Abstract
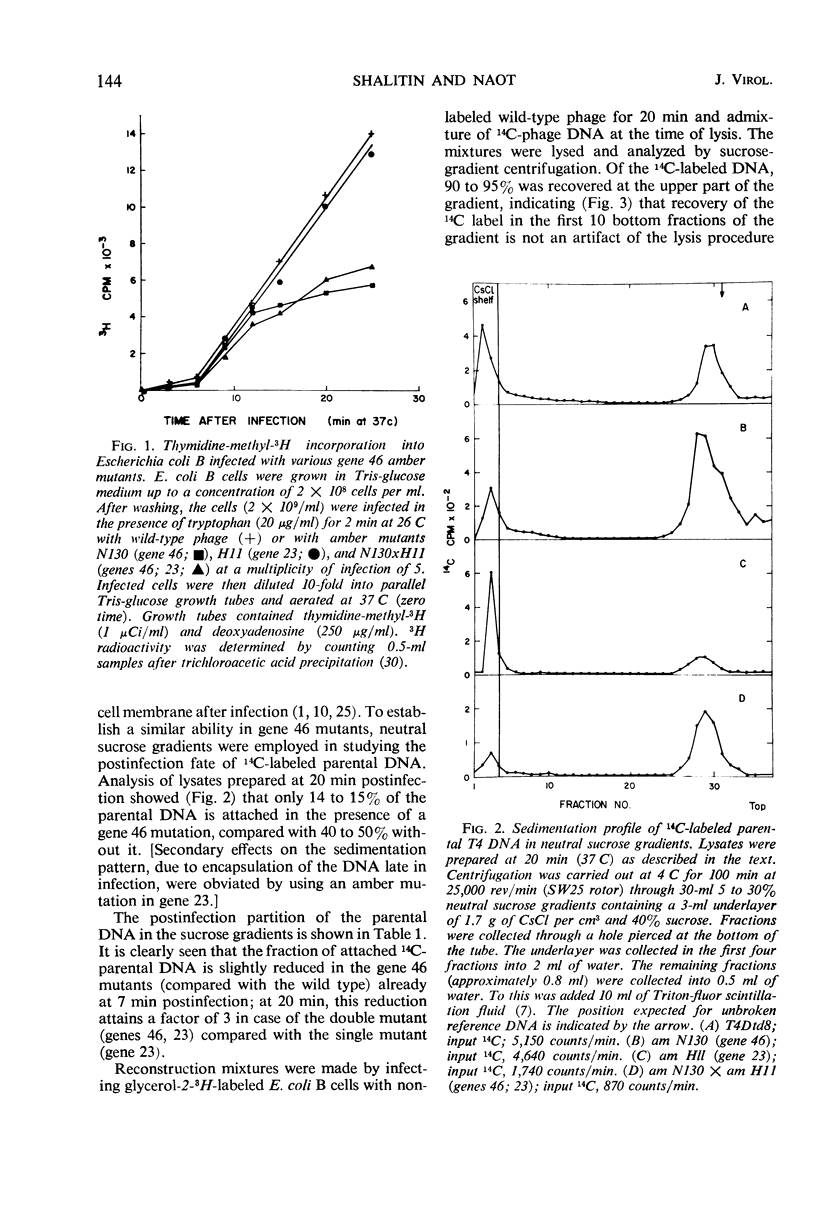
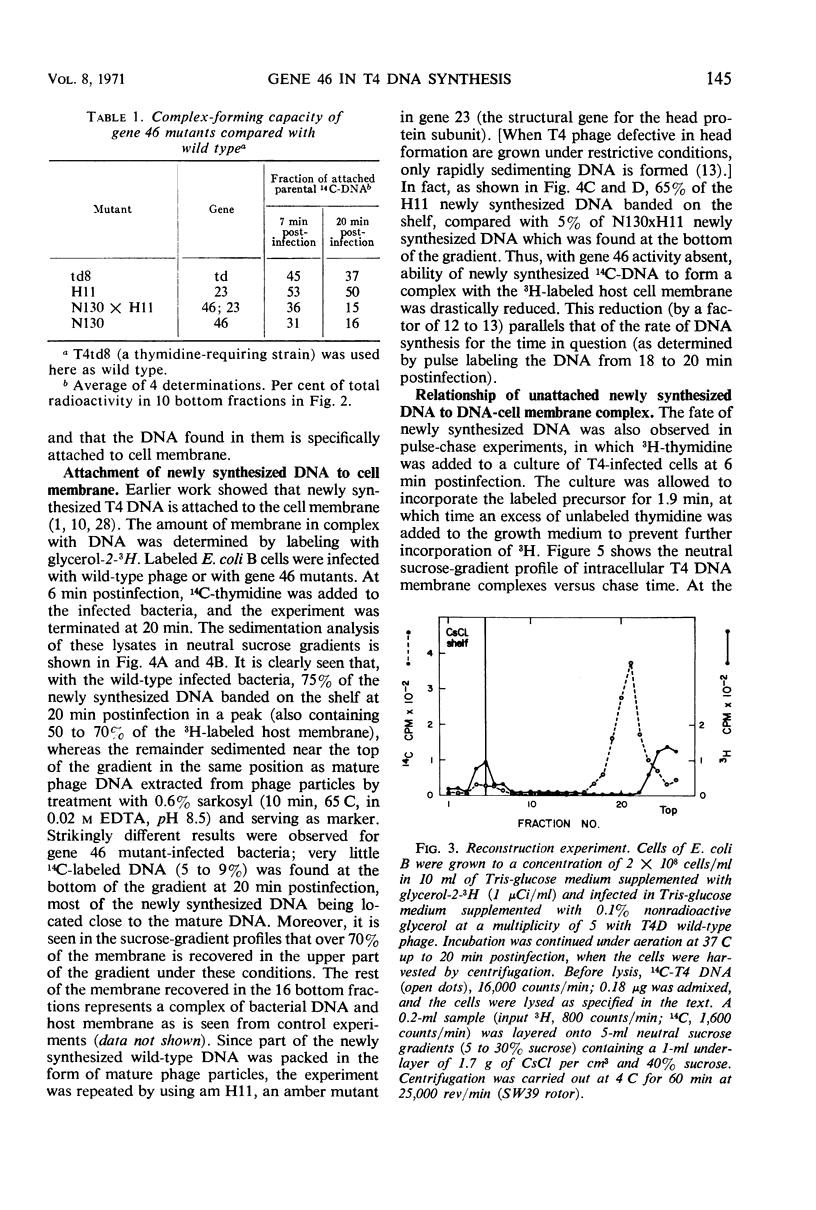
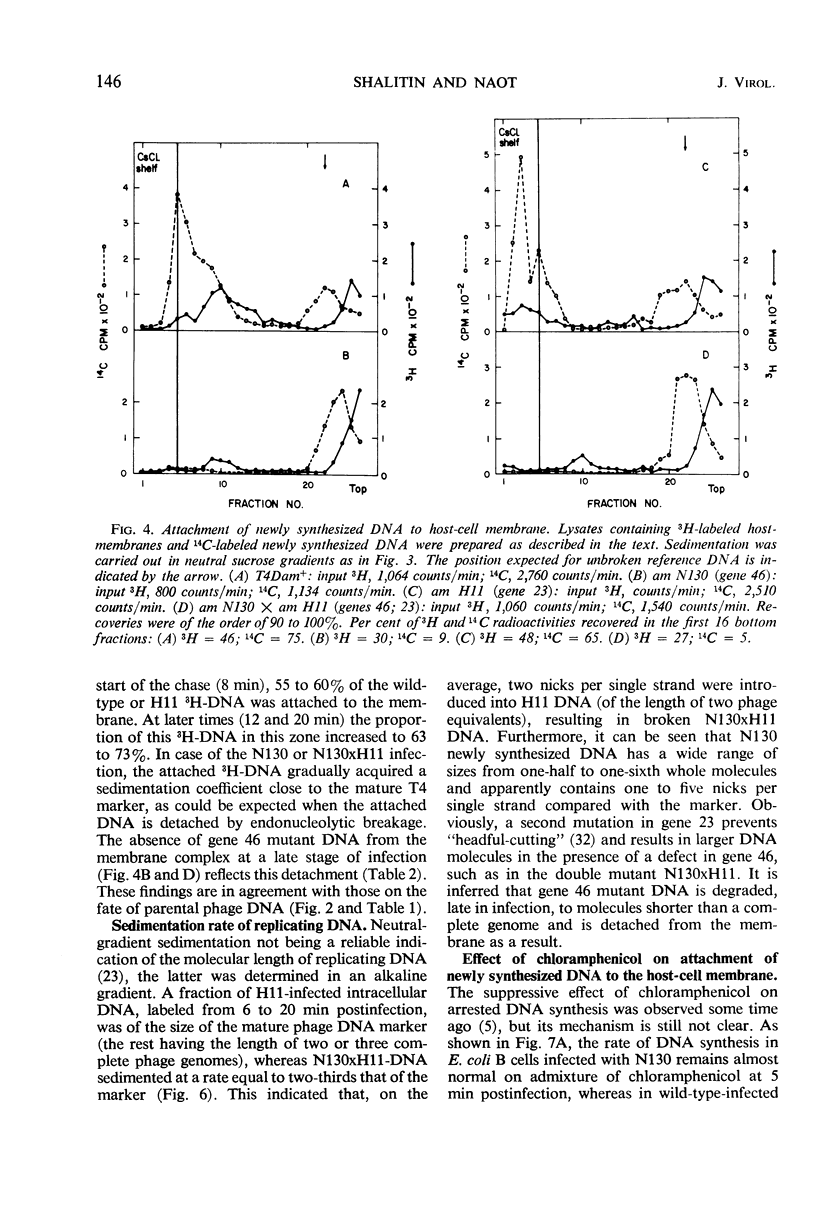
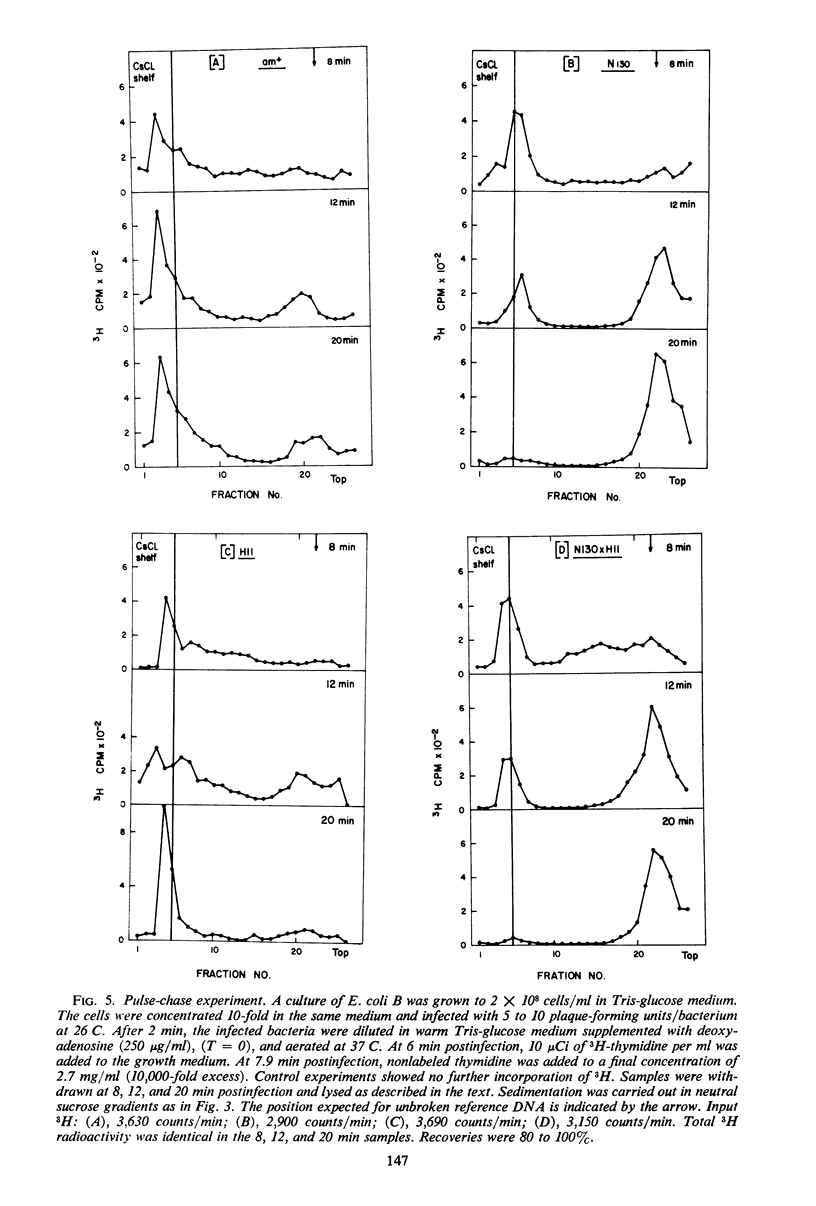
In an attempt to establish whether Escherichia coli B infected with N130 (an amber mutant defective in gene 46) is recombination-deficient, the postinfection fate of 14C-labeled N130 parental deoxyribonucleic acid (DNA) was followed, its amount in complex with the host cell membrane being determined in sucrose gradients after mild lysis of the infected cells. The parental DNA was found to undergo gradual detachment from the membrane during infection. Pulse-chase experiments similarly showed that newly synthesized DNA is normally attached to the host cell membrane and is detached by endonucleolytic breakage at a late stage of infection. The conclusion is that only attached DNA molecules are replicated by membrane-bound replicase, whereas those detached by endonucleolytic breakage are not. It thus seems that the gene 46 product controls the activity of a nuclease whose main function is recombination of DNA nicked by endonuclease, thereby attaching it to the host cell membrane. The rate of T4 DNA synthesis is apparently governed by the efficiency of recombination. Supporting evidence was found in experiments with the double mutant N130 × N134 (genes 46, 33).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman S., Lerman L. S. Kinetics and intermediates in the intracellular synthesis of bacteriophage T4 deoxyribonucleic acid. J Mol Biol. 1970 Jun 14;50(2):235–261. doi: 10.1016/0022-2836(70)90190-7. [DOI] [PubMed] [Google Scholar]
- Altman S., Meselson M. A T4-induced endonuclease which attacks T4 DNA. Proc Natl Acad Sci U S A. 1970 Jul;66(3):716–721. doi: 10.1073/pnas.66.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldy M. W. Repair and recombination in phage T4. II. Genes affecting UV sensitivity. Cold Spring Harb Symp Quant Biol. 1968;33:333–338. doi: 10.1101/sqb.1968.033.01.038. [DOI] [PubMed] [Google Scholar]
- Berger H., Warren A. J., Fry K. E. Variations in genetic recombination due to amber mutations in T4D bacteriophage. J Virol. 1969 Feb;3(2):171–175. doi: 10.1128/jvi.3.2.171-175.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein H. Repair and recombination in phage T4. I. Genes affecting recombination. Cold Spring Harb Symp Quant Biol. 1968;33:325–331. doi: 10.1101/sqb.1968.033.01.037. [DOI] [PubMed] [Google Scholar]
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: requirements for late messenger synthesis. J Mol Biol. 1968 Apr 28;33(2):339–362. doi: 10.1016/0022-2836(68)90193-9. [DOI] [PubMed] [Google Scholar]
- Bose S. K., Warren R. J. Bacteriophage-induced inhibition of host functions. II. Evidence for multiple, sequential bacteriophage-induced deoxyribonucleases responsible for degradation of cellular deoxyribonucleic acid. J Virol. 1969 Jun;3(6):549–556. doi: 10.1128/jvi.3.6.549-556.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody E. N., Diggelmann H., Geiduschek E. P. Transcription of the bacteriophage T4 template. Obligate synthesis of T4 prereplicative RNA in vitro. Biochemistry. 1970 Mar 17;9(6):1289–1299. doi: 10.1021/bi00808a001. [DOI] [PubMed] [Google Scholar]
- Earhart C. F., Tremblay G. Y., Daniels M. J., Schaechter M. DNA replication studied by a new method for the isolation of cell membrane-DNA complexes. Cold Spring Harb Symp Quant Biol. 1968;33:707–710. doi: 10.1101/sqb.1968.033.01.079. [DOI] [PubMed] [Google Scholar]
- Frankel F. R. Evidence for long DNA strands in the replicating pool after T4 infection. Proc Natl Acad Sci U S A. 1968 Jan;59(1):131–138. doi: 10.1073/pnas.59.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel F. R., Majumdar C., Weintraub S., Frankel D. M. DNA polymerase and the cell membrane after T4 infection. Cold Spring Harb Symp Quant Biol. 1968;33:495–500. doi: 10.1101/sqb.1968.033.01.057. [DOI] [PubMed] [Google Scholar]
- Frankel F. R. Studies on the nature of replicating DNA in T4-infected Escherichia coli. J Mol Biol. 1966 Jun;18(1):127–143. doi: 10.1016/s0022-2836(66)80081-5. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Hanawalt P. Isolation and characterization of the DNA replication complex from Escherichia coli. J Mol Biol. 1970 Sep 14;52(2):301–322. doi: 10.1016/0022-2836(70)90032-x. [DOI] [PubMed] [Google Scholar]
- Hosoda J., Levinthal C. Protein synthesis by Escherichia coli infected with bacteriophage T4D. Virology. 1968 Apr;34(4):709–727. doi: 10.1016/0042-6822(68)90092-5. [DOI] [PubMed] [Google Scholar]
- Hosoda J., Mathews E. DNA replication in vivo by polynucleotide-ligase defective mutants of T4. II. Effect of chloramphenicol and mutations in other genes. J Mol Biol. 1971 Jan 28;55(2):155–179. doi: 10.1016/0022-2836(71)90189-6. [DOI] [PubMed] [Google Scholar]
- KANFER J., KENNEDY E. P. METABOLISM AND FUNCTION OF BACTERIAL LIPIDS. I. METABOLISM OF PHOSPHOLIPIDS IN ESCHERICHIA COLI B. J Biol Chem. 1963 Sep;238:2919–2922. [PubMed] [Google Scholar]
- Kozinski A. W., Felgenhauer Z. Z. Molecular recombination in T4 bacteriophage deoxyribonucleic acid. II. Single-strand breaks and exposure of uncomplemented areas as a prerequisite for recombination. J Virol. 1967 Dec;1(6):1193–1202. doi: 10.1128/jvi.1.6.1193-1202.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozinski A. W., Kozinski P. B., James R. Molecular recombination in T4 bacteriophage deoxyribonucleic acid. I. Tertiary structure of early replicative and recombining deoxyribonucleic acid. J Virol. 1967 Aug;1(4):758–770. doi: 10.1128/jvi.1.4.758-770.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozinski A. W. Molecular recombination in the ligase negative T4 amber mutant. Cold Spring Harb Symp Quant Biol. 1968;33:375–391. doi: 10.1101/sqb.1968.033.01.044. [DOI] [PubMed] [Google Scholar]
- Kutter E. M., Wiberg J. S. Biological effects of substituting cytosine for 5-hydroxymethylcytosine in the deoxyribonucleic acid of bacteriophage T4. J Virol. 1969 Oct;4(4):439–453. doi: 10.1128/jvi.4.4.439-453.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutter E. M., Wiberg J. S. Degradation of cytosin-containing bacterial and bacteriophage DNA after infection of Escherichia coli B with bacteriophage T4D wild type and with mutants defective in genes 46, 47 and 56. J Mol Biol. 1968 Dec;38(3):395–411. doi: 10.1016/0022-2836(68)90394-x. [DOI] [PubMed] [Google Scholar]
- Lembach K. J., Kuninaka A., Buchanan J. M. The relationship of DNA replication to the control of protein synthesis in protoplasts of T4-infected Escherichia coli B. Proc Natl Acad Sci U S A. 1969 Feb;62(2):446–453. doi: 10.1073/pnas.62.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. C., Jr, Kozinski A. W. Early intracellular events in the replication of bacteriophage T4 deoxyribonucleic acid. V. Further studies on the T4 protein-deoxyribonucleic acid complex. J Virol. 1970 Apr;5(4):490–501. doi: 10.1128/jvi.5.4.490-501.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosig G., Werner R. On the replication of incomplete chromosomes of phage T4. Proc Natl Acad Sci U S A. 1969 Oct;64(2):747–754. doi: 10.1073/pnas.64.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulitzer J. F., Geiduschek E. P. Function of T4 gene 55. II. RNA synthesis by temperature-sensitive gene 55 mutants. J Mol Biol. 1970 Apr 28;49(2):489–507. doi: 10.1016/0022-2836(70)90259-7. [DOI] [PubMed] [Google Scholar]
- Rouvière J., Lederberg S., Granboulan P., Gros F. Structural sites of RNA synthesis in Escherichia coli. J Mol Biol. 1969 Dec 28;46(3):413–430. doi: 10.1016/0022-2836(69)90185-5. [DOI] [PubMed] [Google Scholar]
- Salser W., Bolle A., Epstein R. Transcription during bacteriophage T4 development: a demonstration that distinct subclasses of the "early" RNA appear at different times and that some are "turned off" at late times. J Mol Biol. 1970 Apr 28;49(2):271–295. doi: 10.1016/0022-2836(70)90246-9. [DOI] [PubMed] [Google Scholar]
- Shalitin C., Kahana S. Conversion of T4 gene 46 mutant deoxyribonucleic acid into nonviable bacteriophage particles. J Virol. 1970 Sep;6(3):353–362. doi: 10.1128/jvi.6.3.353-362.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalitin C., Sarid S. Differential effect of polyamines on T4 morphogenesis. J Virol. 1967 Jun;1(3):559–568. doi: 10.1128/jvi.1.3.559-568.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streisinger G., Emrich J., Stahl M. M. Chromosome structure in phage t4, iii. Terminal redundancy and length determination. Proc Natl Acad Sci U S A. 1967 Feb;57(2):292–295. doi: 10.1073/pnas.57.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIBERG J. S., DIRKSEN M. L., EPSTEIN R. H., LURIA S. E., BUCHANAN J. M. Early enzyme synthesis and its control in E. coli infected with some amber mutants of bacteriophage T4. Proc Natl Acad Sci U S A. 1962 Feb;48:293–302. doi: 10.1073/pnas.48.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R. J., Bose S. K. Bacteriophage-induced inhibition of host functions. I. Degradation of Escherichia coli deoxyribonucleic acid after T4 infection. J Virol. 1968 Apr;2(4):327–334. doi: 10.1128/jvi.2.4.327-334.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg J. S. Mutants of bacteriophage T4 unable to cause breakdown of host DNA. Proc Natl Acad Sci U S A. 1966 Mar;55(3):614–621. doi: 10.1073/pnas.55.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]


