Abstract
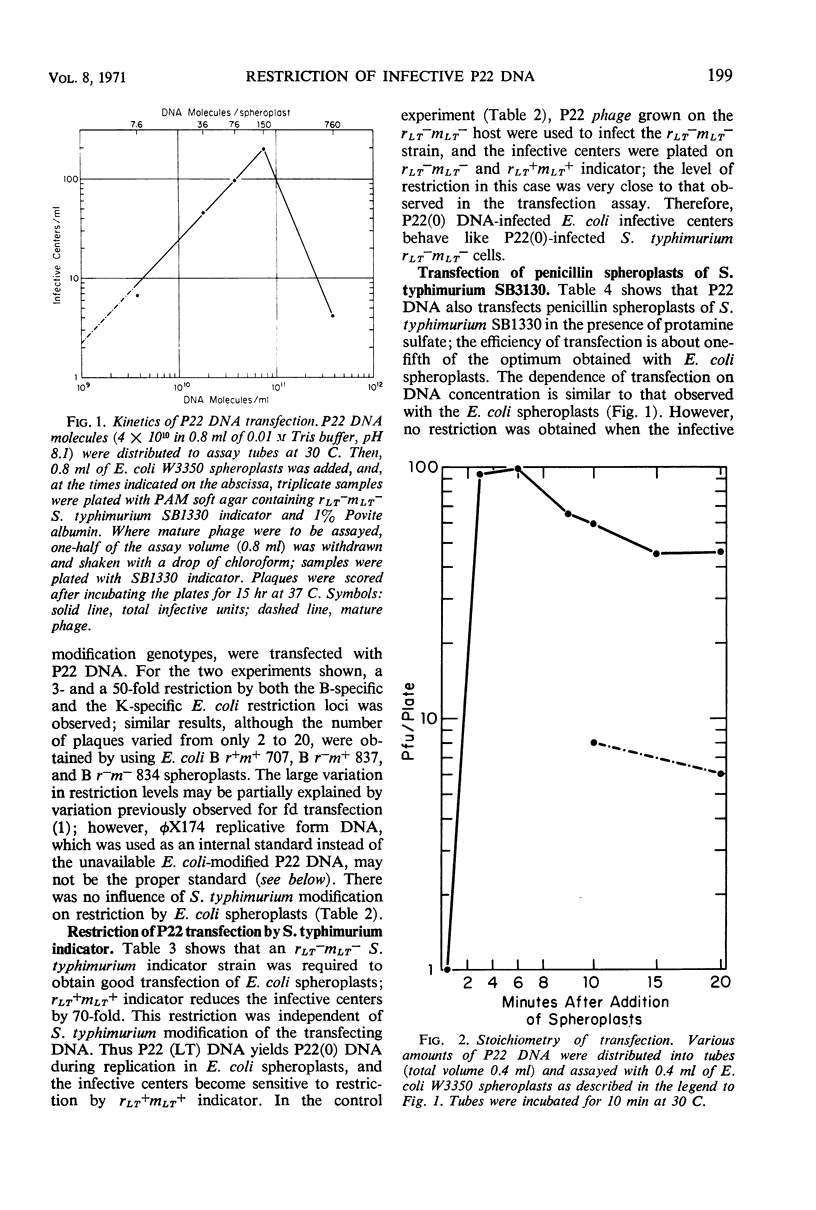
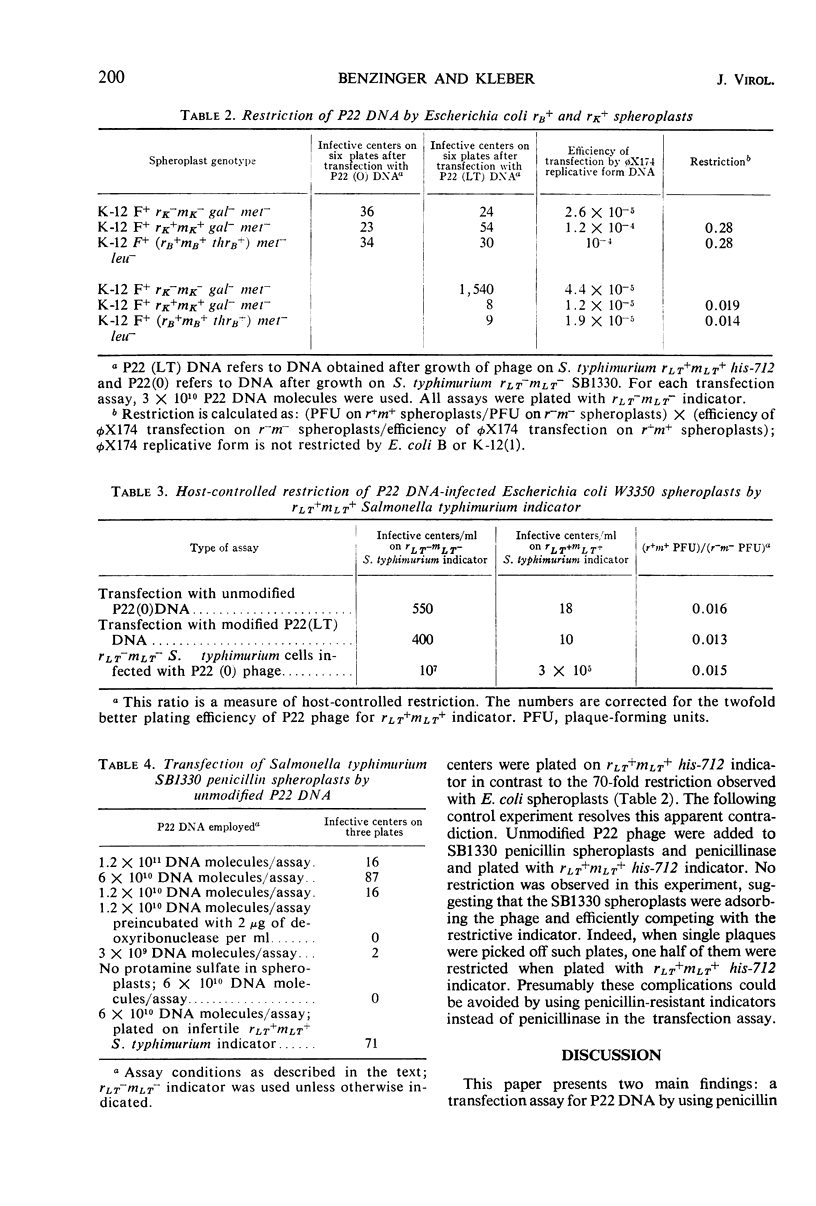
Under proper conditions, one infective center was obtained for 3 × 108 molecules of P22 phage deoxyribonucleic acid (DNA) when lysozyme-ethylenediaminetetraacetic acid spheroplasts of Escherichia coli were transfected in the presence of 25 μg of protamine sulfate per ml. A 3- to 50-fold B-specific and K-specific E. coli restriction of the incoming P22 DNA was observed. When P22 DNA-infected E. coli spheroplasts were plated with infertile rLT+mLT+Salmonella typhimurium indicator, an additional 70-fold restriction was observed. In the presence of protamine sulfate, penicillin spheroplasts of S. typhimurium SB1330 could be transfected b P22 DNA with efficiencies sometimes approaching those obtained with the E. coli spheroplasts; thus, facilitation of transfection by protamine sulfate is not limited to E. coli or to lysozyme-ethylenediaminetetraacetic acid spheroplasts. The application of these results to studies of transfection among other genuses and to studies of in vitro host-controlled restriction and modification for the two loci in S. typhimurium and the one locus in E. coli is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benzinger R., Kleber I., Huskey R. Transfection of Escherichia coli spheroplasts. I. General facilitation of double-stranded deoxyribonucleic acid infectivity by protamine sulfate. J Virol. 1971 May;7(5):646–650. doi: 10.1128/jvi.7.5.646-650.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzinger R. Restriction of infectious bacteriophage fd DNA's and an assay for in vitro host-controlled restriction and modification. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1294–1299. doi: 10.1073/pnas.59.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein D., Matz M. J. A recombination function essential to the growth of bacteriophage P22. J Mol Biol. 1970 Dec 28;54(3):417–440. doi: 10.1016/0022-2836(70)90119-1. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Colson A. M., Colson C., Van Pel A. Host-controlled restriction mutants of Salmonella typhimurium. J Gen Microbiol. 1969 Sep;58(1):57–64. doi: 10.1099/00221287-58-1-57. [DOI] [PubMed] [Google Scholar]
- Colson C., Colson A. M., Van Pel A. Chromosomal location of host specificity in Salmonella typhimurium. J Gen Microbiol. 1970 Feb;60(2):265–271. doi: 10.1099/00221287-60-2-265. [DOI] [PubMed] [Google Scholar]
- Garrick-Silversmith L., Hartman P. E. Histidine-requiring mutants of Escherichia coli K12. Genetics. 1970 Oct;66(2):231–244. doi: 10.1093/genetics/66.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattman S. Variation of 6-methylaminopurine content in bacteriophage P22 deoxyribonucleic acid as a function of host specificity. J Virol. 1971 May;7(5):690–691. doi: 10.1128/jvi.7.5.690-691.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotz G., Mauser R. Infectious DNA from coliphage T1. I. Some properties of the spheroplast assay system. Mol Gen Genet. 1969;104(2):178–194. doi: 10.1007/BF00272800. [DOI] [PubMed] [Google Scholar]
- Miyake T. Exchange of Genetic Material between Salmonella Typhimurium and Escherichia Coli K-12. Genetics. 1962 Aug;47(8):1043–1052. doi: 10.1093/genetics/47.8.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Thomas C. A., Jr The arrangement of information in DNA molecules. J Gen Physiol. 1966 Jul;49(6):143–169. doi: 10.1085/jgp.49.6.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wais A. C., Goldberg E. B. Growth and transformation of phage T4 in Escherichia coli B-4, Salmonella, Aerobacter, Proteus, and Serratia. Virology. 1969 Oct;39(2):153–161. doi: 10.1016/0042-6822(69)90035-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]



