (1.6) | |
| College graduate and beyond | 23.9 (1.7) | 32.4 (2.5) | 41.2 (3.3) | 55.8 (7.8) | 20.3 (1.9) | |
| Annual household income, $ | <0.0001 | |||||
| < 20K | 13.7 (1.1) | 11.7 (1.3) | 11.0 (2.1) | 4.2 (1.5) | 18.0 (1.4) | |
| 20K–44,999 | 26.1 (1.6) | 24.7 (1.9) | 19.8 (2.5) | 12.5 (2.8) | 30.6 (1.8) | |
| 45K–74,999 | 22.5 (1.2) | 21.5 (2.3) | 24.2 (2.6) | 34.6 (5.0) | 24.1 (1.6) | |
| 75K up | 35.3 (2.1) | 39.9 (3.0) | 43.2 (3.4) | 45.2 (5.5) | 24.5 (2.0) | |
| > 20K§ | 2.5 (0.5) | 2.2 (0.6) | 1.7 (0.8) | 3.5 (1.9) | 2.7 (0.7) |
Emmetropia (−0.99 to +0.99 D), mild myopia (−1.00 to −2.99 D), moderate myopia (−3.00 to −5.99 D), severe myopia (> −6.00 D), hyperopia (> +1.00 D).
All means, proportions, and SEs are weighted estimates of the US population characteristics, taking into account NHANES' complex sampling design.
All P values are unadjusted. P values were calculated using Wald test for continuous variables and design-adjusted Rao-Scott Pearson χ2 test for categorical variables.
Participants who were unable to provide a more specific annual household income were asked to indicate whether the household income exceeded $20,000 per year.
Glaucoma Outcome Variables
Table 2 presents rates of self-reported glaucoma, vertical cup-to-disc-ratio greater than or equal to 0.7 and any visual field defects in subjects within each category of refractive error, unadjusted and adjusted for potential confounders.
Table 2. .
Odds Ratios and 95% Confidence Intervals for Self-Reported Glaucoma, Vertical Cup-to-Disc Ratio Greater than or Equal to 0.7, and Any Visual Field Defect Among Subjects with Mild Myopia, Moderate Myopia, Severe Myopia, and Hyperopia Compared with Subjects with Emmetropia, Unadjusted and Adjusted for Potential Confounders
|
|
Emmetropia* |
Mild Myopia* |
Moderate Myopia* |
Severe Myopia* |
Hyperopia* |
| Self-reported glaucoma | |||||
| Unadjusted | 1.00 (reference) | 0.88 (0.58–1.35) | 1.10 (0.50–2.45) | 0.18 (0.05–0.58) | 1.89 (1.19–3.01) |
| Adjusted† | 1.00 (reference) | 0.90 (0.56–1.45) | 1.40 (0.62–3.16) | 0.26 (0.08–0.80) | 1.17 (0.76–1.79) |
| Cup-to-disc ratio ≥ 0.7 | |||||
| Unadjusted | 1.00 (reference) | 0.91 (0.38–2.21) | 0.24 (0.03–2.06) | 0.38 (0.06–2.35) | 0.52 (0.20–1.40) |
| Adjusted† | 1.00 (reference) | 0.84 (0.31–2.25) | 0.37 (0.04–3.57) | 0.85 (0.09–8.42) | 0.30 (0.12–0.74) |
| Any visual field defect | |||||
| Unadjusted | 1.00 (reference) | 1.86 (1.21–2.87) | 2.13 (1.03–4.39) | 6.76 (3.05–14.97) | 1.78 (1.09–2.92) |
| Adjusted† | 1.00 (reference) | 2.02 (1.28–3.19) | 3.09 (1.42–6.72) | 14.43 (5.13–40.61) | 1.02 (0.58–1.81) |
* Emmetropia (−0.99 to +0.99 D), mild myopia (−1.00 to −2.99 D), moderate myopia (−3.00 to −5.99 D), severe myopia (> −6.00 D), hyperopia (> +1.00 D).
Confounders include age, sex, ethnicity, income, and education.
Self-Reported Glaucoma.
The adjusted odds of self-reported glaucoma were not significantly increased in subjects with mild myopia (OR 0.90, 95% CI 0.56–1.45), moderate myopia (OR 1.40, 95% CI 0.62–3.16), severe myopia (OR 0.26, 95% CI 0.08–0.80), or hyperopia (OR 1.17, 95% CI 0.76–1.79) compared with those with emmetropia.
Cup-to-Disc Ratio.
The adjusted odds of vertical cup-to-disc ratio greater than or equal to 0.7 were not significantly increased in subjects with mild myopia (OR 0.84, 95% CI 0.31–2.25), moderate myopia (OR 0.37, 95% CI 0.04–3.57), severe myopia (OR 0.85, 95% CI 0.09–8.42), or hyperopia (OR 0.30, 95% CI 0.12–0.74) compared with those with emmetropia. Multivariate linear regression, which excluded subjects with hyperopia and adjusted for potential confounders did not yield a statistically significant association between refractive error and vertical cup-to-disc ratio (β = 0.0007, P = 0.38).
Visual Field Defect.
The adjusted odds of having any visual field abnormality were significantly increased in subjects with mild myopia (OR 2.02, 95% CI 1.28–3.19), moderate myopia (OR 3.09, 95% CI 1.42–6.72), and severe myopia (OR 14.43, 95% CI 5.13–40.61), but not in those with hyperopia (OR 1.02, 95% CI 0.58–1.81) compared with those with emmetropia.
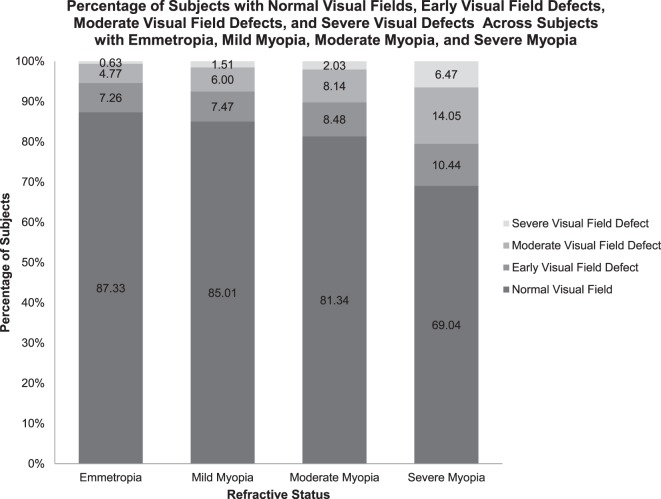
Refractive status was also evaluated with respect to the severity of visual field defects. The Figure presents the proportions of subjects with each category of visual field abnormality amongst subjects with each category of refractive error. The χ2 test indicated a significant difference (P = 0.001) in the distribution of subjects with normal visual fields, early visual field defects, moderate visual field defects, and severe visual defects across subjects with emmetropia, mild myopia, moderate myopia, severe myopia, and hyperopia. The proportion of subjects with moderate and severe visual field defects increased with increasing severity of myopia.
>Figure. .
The percentage of subjects with worse visual field defects increases with increasing severity of myopia.
Discussion
This study of a population-based sample of adults in the US aged 40 years and older found an association between myopia and visual field defects, but failed to find an association between myopia and self-reported glaucoma or vertical cup-to-disc ratio. In subjects with mild, moderate, and severe myopia, the odds of having any visual field defect were increased approximately 2-fold, 3-fold, and 14-fold, respectively, compared with subjects with emmetropia. This pattern suggests an exponential rather than a linear relationship between myopia and visual field defects. In addition, when visual field defects were further categorized into early, moderate, and severe, a pattern emerged suggesting an exponentially increasing proportion of worse visual field defects among subjects with greater myopia.
Our results support findings from previous large population-based studies on myopia and glaucoma. The BMES in Australia found an association between all categories of myopia and glaucoma diagnosed by the presence of matching optic disc cupping with rim thinning (cup-to-disc ratio ≥ 0.7, or cup-to-disc asymmetry ≥ 0.3) and characteristic visual field loss on automated perimetry.27 The Aravind Comprehensive Eye Survey in India and the Tajimi Study in Japan also found an association between myopia greater than −1 D and POAG diagnosed by a comprehensive ophthalmologic exam.28,29 The BES in China found an association between severe myopia greater than −6 D and glaucomatous optic nerve appearance, visual field defects, and elevated IOP.27 The Rotterdam Eye Study in the Netherlands found an association between high myopia greater than −4 D and glaucomatous visual field loss.30 The Los Angeles Latino Eye Study of a Latino population in the US found an association between axial myopia and open angle glaucoma defined by the presence of an open angle and a glaucomatous visual field abnormality and/or evidence of glaucomatous optic nerve damage in at least one eye, particularly in individuals with elevated IOP.31 Our study of a representative sample of the entire US population also found an association between myopia and visual field defects, but not between myopia and self-reported glaucoma or vertical cup to disc ratio greater than or equal to 0.7 with the latter being a possible surrogate for glaucomatous disease; our database did not have information about subjects' IOP.
There are several potential explanations for our findings, including the known possibility that myopia can cause visual field defects independent of those found in glaucomatous disease. Myopia is a known risk factor for numerous retinal diseases, and one significant limitation of our study was the lack of data regarding prior history of retinal detachment. We did, however, repeat our analyses excluding subjects with self-reported macular degeneration and found no substantial difference in our results.
Another factor that may have influenced our results and conclusions is that glaucoma is known to be underdiagnosed both in the developed and developing world. Large population based glaucoma prevalence studies in the United States have estimated that 50% to 75% of Americans are unaware that they have glaucomatous disease.5 Mild to moderate POAG is commonly asymptomatic to patients, even in the presence of visual field defects on exam. It is certainly possible that some subjects in the NHANES population with glaucomatous disease were unaware of their diagnosis and that their visual field abnormality is a more sensitive indicator of the disease than a self-reported diagnosis. Given that glaucoma screening is more common amongst the elderly and myopia is more common amongst the young, the possibility of a systematic ascertainment error or bias is a strong possibility. It is noteworthy, however, that the prevalence of glaucoma found in this study is consistent with that reported in previous studies. Under ideal circumstances, all subjects would have received a complete ophthalmologic examination to determine whether or not glaucoma was present. The lack of such testing also leaves open the possibility that some subjects with self-reported glaucoma were in fact glaucoma suspects or ocular hypertensives. However, it has recently been shown that a self-reported diagnosis of glaucoma, while not a highly sensitive ascertainment method, is quite specific amongst those who state that they have the disease.33
A less likely misclassification error that might explain our findings would be a scenario where subjects with myopia, who often have optic nerves that are difficult to assess with regard to glaucoma, were told that they did not have glaucoma when in fact they did. Given that such patients are often labeled as glaucoma suspects, and that the self-reporting in NHANES would have likely identified such individuals, such a misclassification is unlikely to have been significant in our study.
Another potential limitation of our study is that vertical cup-to-disc ratio may not be an appropriate surrogate measure of glaucomatous disease. This parameter can be especially difficult to judge in myopic subjects, as they are more likely to have tilted optic nerves that are anatomically abnormal.35 Additionally, subjects with high myopia have larger optic nerve heads than subjects with emmetropia,36 which may alter the threshold at which the vertical cup-to-disc ratio should be considered abnormal. Lastly, the range of normal optic disc sizes and cup-to-disc ratios vary among ethnicities37,38 and among individuals, so a numerical threshold value for abnormal cup-to-disc ratio may not be a good proxy for assessing glaucoma risk in a study which includes a diverse, nationally representative population.
Another potential bias in our study could be a nondifferential recall and misclassification with regard to glaucoma diagnosis among subjects with various categories of refractive status, which would most likely result in bias toward the null. In this situation, there would be an underestimation of the strength of the relationship between refractive error and glaucoma diagnosis, a possible explanation for the lack of association between refractive status and self-reported glaucoma in our study.
In addition, rather than using standard automated perimetry for visual field testing, NHANES used a FDT N-30-5 screening protocol with a 2-2-1 scoring algorithm for visual field loss that is highly specific (91.9%) for detecting glaucomatous disease,33 but is unlikely to be the ideal tool for measuring glaucoma severity or progression. One study that compared FDT with standard automated perimetry found the test–retest variability of FDT to be favorable with regard to uniformity over the entire measurement range of the instrument with less variability in the areas of visual field damage.34 While FDT may be as good or better for accurate detection of early glaucomatous disease relative to standard automated perimetry, the latter test is the gold standard for determining disease severity and progression of existing field defects. NHANES used a strict 2-2-1 algorithm that required abnormal results on two FDT exams for visual field defect confirmation. This stricter algorithm is associated with lower sensitivity but greater specificity in detecting subjects with glaucoma relative to single FDT testing. Using this strict 2-2-1 algorithm with a low sensitivity and high specificity, the subjects who were identified as being abnormal were more likely to have glaucomatous visual field loss relative to other algorithms that employ single FDT testing. We have no reason to believe standard automated perimetry or FDT visual field testing would perform differentially based upon refractive status and, thus, expect this underestimation to be nondifferential and bias our results toward the null. Despite this potential bias toward the null, the ORs for any visual field defects was still statistically significantly higher in each myopia group compared with the emmetropia group.
Furthermore, these study results may not reflect associations in smaller ethnic groups such as Asians and other minorities that were not oversampled in NHANES, as were Mexican Americans and Hispanics. Our study population did not have a large enough sample of subjects in each myopia category within each ethnicity category in order to perform sub analyses on each subgroup.
While our study suggests an association between myopia and visual field abnormalities, much additional research is needed before we could advocate for modifying the national screening guidelines to include more aggressive glaucoma screening for individuals with myopia. Since our study is a cross-sectional population-based study, we cannot draw any conclusions about the mechanism by which myopia may cause visual field defects, or whether these defects may eventually progress to glaucoma. Furthermore, we cannot draw any conclusions about the direction of causation, although it would be very unlikely that visual field defects could lead to worsening myopia given what is known about the pathophysiology of these conditions.
In summary, we found that after adjusting for confounding demographic factors, myopia was associated with significantly greater odds of visual field defects compared with emmetropia, but myopia was not associated with a self-reported diagnosis of glaucoma or abnormally elevated vertical cup-to-disc-ratio in a large population-based representative health survey in the US. There was a seemingly exponential relationship between the degree of myopia and the severity of visual field abnormalities. The more than 14-fold greater prevalence of visual field defects among subjects with severe myopia compared with emmetropia suggests an important association that warrants further study. The lack of an association between myopia and glaucoma in this study may reflect the limitations in ascertainment of glaucoma via self-report and reliance on surrogate parameters such as vertical cup-to-disc ratio, and other large population-based studies have confirmed such a relationship in certain specific populations. In addition to epidemiologic confirmation of our findings, further research is needed to elucidate the potential mechanisms by which myopia may cause visual field defects, and to characterize the progression of such defects, particularly in comparison with defects that result from glaucomatous disease.
Footnotes
Supported by grants from the National Eye Institute (EY002162), That Man May See, Inc., Research to Prevent Blindness, and National Center for Advancing Translational Sciences, National Institutes of Health, through University of California-San Francisco Clinical and Translational Science Institute (TL1 TR000144).
Disclosure: M. Qiu, None; S.Y. Wang, None; K. Singh, None; S.C. Lin, None
References
- 1. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006; 90: 262–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Varma R, Lee PP, Goldberg I, Kotak S. An assessment of the health and economic burdens of glaucoma. Am J Ophthalmol. 2011; 152: 515–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nelson P, Aspinall P, Papasouliotis O, Worton B, O'Brien C. Quality of life in glaucoma and its relationship with visual function. J Glaucoma. 2003; 12: 139–150 [DOI] [PubMed] [Google Scholar]
- 4. McKean-Cowdin R, Wang Y, Wu J, Azen SP, Varma R. Impact of visual field loss on health-related quality of life in glaucoma: the Los Angeles Latino Eye Study. Ophthalmology. 2008; 115: 941–948.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tielsch JM, Sommer A, Katz J, et al. Racial variations in the prevalence of primary open-angle glaucoma: the Baltimore Eye Survey. JAMA. 1991; 266: 369–374 [PubMed] [Google Scholar]
- 6. Mitchell P, Smith W, Attebo K, Healey PR. Prevalence of open-angle glaucoma in Australia: the Blue Mountains Eye Study. Ophthalmology. 1996; 103: 1661–1669 [DOI] [PubMed] [Google Scholar]
- 7. Klein BE, Klein R, Sponsel WE, et al. Prevalence of glaucoma: the Beaver Dam Eye Study. Ophthalmology. 1992; 99: 1499–1504 [DOI] [PubMed] [Google Scholar]
- 8. Leske MC, Connell AM, Schachat AP, Hyman L. The Barbados Eye Study: prevalence of open angle glaucoma. Arch Ophthalmol. 1994; 112: 821–829 [DOI] [PubMed] [Google Scholar]
- 9. Dielemans I, Vingerling JR, Wolfs RC, et al. The prevalence of primary open-angle glaucoma in a population-based study in the Netherlands: the Rotterdam Study. Ophthalmology. 1994; 101: 1851–1855 [DOI] [PubMed] [Google Scholar]
- 10. American Academy of Ophthalmology Glaucoma Panel. Preferred Practice Pattern Guidelines. Primary Open-Angle Glaucoma. San Francisco, CA: American Academy of Ophthalmology; 2010. Available at: www.aao.org/ppp. Accessed September 1, 2012. [DOI] [PubMed] [Google Scholar]
- 11. Gemenetzi M, Yang Y, Lotery AJ. Current concepts on primary open-angle glaucoma genetics: a contribution to disease pathophysiology and future treatment. Eye (Lond). 2012; 26: 355–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pasquale LR, Kang JH. Lifestyle, nutrition, and glaucoma. J Glaucoma. 2009; 18: 423–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sacca SC, Bolognesi C, Battistella A, Bagnis A, Izzotti A. Gene-environment interactions in ocular diseases. Mutat Res. 2009; 667: 98–117 [DOI] [PubMed] [Google Scholar]
- 14. Vitale S, Sperduto RD, Ferris FL III. Increased prevalence of myopia in the United States between 1971–1972 and 1999–2004. Arch Ophthalmol. 2009; 127: 1632–1639 [DOI] [PubMed] [Google Scholar]
- 15. Knapp A. Glaucoma in myopic eyes. Arch Ophthalmol. 1926; 55: 35–37 [Google Scholar]
- 16. Moller HU. Excessive myopia and glaucoma. Acta Ophthalmol. 1948; 26: 185–93 [PubMed] [Google Scholar]
- 17. Fong DS, Epstein DL, Allingham RR. Glaucoma and myopia: are they related? Int Ophthalmol Clin. 1990; 30: 215–218 [DOI] [PubMed] [Google Scholar]
- 18. Podos SM, Becker B, Morton WR. High myopia and primary open-angle glaucoma. Am J Ophthalmol. 1966; 62: 1038–1043 [PubMed] [Google Scholar]
- 19. Perkins ES, Phelps CD. Open angle glaucoma, ocular hypertension, low-tension glaucoma, and refraction. Arch Ophthalmol. 1982; 100: 1464–1467 [DOI] [PubMed] [Google Scholar]
- 20. Phelps CD. Effect of myopia on prognosis in treated primary open-angle glaucoma. Am J Ophthalmol. 1982; 93: 622–628 [DOI] [PubMed] [Google Scholar]
- 21. Lotufo D, Ritch R, Szmyd L Jr, Burris JE. Juvenile glaucoma, race, and refraction. JAMA. 1989; 261: 249–252 [PubMed] [Google Scholar]
- 22. Mastropasqua L, Lobefalo L, Mancini A, et al. Prevalence of myopia in open angle glaucoma. Eur J Ophthalmol. 1992; 2: 33–35 [DOI] [PubMed] [Google Scholar]
- 23. Chihara E, Liu X, Dong J, et al. Severe myopia as a risk factor for progressive visual field loss in primary open-angle glaucoma. Ophthalmologica. 1997; 211: 66–71 [DOI] [PubMed] [Google Scholar]
- 24. Daubs JG, Crick RP. Effect of refractive error on the risk of ocular hypertension and open angle glaucoma. Trans Ophthalmol Soc U K. 1981; 101: 121–126 [PubMed] [Google Scholar]
- 25. Wilson MR, Hertzmark E, Walker AM, Childs-Shaw K, Epstein DL. A case-control study of risk factors in open angle glaucoma. Arch Ophthalmol. 1987; 105: 1066–1071 [DOI] [PubMed] [Google Scholar]
- 26. Mitchell P, Hourihan F, Sandbach J, Wang JJ. The relationship between glaucoma and myopia: the Blue Mountains Eye Study. Ophthalmology. 1999; 106: 2010–2015 [DOI] [PubMed] [Google Scholar]
- 27. Xu L, Wang Y, Wang S, Jonas JB. High myopia and glaucoma susceptibility: the Beijing Eye Study. Ophthalmology. 2007; 114: 216–220 [DOI] [PubMed] [Google Scholar]
- 28. Suzuki Y, Iwase A, Araie M, et al. Risk factors for open-angle glaucoma in a Japanese population: the Tajimi Study. Ophthalmology. 2006; 113: 1613–1617 [DOI] [PubMed] [Google Scholar]
- 29. Ramakrishnan R, Nirmalan PK, Krishnadas R, et al. Glaucoma in a rural population of southern India: the Aravind comprehensive eye survey. Ophthalmology. 2003; 110: 1484–1490 [DOI] [PubMed] [Google Scholar]
- 30. Czudowska MA, Ramdas WD, Wolfs RC, et al. Incidence of glaucomatous visual field loss: a ten-year follow-up from the Rotterdam Study. Ophthalmology. 2010; 117: 1705–1712 [DOI] [PubMed] [Google Scholar]
- 31. Jiang X, Varma R, Wu S, et al. Baseline risk factors that predict the development of open-angle glaucoma in a population: the Los Angeles Latino Eye Study. Ophthalmology. 2012; 119: 2245–2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. 2005 Available at: http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm. Accessed March 19, 2012 [Google Scholar]
- 33. Terry AL, Paulose-Ram R, Tilert TJ, et al. The methodology of visual field testing with frequency doubling technology in the National Health and Nutrition Examination Survey, 2005–2006. Ophthalmic Epidemiol. 2010; 17: 411–421 [DOI] [PubMed] [Google Scholar]
- 34. Artes PH, Hutchison DM, Nicolela MT, et al. Threshold and variability properties of matrix frequency-doubling technology and standard automated perimetry in glaucoma. Invest Ophthalmol Vis Sci. 2005; 46: 2451–2457 [DOI] [PubMed] [Google Scholar]
- 35. You QS, Xu L, Jonas JB. Tilted optic discs: the Beijing Eye Study. Eye. 2008; 22: 728–729 [DOI] [PubMed] [Google Scholar]
- 36. Jonas JB. Optic disc size correlated with refractive error. Am J Ophthalmol. 2005; 139: 346–348 [DOI] [PubMed] [Google Scholar]
- 37. Seider MI, Lee RY, Wang D, Pekmezci M, Porco TC, Lin SC. Optic disk size variability between African, Asian, white, Hispanic, and Filipino Americans using Heidelberg retinal tomography. J Glaucoma. 2009; 18: 595–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fansi AA, Papamatheakis DG, Harasymowycz PJ. Racial variability of glaucoma risk factors between African Caribbeans and Caucasians in a Canadian urban screening population. Can J Ophthalmol. 2009; 44: 576–581 [DOI] [PubMed] [Google Scholar]