Abstract
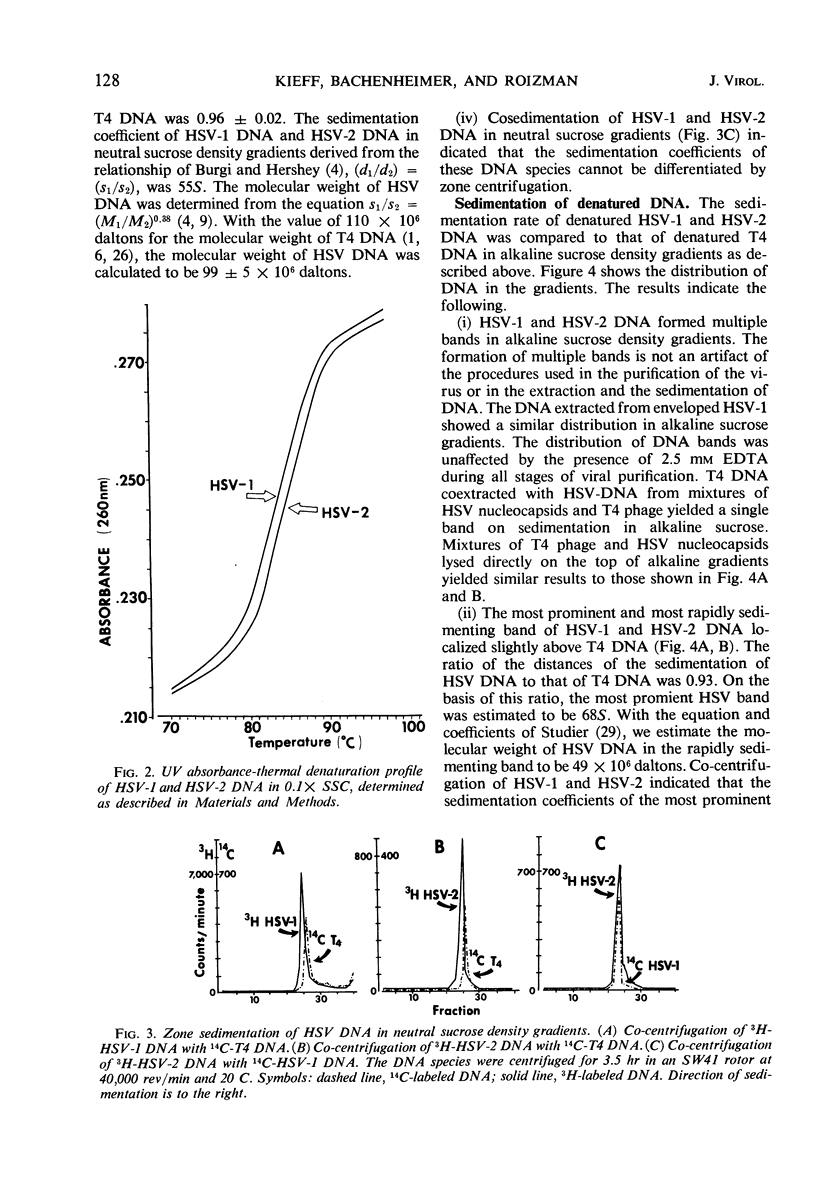
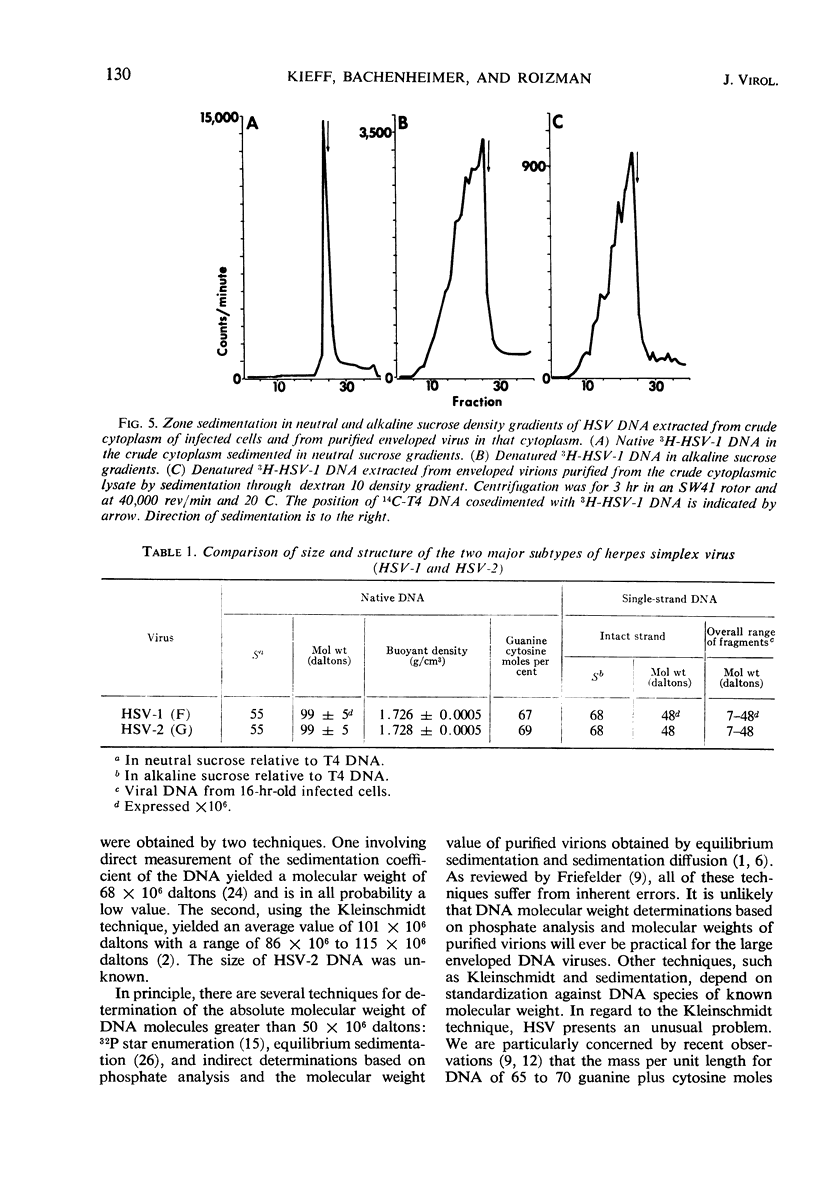
Studies of the size, composition, and structure of the deoxyribonucleic acid (DNA) of the F and G prototypes of herpes simplex virus (HSV) subtypes 1 and 2 (HSV-1 and HSV-2) showed the following. (i) As previously reported by Good-heart et al. HSV-1 and HSV-2 DNA have a buoyant density of 1.726 and 1.728 g/cm3, corresponding to 67 and 69 guanine ± cytosine moles per cent, respectively. The difference in guanine plus cytosine content of the DNA species was confirmed by the finding of a 1 C difference in Tm. (ii) The DNA from purified virus on cocentrifugation with T4 DNA in neutral sucrose density gradients sedimented at 55S, corresponding to 99 ± 5 million daltons in molecular weight. HSV-1 and HSV-2 DNA could not be differentiated with respect to size. (iii) Cosedimentation of alkali-denatured DNA from purified virus with T4 DNA on alkaline sucrose density gradients consistently yielded several bands of single-stranded HSV DNA ranging from fragments 7 × 106 daltons to intact strands 48 × 106 daltons in molecular weight.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft F. C., Freifelder D. Molecular weights of coliphages and coliphage DNA. I. Measurement of the molecular weight of bacteriophage T7 by high-speed equilibrium centrifugation. J Mol Biol. 1970 Dec 28;54(3):537–546. doi: 10.1016/0022-2836(70)90124-5. [DOI] [PubMed] [Google Scholar]
- Becker Y., Dym H., Sarov I. Herpes simplex virus DNA. Virology. 1968 Oct;36(2):184–192. doi: 10.1016/0042-6822(68)90135-9. [DOI] [PubMed] [Google Scholar]
- Berns K. I., Silverman C. Natural occurrence of cross-linked vaccinia virus deoxyribonucleic acid. J Virol. 1970 Mar;5(3):299–304. doi: 10.1128/jvi.5.3.299-304.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdle W. R., Nahmias A. J., Harwell R. W., Pauls F. P. Association of antigenic type of Herpesvirus hominis with site of viral recovery. J Immunol. 1967 Nov;99(5):974–980. [PubMed] [Google Scholar]
- Dubin S. B., Benedek G. B., Bancroft F. C., Freifelder D. Molecular weights of coliphages and colip- hage DNA. II. Measurement of diffusion coefficients using optical mixing spectroscopy, and measurement of sedimentation coefficients. J Mol Biol. 1970 Dec 28;54(3):547–556. doi: 10.1016/0022-2836(70)90125-7. [DOI] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Figueroa M. E., Rawls W. E. Biological markers for differentiation of herpes-virus strains of oral and genital origin. J Gen Virol. 1969 Mar;4(2):259–267. doi: 10.1099/0022-1317-4-2-259. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Molecular weights of coliphages and coliphage DNA. IV. Molecular weights of DNA from bacteriophages T4, T5 and T7 and the general problem of determination of M. J Mol Biol. 1970 Dec 28;54(3):567–577. doi: 10.1016/0022-2836(70)90127-0. [DOI] [PubMed] [Google Scholar]
- Goodheart C. R., Plummer G., Waner J. L. Density difference of DNA of human herpes simplex viruses, types I and II. Virology. 1968 Jul;35(3):473–475. doi: 10.1016/0042-6822(68)90225-0. [DOI] [PubMed] [Google Scholar]
- LEVINTHAL C., THOMAS C. A., Jr Molecular autoradiography: the beta-ray counting from single virus particles and DNA molecules in nuclear emulsions. Biochim Biophys Acta. 1957 Mar;23(3):453–465. doi: 10.1016/0006-3002(57)90363-3. [DOI] [PubMed] [Google Scholar]
- Lando D., de Rudder J., Privat de Garilhe M. A propos de la composition du DNA du virus herpétique. Bull Soc Chim Biol (Paris) 1965;47(6):1033–1042. [PubMed] [Google Scholar]
- Lang D. Molecular weights of coliphages and coliphage DNA. 3. Contour length and molecular weight of DNA from bacteriophages T4, T5 and T7, and from bovine papilloma virus. J Mol Biol. 1970 Dec 28;54(3):557–565. doi: 10.1016/0022-2836(70)90126-9. [DOI] [PubMed] [Google Scholar]
- Lee L. F., Kieff E. D., Bachenheimer S. L., Roizman B., Spear P. G., Burmester B. R., Nazerian K. Size and composition of Marek's disease virus deoxyribonucleic acid. J Virol. 1971 Mar;7(3):289–294. doi: 10.1128/jvi.7.3.289-294.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton S. B., Rubenstein I. Calibration of molecular weight scales for DNA. J Mol Biol. 1969 Dec 14;46(2):313–328. doi: 10.1016/0022-2836(69)90424-0. [DOI] [PubMed] [Google Scholar]
- Nahmias A. J., Dowdle W. R., Naib Z. M., Highsmith A., Harwell R. W., Josey W. E. Relation of pock size on chorioallantoic membrane to antigenic type of herpesvirus hominis. Proc Soc Exp Biol Med. 1968 Apr;127(4):1022–1028. doi: 10.3181/00379727-127-32861. [DOI] [PubMed] [Google Scholar]
- PLUMMER G. SEROLOGICAL COMPARISON OF THE HERPES VIRUSES. Br J Exp Pathol. 1964 Apr;45:135–141. [PMC free article] [PubMed] [Google Scholar]
- Plummer G., Waner J. L., Bowling C. P. Comparative studies of type 1 and type 2 & 'herpes simplex' viruses. Br J Exp Pathol. 1968 Apr;49(2):202–208. [PMC free article] [PubMed] [Google Scholar]
- Plummer G., Waner J. L., Phuangsab A., Goodheart C. R. Type 1 and type 2 herpes simplex viruses: serological and biological differences. J Virol. 1970 Jan;5(1):51–59. doi: 10.1128/jvi.5.1.51-59.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROIZMAN B., AURELIAN L., ROANE P. R., Jr THE MULTIPLICATION OF HERPES SIMPLEX VIRUS. I. THE PROGRAMMING OF VIRAL DNA DUPLICATION IN HEP-2 CELLS. Virology. 1963 Nov;21:482–498. doi: 10.1016/0042-6822(63)90209-5. [DOI] [PubMed] [Google Scholar]
- RUSSELL W. C., CRAWFORD L. V. PROPERTIES OF THE NUCLEIC ACIDS FROM SOME HERPES GROUP VIRUSES. Virology. 1964 Feb;22:288–292. doi: 10.1016/0042-6822(64)90017-0. [DOI] [PubMed] [Google Scholar]
- RUSSELL W. C., CRAWFORD L. V. SOME CHARACTERISTICS OF THE DEOXYRIBONUCLEIC ACID FROM HERPES SIMPLEX VIRUS. Virology. 1963 Nov;21:353–361. doi: 10.1016/0042-6822(63)90196-x. [DOI] [PubMed] [Google Scholar]
- Riva S., Cascino A., Geiduschek E. P. Coupling of late transcription to viral replication in bacteriophage T4 development. J Mol Biol. 1970 Nov 28;54(1):85–102. doi: 10.1016/0022-2836(70)90447-x. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Schmid C. W., Hearst J. E. Molecular weights of homogeneous coliphage DNA's from density-gradient sedimentation equilibrium. J Mol Biol. 1969 Aug 28;44(1):143–160. doi: 10.1016/0022-2836(69)90410-0. [DOI] [PubMed] [Google Scholar]
- Schwartz J., Roizman B. Similarities and Differences in the Development of Laboratory Strains and Freshly Isolated Strains of Herpes Simplex Virus in HEp-2 Cells: Electron Microscopy. J Virol. 1969 Dec;4(6):879–889. doi: 10.1128/jvi.4.6.879-889.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terni M., Roizman B. Variability of herpes simplex virus: isolation of two variants from simultaneous eruptions at different sites. J Infect Dis. 1970 Feb;121(2):212–216. doi: 10.1093/infdis/121.2.212. [DOI] [PubMed] [Google Scholar]


