Abstract
Purpose.
Pathological neovascularization is a crucial component of proliferative retinopathies. Previous studies showed that inactivation of A disintegrin and metalloproteinase 17 (ADAM17), a membrane-anchored metalloproteinase that regulates epidermal growth factor receptor (EGFR) signaling, reduces pathological retinal neovascularization in a mouse model of oxygen-induced retinopathy (OIR). Here, we tested how genetic inactivation of a physiological ADAM17 inhibitor, the tissue inhibitor of matrix metalloproteinases-3 (TIMP3), or intravitreal injection of TIMP3 or of the EGFR inhibitor erlotinib influenced the outcome of OIR.
Methods.
Wild-type mice were subjected to OIR in a chamber with 75% oxygen for 5 days beginning at postnatal day 7 (P7). Upon removal from the oxygen chamber at P12, they received a single intravitreal injection of TIMP3, erlotinib, or control. The central avascular area and neovascular tufts were measured after 5 days in room air (21% oxygen) at P17. Moreover, OIR experiments were performed with Timp3−/− mice and littermate controls.
Results.
Timp3−/− mice showed greater revascularization of the central avascular area and developed equal or fewer neovascular tufts compared to littermate controls, depending on the genetic background. Wild-type mice injected with TIMP3 or erlotinib developed fewer neovascular tufts when compared to untreated littermates. Moreover, vessel regrowth into the avascular area was reduced in TIMP3-injected mice, but not in erlotinib-injected mice.
Conclusions.
These studies demonstrate that TIMP3 and erlotinib inhibit pathological neovascularization in the mouse retina, most likely due to inactivation of ADAM17 and the EGFR, respectively. Thus, TIMP3 and erlotinib emerge as attractive candidate antiangiogenic compounds for prevention and treatment of proliferative retinopathies.
Previous studies have implicated ADAM17, a metalloproteinase that regulates EGFR signaling, in pathological retinal neovascularization. Here, we show that intravitreal injection of an ADAM17 inhibitor (TIMP3) or the EGFR inhibitor erlotinib protects from OIR.
Introduction
Pathological neovascularization is a major cause of blindness and plays a critical role in the development of proliferative retinopathies such as retinopathy of prematurity, diabetic retinopathy, and exudative macular degeneration.1–3 Therefore it is important to understand the mechanism underlying pathological neovascularization in order to identify new targets for treatment of these diseases.4 Previous studies have shown that pathological neovascularization is reduced by inactivation of A disintegrin and metalloproteinase 17 (ADAM17),5 a membrane-anchored metalloproteinase, which is critical for cleaving ligands of the epidermal growth factor receptor (EGFR) and regulating EGFR signaling.6–8 The tissue inhibitor of metalloproteinases-3 (TIMP3) functions as a natural inhibitor of ADAM179–12 and therefore also blocks the release of ligands of the EGFR.13,14
TIMP3 is one of four members of the family of tissue inhibitors of matrix metalloproteinases (TIMPs) and is the only TIMP that can be immobilized in the extracellular matrix.15 Mice lacking TIMP3 have no major spontaneous phenotypes, but they develop pathologies that can be explained by an increase in the activity of ADAM17, such as an enhanced inflammatory response with increased TNFα activity.9–12 TIMP3 also copurifies with ADAM1716 and regulates angiogenesis in three-dimensional tissue culture assays.17 Conditional inactivation of ADAM17 in endothelial cells prevents pathological retinal neovascularization and the growth of heterotopically injected tumors in mice.5 Loss-of-function studies with Timp3−/− mice have shown that TIMP3 regulates choroidal neovascularization,18 as well as VEGF-induced corneal neovascularization and laser-induced choroidal neovascularization,19 and that delivery of TIMP3 by adeno-associated viral vectors ameliorates ischemia-induced neovascularization.20 In addition, TIMP3 regulates angiogenesis by binding directly to the VEGF receptor 2 (VEGFR2).21 Here, we addressed the role of TIMP3 in pathological retinal neovascularization following oxygen-induced retinopathy (OIR) by subjecting Timp3−/− mice to the OIR model and by testing how intravitreal injection of TIMP3 influenced neovascularization after OIR in wild-type mice. Additionally, since ADAM17 is a crucial regulator of EGFR signaling, we tested whether intravitreal injection of the EGFR inhibitor erlotinib (Tarceva)22 affected neovascularization in the OIR model in wild-type mice.
Materials and Methods
Reagents
TIMP3 was kindly provided by Roy Black at Amgen, Inc. (Seattle, WA), and erlotinib (Tarceva) was purchased from Selleck Chemicals (Houston, TX). All other reagents were from Sigma-Aldrich (St. Louis, MO) except for fluorescein-conjugated isolectin B4 (Vector Labs, Burlingame, CA) and fluorescent mounting media (Dako fluorescence mounting medium S3023; Dako, Carpinteria, CA).
Mouse Lines
For experiments involving intraocular injection of TIMP3 or the EGFR inhibitor erlotinib, we used wild-type mice on a mixed genetic background (129/SvJ;C57Bl/6J). Littermates were used to compare eyes injected with inhibitor and carrier control. Timp3−/− mice on a 129/SvJ;C57Bl/6J background were kindly provided by Rama Khokha, PhD,10,23 and heterozygous littermates from matings of Timp3+/− and Timp3−/− parents were used as a control. Moreover, we performed experiments with inbred Timp3−/− C57Bl/6J mice. For this purpose, the mixed-background Timp3−/− mice were backcrossed seven times with C57Bl/6J mice, and the resulting C57BL/6J Timp3−/− mice and wild-type littermate controls were used for OIR experiments.
Oxygen-Induced Retinopathy Mouse Model
In a standardized model of OIR,1,24–26 newborn mice were exposed to 75% oxygen on postnatal day 7 (P7) for the duration of 5 days in a Plexiglas chamber connected to an oxygen controller (Pro-Ox, model 110; Reming Bioinstruments, Redfield, NY) along with their nursing mother. The exposure to high levels of oxygen leads to a regression of the vasculature in the central retina, resulting in a central avascular area. The relative hypoxia, when animals are returned to normoxic conditions (21% oxygen) after 5 days, triggers the production of vascular endothelial growth factor-A (VEGF-A), which increases the proliferative response in the retinal vasculature. This leads to a partial revascularization of the central avascular area and to the development of pathological neovascular tufts on the vitreal side of the internal limiting membrane. On P17, the mice were euthanized, and both eyes were harvested and fixed overnight in 4% paraformaldehyde (PFA). On the next day, the eyes were washed two times with 1× phosphate-buffered saline (PBS); then retinae were dissected, flat-mounted onto microscopic slides, and incubated for 3 hours with LBB (lectin blocking buffer; PBS, 1% BSA, 0.1% Triton X-100, 0.1 M glycine). Subsequently, retinae were incubated with 1:200 fluorescein-labeled isolectin B4 in 0.2× LBB overnight at 4°C. On the next day, retinae were washed twice with PBS and were flat-mounted in fluorescent mounting medium (Dako, Carpenteria, CA). The samples were photographed using a Nikon Eclipse E600 fluorescent microscope (Nikon, Tokyo, Japan) with a 2× objective and a QImaging Retiga EXi camera (QImaging, Surrey, Canada). Images were processed with QCapture 2.68.04 software (QImaging), keeping the exposure and gain constant for all samples. The size of the avascular area and the total retina were measured using National Institutes of Health (NIH) ImageJ software (National Institutes of Health, Bethesda, MD) and Adobe Photoshop (Adobe Systems, Inc., San Jose, CA). Two litters of Timp3−/− and Timp3+/− mixed-background mice were sacrificed at the end of the oxygen treatment (P12), eyes were whole-mounted as described above, and the avascular area was measured. One eye per mouse was used for analysis. The magic wand tool in Adobe Photoshop (Adobe Systems, Inc.) was used to count the neovascular tufts per whole-mounted retina at P17 as previously described.27 All experiments were approved by the Institutional Animal Care and Use Committee of the Hospital for Special Surgery and complied with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Intravitreal Injections
At P12 after OIR, the right eye of wild-type mice was injected intravitreally with 0.5 μL TIMP3 (35 μM) dissolved in PBS or with 0.5 μL erlotinib (20 μM) dissolved in DMSO (dimethyl sulfoxide) or with carrier control. These concentrations were chosen based on an estimated volume of the vitreous in a P12 mouse eye of approximately 5 μL,28 such that a 10-fold dilution would result in a final 100-fold concentration over the half maximal inhibitory concentration (IC50) in cell-based assays for TIMP314 or for erlotinib (Selleck Chemicals). The left eye of each animal remained untreated, according to the approved IACUC protocol. Mice were anesthetized during the injections with a mix of ketamine (100 mg/kg) and xylazine (10 mg/kg) with an intraperitoneal dosage of 0.1 mL per 10 g body weight. During the intravitreal injection, mice were also anesthetized locally with proparacaine hydrochloride 0.5%, and ofloxacin was applied directly after the injection. The intravitreal injections were performed using a microinjecting system (UltraMicroPump III with Micro 4 Controller and NanoFil Syringe; World Precision Instruments, Inc., Sarasota, FL) and a 33-gauge needle (FlexiFil NanoFil beveled Needle 33 gauge; World Precision Instruments, Inc.) penetrating the sclera approximately 1 mm behind the limbus at the 2 o'clock position. To avoid reflux out of the injection site, the needle was retrieved very slowly. Wild-type mice were sacrificed at P17, and the injected eye was whole-mounted as described above. A separately performed toxicity analysis of retina sections from erlotinib- or TIMP3-treated animals and DMSO- or PBS-treated controls that were fixed in 4% PFA and stained with hematoxylin and eosin (H&E) showed no evidence of necrosis or inflammation in any of the samples (erlotinib/DMSO-treated samples: n = 10, DMSO controls: n = 8, TIMP3-treated animals: n = 10, PBS-treated controls: n = 9).
Statistical Analysis
All statistical analyses were performed using Prism 5 software (GraphPad Software, Inc., La Jolla, CA) and Excel (Microsoft, Inc., Redmond, WA). The Wilcoxon-Mann-Whitney test, a nonparametric analog to the independent samples t-test, was used for all comparisons, and a P value of <0.05 was considered statistically significant.
Results
Oxygen-Induced Retinopathy in Timp3−/− Mice
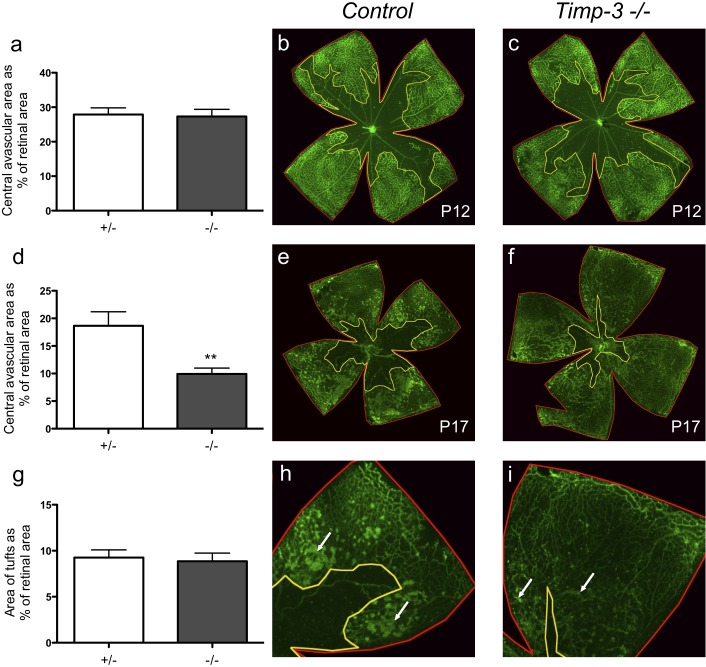
To investigate how loss of TIMP3 function affects pathological neovascularization, we exposed Timp3−/− mice of mixed genetic background (129/SvJ;C57Bl/6J) and control littermates to OIR. After 5 days in 75% oxygen, at P12, Timp3−/− mice had developed a central avascular area similar in size relative to the retina (n = 9, 27.3 ± 2.1%) to that of control littermates (n = 4, 27.8 ± 1.9%; P = 0.94, Figs. 1a–c). After the animals had been exposed to room air between P12 and P17, the revascularization of the avascular area was more pronounced in Timp3−/− mice than in controls, resulting in a smaller central avascular area (9.9 ± 1.1%, n = 25) compared to control littermates (18.7 ± 2.5%, n = 19, Figs. 1d–f, P ≤ 0.003). When we performed similar experiments with inbred C57BL/6J Timp3−/− mice and wild-type littermate controls, we also found a significant increase in the revascularization of the central avascular area at P17 in Timp3−/− mice (2.1 ± 0.45%, n = 20) compared to controls (11.7 ± 1.1%, n = 28, P ≤ 0.001). The effect of inactivation of TIMP3 on the development of neovascular tufts in the retina depended on the genetic background, with comparable tuft development in the mixed-background Timp3−/− mice and controls (Figs. 1g–i, P = 0.84) but decreased formation of neovascular tufts in C57Bl/6J Timp3−/− mice (5.1 ± 0.57%, n = 20) relative to controls (14.5 ± 0.57%, n = 28, P ≤ 0.001), perhaps because the increased perfusion of the central avascular area had a stronger effect on reducing tuft formation in this genetic background.
Figure 1. .
Pathological retinal neovascularization in Timp3−/− mice and littermate controls following exposure to the OIR model. (a–c) Whole-mounted retinas from mixed-background Timp3−/− mice and controls, stained with isolectin B4 FITC at postnatal day 12 (P12) after OIR, showed that the size of the central avascular area was similar in Timp3−/− mice compared to controls (Timp3−/−: 27.3% ± 2.1, n = 9; controls: 27.8% ± 1.9, n = 4). (d–f) Quantification of the size of the central avascular area in retinal flat mounts on P17 after OIR showed a significant increase in revascularization in Timp3−/− mice compared to controls (Timp3−/−: 9.9% ± 1.1, n = 25; controls: 18.7% ± 2.5, n = 19). (g–i) The percentage of the retinal surface covered by neovascular tufts did not differ between Timp3−/− mice and controls on a mixed genetic background (129/SvJ;C57Bl/6J; Timp3−/−: 8.9% ± 0.9, n = 7; controls: 9.3% ± 0.8, n = 9). White arrows represent neovascular tufts. **P < 0.01 in a nonparametric Wilcoxon-Mann-Whitney test.
Intravitreal Injection of TIMP3 Inhibits Pathological Neovascularization
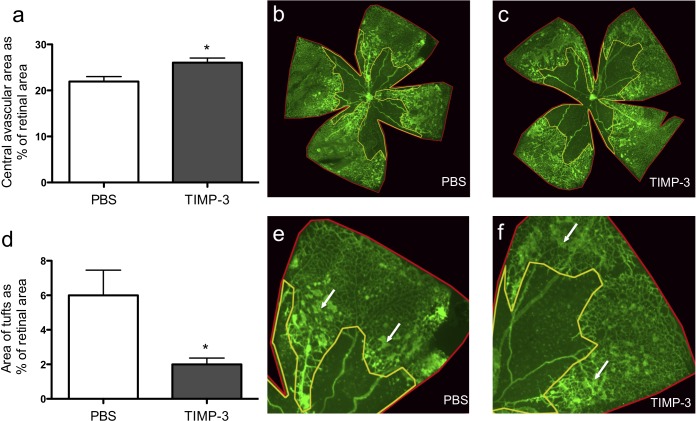
In order to assess whether intraocular application of TIMP3 could be used to reduce retinal neovascularization, wild-type mice were injected intravitreally with TIMP3 or with carrier control at P12 after OIR. Five days after the injection (at P17), TIMP3-treated mice had a significantly larger avascular area compared to littermate controls (Figs. 2a–c, P ≤ 0.02). Furthermore, there were fewer neovascular tufts in the TIMP3-injected eyes compared to the carrier (DMSO)-injected eyes of littermate controls (Figs. 2d–f, P ≤ 0.03).
Figure 2. .
Effect of intravitreal injection of TIMP3 on the outcome of the OIR model. (a–c) Wild-type mice injected with TIMP3 at P12 in the OIR model following removal from the 75% oxygen chamber had a significantly larger central avascular area on P17 than littermates injected with carrier control (TIMP3: 26.1% ± 1.0, n = 15; controls: 22.0% ± 1.1, n = 13). The retinal vasculature on retinal whole mounts was visualized by immunofluorescence analysis using FITC-labeled isolectin B4. (d–f) Wild-type mice injected with TIMP3 developed significantly fewer neovascular tufts at P17 than mice injected with carrier control (TIMP3: 2.0% ± 0.4, n = 9; controls: 6.0% ± 1.5, n = 8). White arrows represent neovascular tufts. *P < 0.05 in a Wilcoxon-Mann-Whitney test.
Intravitreal Injection of Erlotinib Prevents Development of Neovascular Tufts without Interfering with Revascularization of the Central Avascular Area
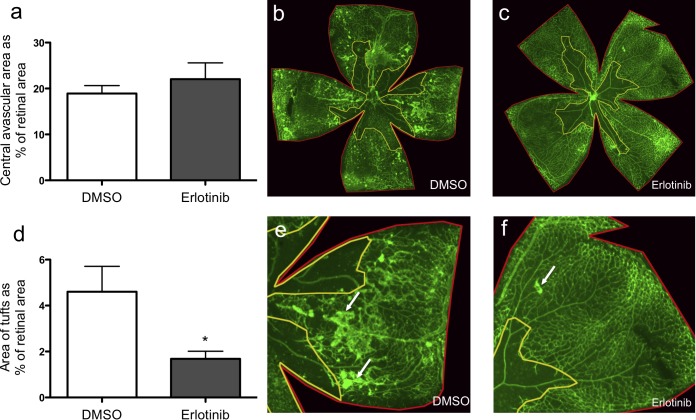
The ability of intravitreally injected TIMP3 to inhibit retinal neovascularization raised questions about whether these effects were caused by TIMP3-dependent changes in ADAM17/EGFR signaling29,30 or in VEGF-A/VEGFR2 signaling.21 To test whether pharmacological inhibition of the EGFR decreases pathological retinal neovascularization, we injected the EGFR inhibitor erlotinib intravitreally in wild-type mice at P12 after OIR. We found that the avascular area in mice injected with erlotinib showed no significant difference when compared to carrier control, suggesting that the revascularization on P17 after OIR was not dependent on the EGFR pathway (Figs. 3a–c, P = 0.96). However, analysis of neovascular tufts in whole-mounted retinae stained with isolectin B4 showed a significant decrease in mice injected with erlotinib when compared to carrier control, suggesting that the EGFR is important for the development of neovascular tufts (Figs. 3d–f, P ≤ 0.03).
Figure 3. .
Intravitreal injection of the EGFR inhibitor erlotinib prevented tuft formation without affecting revascularization of the central avascular area. (a–c) Isolectin B4–stained retinal flat mounts of wild-type mice injected with erlotinib at P12 showed no difference in the revascularization of the avascular area on P17 after OIR compared to controls (erlotinib: 22.0% ± 3.6, n = 9; controls: 19.0% ± 1.7, n = 8). (d–f) Mice injected with erlotinib displayed significantly fewer neovascular tufts at P17 than control-injected animals (1.7% ± 0.3, n = 8; controls: 4.6% ± 1.1, n = 8). White arrows represent neovascular tufts. *P < 0.05 in a Wilcoxon-Mann-Whitney test.
Discussion
The mouse model of OIR allows an assessment of the role of specific signaling pathways in the development of proliferative retinopathies, such as retinopathy of prematurity, diabetic retinopathy, and exudative macular degeneration.1,24–26 Previous studies had implicated the ADAM17/EGFR pathway in pathological retinal neovascularization.5 The principal goals of the current study were to determine the role of TIMP3, a physiological regulator of ADAM17,9–12 in pathological retinal neovascularization using loss- and gain-of-function experiments, and to test the involvement of the EGF-receptor pathway in OIR via pharmacological inhibition of this receptor.
When we evaluated the role of TIMP3 in OIR using loss-of-function experiments, we found that Timp3−/− mice had developed an avascular area similar in size to that of littermate controls immediately after exposure to high oxygen at P12. This suggests that there is no significant difference in vascular regression in the retina between P5 and P12 in the absence of TIMP3. However, at P17, 5 days after OIR, Timp3−/− mice showed significantly increased revascularization of the central avascular area compared to controls, regardless of the genetic background. Interestingly, there was no difference in neovascular tuft formation in Timp3−/− mice compared to littermate controls on a mixed genetic background, whereas tuft formation was significantly reduced in inbred Timp3−/− mice (C57BL/6J). The results obtained with the C57BL/6J Timp3−/− mice are similar to those seen in Adam8−/− mice,31 which had increased revascularization of the central avascular area following OIR but were protected from neovascular tuft formation. Perhaps a more rapid restoration of the central avascular area in Adam8−/− and in inbred Timp3−/− mice on the C57BL/6J background improves perfusion and oxygen delivery to the retina sufficiently to reduce the hypoxia and thus lower VEGF-A levels, thereby removing the stimulus for generation of neovascular tufts.
With respect to the gain-of-function experiments, intravitreal injection of TIMP3 in wild-type mice significantly reduced formation of neovascular tufts compared to controls. Thus, gain-of-function experiments with intravitreally injected TIMP3, which blocked the revascularization of the central avascular area after OIR, had an effect opposite to that in loss-of-function experiments in Timp3−/− mice. Our results are also consistent with those recently reported by Ebrahem et al.,19 who found an increase in laser-induced choroidal neovascularization in Timp3−/− mice, suggesting that TIMP3 regulates different types of pathological neovascularization in the eye in a similar manner. TIMP3 is known to be upregulated in simplex retinitis pigmentosa in photoreceptor-retaining regions, and it is expressed in the RPE,32,33 but it was not among the genes reported to be upregulated in a gene expression analysis during mouse OIR.34 These findings suggest that constitutive expression of TIMP3 is sufficient to control the function of ADAM17 or the VEGFR or both under physiological conditions.
The intravitreally injected TIMP3 likely blocked tuft formation by reducing ADAM17 activity, which is known to be required for activating EGFR-dependent processes in development and disease.6,8,11,35–38 Conditional knockout mice lacking ADAM17 in endothelial cells show decreased revascularization of the central avascular area5; these results are therefore consistent with a model in which the lack of TIMP3 increases the activity of ADAM17, which in turn could increase revascularization, whereas injection of TIMP3 could prevent revascularization by blocking ADAM17. VEGF-A is highly upregulated in mouse OIR,39–41 and previous studies have shown that VEGF-A/VEGFR2 signaling activates ADAM17 to promote crosstalk with the EGFR and stimulate migration of endothelial cells.29,30 Since the injection of TIMP3 might also affect the binding of VEGF-A to the VEGFR2,21 injection of TIMP3 could interfere with pathological neovascularization by inhibiting two crucial components of this pathway, binding of VEGF-A to the VEGFR2 and the activation of ADAM17.
To directly assess the contribution of the EGFR pathway to pathological neovascularization, we injected the EGFR inhibitor erlotinib into the vitreous of wild-type mice subjected to OIR. We found that injection of erlotinib reduced formation of neovascular tufts, similar to findings in mice injected with TIMP3. However, we found no significant difference in the revascularization of the avascular area on P17 after OIR compared to that in carrier control-treated animals, although there was a trend toward slower revascularization of the dropout area. These results suggest that EGFR signaling is important for the development of neovascular tufts, whereas injection of TIMP3 blocks both tuft formation and the revascularization of the central avascular area. As noted above, it is possible that TIMP3 blocks revascularization more efficiently because it also directly blocks VEGFR2 signaling,21 so the combined inhibition of the VEGFR2 and ADAM17 could have a stronger effect than the inhibition of the EGFR alone. However, these differences could also be caused by distinct pharmacokinetic properties of TIMP3, a recombinantly expressed protein, versus erlotinib, a small-molecule tyrosine kinase inhibitor. Further studies with conditional knockout mice for the EGFR in endothelial cells will help address this question.
In summary, mice lacking TIMP3 showed a faster revascularization of the avascular area after OIR whereas intravitreal injection of TIMP3 reduced the revascularization, and both TIMP3 and erlotinib reduced the development of neovascular tufts. These results suggest that erlotinib, which is already approved for treatment of small-cell lung cancer and other tumors, or TIMP3, or perhaps also other inhibitors of the ADAM17/EGFR signaling pathway, could provide novel opportunities for treatment of proliferative retinopathies.
Acknowledgments
We thank Elin Mogollon for excellent technical assistance.
Footnotes
Supported by NIH Grant R01 GM64750 (CPB), NIH Grant R01 EY19474 (MFC), NIH NHLBI T32 Training Grant GM007739-31 (KG), and the Jackstaedt Foundation (NJH). This work was conducted in a facility constructed with support from Research Facilities Improvement Program Grant C06-RR12538-01 from the National Center for Research Resources, National Institutes of Health.
Disclosure: N.J. Hewing, None; G. Weskamp, None; J. Vermaat, None; E. Farage, None; K. Glomski, None; S. Swendeman, None; R.V.P. Chan, None; M.F. Chiang, None; R. Khokha, None; B. Anand-Apte, None; C.P. Blobel, None
References
- 1. Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis. 2007; 10: 133–140 [DOI] [PubMed] [Google Scholar]
- 2. Bradley J, Ju M, Robinson GS. Combination therapy for the treatment of ocular neovascularization. Angiogenesis. 2007; 10: 141–148 [DOI] [PubMed] [Google Scholar]
- 3. Edwards AO, Malek G. Molecular genetics of AMD and current animal models. Angiogenesis. 2007; 10: 119–132 [DOI] [PubMed] [Google Scholar]
- 4. Sherris D. Ocular drug development--future directions. Angiogenesis. 2007; 10: 71–76 [DOI] [PubMed] [Google Scholar]
- 5. Weskamp G, Mendelson K, Swendeman S, et al. Pathological neovascularization is reduced by inactivation of ADAM17 in endothelial cells but not in pericytes. Circ Res. 2010; 106: 932–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peschon JJ, Slack JL, Reddy P, et al. An essential role for ectodomain shedding in mammalian development. Science. 1998; 282: 1281–1284 [DOI] [PubMed] [Google Scholar]
- 7. Sahin U, Weskamp G, Zhou HM, et al. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR-ligands. J Cell Biol. 2004; 164: 769–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blobel CP. ADAMs: key players in EGFR-signaling, development and disease. Nat Rev Mol Cell Bio. 2005; 6: 32–43 [DOI] [PubMed] [Google Scholar]
- 9. Mahmoodi M, Sahebjam S, Smookler D, Khokha R, Mort JS. Lack of tissue inhibitor of metalloproteinases-3 results in an enhanced inflammatory response in antigen-induced arthritis. Am J Pathol. 2005; 166: 1733–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mohammed FF, Smookler DS, Taylor SE, et al. Abnormal TNF activity in Timp3−/− mice leads to chronic hepatic inflammation and failure of liver regeneration. Nat Genet. 2004; 36: 969–977 [DOI] [PubMed] [Google Scholar]
- 11. Murthy A, Defamie V, Smookler DS, et al. Ectodomain shedding of EGFR ligands and TNFR1 dictates hepatocyte apoptosis during fulminant hepatitis in mice. J Clin Invest. 2010; 120: 2731–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smookler DS, Mohammed FF, Kassiri Z, Duncan GS, Mak TW, Khokha R. Tissue inhibitor of metalloproteinase 3 regulates TNF-dependent systemic inflammation. J Immunol. 2006; 176: 721–725 [DOI] [PubMed] [Google Scholar]
- 13. Horiuchi K, Le Gall S, Schulte M, et al. Substrate selectivity of EGF-receptor ligand sheddases and their regulation by phorbol esters and calcium influx. Mol Biol Cell. 2007; 18: 176–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Le Gall S, Bobe P, Reiss K, et al. ADAMs 10 and 17 represent differentially regulated components of a general shedding machinery for membrane proteins such as TGFα, L-Selectin and TNFα. Mol Biol Cell. 2009; 20: 1785–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000; 1477: 267–283 [DOI] [PubMed] [Google Scholar]
- 16. Kwak HI, Mendoza EA, Bayless KJ. ADAM17 co-purifies with TIMP-3 and modulates endothelial invasion responses in three-dimensional collagen matrices. Matrix Biol. 2009; 28: 470–479 [DOI] [PubMed] [Google Scholar]
- 17. Saunders WB, Bohnsack BL, Faske JB, et al. Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J Cell Biol. 2006; 175: 179–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Janssen A, Hoellenriegel J, Fogarasi M, et al. Abnormal vessel formation in the choroid of mice lacking tissue inhibitor of metalloprotease-3. Invest Ophthalmol Vis Sci. 2008; 49: 2812–2822 [DOI] [PubMed] [Google Scholar]
- 19. Ebrahem Q, Qi JH, Sugimoto M, et al. Increased neovascularization in mice lacking tissue inhibitor of metalloproteinases-3. Invest Ophthalmol Vis Sci. 2011; 52: 6117–6123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Auricchio A, Behling KC, Maguire AM, et al. Inhibition of retinal neovascularization by intraocular viral-mediated delivery of anti-angiogenic agents. Mol Ther. 2002; 6: 490–494 [DOI] [PubMed] [Google Scholar]
- 21. Qi JH, Ebrahem Q, Moore N, et al. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat Med. 2003; 9: 407–415 [DOI] [PubMed] [Google Scholar]
- 22. Gridelli C, Maione P, Bareschino MA, et al. Erlotinib in the treatment of non-small cell lung cancer: current status and future developments. Anticancer Res. 2010; 30: 1301–1310 [PubMed] [Google Scholar]
- 23. Leco KJ, Waterhouse P, Sanchez OH, et al. Spontaneous air space enlargement in the lungs of mice lacking tissue inhibitor of metalloproteinases-3 (TIMP-3). J Clin Invest. 2001; 108: 817–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stahl A, Connor KM, Sapieha P, et al. Computer-aided quantification of retinal neovascularization. Angiogenesis. 2009; 12: 297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci U S A. 1995; 92: 905–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith LE, Wesolowski E, McLellan A, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994; 35: 101–111 [PubMed] [Google Scholar]
- 27. Connor KM, Krah NM, Dennison RJ, et al. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc. 2009; 4: 1565–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Remtulla S, Hallett PE. A schematic eye for the mouse, and comparisons with the rat. Vision Res. 1985; 25: 21–31 [DOI] [PubMed] [Google Scholar]
- 29. Maretzky T, Evers A, Zhou W, et al. Migration of growth factor-stimulated epithelial and endothelial cells depends on EGFR transactivation by ADAM17. Nat Commun. 2011; 2: 229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Swendeman S, Mendelson K, Weskamp G, et al. VEGF-A stimulates ADAM17-dependent shedding of VEGFR2 and crosstalk between VEGFR2 and ERK signaling. Circ Res. 2008; 103: 916–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guaiquil VH, Swendeman S, Zhou W, et al. ADAM8 is a negative regulator of retinal neovascularization and of the growth of heterotopically injected tumor cells in mice. J Mol Med. 2010; 88: 497–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zeng Y, Rosborough RC, Li Y, Gupta AR, Temporal Bennett J. and spatial regulation of gene expression mediated by the promoter for the human tissue inhibitor of metalloproteinases-3 (TIMP-3)-encoding gene. Dev Dyn. 1998; 211: 228–237 [DOI] [PubMed] [Google Scholar]
- 33. Jomary C, Neal MJ, Jones SE. Increased expression of retinal TIMP3 mRNA in simplex retinitis pigmentosa is localized to photoreceptor-retaining regions. J Neurochem. 1995; 64: 2370–2373 [DOI] [PubMed] [Google Scholar]
- 34. Sato T, Kusaka S, Hashida N, Saishin Y, Fujikado T, Tano Y. Comprehensive gene-expression profile in murine oxygen-induced retinopathy. Br J Ophthalmol. 2009; 93: 96–103 [DOI] [PubMed] [Google Scholar]
- 35. Franzke CW, Cobzaru C, Triantafyllopoulou A, et al. Epidermal ADAM17 maintains the skin barrier by regulating EGFR ligand-dependent terminal keratinocyte differentiation. J Exp Med. 2012; 209: 1105–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jackson LF, Qiu TH, Sunnarborg SW, et al. Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. Embo J. 2003; 22: 2704–2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sternlicht MD, Sunnarborg SW, Kouros-Mehr H, Yu Y, Lee DC, Werb Z. Mammary ductal morphogenesis requires paracrine activation of stromal EGFR via ADAM17-dependent shedding of epithelial amphiregulin. Development. 2005; 132: 3923–3933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chalaris A, Adam N, Sina C, et al. Critical role of the disintegrin metalloprotease ADAM17 for intestinal inflammation and regeneration in mice. J Exp Med. 2010; 207: 1617–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xiang N, Zhao MJ, Li XY, Zheng HH, Li GG, Li B. Redundant mechanisms for vascular growth factors in retinopathy of prematurity in vitro. Ophthalmic Res. 2011; 45: 92–101 [DOI] [PubMed] [Google Scholar]
- 40. Zhao M, Shi X, Liang J, et al. Expression of pro- and anti-angiogenic isoforms of VEGF in the mouse model of oxygen-induced retinopathy. Exp Eye Res. 2011; 93: 921–926 [DOI] [PubMed] [Google Scholar]
- 41. McColm JR, Geisen P, Hartnett ME. VEGF isoforms and their expression after a single episode of hypoxia or repeated fluctuations between hyperoxia and hypoxia: relevance to clinical ROP. Mol Vis. 2004; 10: 512–520 [PMC free article] [PubMed] [Google Scholar]