Abstract
When the cell cycle is arrested, even though growth-promoting pathways such as mTOR are still active, then cells senesce. For example, induction of either p21 or p16 arrests the cell cycle without inhibiting mTOR, which, in turn, converts p21/p16-induced arrest into senescence (geroconversion). Here we show that geroconversion is accompanied by dramatic accumulation of cyclin D1 followed by cyclin E and replicative stress. When p21 was switched off, senescent cells (despite their loss of proliferative potential) progressed through S phase, and levels of cyclins D1 and E dropped. Most cells entered mitosis and then died, either during mitotic arrest or after mitotic slippage, or underwent endoreduplication. Next, we investigated whether inhibition of mTOR would prevent accumulation of cyclins and loss of mitotic competence in p21-arrested cells. Both nutlin-3, which inhibits mTOR in these cells, and rapamycin suppressed geroconversion during p21-induced arrest, decelerated accumulation of cyclins D1 and E and decreased replicative stress. When p21 was switched off, cells successfully progressed through both S phase and mitosis. Also, senescent mouse embryonic fibroblasts (MEFs) overexpressed cyclin D1. After release from cell cycle arrest, senescent MEFs entered S phase but could not undergo mitosis and did not proliferate. We conclude that cellular senescence is characterized by futile hyper-mitogenic drive associated with mTOR-dependent mitotic incompetence.
Keywords: MTOR, rapamycin, aging, cyclins, cell cycle, regenerative/proliferative potential
Introduction
In cell culture, senescence is characterized by cellular hypertrophy, SA-β-Gal staining, hyper-secretory phenotype and permanent loss of regenerative or replicative potential (RP), meaning that cells cannot restart proliferation after release from cell cycle arrest.1-3 These hallmarks of senescence are promoted by growth-promoting pathways such as mTOR (target of rapamycin), when the cell cycle is arrested.3 For example, while arresting cell cycle, p21 and p16 do not inhibit mTOR, which, in turn, converts p21/p16-induced arrest into irreversible senescence.4-6 Thus, active growth-promoting pathways in resting cells drive a senescent program, a process named gerogenic conversion or geroconversion.3,6 Rapamycin and other inhibitors of mTOR as well as serum starvation decelerate geroconversion, decreasing cellular hypertrophy and preventing loss of regenerative/proliferative potential (RP).4-9 Remarkably, rapamycin slows down aging in mice10-15 and prevents age-related diseases in animals,16-23 suggesting a common basis in cellular senescence and organismal aging.
Yet, organismal aging is associated not only with decreased regeneration, but also with hyper-proliferation, such as hyperplasia, fibrosis, prostate enlargement, atherosclerotic plaques, benign tumors and cancer. Inappropriate re-entry into the cell cycle is involved in many age-related pathologies. This cannot be easily explained by such a hallmark of cellular senescence as loss of regenerative/proliferative potential (RP). Furthermore, it was noticed previously that senescent cells express high levels of cyclins D1 and E.24-29 Here we investigated how these markers of proliferation (cyclins) can be associated with the loss of RP. The second question we address here is how inhibitors of mTOR affect the ability of cells to re-start proliferation after their release from p21-induced cell cycle arrest. Is it initiation of the cell cycle, or its completion affected? We also address the question of how nutlin-3, a Mdm-2 inhibitor and p53 inducer, preserves RP in HT1080-p21–9 cells (HT-p21 cells). In HT-p21 cells, nutlin-3 inhibits mTOR and, like rapamycin, suppresses geroconversion during p21-induced arrest, maintaining quiescence and preserving RP.7,8,30 Using time-lapse video microscopy, we demonstrate that preservation of RP by both rapamycin and nutlin-3 is due to preservation of mitotic competence, an ability to undergo mitosis.
Results
Induction of cyclins D1 and E in senescent cells
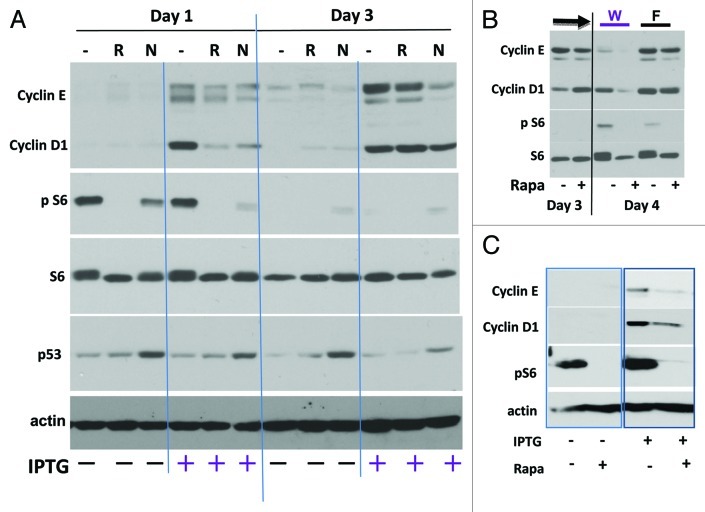
First, we used a well-studied model of cellular senescence: HT-p21 cells with IPTG-inducible p21.31,32 In these cells, IPTG induces p21 and irreversible senescence, whereas nutlin-3 induces p53 and reversible arrest.7,8 Unlike nutlin-3a, IPTG strongly induced cyclin D1 and cyclin E (Fig. 1A). Cyclin E levels continued to rise from day 1 to day 3 in IPTG-treated cells (Fig. 1A). We conclude that elevated levels of cyclins D1 and E were associated with senescence but not with reversible arrest.
Figure 1. Immunoblot analysis of cyclins D1 and E in IPTG-induced senescence in HT-p21–9 and HT-p16 cells. (A) HT-p21–9 cells were treated with 500 nM rapamycin (R) or10 uM nutlin-3 (N) in the presence or absence of IPTG (+). Cells were lysed on day 1 and 3. (B) HT-p21–9 cells were treated with IPTG and co-treated with (+) or without (-) rapamycin (Rapa) for 3 d. On day 3, cells were either washed (W) or cultured in fresh medium with IPTG (F) plus/minus rapamycin (+ -) and lysed the next day (day 4). (C) HT-p16 cells were treated with or without IPTG (+/−) and with or without rapamycin (Rapa +/−) for 1 d before lysis.
Deceleration of cyclin induction by nutlin-3a and rapamycin
In IPTG-treated cells, rapamycin and nutlin-3 decreased levels of cyclin D1 and cyclin E on day 1 and also slightly decreased levels of cyclin E on day 3 (Fig. 1A). This parallels deceleration of geroconversion by inhibitors of mTOR. In fact, rapamycin delayed the appearance of hallmarks of senescence in IPTG-treated HT-p21 cells but did not completely prevent them.33 In agreement with previous reports,7,8 rapamycin and nutlin-3 decreased pS6 in IPTG-treated cells (Fig. 1A). In conditions shown in Figure 1A, dephosphorylation of S6 by rapamycin and nutlin-3a could be detectable on day 1 only, because its phosporylation was spontaneously inhibited by day 3. This spontaneous deactivation of mTOR can be explained by medium exhaustion in relatively dense cell culture used for immunoblots. Furthermore, since IPTG-treated cells grow in size exponentially for 3–4 d, they exhaust and acidify medium as strongly as proliferating cells.34 (In low cell density, S6 remained highly phosphorylated in senescent cells.) To prevent spontaneous deactivation of mTOR, the medium was changed on day 3 (Fig. 1B), and the effect of rapamycin was determined the next day. Even in these conditions, rapamycin only marginally decreased cyclins E and D1 on day 4. Similar results are also shown in Figure 2A. We conclude that inhibition of mTOR decelerated but did not prevent accumulation of cyclins.
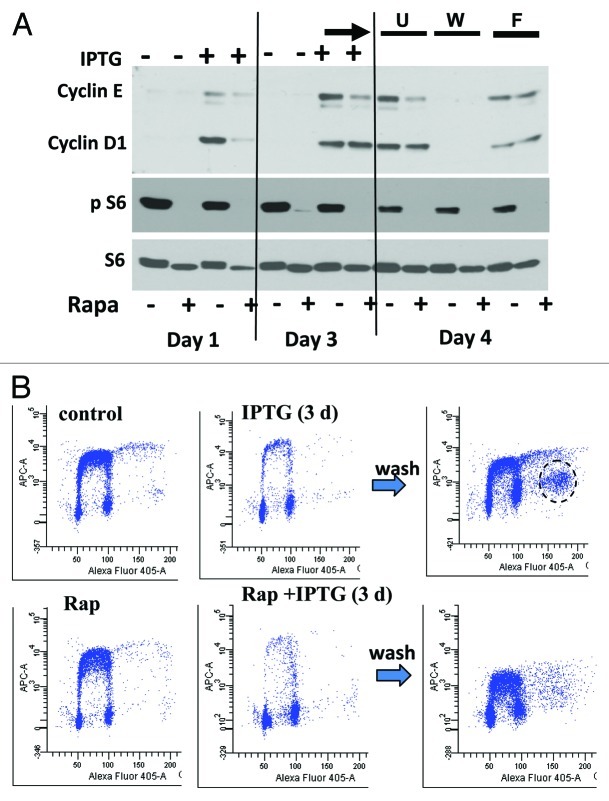
Figure 2. Levels of cyclins and Br-dU incorporation after release from cell cycle arrest. (A) Immunoblot analysis. HT-p21 cells were treated with IPTG and rapamycin (as indicated +/−) for 1 and 3 d and lysed for immunoblot. In addition, on day 3, IPTG-treated cells (+/− Rapa) were either washed and further cultured without IPTG (W) or placed in fresh medium with IPTG (F) and left unchanged (U). Rapamycin was present as indicated (+/−) at the bottom. (B) Flow cytometry. HT-p21 cells treated with IPTG and rapamycin (Rap) for 3 d and then labeled with BrdU for 1 h. In parallel, HT-p21 cells treated for 3 d with IPTG or IPTG +Rap were washed (wash) and incubated overnight without drugs and then labeled with BrdU. Fixed cells were then stained using anti-BrdU antibody and propidium iodide and analyzed by flow cytometry as described in Materials and Methods.
Similarly, cyclin D1 and E accumulated in HT-p16 cells, in which senescence was triggered by p16 instead of p21 (Fig. 1C). In p16-induced senescence, rapamycin also diminished the accumulation of cyclin D1 and especially cyclin E.
Hyper-mitogenic drive and cellular senescence was associated with the appearance of a proportion of TUNEL-positive cells (Fig. S1), which was partially inhibited by rapamycin.
Normalization of cyclins D1 and E after cell cycle release
When medium was not only changed to fresh medium (fresh “F”) but IPTG was also washed out (W), then levels of cyclins decreased (Fig. 1B, W). This decrease was accelerated by rapamycin (Figs. 1B and 2A). In lower cell density, pS6 was detectable by day 4 (Fig. 2A, U) and was not affected by neither wash (W) nor change (F) of the medium (Fig. 2A). Cyclins D1 and E disappeared when IPTG was washed out (W). These cyclins remained highly overexpressed in the presence of IPTG though (F). This disappearance of cyclins may be explained by cell progression through S-phase (Fig. 2B), when IPTG was washed out. Importantly, senescent cells not only entered S phase, but also some cells entered a second S phase without cell division, as evidenced by cells with 8N DNA (Fig. 2B, circle). This indicates a powerful replicative drive. Notably, these hyperploid cells showed intermittent levels of Br-dU incorporation, consistent with abnormal cell cycle progression. Rapamycin did not prevent S phase progression but diminished a number of hyperploid cells (Fig. 2B).
Mitotic catastrophe and lacerosis limits proliferation of senescent cells
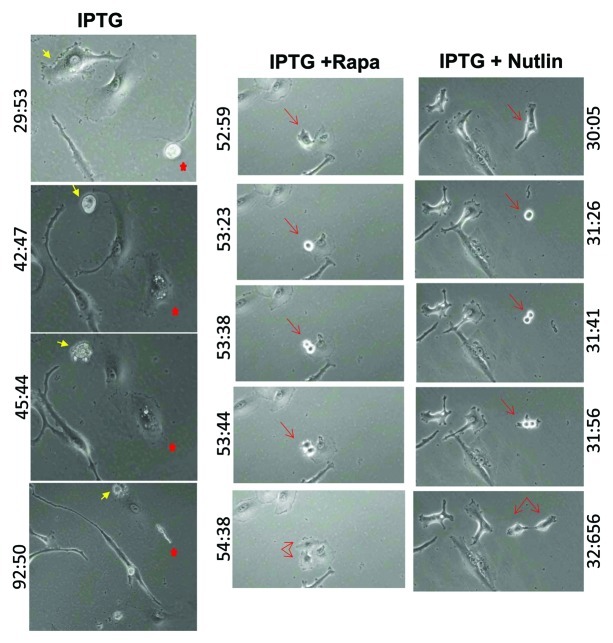
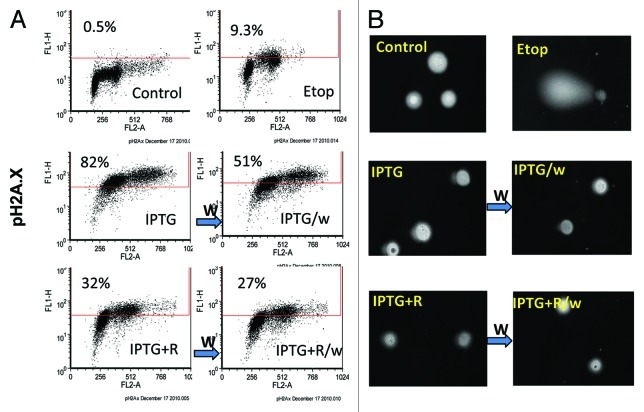
It was previously shown that following removal of IPTG, senescent HT-p21 cells enter mitosis and die.32 Here, we employed time-lapse video microscopy to observe whether treatment with rapamycin and nutlin-3 during geroconversion (IPTG treatment) affects mitotic events. HT-p21 cells were treated with IPTG for 3 d to induce senescence, and then IPTG was washed out. In IPTG-treated cells, at the time when IPTG was just washed out (time = 0), IPTG-treated cells were flat and large (Fig. 3). After 4 d, a number of cells in the same field decreased, consistent with dead cells in the view (Fig. 3). The remaining alive cells included two very large flat cells and a thread-like cell (Fig. 3). Time-lapse video microscopy (Vid. S1) revealed three main scenarios. Some cells entered mitosis, becoming round and remaining round for 8–12 h and then underwent morphological apoptosis (Fig. 4, yellow arrows). Other cells exited mitosis as morphologically multi-nucleated cells (red points), some of which eventually died. These two scenarios are consistent with mitotic catastrophe.32 The third scenario is unusual and according to our knowledge has not described previously. Without rounding (mitosis), cells became increasingly long and thin, thread-like, tearing themselves apart (Vid. S1, thread-like cell at time 92:50, Fig. 4A) and die after several hours. Although this needs additional investigation, we can interpret this as attempted division before cells actually entered mitosis. Since “lacero” in Latin means “tear” we named this kind of cell death “lacerosis.”
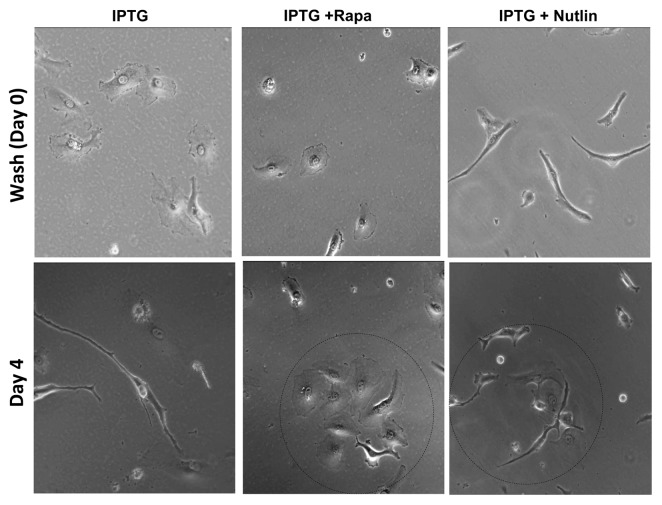
Figure 3. Time-lapse video microscopy of HT-p21 cells released from cell cycle arrest. HT-p21 cells were treated with IPTG with or without rapamycin and nutlin-3 for 3 d, and then drugs were washed out (day 0) and cells were allowed to re-grow in the fresh medium for 4 d. Video was recorded throughout the incubation time. Microphotographs on day 0 and day 4 are shown.
Figure 4. Mitotic cell fate revealed by time-lapse video microscopy. HT-p21 cells were treated as described in Figure 3 legend. Microphotographs at indicated time are shown. Individual cells are marked with arrows and stars of the same color and shape. Microphotographs were taken at indicated time (hours and minutes on the left or right of each panel).
Rapamycin and nutlin-3 preserves mitotic competence
Treatment with rapamycin and nutlin-3a during geroconversion preserved RP, allowing these cells to proliferate and form colonies when IPTG was washed out.7 Initially (at time 0), there were only a few cells in a view field (Fig. 3). Rapa+IPTG-treated cells had similar morphology (large and flat, although smaller than IPTG-treated cells) to IPTG-treated cells (although smaller), whereas Nutlin+IPTG-cells were elongated and fibroblast-like (Fig. 3). After 4 d, cells started to form colonies (Fig. 3, encircled), suggesting either successful replication or conglomeration or both. Time-lapse video microscopy (Vid. S2: rapamycin) and nutlin-3 (Vid. S3) showed that all cells that entered mitosis in fact divided and survived. As examples, successful mitoses are shown (Fig. 4). No single cell died (in view fields) neither via mitotic catastrophe nor via lacerosis.
DNA damage response is not increased upon cell cycle progression
Next we tested whether DNA damage could explain mitotic incompetence of senescent cells. We have shown that senescence, triggered by agents that do not damage DNA, is still manifested by atypical DNA damage response (pseudo-DDR).35 This may be a marker of hyper-activation of signaling pathways.35 Not mutually exclusive, this may be a marker of replicative stress,36 which is observed during mitogenic activation.37 Pseudo-DDR is decreased by rapamycin35 and metformin,38 inhibitors of “proaging” signaling pathways. It is noteworthy that metformin can extend lifespan in mice.39 Therefore, pseudo-DDR may be a marker of hyper-mitogenic drive in senescent HT-p21 and HT-p16 cells. Actual DNA damage is not detectable.35 In theory, actual DNA damage may arise after IPTG is washed out, so cells enter S-phase. Here we measured phospho-H2AX and direct DNA damage (comet assay) after IPTG was washed out (Fig. 5). In agreement with a previous report,35 gamma-H2AX was elevated in senescent (IPTG-treated cells) without detectable DNA damage by comet assay (Fig. 5A). Rapamycin partially decreased gamma-H2AX (Fig. 5A; Fig. S1B). When IPTG was washed out, phospho-H2AX levels slightly decreased. Most importantly, comet assay still did not reveal detectable DNA damage (Fig. 5B). Based on these data, we cannot explain mitotic incompetence of senescent cells by DNA damage during progression through S phase.
Figure 5. Effects of cell cycle release on pseudo-DDR in senescent HT-p21 cells. (A) Levels of gamma-H2A.X by flow cytometry. HT-p21 cells were treated with IPTG +/− rapamycin for 4 d (left column) and cells were washed off drugs (wash/W) and incubated in fresh medium for 14 h (right column). Fixed cells were stained using anti-phospho H2A.X antibody and propidium iodide and analyzed by flow cytometry. As a positive control, cells treated for 1 h with10 ug/ml etoposide (Etop). (B) Comet assay. Cells were treated as in (A) and subjected to single-cell electrophoresis under denaturing conditions (comet assay).
Mitogenic drive and mitotic incompetence in senescent MEFs
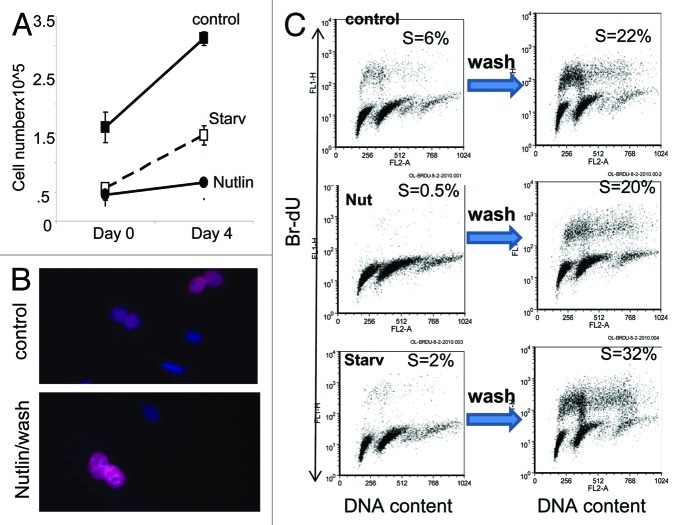
We next investigated whether hyper-mitogenic drive is coupled with mitotic incompetence in senescent normal cells. In mouse embryonic fibroblasts (MEFs), senescence can be caused by nutlin-3, which arrests cell cycle but does not inhibit the mTOR pathway in these cells.8 MEFs become hypertrophic, flat, β-Gal-positive8 and highly positive for nuclear cyclin D1 (Fig. S1C). This is a convenient model of senescence, because nutlin-3 can be easily washed out, revealing irreversible loss of RP. In fact, there was no cell proliferation after nutlin-3 was washed out (Fig. 6A). For comparison, serum starvation caused a reversible arrest (quiescence): MEFs resumed proliferation when serum was re-added (Fig. 6A). Yet, nutlin-3-induced senescent MEFs incorporated Br-dU when nutlin-3 was removed (Fig. 6B). Both senescent and quiescent cells retained the ability to enter and progress through S phase, as evidenced by incorporation of Br-dU (Fig. 6C). Furthermore, removal of nutlin-3 caused 40-fold induction in Br-dU incorporation compared with 16-fold induction caused by re-addition of serum to starved cells (Fig. 6C). Therefore, hyper-mitogenic drive in senescent MEFs ensured their robust re-entry in cell cycle but not proliferation.
Figure 6. Regenerative/proliferative potential in senescent MEFs. (A) Regenerative/proliferative potential. MEFs were treated with 10 uM nutlin-3 (Nutlin) or starved (starv) in 0.2% FBS for 6 d. Then MEFs were washed (day 0) and allowed to re-grow in drug-free 10% FBS medium for 4 d and counted. (B) EdU incorporation. MEFs were treated with 10 uM nutlin-3 for 4 d, then washed and incubated in fresh medium for 1 d and labeled with EdU. Fixed cells were stained for EdU and counterstained with DAPI and microphotographed. Merged images are shown. (C) Br-dU incorporation by flow cytometry. MEFs were incubated with 10 uM nutlin-3 or starved (Starv) in 0.2% FBS for 6 d and then were washed and incubated in fresh 10% FBS medium without drugs for 24 h. Cells were labeled with BrdU immediately after treatment and 24 h after wash. Fixed cells were stained with anti-BrdU antibody and propidium iodide and analyzed by flow cytometry.
Discussion
The precise link between organismal aging and cellular senescence remains to be elucidated. Here we demonstrated that cellular senescence is associated with both hyper-mitogenic drive (during arrest) and failure to undergo mitosis (after release). As theoretically predicted,40 when the cell cycle is blocked by CDK inhibitors in the presence of mitogenic stimulation, then cells grow in size without cell cycle progression, becoming senescent. Mitogenic and growth stimulation induces cyclins in a futile attempt to override cell cycle block. Cyclins D1 and E are highly expressed in senescent cells, even compared with proliferating cells. Elevated cyclin D1 and active mTOR cause cellular hypertrophy, especially given that not only mTOR but also cyclin D1 is involved in metabolic switch and cell growth in size.41-46 Also, cyclin E induces HIF-1,47 and this may contribute to the hyper-secretory phenotype of senescent cells. Both cyclins E and D1 induce chromosomal instability.48-50 Finally, cyclins push cells into S-phase. This may explain an inappropriate cell cycle entry of post-mitotic cells in aging. For example, in Alzheimer disease and other neurodegenerative disorders, neurons re-enter S phase and die.51-55 Aging arterial muscle cells56,57 and liver cells58 become polyploid. In agreement with previous reports,32 when the cell cycle was released (removal of p21), some cells entered mitosis and underwent mitotic catastrophe. As demonstrated by time-lapse video microscopy, overactivated and hypermitogenic cells attempted to undergo cell division without even entering mitosis, becoming thin and very long, damaging and tearing themselves to death. Treatment with rapamycin or nutlin-3a during geroconversion prevented mitotic catastrophe: cells that entered mitosis completed it and then underwent second mitosis, eventually forming colonies. Also, cells were not hyper-active and did not attempt to divide without entering mitosis. Here we not only confirmed paradoxical suppression of senescence by p53, but also demonstrated the mechanism: preservation of mitotic competence. Similarly, rapamycin preserved mitotic competence, supporting the notion that inhibition of mTOR by p53 may account for its gerosuppressive effect. Still, as evidenced by time-lapse video microscopy, both morphology and migratory activities of cells pretreated with rapamycin and nutlin-3 were rather different, indicating additional pathways affected by nutlin-3. Neither rapamycin nor nutlin-3 suppressed hypertrophy completely, but rather decelerated the process.5 Here we showed that inhibition of mTOR did not prevent accumulation of cyclins but only decelerated this accumulation. Some questions remain. May this explain why rapamycin decreases but does not prevent hypertrophy? Is there any mechanistic link between deceleration of cyclin D and E accumulation and preservation of mitotic competence? Or are these markers independent? And most importantly, are any other signal transduction pathways (besides mTOR) responsible for markers of senescence? Our additional study demonstrated that U0126, which targets both MEK and mTOR/pS6,59,60 especially ponently prevented cyclin D1 accumulation, suggesting the involvement of the MEK/ERK pathway in senescence-associated overexpression of cyclin D1 (MS in preparation).
Material and Methods
Cell lines and reagents
HT-p21 cells and HT-p16, derived from HT1080 human fibrosarcoma cells (ATCC), provided by Igor Roninson, were previously described.4,31-33 HT-p21 and HT-p16 cells were cultured in high-glucose DMEM without pyruvate supplemented with FC2 serum (HyClone FetalClone II from Thermo Scientific). HT-p21 and HT-p16 cells, p21 and p16 expression can be turned on or off using isopropyl-thio-galactosidase (IPTG).31,32 Mouse embryonic fibroblasts (MEFs) were provided by Dr. N. Venkatesh (Dr. A. Gudkov’s laboratory, Roswell Park Cancer Institute). Rapamycin was obtained from LC Laboratories and dissolved in DMSO as 5 mM solution. IPTG (Invitrogen) was dissolved in water as 50 mg/ml stock solution and used in cell culture at final concentration of 10–50 µg/ml. Nutlin-3 was from Sigma-Aldrich, dissolved in DMSO.
Immunoblot analysis
Whole-cell lysates were prepared using boiling lysis buffer (1% SDS, 10 mM Tris.HCl, pH 74.). Equal amounts of proteins were separated using 10% SDS-polyacrylamide minigels and transferred to nitrocellulose membranes. The following antibodies were used: rabbit anti-phospho-S6 (Ser235/236) and mouse anti-S6, anti-actin antibody from Sigma-Aldrich and mouse anti-p53 (Ab-6) from Oncogene, mouse anti-cyclins D1 and E were from Santa Cruz Biotechnology. Secondary anti-rabbit and anti-mouse HRP conjugated antibodies were from Cell Signaling Biotechnology.
Levels of gamma-H2A.X
Treated cells were fixed in 1% paraformaldehyde/80% ethanol (at -20°C), stained with mouse anti-phospho histone H2A.X (Ser 139) antibody (clone JBW301, Millipore), and followed by incubation with anti-mouse Alexa Flour 488 secondary antibody (Invitrogen). Cells were counterstained with propidium iodide and analyzed by FACScan.
Br-dU incorporation
Treated cells were labeled with 100 uM BrdU for 1 h and fixed in ice-cold 70% ethanol, stained with rat anti-BrdU antibody (Accurate Chemicals and Scientific Corp), followed by incubation with anti-rat Alexa Flour 488 antibody (Invitrogen). Cells were counterstained with propidium iodide and analyzed by FACScan (Fig. 6B). Br-du incorporation shown in Figure 2A was performed as described previously.61
Comet assay
Comet assay was performed using Trevigen CometAssay kit according to the manufacturer’s instructions.
EdU assay
Cells were labeled with Edu overnight, fixed and stained for EdU and DAPI using Click-It EdU HCS assay kit (Invitrogen).
TUNEL assay
TUNEL assay was performed as described previously.61
Live-cell multimode time-lapse imaging (video microscopy)
Phase contrast time-lapse sequences were collected as previously described.61
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/22937
References
- 1.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–40. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 2.Rodier F, Muñoz DP, Teachenor R, Chu V, Le O, Bhaumik D, et al. DNA-SCARS: distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J Cell Sci. 2011;124:68–81. doi: 10.1242/jcs.071340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blagosklonny MV. Cell cycle arrest is not yet senescence, which is not just cell cycle arrest: terminology for TOR-driven aging. Aging (Albany NY) 2012;4:159–65. doi: 10.18632/aging.100443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demidenko ZN, Blagosklonny MV. Growth stimulation leads to cellular senescence when the cell cycle is blocked. Cell Cycle. 2008;7:3355–61. doi: 10.4161/cc.7.21.6919. [DOI] [PubMed] [Google Scholar]
- 5.Demidenko ZN, Blagosklonny MV. Quantifying pharmacologic suppression of cellular senescence: prevention of cellular hypertrophy versus preservation of proliferative potential. Aging (Albany NY) 2009;1:1008–16. doi: 10.18632/aging.100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leontieva OV, Blagosklonny MV. DNA damaging agents and p53 do not cause senescence in quiescent cells, while consecutive re-activation of mTOR is associated with conversion to senescence. Aging (Albany NY) 2010;2:924–35. doi: 10.18632/aging.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demidenko ZN, Korotchkina LG, Gudkov AV, Blagosklonny MV. Paradoxical suppression of cellular senescence by p53. Proc Natl Acad Sci USA. 2010;107:9660–4. doi: 10.1073/pnas.1002298107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korotchkina LG, Leontieva OV, Bukreeva EI, Demidenko ZN, Gudkov AV, Blagosklonny MV. The choice between p53-induced senescence and quiescence is determined in part by the mTOR pathway. Aging (Albany NY) 2010;2:344–52. doi: 10.18632/aging.100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leontieva OV, Natarajan V, Demidenko ZN, Burdelya LG, Gudkov AV, Blagosklonny MV. Hypoxia suppresses conversion from proliferative arrest to cellular senescence. Proc Natl Acad Sci USA. 2012;109:13314–8. doi: 10.1073/pnas.1205690109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–5. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, et al. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle. 2011;10:4230–6. doi: 10.4161/cc.10.24.18486. [DOI] [PubMed] [Google Scholar]
- 13.Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, et al. Rapamycin extends maximal lifespan in cancer-prone mice. Am J Pathol. 2010;176:2092–7. doi: 10.2353/ajpath.2010.091050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, et al. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–82. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spong A, Bartke A. Rapamycin slows aging in mice. Cell Cycle. 2012;11:845. doi: 10.4161/cc.11.5.19607. [DOI] [PubMed] [Google Scholar]
- 16.Blagosklonny MV. Aging and immortality: quasi-programmed senescence and its pharmacologic inhibition. Cell Cycle. 2006;5:2087–102. doi: 10.4161/cc.5.18.3288. [DOI] [PubMed] [Google Scholar]
- 17.Tsang CK, Qi H, Liu LF, Zheng XFS. Targeting mammalian target of rapamycin (mTOR) for health and diseases. Drug Discov Today. 2007;12:112–24. doi: 10.1016/j.drudis.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Blagosklonny MV. An anti-aging drug today: from senescence-promoting genes to anti-aging pill. Drug Discov Today. 2007;12:218–24. doi: 10.1016/j.drudis.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Blagosklonny MV. Validation of anti-aging drugs by treating age-related diseases. Aging (Albany NY) 2009;1:281–8. doi: 10.18632/aging.100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dazert E, Hall MN. mTOR signaling in disease. Curr Opin Cell Biol. 2011;23:744–55. doi: 10.1016/j.ceb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Bjedov I, Partridge L. A longer and healthier life with TOR down-regulation: genetics and drugs. Biochem Soc Trans. 2011;39:460–5. doi: 10.1042/BST0390460. [DOI] [PubMed] [Google Scholar]
- 22.Zheng XF. Chemoprevention of age-related macular regeneration (AMD) with rapamycin. Aging (Albany NY) 2012;4:375–6. doi: 10.18632/aging.100469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blagosklonny MV. Prospective treatment of age-related diseases by slowing down aging. Am J Pathol. 2012;181:1142–6. doi: 10.1016/j.ajpath.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 24.Dulić V, Drullinger LF, Lees E, Reed SI, Stein GH. Altered regulation of G1 cyclins in senescent human diploid fibroblasts: accumulation of inactive cyclin E-Cdk2 and cyclin D1-Cdk2 complexes. Proc Natl Acad Sci USA. 1993;90:11034–8. doi: 10.1073/pnas.90.23.11034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucibello FC, Sewing A, Brüsselbach S, Bürger C, Müller R. Deregulation of cyclins D1 and E and suppression of cdk2 and cdk4 in senescent human fibroblasts. J Cell Sci. 1993;105:123–33. doi: 10.1242/jcs.105.1.123. [DOI] [PubMed] [Google Scholar]
- 26.Gong J, Traganos F, Darzynkiewicz Z. Growth imbalance and altered expression of cyclins B1, A, E, and D3 in MOLT-4 cells synchronized in the cell cycle by inhibitors of DNA replication. Cell Growth Differ. 1995;6:1485–93. [PubMed] [Google Scholar]
- 27.Wong H, Riabowol K. Differential CDK-inhibitor gene expression in aging human diploid fibroblasts. Exp Gerontol. 1996;31:311–25. doi: 10.1016/0531-5565(95)00025-9. [DOI] [PubMed] [Google Scholar]
- 28.Burton DG, Sheerin AN, Ostler EL, Smith K, Giles PJ, Lowe J, et al. Cyclin D1 overexpression permits the reproducible detection of senescent human vascular smooth muscle cells. Ann N Y Acad Sci. 2007;1119:20–31. doi: 10.1196/annals.1404.026. [DOI] [PubMed] [Google Scholar]
- 29.Atadja P, Wong H, Veillete C, Riabowol K. Overexpression of cyclin D1 blocks proliferation of normal diploid fibroblasts. Exp Cell Res. 1995;217:205–16. doi: 10.1006/excr.1995.1080. [DOI] [PubMed] [Google Scholar]
- 30.Darzynkiewicz Z. Another “Janus paradox” of p53: induction of cell senescence versus quiescence. Aging (Albany NY) 2010;2:329–30. doi: 10.18632/aging.100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang BD, Xuan Y, Broude EV, Zhu H, Schott B, Fang J, et al. Role of p53 and p21waf1/cip1 in senescence-like terminal proliferation arrest induced in human tumor cells by chemotherapeutic drugs. Oncogene. 1999;18:4808–18. doi: 10.1038/sj.onc.1203078. [DOI] [PubMed] [Google Scholar]
- 32.Chang BD, Broude EV, Fang J, Kalinichenko TV, Abdryashitov R, Poole JC, et al. p21Waf1/Cip1/Sdi1-induced growth arrest is associated with depletion of mitosis-control proteins and leads to abnormal mitosis and endoreduplication in recovering cells. Oncogene. 2000;19:2165–70. doi: 10.1038/sj.onc.1203573. [DOI] [PubMed] [Google Scholar]
- 33.Demidenko ZN, Zubova SG, Bukreeva EI, Pospelov VA, Pospelova TV, Blagosklonny MV. Rapamycin decelerates cellular senescence. Cell Cycle. 2009;8:1888–95. doi: 10.4161/cc.8.12.8606. [DOI] [PubMed] [Google Scholar]
- 34.Leontieva OV, Blagosklonny MV. Yeast-like chronological senescence in mammalian cells: phenomenon, mechanism and pharmacological suppression. Aging (Albany NY) 2011;3:1078–91. doi: 10.18632/aging.100402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pospelova TV, Demidenko ZN, Bukreeva EI, Pospelov VA, Gudkov AV, Blagosklonny MV. Pseudo-DNA damage response in senescent cells. Cell Cycle. 2009;8:4112–8. doi: 10.4161/cc.8.24.10215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Darzynkiewicz Z. When senescence masquerades as DNA damage: is DNA replication stress the culprit? Cell Cycle. 2009;8:3810–1. doi: 10.4161/cc.8.23.10368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka T, Kajstura M, Halicka HD, Traganos F, Darzynkiewicz Z. Constitutive histone H2AX phosphorylation and ATM activation are strongly amplified during mitogenic stimulation of lymphocytes. Cell Prolif. 2007;40:1–13. doi: 10.1111/j.1365-2184.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halicka HD, Zhao H, Li J, Traganos F, Zhang S, Lee M, et al. Genome protective effect of metformin as revealed by reduced level of constitutive DNA damage signaling. Aging (Albany NY) 2011;3:1028–38. doi: 10.18632/aging.100397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anisimov VN. Metformin for aging and cancer prevention. Aging (Albany NY) 2010;2:760–74. doi: 10.18632/aging.100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blagosklonny MV. Cell senescence and hypermitogenic arrest. EMBO Rep. 2003;4:358–62. doi: 10.1038/sj.embor.embor806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nelsen CJ, Rickheim DG, Tucker MM, Hansen LK, Albrecht JH. Evidence that cyclin D1 mediates both growth and proliferation downstream of TOR in hepatocytes. J Biol Chem. 2003;278:3656–63. doi: 10.1074/jbc.M209374200. [DOI] [PubMed] [Google Scholar]
- 42.Zacharek SJ, Xiong Y, Shumway SD. Negative regulation of TSC1-TSC2 by mammalian D-type cyclins. Cancer Res. 2005;65:11354–60. doi: 10.1158/0008-5472.CAN-05-2236. [DOI] [PubMed] [Google Scholar]
- 43.Wang C, Lisanti MP, Liao DJ. Reviewing once more the c-myc and Ras collaboration: converging at the cyclin D1-CDK4 complex and challenging basic concepts of cancer biology. Cell Cycle. 2011;10:57–67. doi: 10.4161/cc.10.1.14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Icreverzi A, de la Cruz AF, Van Voorhies WA, Edgar BA. Drosophila cyclin D/Cdk4 regulates mitochondrial biogenesis and aging and sensitizes animals to hypoxic stress. Cell Cycle. 2012;11:554–68. doi: 10.4161/cc.11.3.19062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanse EA, Mashek DG, Becker JR, Solmonson AD, Mullany LK, Mashek MT, et al. Cyclin D1 inhibits hepatic lipogenesis via repression of carbohydrate response element binding protein and hepatocyte nuclear factor 4α. Cell Cycle. 2012;11:2681–90. doi: 10.4161/cc.21019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knudsen KE. Cyclin D1 goes metabolic: Dual functions of cyclin D1 in regulating lipogenesis. Cell Cycle. 2012;11:3534. doi: 10.4161/cc.22039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sengupta T, Abraham G, Xu Y, Clurman BE, Minella AC. Hypoxia-inducible factor 1 is activated by dysregulated cyclin E during mammary epithelial morphogenesis. Mol Cell Biol. 2011;31:3885–95. doi: 10.1128/MCB.05089-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bagheri-Yarmand R, Biernacka A, Hunt KK, Keyomarsi K. Low molecular weight cyclin E overexpression shortens mitosis, leading to chromosome missegregation and centrosome amplification. Cancer Res. 2010;70:5074–84. doi: 10.1158/0008-5472.CAN-09-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bagheri-Yarmand R, Nanos-Webb A, Biernacka A, Bui T, Keyomarsi K. Cyclin E deregulation impairs mitotic progression through premature activation of Cdc25C. Cancer Res. 2010;70:5085–95. doi: 10.1158/0008-5472.CAN-09-4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Casimiro MC, Pestell RG. Cyclin d1 induces chromosomal instability. Oncotarget. 2012;3:224–5. doi: 10.18632/oncotarget.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bonda DJ, Evans TA, Santocanale C, Llosá JC, Viña J, Bajic VP, et al. Evidence for the progression through S-phase in the ectopic cell cycle re-entry of neurons in Alzheimer disease. Aging (Albany NY) 2009;1:382–8. doi: 10.18632/aging.100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frade JM. Unscheduled re-entry into the cell cycle induced by NGF precedes cell death in nascent retinal neurones. J Cell Sci. 2000;113:1139–48. doi: 10.1242/jcs.113.7.1139. [DOI] [PubMed] [Google Scholar]
- 53.Song B, Davis K, Liu XS, Lee HG, Smith M, Liu X. Inhibition of Polo-like kinase 1 reduces beta-amyloid-induced neuronal cell death in Alzheimer’s disease. Aging (Albany NY) 2011;3:846–51. doi: 10.18632/aging.100382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frade JM, López-Sánchez N. A novel hypothesis for Alzheimer disease based on neuronal tetraploidy induced by p75 (NTR) Cell Cycle. 2010;9:1934–41. doi: 10.4161/cc.9.10.11582. [DOI] [PubMed] [Google Scholar]
- 55.Futatsugi A, Utreras E, Rudrabhatla P, Jaffe H, Pant HC, Kulkarni AB. Cyclin-dependent kinase 5 regulates E2F transcription factor through phosphorylation of Rb protein in neurons. Cell Cycle. 2012;11:1603–10. doi: 10.4161/cc.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hixon ML, Gualberto A. Vascular smooth muscle polyploidization--from mitotic checkpoints to hypertension. Cell Cycle. 2003;2:105–10. doi: 10.4161/cc.2.2.341. [DOI] [PubMed] [Google Scholar]
- 57.Jones MR, Ravid K. Vascular smooth muscle polyploidization as a biomarker for aging and its impact on differential gene expression. J Biol Chem. 2004;279:5306–13. doi: 10.1074/jbc.M308406200. [DOI] [PubMed] [Google Scholar]
- 58.Enesco HE, Shimokawa I, Yu BP. Effect of dietary restriction and aging on polyploidy in rat liver. Mech Ageing Dev. 1991;59:69–78. doi: 10.1016/0047-6374(91)90074-A. [DOI] [PubMed] [Google Scholar]
- 59.Fukazawa H, Uehara Y. U0126 reverses Ki-ras-mediated transformation by blocking both mitogen-activated protein kinase and p70 S6 kinase pathways. Cancer Res. 2000;60:2104–7. [PubMed] [Google Scholar]
- 60.Demidenko ZN, Shtutman M, Blagosklonny MV. Pharmacologic inhibition of MEK and PI-3K converges on the mTOR/S6 pathway to decelerate cellular senescence. Cell Cycle. 2009;8:1896–900. doi: 10.4161/cc.8.12.8809. [DOI] [PubMed] [Google Scholar]
- 61.Demidenko ZN, Kalurupalle S, Hanko C, Lim CU, Broude E, Blagosklonny MV. Mechanism of G1-like arrest by low concentrations of paclitaxel: next cell cycle p53-dependent arrest with sub G1 DNA content mediated by prolonged mitosis. Oncogene. 2008;27:4402–10. doi: 10.1038/onc.2008.82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.