Abstract
Identification and quantification of immunogenic peptides and tumor-derived epitopes presented on MHC-I molecules are essential for basic studies and vaccines generation. Although lymphocytes derived from transgenic mice can serve as sensitive detectors of processes of antigen presentation and recognition, they are not always available. The use of cell lines might be extremely useful. In this study, we generated a lacZ inducible CD8+ hybridoma (BUSA14) capable of recognizing both human and mouse gp10025–33 melanoma antigens presented on dendritic and tumor cell lines. This hybridoma expresses a variety of membranal T cell markers and secretes IL-2 and TNFα. Thus, BUSA14 offers a quantifiable, cheap and straightforward tool for studying peptide presentation by MHC-I molecules on the cell surface.
Introduction
The key event in T cell activation is the recognition of a peptide bound to major histocompatibility complex (MHC) molecules on the surface of antigen presenting cells (APCs). The enormous pool of peptides displayed on MHC makes it almost impossible to detect a given peptide-MHC complex on the surface of APCs by using conventional indirect methods. On the other hand, direct recognition of a selected peptide by the TCR results in generation of intracellular signals leading to initiation of the primary stages of T cell activation [1]. To facilitate measurement of T cell activation and to enable identification of individual clones, β-galactosidase (lacZ) inducible CD4+ and CD8+ T cell hybrids were developed. Previous studies showed that heterologous Escherichia coli β-galactosidase (lacZ) gene, under control of the IL-2 entire enhancer region or the nuclear factor in activated T cells (NFAT) element alone, is specifically induced in transfected and activated T cells [2] [3] [4]. Thus, activation of transfected T cells, results in synthesis of both IL-2 and lacZ gene products. Moreover, since the lacZ remains sequestered within the activated cells, chromogenic or fluorogenic substrate enables measurement of an activating event in a single T cell [5] [3] [4]. Generation of the hybrids is relatively easy and allows maintenance in culture and the lacZ assay provides a rapid, sensitive and non-radioactive method for measuring T cell activation [1]. In this study we isolated T cells from Pmel-1 mice and generated a lacZ inducible CD8+ T cell hybridoma. The hybridoma possesses a TCR specific for the H-2Db derived human and mouse gp10025–33 peptides, recognizes specific Ag-MHC complex on the surface of a dendritic cell line (DCs) or tumor cells, secretes T cell related cytokines and expresses a variety of membranal T cell markers.
Materials and Methods
Mice
Pmel-1 mice carry a rearranged T cell receptor transgene specific for the H-2Db restricted, human gp10025–33 peptide [6] were originally purchased from the Jackson laboratory (Bar Harbor, ME, USA). Animals were maintained and treated according to the Weizmann Institute of Science and National Institute of Health guidelines. All experiments in mice were approved by the Institutional Use and Care Committee (IACUC) of the Weizmann Institute of Science.
Cells
The OVA257–264–specific, H-2Kb-restricted CTL hybridoma, B3Z [7], and the BWZ.36/CD8α fusion partner [2] were kindly provided by Dr. N. Shastri, University of California, Berkeley, USA. Cells were grown in lymphocyte medium containing RPMI 1640+HEPES (Invitrogen, Carlsbad, CA, USA), 10% FCS (HyClone, Bonn, Germany), 2 mM glutamine (Invitrogen), 1% Sodium pyruvate (Invitrogen), 1% non-essential amino acids (Invitrogen), 5×10−5 M β-mercaptoethanol (βME) and Penicillin-Streptomycin combined antibiotics. To avoid loss of CD8 expression, lymphocyte medium was supplemented with 1 mg/ml G418 (Invitrogen) for selection. Both B3Z and BWZ.36/CD8α harbor the NFAT-lacZ inducible reporter gene for T cell activation. The C57BL/6 (H-2b)-derived immortalized DC line DC2.4 [8] was kindly provided by Dr. K. Rock, UMass Medical School, North Worcester, MA, USA. DC2.4 cells were cultured in RPMI 1640 medium supplemented with 10% FCS, 2 mM L-glutamine and combined antibiotics. The C57Bl/6 derived, highly metastatic, poorly immunogenic, low MHC class-I expressing cell line B16-F10.9 (F10.9) [9], the high-metastatic, low-immunogenic D122 clone of the 3LL carcinoma, of C57BL/6 (H-2b) origin and the carcinogen-induced T cell lymphoma EL4 cells (H-2b) were grown in DMEM (Invitrogen) containing 10% FCS, 2 mM L-glutamine, 1 mM sodium pyruvate, 1% nonessential amino acids and 1% Penicillin-Streptomycin combined antibiotics. The C57Bl/6 derived, chicken Ovalbumin transfected, highly metastatic B16-MO5 [10] cell line was cultured in B16-F10.9 medium supplemented with 2 mg/ml G-418. Both F10.9 and B16-MO5 over express the tumor associated murine gp100 protein.
Generation of T cell hybridomas
Total splenocytes were isolated from spleens of Pmel-1 mice. Cells were washed once with PBS and resuspended in 6 ml OptiMEM medium (Invitrogen). Four ml were transferred into flasks containing 40 ml of lymphocyte medium and incubated at 37°C. As sensitizing cells, Two ml were incubated with 30 µg/ml hgp10025–33 peptide for 2 hours, diluted in 10 ml lymphocyte medium and added to the flasks. Four days later, Cells were washed once with PBS, separated on Lympholyte-M (Cedarlane, Burlington, NC, USA) and fused with the BWZ.36/CD8α cells using polyethylene glycol (PEG1500; Boehringer Mannhiem, Indianapolis, IN, USA) as described before [1]. Briefly, equal numbers (10×106) of lymphocytes and BWZ.36/CD8α cells were mixed in a 50 ml conical centrifuge tube and washed once in pre-warmed serum-free RPMI 1640 medium. The supernatant was aspirated and the pellet was loosened by gentle tapping. One ml of 50% PEG was slowly added during 90 seconds. The PEG was then diluted with 10 ml warm serum-free medium and the tube was placed in a 37°C water bath for 8 minutes. Then, cells were centrifuged, resuspended in lymphocyte medium to 3×105/ml, and added (0.1 ml) to each well of 96 well plates. Twenty-four hours later, HAT and hygromycin were added (final concentrations of 1.36 mg/ml hypoxanthine (Sigma), 17.6 mg/ml aminopterin (Sigma), 388 mg/ml thymidine (Sigma), and 400 U/ml hygromycin (Invitrogen). Resistant clones were observed starting 10–15 days later. All clones were tested for antigen recognition in T cell activation assay as described in section 2.6.
Antibodies and peptides
The anti mouse APC-CD279, APC-CD44, FITC-CD69, FITC-CD8, FITC-CD62L, PerCp-Cy5.5-IFNγ, eFluor450-TNFα, PE-IL-4, PE-TCRVβ13, PE-Cy7-CD8 and AlexaFluor 488-CD107a were all purchased from eBioscience (San Diego, CA, USA). Antibodies against mouse CTLA4 and FcγII/III receptors (2.4G2 hybridoma, Fc Block) were purchased from BioXcell (West Lebanon, NH, USA). The APC conjugated anti mouse IL-2 was purchased from BD (Becton Dickinson, San Jose, CA, USA). The FITC-conjugated F(ab′)2 fragment donkey anti-mouse IgG (H+L) was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). The OVA257–264 (SIINFEKL), human gp10025–33 (KVPRNQDWL) and mouse gp10025–33 (EGSRNQDWL) peptides were synthesized by Sigma-Aldrich (Rehovot, Israel).
Flow cytometry
Indirect staining
One million cells were harvested, washed twice using 3 ml FACS buffer (0.5% BSA, 0.1% sodium azide in PBS) and incubated with 1 µg/ml of primary antibody (Ab) for 1 hour at 4°C. Samples were washed twice with FACS buffer and stained with 1 µg/ml of FITC-labeled secondary Ab for 1 hour at 4°C. Then washed twice, resuspended in 0.5 ml cold PBS with 0.1% sodium azide and analyzed.
Direct staining
Cells were harvested, washed once with cold FACS buffer, and incubated for 30 minutes at 4°C in the dark with antibodies (at the concentrations recommended by the manufacturer). Cells were incubated for and washed once using 3 ml FACS buffer, resuspended in 0.5 ml PBS with 0.1% sodium azide and analyzed by flow cytometry.
Intracellular cytokine staining
DC2.4 cells, 2.5×106, were harvested and incubated with the relevant peptides at 10 µg/ml in OptiMEM for 1 hour. Then, 6×105 peptide loaded DC2.4 and 2×106 hybridoma cells were added to a 24 well plate followed by centrifugation at 1000 rpm for 5 minutes at 18°C. As positive control for hybridoma activation, 50 ng/ml PMA (Phorbol 12-Myristate 13-Acetate, Sigma) and 750 ng/ml ionomycin (Sigma) were add to some wells. The plates were incubated for 2 hrs at 37°C, 5% CO2. Brefeldin A (BFA, eBioscience), at a final concentration of 3 µg/ml was added to all wells and the plates were centrifuged at 500 rpm, 5 min at 18°C. Following incubation for 4 additional hours, cultures were harvested, washed with staining buffer and fixated with 0.1 ml fixation buffer (PBS w/o Ca and Mg+4% paraformaldehyde) at 4°C for 20 minutes. Then washed twice and resuspended in 1 ml permeabilizing solution (PBS (w/o Ca and Mg supplemented with 0.1% Saponin, 5% FCS and 0.1% azide) for 15 minutes at 4°C. Samples were then washed and stained for cell surface markers and intracellular cytokines. All samples were analyzed by SORP LSRII (Becton Dickinson, San Jose, CA, USA) and FlowJo software (ThreeStar, San Carlos, CA, USA).
T cell hybridoma activation assay
A. DC2.4 cells were loaded with varying concentrations (0.001–100 µg/ml) of human or mouse gp10025–33. Culture were set in 96-well plates by adding equal numbers (6×104) of peptide-loaded DC2.4 and hybridoma cells for 12 hours.
B. DC2.4 cells were loaded with 50 µg/ml hgp10025–33 or control peptide. Cultures were set in 96-well plates by mixing 6×104 of peptide-loaded DC2.4 and 4.7×102–6×104 hybridoma cells for 12 hours.
For both assays, growth medium was removed and cells were washed once with 100 µl PBS. For lysis, 100 µl of lysis buffer (PBS with 9 mM MgCl2, 0.125% NP40) containing 0.3 mM chlorophenol red β-D galactopyranoside (CPRG (Sigma) were added to each well, mixed, and clear lysates were transferred into new 96 well plates. One to twenty four hours later, the optical density of each well was detected with a Synergy HT Multi-Mode Microplate reader (BioTek Winooski, VT, USA) at 570 nm using 630 nm as reference.
Cytotoxicity assays
Hybridomas were washed and incubated for 4 hours with L-[35S]methionine (PerkinElmer, Waltham, MA, USA) labelled target cells at different Effector∶Target ratios [11]. The percentage of specific lysis of triplicates was calculated as follows: (average experimental cpm - average spontaneous cpm)/(average maximum cpm - average spontaneous cpm)×100. Maximal L-[35S]methionine containing protein release was obtained by lysis of target cells with 0.1 M NaOH.
Results
Generation of human and mouse gp10025–33 specific T cell hybridoma
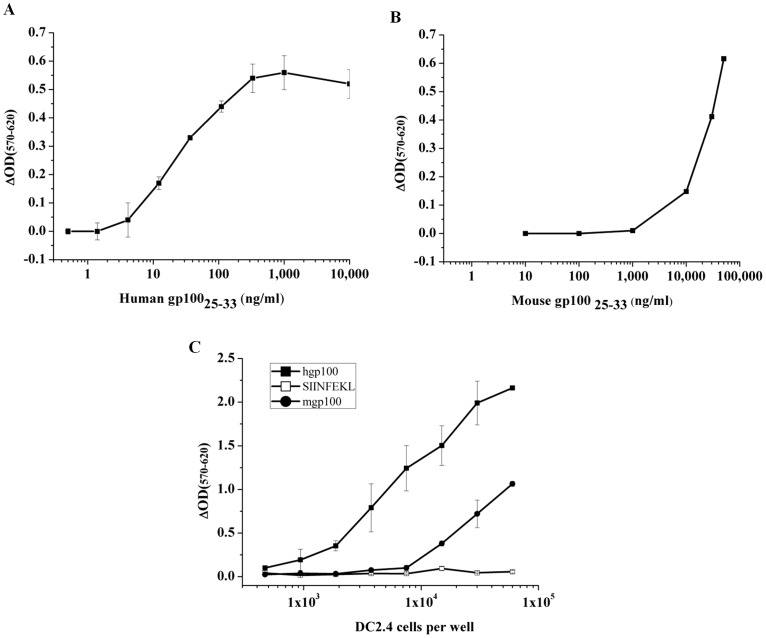
In order to produce LacZ inducible T cell hybrids, specific to human and mouse gp10025–33 peptides, we took advantage of CD8+ T cells isolated from Pmel-1 mice [6], which carry a transgenic TCR specific for the Pmel17/gp100 derived, H-2Db restricted gp10025–33 peptide. Lymphocytes isolated from Pmel-1 mice were activated in-vitro by peptide and fused with the BWZ.36/CD8α cells [2] harboring the NFAT-lacZ inducible reporter gene for T cell activation. After drug selection, hybrids were subjected to screening assays in order to identify hgp10025–33 specific clones (data not shown). Few positive clones were detected and one of them, named BUSA14, was selected for further functional and morphological characterizations. First, we measured the antigen induced LacZ response to mouse and human gp10025–33 peptides. As APC we used DC2.4 [8], a C57BL/6 derived immortalized DC line, which expresses both H-2Kb and H-2Db molecules at high levels on the cell surface, thus allowing efficient peptide loading and presentation. DC2.4 cells were loaded with varying amounts of mouse or human gp10025–33 and subjected to hybridoma activation assays with BUSA14 cells. As shown in Fig. 1A and B, both mouse and human gp10025–33 loaded DC2.4 cells elicited activation of BUSA14 cells. Major differences can be seen in the dose response to the two peptides, presumably as a result of the increased affinity of hgp10025–33 to H-2Db molecules, allowing long lasting complexes on the cell surface of DC2.4 cells. In another experiment, serial dilutions of DC2.4 cells, pre- loaded with 50 µg/ml mouse or human gp10025–33, were incubated with a constant number of BUSA14 cells. As shown in Fig. 1C, hgp10025–33 loaded DC2.4 cells are by far more efficient in activating BUSA14 cells.
Figure 1. BUSA14 cells are efficiently activated by mouse and human gp10025–33 peptides.
DC2.4 cells were loaded with hgp10025–33 at doses ranging from 0.5 to 10000 ng/ml (A) or mgp10025–33 (10 to 50000 ng/ml)(B). Then co-cultured with BUSA14 for 12 hours. Cells were then lysed and β-Gal enzymatic activity was detected with CPRG. Representative results (1 of 3 experiments) are presented as ΔOD (sample OD-background OD) measured after 3 hours. C. Sixty thousand BUSA14 cells/well were incubated in triplicate overnight with serial dilutions of DC2.4 cells loaded with 50 µg/ml hgp10025–33 or mgp10025–33. SIINFEKL loaded DC2.4 cells were used as negative control. Assays were done as before and presented as ΔOD measured after 24 hours.
Surface molecules expressed by resting and activated BUSA14 cells
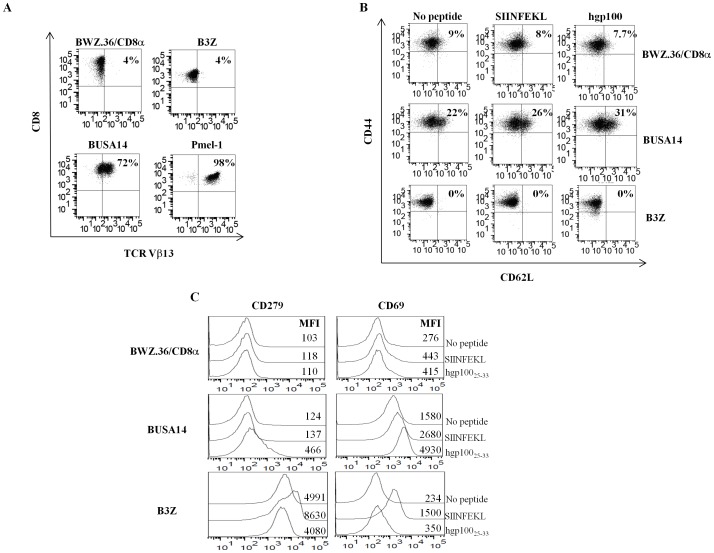
To further characterize the generated hybridoma, cells were analyzed for surface expression of CD8 and the Pmel-1 specific TCR Vβ13 chain. The results presented in Fig. 2A clearly indicate that only BUSA14 and not B3Z or BWZ.36/CD8α express TCRVβ13. In order to test whether restimulation with hgp10025–33 promotes the differentiation of BUSA14 cells towards later activation stages, we performed an activation assay. DC2.4 cells were loaded with hgp10025–33 and co-incubated for 12 hours with BUSA14, B3Z or BWZ.36/CD8α cells. The mixed cultures were analyzed by flow cytometry for surface expression of CD69, CD279, CD62L and CD44. As shown in Fig. 2B, all 3 hybridomas retained their effector phenotype (CD44hi/CD62Llow) following peptide stimulation. As shown in Fig. 2C, CD69 levels were up-regulated both in activated BUSA14 and B3Z cells while CD279 up-regulation occurred only in B3Z cells.
Figure 2. T cell markers expressed by BUSA14 cells.
A. BUSA14, B3Z and BWZ.36/CD8α cells were analyzed by flow cytometry and monoclonal antibodies against CD8 and TCR Vβ13 to confirm the Pmel-1 TCR expression. B. BUSA14, BWZ.36/CD8α and B3Z cells were co-cultured with hgp10025–33 or SIINFEKL for 12 hours, or remained untreated. Cells were stained with antibodies to CD8, CD62L and CD44 and analyzed by flow cytometry. Cells were gated for CD8 to exclude DC2.4 cells. C. Two million BUSA14, BWZ.36/CD8α and B3Z cells were co-cultured with 6×105 DC2.4 loaded with hgp10025–33 or SIINFEKL for 12 hours, or remained untreated. Then stained with antibodies against CD8, CD69, CD279 and analyzed by flow cytometry. Cells were gated for CD8 to exclude DC2.4 cells. Mean fluorescent intensity (MFI) values are presented in the figure. This figure is a representative of three experimental repeats.
Detection of cytokines produced by resting and activated BUSA14 cells
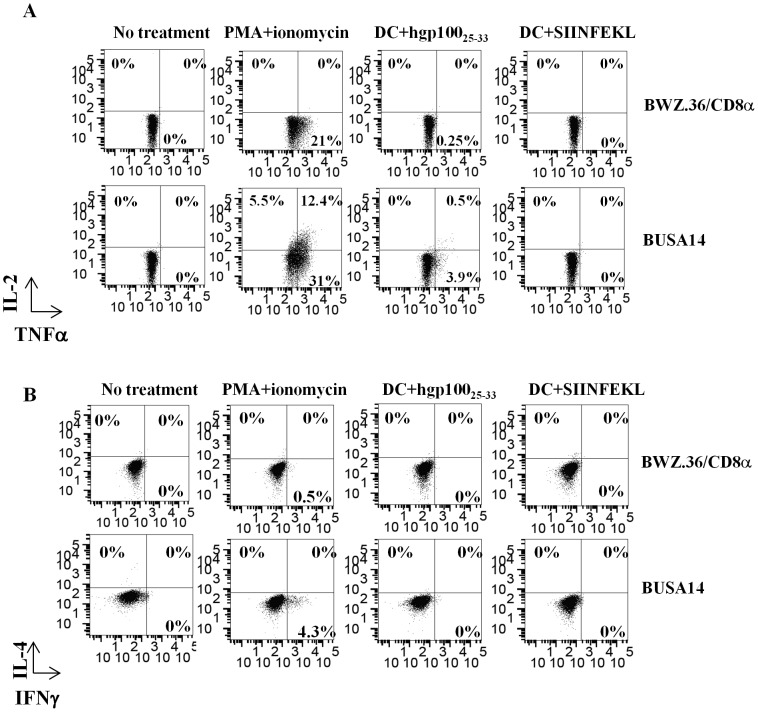
BUSA14 and BWZ.36/CD8α Cells were co-incubated with DC2.4 loaded with hgp10025–33 or SIINFEKL for 6 hours and intracellular stained with antibodies against CD8, IL-2, IL-4, TNFα, IFNγ and CD107a. Cells co-cultured with unloaded DC2.4 or PMA and ionomycin served as negative and positive controls, respectively. As shown in Fig. 3, BUSA14 cells produced a variety of cytokines following activation with PMA and ionomycin. Thirty percent of BUSA14 cells produced TNFα, 12% produced TNFα and IL-2, 6% and 4% produced IL-2 and IFNγ respectively. Following activation with hgp10025–33 loaded DC2.4, only 4% of the cells generated low amounts of TNFα. BWZ.36/CD8α cells showed moderate TNFα expression only following activation by PMA and ionomycin. We could not detect CD107a on BUSA14 or BWZ.36/CD8α following incubation in presence of peptide or PMA (data not shown).
Figure 3. Detection of cytokines produced by BUSA14 cells.
BUSA14 or BWZ.36/CD8α were co-incubated with DC2.4 cells loaded with hgp10025–33 or SIINFEKL. Cells alone or co-cultured with unloaded DC2.4 or with PMA and ionomycin served as negative and positive controls, respectively. All cells were intracellulary stained with antibodies to CD8, IL-2/TNFα (A), IL-4/IFNγ (B) and analyzed by flow cytometry. Cells were gated for CD8 to exclude DC2.4 cells.
Activation of BUSA14 cells did not result in cytotoxic capacity
Aiming at further investigating whether BUSA14 cells are activated following presentation of mgp100 by melanoma cells, we incubated the hybrids with F10.9 or B16-MO5. Co-culturing with D122 clone of 3LL lung carcinoma served as negative control. Since gp10025–33 is presented on H-2Db, these tumor lines were analyzed for membranal MHC-I (Fig. 4A, EL4 cells served as positive control). In another experiment, the tumor cells were loaded with hgp10025–33 or SIINFEKL before co-culturing with BUSA14 or BWZ.36/CD8α As shown in Fig. 4B, incubation in presence of all three hgp10025–33 loaded tumor lines resulted in activation of BUSA14 as detected by CPRG assays. We than tested whether BUSA14 cells are activated by the endogenously processed mgp10025–33 peptide on the surface of B16-MO5 and F10.9 melanoma lines. CPRG assays were done following 12 hours of culturing. Co-incubation with D122 cells served as reference for CPRG background levels. As shown in Fig. 4C, BUSA14 cells recognized the mgp10025–33 on the surface of both B16-MO5 and F10.9 tumor lines. Although BUSA14 cells were activated by mgp10025–33 presented on melanoma lines, we could not detect any killing of these cells by BUSA hybrids (data not shown).
Figure 4. BUSA14 are activated by hgp10025–33 and mgp10025–33 presented on melanoma cell lines.
A. B16-MO5, F10.9, D122 and EL4 tumor cell lines were analyzed by flow cytometry with monoclonal antibodies to H-2Kb and H-2Db to analyze MHC-I membranal expression. MFI values are presented in the figure. B. Twenty thousand B16-MO5, F10.9 and D122 cells were loaded with 30 µg/ml hgp10025–33 or SIINFEKL peptides. Cells were washed and co-incubated with 6×104 BUSA14 and BWZ.36/CD8α for 12 hours. Cells were then lysed and β-Gal enzymatic activity was monitored with CPRG. Cultures with D122 served as reference for CPRG background levels. Representative results (1 of 3 experiments) are presented as ΔOD (sample OD-background OD) measured after 12 hours. C. Sixty thousand BUSA14 and BWZ.36/CD8α cells/well were incubated overnight, in triplicates, with 2×104 B16-MO5, F10.9 or D122 tumor cell lines. Representative results (1 of 2 experiments) are presented as ΔOD (sample OD-background OD) measured after 24 hours. Statistical analysis was done using student T test (*p<0.05, **p<0.01, ***p<0.001).
Discussion
In this study we generated a LacZ inducible T cell hybridoma specific for mouse and human gp10025–33 peptides. The hybridoma, named BUSA14, specifically recognize peptide-MHC class I complexes and is specifically activated by APCs and tumor cell lines presenting the gp10025–33 peptide. BUSA14 expresses cell surface markers similar to the set expressed on activated T cells. Although activated by melanoma lines, this hybridoma did not exhibit cytotoxic activity against these tumors.
The different affinities of mgp10025–33 and hgp10025–33 to H-2Db molecules can be used to study MHC-TCR interactions of low and high affinity peptides using the BUSA14 cells. The fact that BUSA14 can recognize the mgp10025–33 on the surface of tumor cells can offer an easy and accurate system for screening tumor lines for peptide presentation, or to study escape mechanisms involving MHC down regulation and inhibition of antigen processing in tumor cells.
To summarize, we offer a peptide specific hybridoma that is straightforward, sensitive, accurate and easy to maintain. It is advantageous when compared to other cellular assays designed for measuring peptide presentation that are expensive, time consuming and require primary cells. BUSA14 cells can serve as a highly applicable tool for studying TCR-MHC interactions at both high and low affinity peptide systems.
Funding Statement
The authors have no support or funding to report.
References
- 1. Malarkannan S, Mendoza LM, Shastri N (2001) Generation of Antigen-Specific, LacZ-Inducible T-Cell Hybrids. Methods in molecular biology 156: 265–272. [DOI] [PubMed] [Google Scholar]
- 2. Sanderson S, Shastri N (1994) LacZ inducible, antigen/MHC-specific T cell hybrids. International immunology 6: 369–376 Available: http://www.ncbi.nlm.nih.gov/pubmed/8186188. Accessed 2013 Jan 9 [DOI] [PubMed] [Google Scholar]
- 3. Shaw JP, Utz PJ, Durand DB, Toole JJ, Emmel EA, et al. (1988) Identification of a putative regulator of early T cell activation genes. Science 241: 202–205. [DOI] [PubMed] [Google Scholar]
- 4. Durand DB, Bush MR, Morgan JG, Weiss A, Crabtree GR (1987) A 275 basepair fragment at the 5′ end of the interleukin 2 gene enhances expression from a heterologous promoter in response to signals from the T cell antigen receptor. Journal of experimental medicine 165: 395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karttunen J, Shastri N (1991) Measurement of ligand-induced activation in single viable T cells using the lacZ reporter gene. Proceedings of the National Academy of Sciences of the United States of America 88: 3972–3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Overwijk WW, Tsung A, Irvine KR, Parkhurst MR, Goletz TJ, et al. (1998) gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. Journal of experimental medicine 188: 277–286 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2212458&tool=pmcentrez&rendertype=abstract. Accessed 2010 Aug 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karttunen J, Sanderson S, Shastri N (1992) Detection of rare antigen-presenting cells by the lacZ T-cell activation assay suggests an expression cloning strategy for T-cell antigens. Proceedings of the National Academy of Sciences of the United States of America 89: 6020–6024 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=402130&tool=pmcentrez&rendertype=abstract. Accessed 2011 Jun 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shen Z, Reznikoff G, Dranoff G, Rock K (1997) Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. Journal of Immunology 158: 2723–2730 Available: http://www.jimmunol.org/cgi/content/abstract/158/6/2723. Accessed 2011 Oct 4 [PubMed] [Google Scholar]
- 9. Porgador A, Tzehoval E, Vadai E, Feldman M, Eisenbach L (1995) Combined vaccination with major histocompatibility class I and interleukin 2 gene-transduced melanoma cells synergizes the cure of postsurgical established lung metastases. Cancer research 55: 4941–4949 Available: http://www.ncbi.nlm.nih.gov/pubmed/7585534. Accessed 2011 Oct 17 [PubMed] [Google Scholar]
- 10. Falo LD, Kovacsovics-Bankowski M, Thompson K, Rock KL (1995) Targeting antigen into the phagocytic pathway in vivo induces protective tumour immunity. Nature Medicine 1: 649–653 Available: http://dx.doi.org/10.1038/nm0795-649. Accessed 2011 Oct 17 [DOI] [PubMed] [Google Scholar]
- 11. Katz A, Feldman M, Eisenbach L (1992) The use of [35S]methionine as a target cell label in long term cytotoxic assays. Journal of immunological methods 149: 255–260 Available: http://www.ncbi.nlm.nih.gov/pubmed/1593137. Accessed 2012 Jan 9 [DOI] [PubMed] [Google Scholar]