Abstract
Lassa fever (LF) is one of the most prevalent viral hemorrhagic fevers in West Africa responsible for thousands of deaths annually. The BSL-4 containment requirement and lack of small animal model to evaluate Lassa virus (LASV)-specific cell-mediated immunity (CMI) complicate development of effective LF vaccines. Here we have described a CBA/J-ML29 model allowing evaluation of LASV-specific CMI responses in mice. This model is based on Mopeia virus reassortant clone ML29, an attractive immunogenic surrogate for LASV. A single intraperitoneal (i.p.) immunization of CBA/J mice with ML29 protected animals against a lethal homologous intracerebral (i.c.) challenge with 588 LD50. The ML29-immunized mice displayed negligible levels of LASV-specific antibody titers, but LASV-specific CMI responses were detectable early and peaked on day 8–10 after immunization. A T cell cytotoxicity assay in vivo showed a correlation between LASV-specific cytotoxicity and the timing of protection induced by the ML29 immunization. Notably, CBA/J mice that received CD8+ T cell-depleted splenocytes from ML29-immunized donors all succumbed to a lethal i.c. challenge, demonstrating that CD8+ T cells are critical in protection. The CBA/J-ML29 model can be useful immunological tool for the preliminary evaluation of immunogenicity and efficacy of vaccine candidates against LASV outside of BSL-4 containment facilities.
Keywords: Lassa virus, Reassortant ML29, LCMV, Cell-mediated immunity, Vaccine development
1. Introduction
Lassa virus (LASV) is transmitted to humans by a rodent reservoir, Mastomys natalensis, and is capable of causing lethal Lassa fever (LF) disease. There is no licensed vaccine for the prevention of LF and vaccine development efforts are hampered by both the high cost of non-human primate (NHP) animal models and biocontainment requirements (BSL-4). In experimental mice, LASV and the closely related prototype arenavirus lymphocytic choriomeningitis virus, LCMV, induce a fatal immunopathological disease after intracerebral (i.c.) inoculation. In contrast, in humans LASV infection is not associated with immunopathology. Outcome of the disease correlates with viral loads in blood and tissues. In progressed LF cases uncontrolled virus replication and virus-induced immunodeficiency result in multi-system organ failure and death. Notably, in LCMV-infected mice and LASV-exposed humans protection and recovery are primarily dependent on CMI responses with minimal, if any, antibody involvement [1–7].
LASV has the highest human impact of any of the hemorrhagic fever viruses (with the exception of dengue fever) with an estimated 100,000–300,000 infections and 5000–10,000 deaths annually in western Africa [1,8–11]. It seems that LASV burden in endemic areas is much greater than previously reported. Based on available sero-epidemiological data from four of the most affected countries, Guinea, Sierra-Leone, Liberia, and Nigeria, Richmond and Baglole [11] estimated that 59 million people are at risk of primary LASV infections with an annual incidence of disease as high as 3 million and as many as 67,000 deaths per year. The current LF predicted areas cover approximately 80% of each of Sierra-Leone and Liberia, 50% of Guinea, 40% of Nigeria, 30% of each of Côte d]Ivoire, Togo and Benin, and 10% of Ghana [9]. Recently two new LASV-like arenaviruses, Lujo [12,13] and Luna [14], were found in South Africa and Lujo virus caused nosocomial outbreak with unprecedented high case fatality rate of 80%.
Fortunately, only ~80% of LASV-infected individuals expressed clinical manifestations and overall case-fatality rate is ~1–2%, but in some risk groups (pregnant women, children <5 years old, immunocompromised individuals) this rate can be as high as 50% or higher [1]. In 29% of patients, acute LF is accompanied by a sensorineural hearing deficit, which accounts for a permanent hearing loss in 17.6% of survivors [15,16]. The sizeable disease burden, numerous imported cases of LF in non-endemic countries [17,18], and the possibility that LASV can be used as an agent of biological warfare [19] make a strong case for vaccine development.
Presently there is no licensed vaccine against LASV. Efficacy trials in humans for LASV vaccine candidates are not feasible for ethical reasons and vaccine development must rely on the FDA animal rule [20]. These guidelines allow for the approval of potential vaccine candidates if appropriate safety and efficacy criteria are met in suitable animal models. Currently models of LF disease include NHP, strain 13 guinea pigs, and hamsters [21–27]. NHP animal models are expensive and logistically difficult to house and utilize. While guinea pigs and hamsters prove more economical, final efficacy studies of vaccine candidates still necessitate BSL-4 biocontainment facilities. For these reasons, development of LASV vaccine candidates requires a small animal model outside of BSL-4 containment.
For the evaluation of immunogenicity of LASV vaccine candidates we employed Mopeia virus (MOPV) reassortant clone ML29 [28]. The MOPV ML29 virus contains the large (L) genomic segment from the non-pathogenic MOPV (AN20410), encoding a viral RNA-dependent RNA-polymerase and a RING finger matrix protein (Z), and the small (S) genomic segment from LASV (Josiah), encoding the major LASV immunogens, the nucleoprotein (NP) and glycoprotein complex (GPC). Additional characterization of ML29 has shown that it contains eighteen mutations that distinguish its genome from that of the parental strains and these mutations are likely to contribute to its attenuated phenotype [29,30]. The MOPV ML29 has proven non-pathogenic in mice, guinea pigs, NHP (rhesus, marmosets, SIV-infected rhesus macaques) [2,31] and can completely protect experimental animals including NHP against a fatal LASV challenge [30]. In spite of its safety profile in NHP [21], MOPV is still classified by the CDC as a risk group 3 agent, while according to the EU regulations MOPV is a biosafety level 2 agent. Because two thirds of the ML29 genome is derived from MOPV, the ML29 also belongs to the risk group 3 in the US but it is not considered a select agent according to the CDC [29].
Here we describe the immunogenicity of ML29 in CBA/J mice. In these mice intraperitoneal (i.p.) inoculation of LASV or ML29 results in a non-manifested infection that is effectively cleared by CMI responses, while an intracerebral (i.c.) inoculation results in LCMV-like manifestations and death due to T cell-mediated acute inflammatory response [6,21,23,28,29,32]. This route-dependent outcome facilitates the use of the reassortant virus ML29 as both, an effective immunogen encoding major LASV antigens, and an i.c. challenge agent causing fatal T cell-mediated immunopathology in mice. We have shown that in CBA/J mice LASV-specific protection is correlated with T cell responses assayed by IFN-γ ELISPOT, by intracellular staining (IFN-γ/TNF-α), and by in vivo CTL assay. Notably, in splenocyte transfer experiments protection of recipient mice was fully dependent on CD8+ T cell population providing additional evidence that CD8+ CTL responses are plying the crucial role in protection.
2. Materials and methods
2.1. Viruses and cells
MOPV reassortant clone ML29 has been previously described [28,29]. Virus was propagated in Vero E6 cells (ATCC, CRL-1586), cultured in minimum essential medium (MEM, GIBCO) with 2% fetal bovine serum (FBS), 1% penicillin-streptomycin and L-glutamine (2 mM) at 37 °C in 5% CO2 incubator by using a multiplicity of infection (MOI) of 0.01. Supernatants were collected at 72 h post-infection, titrated on Vero E6 cells and virus stocks (1 × 107 PFU/ml) were stored at −70 °C.
2.2. Immunization protocols
CBA/J mice were purchased from Harlan® Laboratories (Indianapolis, IN). For ML29 immunogenicity studies, mice (n = 5) were immunized with 1 × 103 PFU of ML29 i.p. in 100 μl of MEM media, or with 1 × 107 IU (infectious units) alphavirus replicon virus-like-particle-vectors (VLPV) expressing modified LASV GPC (kindly provided by Dr. P. Pushko, Medigen, Inc., Frederick, MD) [33], or with 100 μl of conditioned MEM media (mock-vaccination control). On day 7 VLPV-immunized mice were boosted at the same dose, 1 × 107 IU. To study LASV-specific T cell responses, ML29-immunized mice were euthanized at 2-day interval during 14 days after immunization and spleens were harvested. Erythrocyte-free splenocytes were subsequently used for IFN-γ ELISPOT, intracellular cytokine staining, in vivo CTL assay, and for splenocyte transfers.
2.3. Detection of anti-LASV antibodies
Antibody responses were measured by IgG ELISA and plaque reduction neutralization (PRNT) assay as previously described [34]. In brief, microtiter plates were coated with 5 × 105 PFU/well of sonicated ML29 in 100 μl of carbonate–bicarbonate buffer, washed with PBS-0.05% Tween 20 (PBST), and blocked with 10% non-fat dry milk. Serial dilutions of plasma samples were added in duplicates to plates and incubated for 1 h at 37 °C. After incubation plates were washed with PBST, goat anti-mouse IgG was added to each well and incubated for 1 h at 37 °C. After incubation plates were washed, TMB substrate (KPL, 52-00-01) was added to all wells and, color development was read at A450. Neutralization antibody titers were measured by PRNT using a constant dose of ML29, Vero cell monolayers, and serial 1-log dilutions of plasma. Incubation of virus with serum was performed at 37 °C for 1 h. As controls, samples collected from mice before ML29 infection, or samples from LASV-infected individuals [35] were used. End points were calculated from the highest serum dilution inducing 50% plaque reduction.
2.4. LASV GPC immunodominant epitope mapping
An overlapping peptide library derived from LASV (Josiah) GPC contained sixty nine 21-mers peptides (Mimotopes, Australia). In the initial experiments small groups of adjacent peptides were pooled and used as antigen-specific stimuli in IFN-γ ELISPOT [36]. IFN-γ positive pools were subsequently further divided into individual peptides to map the immunodominant regions of the LASV GPC in CBA/J mice. Mouse IFN-γ ELISPOT assay (Mabtech AB, Sweden) was performed according to manufacturer’s protocol. Briefly, erythrocyte-free splenocytes (6 × 106 cells/ml in 100 μl) were added to 96-well filter plate (Millipore, MSIPS4510) pre-coated with anti-mouse monoclonal IFN-γ antibodies in triplicate at dilutions of 3 × 105 cells/well or 1.5 × 105 cells/well. Cells were stimulated overnight at 37 °C with cocktail of 10 μM GPC 21-mer peptides. After stimulation, cells were washed, biotinylated anti-mouse IFN-γ antibody was added, and plates were incubated for 2 h at RT. After additional incubation with streptavidin-horseradish peroxidase, spot-forming cells (SFC) were developed with TMB substrate (Mabtech AB) and counted using C.T.L. Ltd. Immunospot® S5 Micro-analyzer and Immunospot® V 4.0 software.
2.5. Intracellular cytokine staining
For intracellular cytokine staining, 1 × 106 cells per sample were stimulated for 12 h at 37 °C with 10 μM of optimized GPC peptide cocktail. Cells were washed with PBS and surface staining was performed using the following markers: CD3 (eBioscience, Cat. no. 45-0031, clone 145-2C11, PerCP-Cy5.5) and CD4 (eBioscience, Cat. no. 17-0042, clone RM4-5, APC) or CD3 and CD8 (eBioscience, Cat. no. 17-0081, clone 53-6.7, APC). Cells were then washed with PBS, fixed/permeabilized using Cytofix/Cytoperm™ Plus kit (BD Bioscience, Cat. no. 555028) and stained for IFN-γ (eBioscience, Cat. no. 12-7311, clone XMG1.2, PE) and TNF-α (eBioscience, Cat. no. 11-7321, clone MP6-XT22, FITC). After stimulation cells were washed with PBS and stained for surface markers, CD3 and CD8 (eBio-science, Cat. no. 17-0081, clone 53-6.7, APC). Stained cells were analyzed by FACScalibur (BD Bioscience, San Diego, CA) and flow cytometry data were analyzed with FlowJo software (Tree Star, San Carlos, CA).
2.6. In vivo CTL assay
Splenocytes from naïve mice were stained with either 0.5 μM CFSE or with 2.5 μM of Cell Tracker™ Far Red (Molecular Probes, Eugene, OR) for 15 min at 37 °C. CFSE-stained splenocytes were subsequently labeled with 10 μM of immunodominant LASV GPC peptide cocktail for 1 h at 37 °C. Splenocytes from each of the peptide-pulsed CFSE-stained population and the non-pulsed Cell Tracker™ Far Red stained population (5 × 106 cells each) were transferred intravenously (i.v.) into mice that were previously immunized with ML29 (infected) or naïve (uninfected). At 6 h after transfer splenocytes from recipient mice were harvested and analyzed by flow cytometer. Transferred target populations of cells were distinguished from one another based on CFSE and Cell Tracker™ Far Red staining and peptide-pulsed CFSE numbers were compared to non-pulsed control targets. Percent specific lysis was calculated as: 100 − ([(# peptide pulsed in infected/# unpulsed in infected)/(# peptide pulsed in uninfected/# unpulsed in uninfected)] × 100) [37]. Only samples from recipient mice where a minimum of 5000 CFSE positive events were collected were used for data analysis.
2.7. Splenocyte transfer protocol
This protocol was essential the same as previously described [28,29]. The only difference was that instead of LASV we used a challenge of recipient mice with ML29 (i.c.). Using the standard Reed-Muench calculations 1 LD50 was 1.7 PFU of ML29 (i.c.) and 1 PD50 (protective dose) was 1.47 PFU of ML29 (i.p.). In good confirmation with these calculations the ML29 immunization with 150 PFU of ML29 (i.p.) produced immune splenocytes that completely protected CBA mice against a fatal LASV challenge (i.c.) [29]. For splenocyte transfers mice were immunized i.p. with 1 × 103 PFU of ML29 and at different time points after immunization 5 mice per time-point were euthanized and donor splenocytes were prepared as previously described. Recipient mice were sedated and challenged with 855 LD50 of ML29 in 50 μl of MEM media i.c. at time 0. Two hours after challenged recipient mice (n = 5) received 3.0 × 107 splenocytes i.v. from immunized donors at different time points. Mice were monitored during 21 days post-challenge. To evaluate contribution of different T cell populations in the protection, splenocytes from ML29-immunized mice were depleted of either CD4+ T cells or CD8+ T cells using magnetic bead technology (CD4: MACS® Cat. no. 130-049-201; CD8: MACS® Cat. no. 130-049-401).
2.8. Data analysis
Statistical analyses (mean, SD, T-test) and graphics were performed using the Origin 6.0 package (Microcal Software, Inc., Northampton, MA).
3. Results
3.1. Humoral responses against MOPV ML29 do not contribute to protection against a lethal challenge
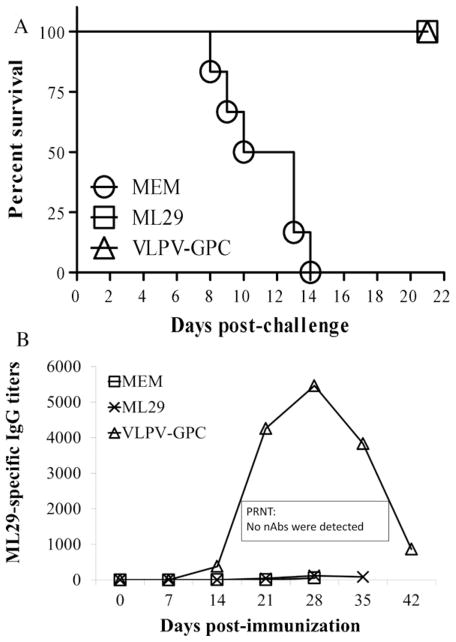
An i.c. inoculation of CBA/J mice with ML29 (588 LD50) results in death of 100% of mice, while an i.p. administration of the same dose did not induce clinical manifestation and all mice survived. Importantly, i.p.-inoculated mice were fully protected against a lethal i.c. challenge confirming previously published results with LASV challenge [28,29]. To determine the mechanism of protection in this model we aimed to evaluate humoral and CMI responses. CBA/J mice were immunized i.p. with ML29 or with alphavirus VLPV expressing modified LASV GPC [33] and challenged with ML29 (i.c.). Plasma samples were collected from immunized-challenged mice at designated time points after immunization and LASV antibodies were determined by IgG ELISA and by PRNT.
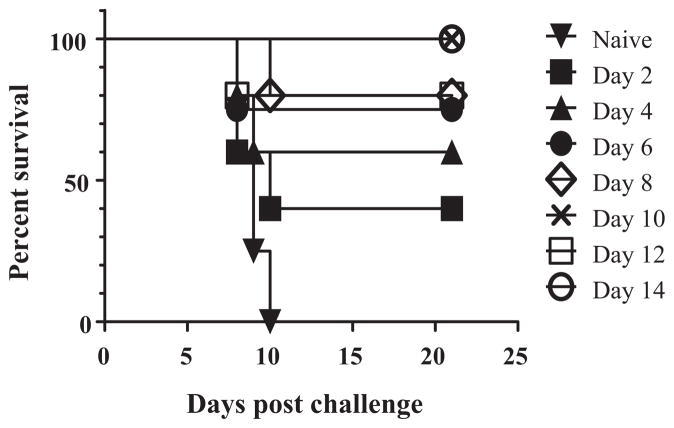
As seen in Fig. 1, all mice immunized either with a single-shot of ML29 or with VLPV-GPC using a prime-boost strategy were fully protected against lethal i.c. challenge, monitored for 21 days after challenge. Observation during the extended period of time (42 days after challenge) did not change survival kinetics of immunized animals [29]. While the VLPV-GPC immunization induced IgG antibody responses that peaked on week 4 and rapidly dropped at the end of the experiment, the ML29 immunization induced negligible antibody responses in IgG ELISA. Neutralizing antibodies were not detectable in both experimental groups, immunized with ML29 or with VLPV-GPC. These results are in line with previous observations indicating that protection and survival in animal models and in LF patients are not associated with antibody responses [2,7,31].
Fig. 1.
ML29 immunization does not elicit significant titers of IgG antibodies. (A), survival kinetics of immunized and challenged CBA/J mice. Mice were immunized with a single dose of ML29 (1 × 103 PFU, i.p.). VLPV-GPC group was primed with 107 IU of VLPV and boosted on day 7 with the same dose [33]. All mice were challenged on day 14 with ML29 (588 LD50, i.c.) and bled through day 21 after challenge. (B) Plasma samples were analyzed by IgG ELISA or PRNT as described in M&M.
3.2. Immunodominant epitope mapping of LASV GPC for optimization of IFN-γ ELISPOT assay and intracellular staining
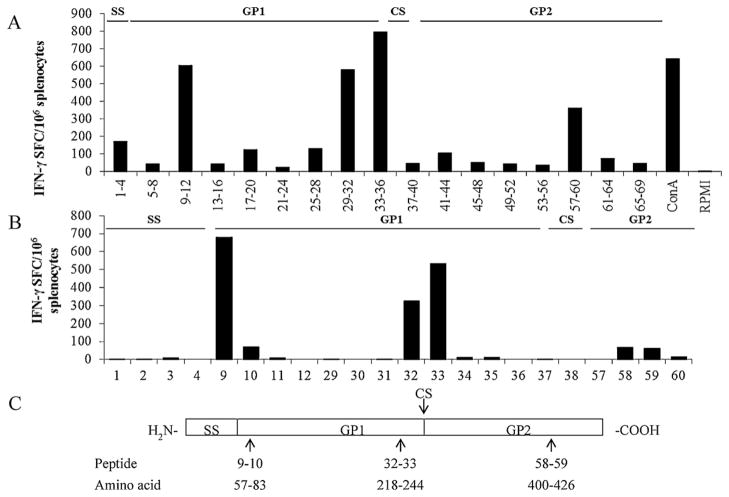
Initially, 69 peptides were tested in 17 pools and 5 pools with the highest responses in IFN-γ ELISPOT were additionally tested to identify individual peptides inducing the highest IFN-γ secretion. As seen in Fig. 2, analysis with individual peptides identified one region (peptides 9, 10) at the N-terminus of the GP1 sequence, two positive peptides (32 and 33) were found within the C-terminus of the GP1 (linked to cleavage sequence) and two positive peptides (58 and 59) were detected within the C-terminus of the GP2. These peptides corresponded to amino acid GPC sequences at positions 57–83, 218–44, and 400–426 (Fig. 2). Use of these peptides in ELISPOT and in intracellular staining significantly reduced secretion background caused by “non-specific” peptides within the library.
Fig. 2.
Identification of immunodominant regions within the GPC amino acid sequence. (A) Mapping of GPC H2k-restricted epitopes. Splenocytes collected on day 14 after ML29 immunization were stimulated with a peptide library (Mimotopes, Australia) consisting of overlapping 21-mer peptides derived from LASV GPC. Cytokine-secreting cells were enumerated in IFN-γ ELISPOT (U-CyTech biosciences, Utrecht, The Netherlands) and expressed as spot-forming cells, SFC. Responses to peptides derived from GP1 and GP2 are indicated by black bars. (B), secretion of IFN-γ by ML29-immune splenocytes after stimulation with individual peptides from Mimotopes library. (C) Mapping of LASV GPC epitopes. Immunogenic regions (arrows) are identified by peptide number within the library and the corresponding amino acid location within the GPC. SS, signal sequence; GP1, glycoprotein 1; CS, cleavage site; GP2, glycoprotein 2.
3.3. A single immunization with MOPV ML29 induces T cell responses detected by IFN-γ ELISPOT and by intracellular cytokine staining
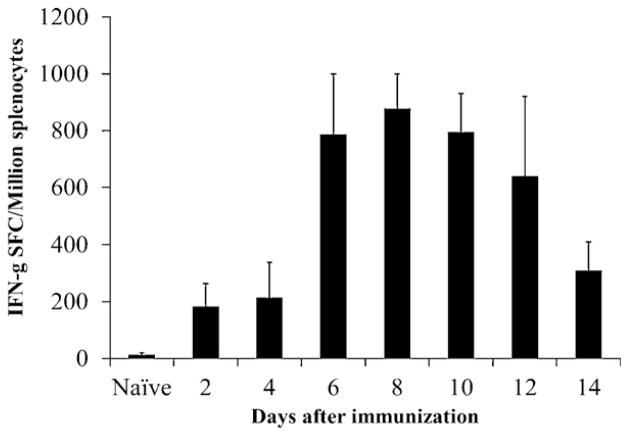
The lack of LASV-specific antibody responses in CBA/J mice survived ML29 i.c. challenge implicates CMI responses in protection. To prove that, CBA/J mice were immunized with 1 × 103 PFU of ML29 (i.p.), sacrificed at 2-day intervals during a 14 day time period, and splenocytes were stimulated overnight with the optimized LASV GPC cocktail. As seen in Fig. 3, IFN-γ SFC were detected as early as 2 days after immunization, rapidly increased at day 6, and peaked at day 8. After day 8, the number of IFN-γ SFC began gradually decreasing through the end of the experiment (day 14).
Fig. 3.
LASV-specific T cell responses detected in IFN-γ ELISPOT. CBA/J mice (n = 5) were immunized with 1 × 103 PFU of ML29 and sacrificed at designated time points. Erythrocyte-free splenocytes were prepared and incubated overnight with GPC peptides in IFN-γ ELISPOT assay. Error bars indicate standard deviations.
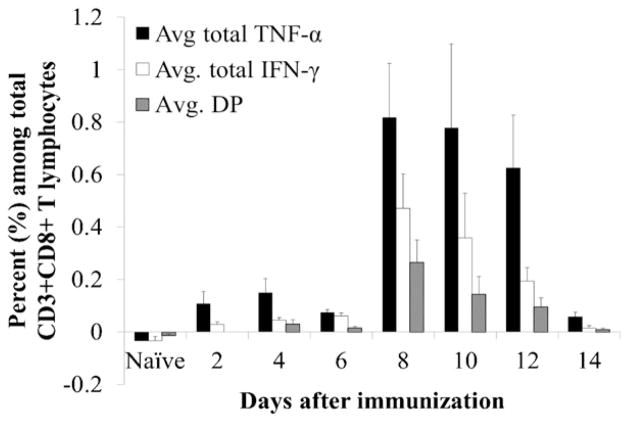
In addition to IFN-γ ELISPOT, we applied intracellular cytokine staining to quantitate numbers of CD8+ and CD4+ T cells secreting IFN-γ and TNF-α after stimulation with immunodominant LASV GPC-specific peptides. While we confirmed detection of LASV-specific CD3+CD8+ T cells by intracellular staining for TNF-α as early as 2 day after immunization (Fig. 4), the kinetic of this response was delayed in comparison to ELISPOT data. Numbers of LASV-specific IFN-γ+, TNF-α+, and double-positive cells did not increase until day 8, when they peaked reaching 0.4%, 0.8% and 0.3% of CD3+CD8+ T cells and subsequently decreased through the end of the experiment on day 14.
Fig. 4.
LASV-specific T cell responses detected by intracellular cytokine staining. The ML29-immune splenocytes were collected from mice (n = 5) at different time points after immunization, incubated with GPC peptides and then stained for either CD3 and CD4 surface markers or CD3 and CD8 surface markers to evaluate the LASV-specific responses in these two T cell populations. Subsequently, cells were permeabilized and stained for IFN-γ and TNF-α to evaluate the ability of these T lymphocytes to produce pro-inflammatory cytokines in response to LASV-GPC stimulation. Frequencies shown are based on CD3+CD4+ gated T lymphocytes. DP, double-positive for IFN-γ and TNF-α.
3.4. LASV-specific T cell cytotoxic activity in vivo
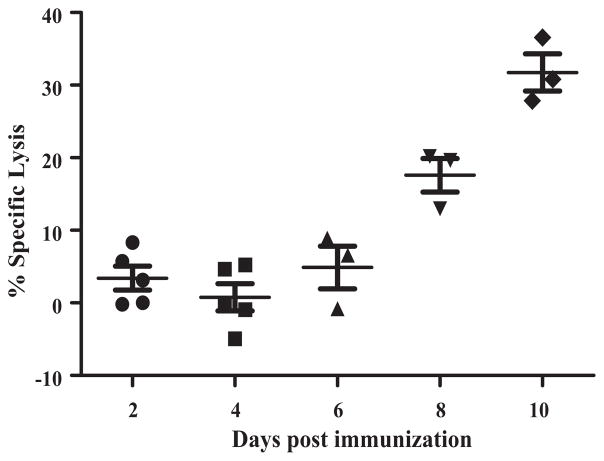
ELISPOT and intracellular staining are reliable indirect tools for evaluation of contribution of T cell responses in recovery and protection. To directly detect LASV-specific cytotoxic activity among the CD8+ population of T lymphocytes we conducted CTL assay in vivo. Splenocytes from naïve mice were stained with either CFSE or Cell Tracker™ Far Red. CFSE stained cells were subsequently pulsed with immunodominant LASV GPC peptides while Cell Tracker™ Far Red cells remained unstimulated. A 1:1 ratio of peptide-pulsed:peptide-unpulsed target splenocytes were transferred into recipient mice that were immunized with ML29 at the designated time points before transfer. Six hours after transfer, recipient mice were sacrificed and spleens were harvested and processed and the two different populations of target cells were enumerated by flow cytometry. As seen in Fig. 5, in good confirmation with previous results, the specific lysis was not significantly distinguishable from background levels on days 2–6. On day 8, specific lysis was ~20% and peaked on day 10 after immunization with a maximum lysis of peptide-pulsed targets reaching above 30%. These results confirm the presence of LASV-specific CD8+ cytolytic activity among splenocytes in response to a single ML29 immunization.
Fig. 5.
Detection of LASV-specific CTL activity in vivo. CBA/J mice (n = 5) were immunized with a single dose of ML29 at the designated time points prior to CTL assay. At each time point, immunized mice received 5 × 106 target splenocytes from naïve mice stained with CFSE and pulsed with LASV GPC peptides (Ag+) and 5 × 106 target splenocytes stained with Cell Tracker™ Far Red and left unpulsed (Ag−). Percent specific lysis was determined by the ratio of recovered Ag-labeled and non-labeled target cells and adjusted for background from naïve recipients. Only mice with >5000 recovered CFSE events were used for data analysis.
3.5. Protective efficacy of splenocytes from MOPV ML29 immunized mice
In the next experiments a splenocyte transfer protocol was employed to evaluate protective efficacy of donor splenocytes from ML29-immunized mice. Donor mice were immunized with ML29 and spleens were harvested at different time points. Recipient mice were challenged i.c. with ML29 (i.c.) and received donor splenocytes by i.v. inoculation. As seen in Fig. 6, donor splenocytes from mice 2 days after immunization protected only 40% of recipient mice. Survival percentage increased over time reaching full protection by day 10 (Fig. 6). With the exception of one (technical) death in the group receiving day 12 splenocytes, the kinetics of survival correlated with data from both IFN-γ ELISPOT, intracellular cytokine staining, and in vivo CTL assays, peaking between day 8 and 10.
Fig. 6.
Protective efficacy of splenocytes from ML29-immunized mice in transfer experiments. Donor mice were immunized with ML29 and sacrificed at indicated time points. Naïve recipient mice were challenged with 855 LD50 of ML29 (i.c.) and received 3.0 × 107 splenocytes from the indicated donor mice during 2 hours after challenge. Mice (n = 5) were monitored for lethality for 21 days after challenge. Data represented as percent (%) survival by standard Kaplan–Meier curve.
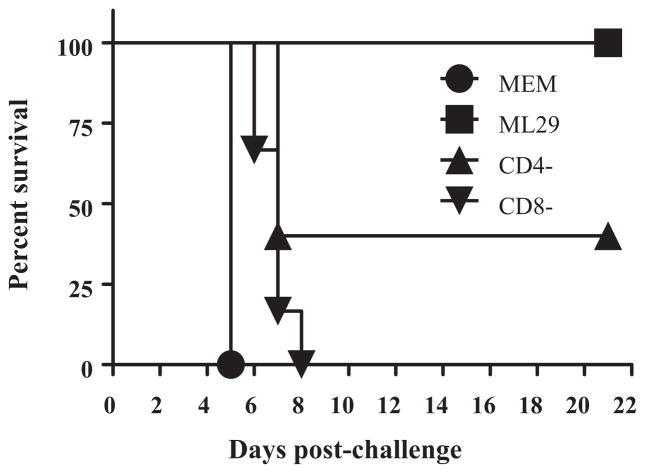
3.6. Recipient splenocytes depleted of CD8+ T cells did not protect mice against lethal challenge
To further evaluate the role of T cells in protection against a lethal i.c. challenge, adoptive transfer experiments were performed using recipient splenocytes depleted of CD4+ or CD8+ T cells. As seen in Fig. 7, all recipient mice receiving CD8-depleted splenocytes died after i.c. challenge by day 8. Recipient mice receiving CD4-depleted splenocytes were partially protected implicating a contribution of CD4+ T cells in LASV protection in mice as it was previously described [38].
Fig. 7.
CD8+ T lymphocytes are critical for protection against lethal challenge. CBA/J mice were immunized with 1 × 103 PFU of ML29 and sacrificed on day 10 and erythrocyte-free splenocytes were prepared. For ML29-immune splenocytes, fractions were depleted of CD4+ cells or CD8+ cells by MACS® magnetic beads. Recipient mice (n = 5–6 per group) were challenged with 855 LD50 of ML29 (i.c.) and received 3 × 107 splenocytes (no depletion, CD8-depleted, CD4+-depleted). Survival was monitored for 21 days post-challenge. MEM, donor splenocytes from mice injected with conditioned media (mock-immunization); ML29, total splenocytes from ML29-immunized mice; CD4-, donor splenocytes from ML29-immunized mice depleted of CD4 T lymphocytes; CD8-, donor splenocytes from ML29-immunized mice depleted of CD8 T lymphocytes.
4. Discussion
The goal of this study was to characterize LASV-specific CMI response in ML29-immunized mice and to figure out if these responses correlate with protection in these mice. Although mice do not accurately model human LF disease, they can provide an economical assay for vaccine potency in terms of capacity of vaccine candidates to elicit protective CMI responses. This type of small animal model for evaluation of CMI responses is especially needed when promising vaccine technology will be transferred from the laboratory to the manufacturing stage. An additional benefit afforded by this model is a lower level of biocontainment. This level must be lower than BSL-4 because pre-clinical vaccine development in the BSL-4 maximum containment laboratory is not practical.
Previously we screened different strains of mice on their susceptibility to LASV infection and have found that outcome of the infection depends critically on mouse genotype, age, and routes of virus inoculation [23,32,39]. During this screening we also found that i.c. inoculation of young adult CBA mice with reassortant ML29 as well as with both parental viruses, LASV and MOPV, resulted in a fatal LCMV-like convulsive immunopathological disease [28]. However, i.p. inoculation did no induce clinical manifestation and death. In contrast, this route of inoculation resulted in production of T cells capable of protecting recipient mice against subsequent lethal i.c. challenge with homologous virus in splenocyte transfer experiments. This adoptive transfer model is exquisitely specific in arenavirus-infected mice [40]. In good confirmation with this observation we did not see cross-protection between LASV and MOPV in splenocyte transfer experiments [28]. However, the classical immunization-challenge experiments revealed a partial cross-protection when 70% of mice immunized with LASV (i.p.) were protected against MOPV challenge (i.c.) and i.p. inoculation of MOPV protected 45% of mice against LASV [39]. Importantly, in immunization-challenge experiments and in splenocyte transfers ML29-immunized mice behaved like LASV-immunized mice indicating that the ML29 S RNA-encoded immunogens (LASV GPC and NP) induced LASV-specific protective CMI responses [28,29,31].
In our ML29-CBA/J model, survival of immunized mice was not associated with induction of detectable levels of anti-LASV antibodies (Fig. 1) in good confirmation with clinical and experimental observations that recovery from LASV infection occurred before the appearance of neutralizing antibodies. In this support, controlled clinical trials with human convalescent plasma containing high titers of antibodies against major LASV immunogens, NP and GPC, failed to show protective effects [41]. These observations indicate that CMI responses play a primary role in viral clearance. Indeed, injection of the ML29-immune splenocytes effectively eliminated LASV from tissues of challenged animals and on day 3 after immune transfer LASV was not detectable in tissues [29].
To optimize assays for evaluation of CMI responses we mapped H2k-restricted immunodominant epitopes and found that they were located at three amino acid positions: 57–83, 218–244, and 400–426. Interestingly enough, two of three recently detected HLA-A*0201-restricted epitopes were found at the same sites of LASV GPC, peptides GPC 60–68, SLYKGVYEL, and GPC 441–449, YLISIFLHL [42,43]. These peptides displayed high-affinity binding to HLA-A*0201, induced CD8+ T-cell responses of high functional avidity in HLA-A*0201 transgenic mice, and were naturally processed from native LASV GPC in human HLA-A*0201-positive target cells. Future studies are aimed at identifying H2k-restricted immunodominant epitopes more precisely to develop tetramer staining assay for enumeration of CD8+ T cell in our model. Flow cytometric experiments showed that the IFN-γ producing cells encompass both CD8+ T and CD4+ T cell populations. Intracellular cytokine staining also revealed poly-functional cells among the CD8+ T cell populations. These cells produced IFN-γ, TNF-α, or both cytokines after stimulation with LASV GPC-derived peptides (Fig. 4).
In order to detect LASV-specific cytotoxicity we employed an in vivo CTL assay to demonstrate that target cells pulsed with LASV-specific peptides were killed at a significantly higher rate than non-pulsed targets that were transferred into ML29 immunized mice. In this assay we observed clear evidence of LASV-specific lysis in immunized animals that began to increase by day 8 and peaked at day 10 as compared to naïve recipients (Fig. 5). These data correlate with IFN-γ ELISPOT and intracellular cytokine staining experiments in which responses also peaked between days 8 and 10 (Figs. 3 and 4). These in vivo CTL results provide an evidence of antigen-specific cytolytic activity in ML29-immunized mice.
To further evaluate the role of protective CMI responses in the ML29-CBA/J mouse model, we performed a kinetic analysis of protective potential of ML29-immune splenocytes. In these experiments ML29-immune splenocytes were collected at different time points after i.p. immunization and were used to rescue challenged mice. Protective T lymphocytes were detected in the spleen as early as 2 days after vaccination in good confirmation with our previous results in LASV-CBA model [29]. The cells collected on days 7 and 10 fully protected animals against challenge with LASV [29] and ML29 (Fig. 6), respectively. Taken together these results indicate that development of protective CMI responses is slightly delayed in ML29-CBA mice in comparison with LASV-CBA model and with classical LCMV infection in mice.
As seen in Fig. 7, all mice that received CD8-depleted splenocytes from ML29-immunized donors succumbed to an i.c. challenge, in line with the prediction that cytotoxic CD8 T cell populations play a crucial role in viral clearance and protection. Worth noting, mice that received CD4-depleted splenocytes from ML29 immunized donors displayed a 40% survival rate after a lethal i.c. challenge. These results indicate contribution of CD4+ T cells in development of protective CMI responses in mice. Previously, cross-protection against LCMV challenge mediated by a CD4+ T-cell clone specific for LASV GP2 epitope (403–417) was demonstrated in C3H/HeJ mice [38]. In LASV-infected individuals a highly conserved memory CD4+ epitope was also mapped to LASV GP2 glycoprotein [44]. The role of LASV-specific CD4+ cells in protection against acute infection and in the development of effective memory has to be further evaluated and the proposed ML29-CBA model can contribute to this field.
LCMV infection has been widely studied as a model for of CD8+ CTL-mediated protection and a replication-deficient LCMV has been recently proposed as a promising vector for the induction of potent CD8+ T cell immunity to control infections (e.g., HIV, HCV, tuberculosis and malaria) and for cancer immunotherapy [45]. The close relationship between LCMV, LASV and ML29 [28,29,46] and the similar behavior of these viruses in mice suggest that a ML29-CBA model can be a useful experimental tool.
The relative resistance of commonly used strains of laboratory mice to non-i.c. LASV challenge represents a major drawback in LF research. However, some attractive similarities between the LCMV murine model and LASV infection in mice can be very helpful for LASV research. First, CD8+ CTL responses are playing the major role in prevention and protection of experimental diseases caused by both viruses in mice, guinea pigs, and in NHP. CBA mice i.p. inoculated with LASV can serve as a promising model for study induction of protective immune responses. Substitution of LASV with MOPV reassortant clone ML29, an “immunological surrogate” of LASV, will allow conducting these experiments outside of BSL4 environment which is currently a major obstacle for LASV research. Identification of protective LASV CD8+ CTL epitopes restricted by HLA-A2 has allowed for studies in a HLA Tg mouse model [42,43]. Interestingly, in a small proportion of humanized MHC-I tg mice i.v. inoculated with LASV strain isolated from M. natalensis, mice developed some manifestations resembling those of human LF [47]. Thus, MHC-1 Tg mice can be potentially very useful in LF research as well. Second, vertical transmission of LASV seems to be involved in virus circulation in M. natalensis in West Africa. Testing of the existing models of circulation of rodent-borne viruses in LASV-murine systems will be helpful to elucidate the rodent-to-rodent transmission in endemic areas of LASV in West Africa. Third, knockout mice provide valuable tools to offer insight into the role of innate and adaptive immune responses in the pathogenesis and protection. Preliminary results indicate that STAT-1 and IFN-α/βR KO mice have had a partial susceptibility to LASV infection. The TLR2-MyD88-dependent signaling is involved in induction of pro-inflammatory cytokines, innate and adaptive immune responses during acute LCMV infection [48–52].
In summary, results presented in this manuscript indicate that CBA-ML29 model, with some limitations, can be potentially useful to assess protective potency of LASV-specific CMI responses outside of BSL-4 containment. This model has to be further validated in non-human primates.
Acknowledgments
This work was supported by RO1 grants AI052367 and AI093450 (to I.S.L.) and F31 AI082993 (to M.A.G) from the National Institute of Allergy and Infectious Disease. We would like to thank Peter Pushko for kindly providing the VLPV vectored LASV vaccine candidates. We would also like to acknowledge the Institute of Human Virology Animal Core Facility with a special thanks to Lanea George for the assistance in conducting animal studies.
References
- 1.McCormick JB, Fisher-Hoch SP. Lassa fever. Curr Top Microbiol Immunol. 2002;262:75–109. doi: 10.1007/978-3-642-56029-3_4. [DOI] [PubMed] [Google Scholar]
- 2.Salvato MS, Lukashevich IS. Vaccines against Lassa fever. In: Levine MM, editor. New generation vaccines. 4. Marcel Dekker, Inc; 2009. pp. 895–904. [Google Scholar]
- 3.Moraz M-L, Kunz S. Pathogenesis of arenavirus hemorrhagic fevers. Expert Rev Anti-infect Ther. 2011;9(1):49–59. doi: 10.1586/eri.10.142. 2011/08/23. [DOI] [PubMed] [Google Scholar]
- 4.Droniou-Bonzom ME, Reignier T, Oldenburg JE, Cox AU, Exline CM, Rathbun JY, et al. Substitutions in the glycoprotein (GP) of the Candid#1 vaccine strain of Junin virus increase dependence on human transferrin receptor 1 for entry and destabilize the metastable conformation of GP. J Virol. 2011 Oct 5;2011 doi: 10.1128/JVI.05616-11. JVI.05616-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salvato MS, Clegg JCS, Buchmeier MJ, Charrel RN, Gonzalez JP, Lukashevich IS, et al. Arenaviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus taxonomy, IXth report of the ICTV. London: Elsevier/Academic Press; 2005. pp. 715–23. [Google Scholar]
- 6.Oldstone M. Biology and pathogenesis of lymphocytic choriomeningitis virus infection. Curr Top Microbiol Immunol. 2002;263:83–117. doi: 10.1007/978-3-642-56055-2_6. [DOI] [PubMed] [Google Scholar]
- 7.Grant-Klein R, Altamura LA, Schmaljohn CS. Progress in recombinant DNA-derived vaccines for Lassa virus and filoviruses. Antivir Res. 2011;162(1–2):148–61. doi: 10.1016/j.virusres.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Khan SH, Goba A, Chu M, Roth C, Healing T, Marx A, et al. New opportunities for field research on the pathogenesis and treatment of Lassa fever. Antiviral Res. 2008;78(1):103–15. doi: 10.1016/j.antiviral.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Fichet-Calvet E, Rogers DJ. Risk maps of Lassa fever in West Africa. PLoS Negl Trop Dis. 2009;3(3):e38810. doi: 10.1371/journal.pntd.0000388. 1371/journal. pntd. 0000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loureiro ME, Wilda M, Levingston Macleod JM, D’Antuono A, Foscaldi S, Marino Buslje C, et al. Molecular determinants of Arenavirus Z protein homo-oligomerization and L polymerase binding. J Virol. 2011 Sep 28;2011 doi: 10.1128/JVI.05691-11. JVI. 05691-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richmond JK, Baglole D. Lassa fever: epidemiology, clinical features, and social consequences. BMJ. 2003 Nov;327(7426):1271–5. doi: 10.1136/bmj.327.7426.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paweska J, Sewlall NH, Ksiazek TG, Blumberg LH, Hale MJ, Lipkin WI. Nosocomial outbreak of novel arenavirus infection, southern Africa. Emerg Infect Dis. 2009;15:1598–602. doi: 10.3201/eid1510.090211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Briese T, Paweska JT, McMullan LK, Hutchison SK, Street C, Palacios G, et al. Genetic detection and characterization of lujo virus, a new hemorrhagic fever-associated arenavirus from Southern Africa. PLoS Pathog. 2009;4(5):e1000455. doi: 10.1371/journal.ppat.1000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishii A, Thomas Y, Moonga L, Nakamura I, Ohnuma A, Hang’ombe B, et al. Novel arenavirus Zambia. Emerg Infect Dis. 2011;17(10):1921–4. doi: 10.3201/eid1710.10452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cummins D, McCormick J, Bennett D, Samba J, Farrar B, Machin S, et al. Acute sensorineural deafness in Lassa fever. JAMA. 1990;264(16):2093–6. [PubMed] [Google Scholar]
- 16.Liao B, Byl FM, Adour KK. Audiometric comparison of Lassa fever hearing loss and idiopathic sudden hearing loss: evidence for viral cause. Otolaryngol Head Neck Surg. 1992;106(3):226–9. doi: 10.1177/019459989210600303. [DOI] [PubMed] [Google Scholar]
- 17.Macher AB, Wolfe MS. Historical Lassa fevers reports and 30-year clinical update. Emerg Infect Dis. 2006;12(5):835–7. doi: 10.3201/eid1205.050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neuman BW, Bederka LH, Stein DA, Ting JPC, Moulton HM, Buchmeier MJ. Development of peptide-conjugated morpholino oligomers as panarenavirus inhibitors. Antimicrob Agents Chemother. 2011 Oct;55(10):4631–8. doi: 10.1128/AAC.00650-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borio L, Inglesby T, Peters CJ, Schmaljohn AL, Hughes JM, Jahrling PB, et al. Hemorrhagic fever viruses as biological weapons: medical and public health management. JAMA. 2002;287(18):2391–405. doi: 10.1001/jama.287.18.2391. [DOI] [PubMed] [Google Scholar]
- 20.Cuevas CD, Lavanya M, Wang E, Ross SR. Junin virus infects mouse cells and induces innate immune responses. J Virol. 2011 Nov;85(21):11058–68. doi: 10.1128/JVI.05304-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters CJ, Jahrling PB, Liu CT, Kenyon RH, McKee KT, Jr, Oro JGB. Experimental studies of arenaviral hemorrhagic fevers. Curr Top Microbiol Immunol. 1987;134:5–68. doi: 10.1007/978-3-642-71726-0_2. [DOI] [PubMed] [Google Scholar]
- 22.Gowen BB, Holbrook MR. Animal models of highly pathogenic RNA viral infections: hemorrhagic fever viruses. Antivir Res. 2008;78:79–90. doi: 10.1016/j.antiviral.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Lukashevich IS, Vela EM. Pathogenesis of Lassa virus infection in experimental animals. In: Vela EM, editor. Molecular pathogenesis of hemorrhagic fever viruses. Kerala, India: Transw Res Net; 2010. pp. 101–42. [Google Scholar]
- 24.Carrion R, Jr, Brasky K, Mansfield K, Johnson C, Gonzales M, Ticer A, et al. Lassa virus infection in experimentally infected marmosets: liver pathology and immunophenotypic alterations in target tissues. J Virol. 2007 Apr;81(12):6482–90. doi: 10.1128/JVI.02876-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baize S, Marianneau P, Loth P, Reynard S, Journeaux A, Chevallier M, et al. Early and strong immune responses are associated with control of viral replication and recovery in Lassa virus-infected cynomolgus monkeys. J Virol. 2009 Jun;83(11):5890–903. doi: 10.1128/JVI.01948-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sbrana E, Mateo RI, Xiao S-Y, Popov VL, Newman PC, Tesh RB. Clinical laboratory, virologic, and pathologic changes in hamsters experimentally infected with pirital virus (arenaviridae): a rodent model of Lassa fever. Am J Trop Med Hyg. 2006 Jun;74(6):1096–102. [PubMed] [Google Scholar]
- 27.Zapata JC, Pauza CD, Djavani MM, Rodas JD, Moshkoff D, Bryant J, et al. Lymphocytic choriomeningitis virus (LCMV) infection of macaques: a model for Lassa fever. Antivir Res. 2011;92(2):125–38. doi: 10.1016/j.antiviral.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lukashevich IS. Generation of reassortants between African arenaviruses. Virology. 1992;188(2):600–5. doi: 10.1016/0042-6822(92)90514-p. [DOI] [PubMed] [Google Scholar]
- 29.Lukashevich IS, Patterson J, Carrion R, Moshkoff D, Ticer A, Zapata J, et al. A live attenuated vaccine for Lassa fever made by reassortment of Lassa and Mopeia viruses. J Virol. 2005;79(22):13934–42. doi: 10.1128/JVI.79.22.13934-13942.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lukashevich IS, Patterson J, Carrion R, Jr, Brasky K, Mansfield K, Zapata J, et al. Safety, immunogenicity, and efficacy of the ML29 vaccine for Lassa fever in small non-human primates. Vaccine. 2008;26:5246–54. doi: 10.1016/j.vaccine.2008.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lukashevich IS. A live reassortant vaccine for Lassa fever. In: Vela EM, editor. Molecular pathogenesis of hemorrhagic fever viruses. Kerala, India: Transw Res Net; 2010. pp. 143–70. [Google Scholar]
- 32.Lukashevich IS. Lassa virus lethality for inbred mice. Ann Soc Belg Med Trop. 1985;65(2):207–9. [PubMed] [Google Scholar]
- 33.Pushko P, Geisbert J, Parker M, Jahrling P, Smith J. Individual and bivalent vaccines based on alphavirus replicons protect guinea pigs against infection with Lassa and Ebola viruses. J Virol. 2001 Dec;75(23):11677–85. doi: 10.1128/JVI.75.23.11677-11685.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lukashevich IS, Rodas JD, Tikhonov II, Zapata JC, Yang Y, Djavani M, et al. LCMV-mediated hepatitis in rhesus macaques: WE but not ARM strain activates hepatocytes and induces liver regeneration. Arch Virol. 2004;149(12):2319–36. doi: 10.1007/s00705-004-0385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lukashevich IS, Clegg JC, Sidibe K. Lassa virus activity in Guinea: distribution of human antiviral antibody defined using enzyme-linked immunosorbent assay with recombinant antigen. J Med Virol. 1993;40(3):210–7. doi: 10.1002/jmv.1890400308. [DOI] [PubMed] [Google Scholar]
- 36.Jiang X, Dalebout TJ, Bredenbeek PJ, Carrion R, Jr, Brasky K, Patterson J, et al. Yellow fever 17D-vectored vaccines expressing Lassa virus GP1 and GP2 glycoproteins provide protection against fatal disease in guinea pigs. Vaccine. 2011;29(6):1248–57. doi: 10.1016/j.vaccine.2010.11.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barber DL, Wherry EJ, Ahmed R. Cutting edge: rapid in vivo killing by memory CD8 T cells. J Immunol. 2003 Jul;171(1):27–31. doi: 10.4049/jimmunol.171.1.27. [DOI] [PubMed] [Google Scholar]
- 38.La Posta VJ, Auperin DD, Kamin-Lewis R, Cole GA. Cross-protection against lymphocytic choriomeningitis virus mediated by a CD4+ T-cell clone specific for an envelope glycoprotein epitope of Lassa virus. J Virol. 1993;67(6):3497–506. doi: 10.1128/jvi.67.6.3497-3506.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barkar ND, Lukashevich IS. Lassa and Mozambique viruses: cross protection in experiments on mice and action of immunosuppressants on experimental infections. Vopr Virusol. 1989;34:598–603. [PubMed] [Google Scholar]
- 40.McIntyre KM, Bukovski JF, Welsh RM. Exquisite specificity of adoptive immunization in arenavirus-infected mice. Antivir Res. 1985;5:299–305. doi: 10.1016/0166-3542(85)90044-0. [DOI] [PubMed] [Google Scholar]
- 41.McCormick JB, King IJ, Webb PA, Scribner CL, Craven RB, Johnson KM, et al. Lassa fever, effective therapy with ribavirin. N Engl J Med. 1986;314(1):20–6. doi: 10.1056/NEJM198601023140104. [DOI] [PubMed] [Google Scholar]
- 42.Botten J, Alexander J, Pasquetto V, Sidney J, Barrowman P, Ting J, et al. Identification of protective Lassa virus epitopes that are restricted by HLA-A2. J Virol. 2006 Sep;80(17):8351–61. doi: 10.1128/JVI.00896-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boesen A, Sundar K, Coico R. Lassa fever virus peptides predicted by computational analysis induce epitope-specific cytotoxic-T-lymphocyte responses in HLA-A2. 1 Transgenic Mice. Clin Diagn Lab Immunol. 2005 Oct;12(10):1223–30. doi: 10.1128/CDLI.12.10.1223-1230.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meulen J, Badusche M, Satoguina J, Strecker T, Lenz O, Loeliger C, et al. Old and New World arenaviruses share a highly conserved epitope in the fusion domain of the glycoprotein 2, which is recognized by Lassa virus-specific human CD4+ T-cell clones. Virology. 2004;321(1):134–43. doi: 10.1016/j.virol.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 45.Kotturi MF, Swann JA, Peters B, Arlehamn CL, Sidney J, Kolla RV, et al. Human CD8+ and CD4+ T cell memory to lymphocytic choriomeningitis virus infection. J Virol. 2011 Nov;85(22):11770–80. doi: 10.1128/JVI.05477-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hastie KM, Liu T, Li S, King LB, Ngo N, Zandonatti MA, et al. Crystal structure of the Lassa virus nucleoprotein–RNA complex reveals a gating mechanism for RNA binding. Proceedings of the National Academy of Sciences. 2011 doi: 10.1073/pnas.1108515108. %R 101073/pnas1108515108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flatz L, Rieger T, Merkler D, Bergthaler A, Regen T, Schedensack M, et al. T cell-dependence of Lassa fever pathogenesis. PLoS Pathogen. 2010;6(3):e1000836. doi: 10.1371/journal.ppat.1000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou S, Kurt-Jones EA, Mandell L, Cerny A, Chan M, Golenbock DT, et al. MyD88 is critical for the development of innate and adaptive immunity during acute lymphocytic choriomeningitis virus infection. Eur J Immunol. 2005;35(3):822–30. doi: 10.1002/eji.200425730. [DOI] [PubMed] [Google Scholar]
- 49.Zhou S, Cerny AM, Zacharia A, Fitzgerald KA, Kurt-Jones EA, Finberg RW. Induction and inhibition of type I interferon responses by distinct components of lymphocytic choriomeningitis virus. J Virol. 2010 Sep;84(18):9452–62. doi: 10.1128/JVI.00155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou S, Kurt-Jones EA, Cerny AM, Chan M, Bronson RT, Finberg RW. MyD88 intrinsically regulates CD4 T-cell responses. J Virol. 2009 Feb;83(4):1625–34. doi: 10.1128/JVI.01770-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jung A, Kato H, Kumagai Y, Kumar H, Kawai T, Takeuchi O, et al. Lymphocytoid choriomeningitis virus activates plasmacytoid dendritic cells and induces a cytotoxic T-cell response via MyD88. J Virol. 2008 Jan;82(1):196–206. doi: 10.1128/JVI.01640-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou S, Halle A, Kurt-Jones EA, Cerny AM, Porpiglia E, Rogers M, et al. Lymphocytic choriomeningitis virus (LCMV) infection of CNS glial cells results in TLR2-MyD88/Mal-dependent inflammatory responses. J Neuroimmunol. 2008;194(1–2):70–82. doi: 10.1016/j.jneuroim.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]