Abstract
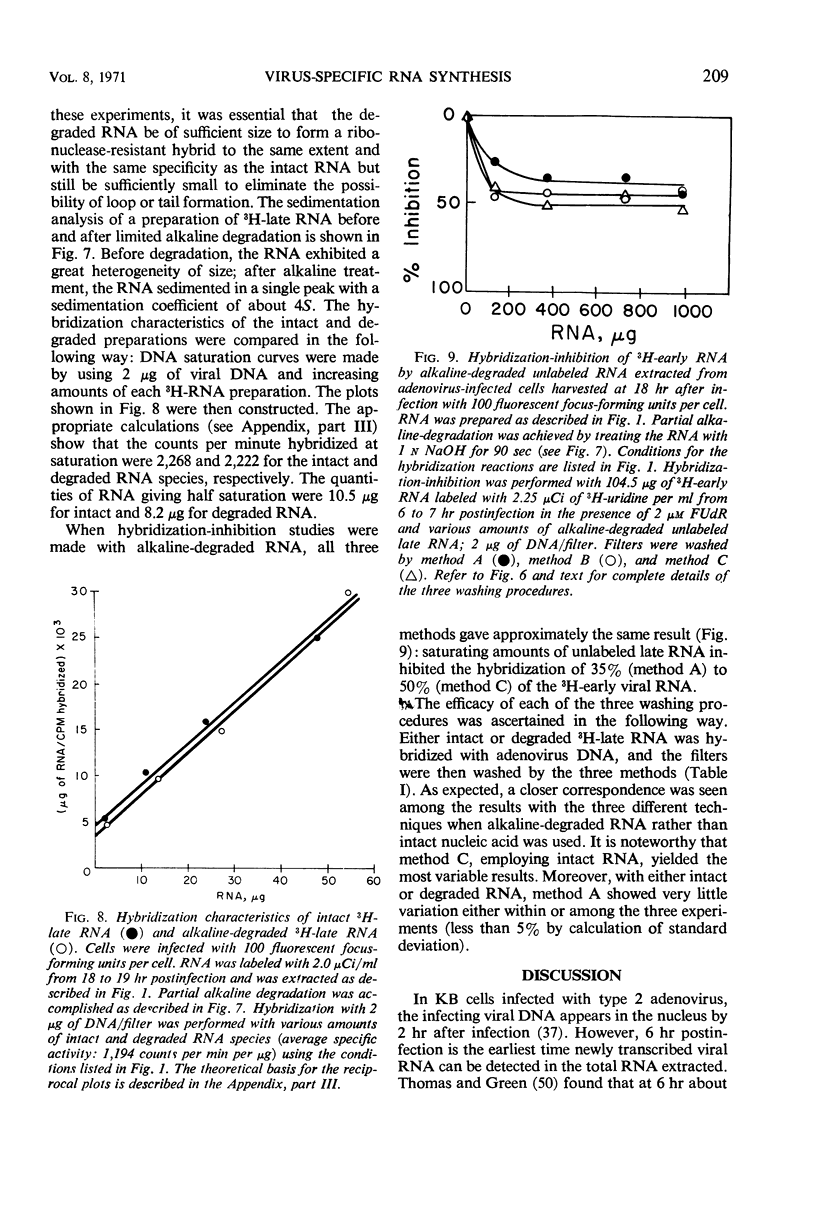
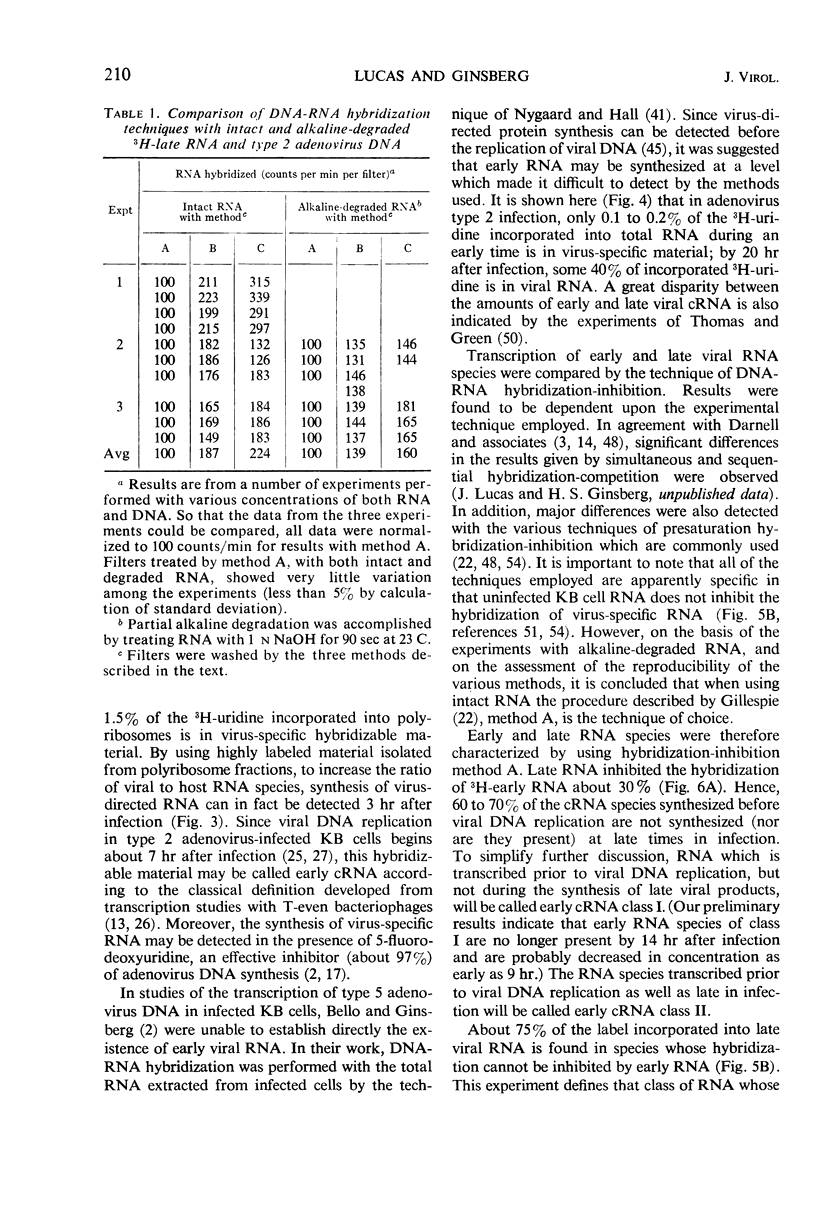
By using the technique of deoxyribonucleic acid (DNA)-ribonucleic acid (RNA) hybridization, virus-specific RNA (cRNA) was detected 6 hr after infection in preparations of total RNA from cells infected with type 2 adenovirus in the presence of 2 μm 5-fluorodeoxyuridine. In the absence of 5-fluorodeoxyuridine, there was a continuous increase in the incorporation of 3H-uridine into viral cRNA until 20 hr after infection, at which time approximately 40% of the 3H-uridine entering RNA was found in virus-specific RNA. When RNA was prepared from polyribosome fractions obtained from cytoplasmic extracts of infected cells, virus-directed transcription was detected at 3 hr after infection (i.e., 3 to 4 hr before the initiation of viral DNA synthesis). Viral cRNA species synthesized at different times after infection were compared by the technique of DNA-RNA hybridization-inhibition (“presaturation” hybridization-competition). Three hybridization-inhibition techniques were compared. The techniques differed in the manner in which the DNA-RNA complex was isolated after the first hybridization reaction. Depending on the procedure employed, various degrees of inhibition were measured. The variation could be essentially eliminated if prior to hybridization the inhibitory RNA species were alkali-degraded to a uniform size of about 4S. Undegraded RNA could be used if the DNA-RNA complex was isolated by using a procedure involving rigorous washing (preferably including ribonuclease treatment) before the second hybridization with labeled RNA. When a rigorous hybridization-inhibition procedure was used, three classes of virus-specific RNA species could be distinguished: (i) early RNA class I whose synthesis began prior to viral DNA replication and stopped at some time after the initiation of viral DNA replication—it comprised about 70% of the early RNA species and was apparently degraded by 18 hr after infection; (ii) early RNA class II whose synthesis began prior to viral DNA replication and apparently continued at an enhanced rate late in infection; and (iii) late RNA whose synthesis began after the initiation of viral DNA synthesis.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avery R. J., Midgley J. E. A new approach to the analysis of hybridization of bacterial nucleic acids. Analysis of the ribosomal ribonucleic acids of Bacillus subtilis. Biochem J. 1969 Nov;115(3):383–394. doi: 10.1042/bj1150383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello L. J., Ginsberg H. S. Relationship between deoxyribonucleic acid-like ribonucleic acid synthesis and inhibition of host protein synthesis in type 5 adenovirus-infected KB cells. J Virol. 1969 Feb;3(2):106–113. doi: 10.1128/jvi.3.2.106-113.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Pène J. J., Darnell J. E. Studies on HeLa cell nuclear DNA-like RNA by RNA-DNA hybridization. Proc Natl Acad Sci U S A. 1967 Jul;58(1):320–327. doi: 10.1073/pnas.58.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. O., Robertson F. W., Burns J. A., Melli M. Methods for the analysis of deoxyribonucleic acid-ribonucleic acid hybridization data. Biochem J. 1969 Nov;115(3):361–370. doi: 10.1042/bj1150361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. O., Robertson F. W. Transcription of bacteriophage T4 deoxyribonucleic acid in vitro. Biochem J. 1969 Nov;115(3):353–361. doi: 10.1042/bj1150353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: synthesis and relative stability of early and late RNA. J Mol Biol. 1968 Feb 14;31(3):325–348. doi: 10.1016/0022-2836(68)90413-0. [DOI] [PubMed] [Google Scholar]
- Brody E. N., Diggelmann H., Geiduschek E. P. Transcription of the bacteriophage T4 template. Obligate synthesis of T4 prereplicative RNA in vitro. Biochemistry. 1970 Mar 17;9(6):1289–1299. doi: 10.1021/bi00808a001. [DOI] [PubMed] [Google Scholar]
- Brody E. N., Geiduschek E. P. Transcription of the bacteriophage T4 template. Detailed comparison of in vitro and in vivo transcripts. Biochemistry. 1970 Mar 17;9(6):1300–1309. doi: 10.1021/bi00808a002. [DOI] [PubMed] [Google Scholar]
- Bruner R., Cape R. E. The expression of two classes of late genes of bacteriophage T4. J Mol Biol. 1970 Oct 14;53(1):69–89. doi: 10.1016/0022-2836(70)90046-x. [DOI] [PubMed] [Google Scholar]
- Carp R. I., Sauer G., Sokol F. The effect of actinomycin D on the transcription and replication of simian virus 40 deoxyribonucleic acid. Virology. 1969 Feb;37(2):214–226. doi: 10.1016/0042-6822(69)90201-3. [DOI] [PubMed] [Google Scholar]
- Church R. B., McCarthy B. J. Related base sequences in the DNA of simple and complex organisms. II. The interpretation of DNA-RNA hybridization studies with mammalian nucleic acids. Biochem Genet. 1968 Jun;2(1):55–73. doi: 10.1007/BF01458451. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Ribonucleic acids from animal cells. Bacteriol Rev. 1968 Sep;32(3):262–290. doi: 10.1128/br.32.3.262-290.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Propagation in a fluid medium of a human epidermoid carcinoma, strain KB. Proc Soc Exp Biol Med. 1955 Jul;89(3):362–364. doi: 10.3181/00379727-89-21811. [DOI] [PubMed] [Google Scholar]
- FLANAGAN J. F., GINSBERG H. S. Synthesis of virus-specific polymers in adenovirus-infected cells; effect of 5-fluorodeoxyuridine. J Exp Med. 1962 Aug 1;116:141–157. doi: 10.1084/jem.116.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M. P., Lyons M. J., Ginsberg H. S. Biochemical consequences of type 2 adenovirus and Simian virus 40 double infections of African green monkey kidney cells. J Virol. 1970 May;5(5):586–597. doi: 10.1128/jvi.5.5.586-597.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILBERT W. Polypeptide synthesis in Escherichia coli. II. The polypeptide chain and S-RNA. J Mol Biol. 1963 May;6:389–403. doi: 10.1016/s0022-2836(63)80051-0. [DOI] [PubMed] [Google Scholar]
- GIRARD M., LATHAM H., PENMAN S., DARNELL J. E. ENTRANCE OF NEWLY FORMED MESSENGER RNA AND RIBOSOMES INTO HELA CELL CYTOPLASM. J Mol Biol. 1965 Feb;11:187–201. doi: 10.1016/s0022-2836(65)80050-x. [DOI] [PubMed] [Google Scholar]
- GREEN M. Biochemical studies on adenovirus multiplication. III. Requirement for DNA synthesis. Virology. 1962 Dec;18:601–613. doi: 10.1016/0042-6822(62)90063-6. [DOI] [PubMed] [Google Scholar]
- Gage L. P., Geiduschek E. P. Repression of early messenger transcription in the development of a bacteriophage. J Mol Biol. 1967 Dec 14;30(2):435–440. [PubMed] [Google Scholar]
- Geiduschek E. P., Wilson D. L., Gage L. P. Determinants of gene expression during viral development. J Cell Physiol. 1969 Oct;74(2 Suppl):81–86. doi: 10.1002/jcp.1040740407. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Green M. Oncogenic viruses. Annu Rev Biochem. 1970;39:701–756. doi: 10.1146/annurev.bi.39.070170.003413. [DOI] [PubMed] [Google Scholar]
- Green M., Piña M., Kimes R., Wensink P. C., MacHattie L. A., Thomas C. A., Jr Adenovirus DNA. I. Molecular weight and conformation. Proc Natl Acad Sci U S A. 1967 May;57(5):1302–1309. doi: 10.1073/pnas.57.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL B. D., NYGAARD A. P., GREEN M. H. CONTROL OF T2-SPECIFIC RNA SYNTHESIS. J Mol Biol. 1964 Jul;9:143–153. doi: 10.1016/s0022-2836(64)80096-6. [DOI] [PubMed] [Google Scholar]
- HALL B. D., SPIEGELMAN S. Sequence complementarity of T2-DNA and T2-specific RNA. Proc Natl Acad Sci U S A. 1961 Feb 15;47:137–163. doi: 10.1073/pnas.47.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOMMA M., GRAHAM A. F. SYNTHESIS OF RNA IN L CELLS INFECTED WITH MENGO VIRUS. J Cell Physiol. 1963 Oct;62:179–192. doi: 10.1002/jcp.1030620207. [DOI] [PubMed] [Google Scholar]
- Hansen J. N., Spiegelman G., Halvorson H. O. Bacterial spore outgrowth: its regulation. Science. 1970 Jun 12;168(3937):1291–1298. doi: 10.1126/science.168.3937.1291. [DOI] [PubMed] [Google Scholar]
- Hudson J., Goldstein D., Weil R. A study on the transcription of the polyoma viral genome. Proc Natl Acad Sci U S A. 1970 Jan;65(1):226–233. doi: 10.1073/pnas.65.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavallé R., De Hauwer G. Messenger RNA synthesis during amino acid starvation in Escherichia coli. J Mol Biol. 1968 Oct 28;37(2):269–288. doi: 10.1016/0022-2836(68)90267-2. [DOI] [PubMed] [Google Scholar]
- Lawrence W. C., Ginsberg H. S. Intracellular uncoating of type 5 adenovirus deoxyribonucleic acid. J Virol. 1967 Oct;1(5):851–867. doi: 10.1128/jvi.1.5.851-867.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Philipson L. Early events of virus-cell interaction in an adenovirus system. J Virol. 1969 Oct;4(4):323–338. doi: 10.1128/jvi.4.4.323-338.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukage A., Minagawa T. Shut-off of early messenger RNA synthesis in E. coli infected with phage T2. Biochem Biophys Res Commun. 1967 Oct 11;29(1):39–44. doi: 10.1016/0006-291x(67)90537-2. [DOI] [PubMed] [Google Scholar]
- McCarthy B. J., Church R. B. The specificity of molecular hybridization reactions. Annu Rev Biochem. 1970;39:131–150. doi: 10.1146/annurev.bi.39.070170.001023. [DOI] [PubMed] [Google Scholar]
- Melli M., Bishop J. O. Hybridization between rat liver DNA and complementary RNA. J Mol Biol. 1969 Feb 28;40(1):117–136. doi: 10.1016/0022-2836(69)90300-3. [DOI] [PubMed] [Google Scholar]
- NYGAARD A. P., HALL B. D. A method for the detection of RNA-DNA complexes. Biochem Biophys Res Commun. 1963 Jul 18;12:98–104. doi: 10.1016/0006-291x(63)90242-0. [DOI] [PubMed] [Google Scholar]
- Oda K. I., Joklik W. K. Hybridization and sedimentation studies on "early" and "late" vaccinia messenger RNA. J Mol Biol. 1967 Aug 14;27(3):395–419. doi: 10.1016/0022-2836(67)90047-2. [DOI] [PubMed] [Google Scholar]
- Oda K., Dulbecco R. Regulation of transcription of the SV40 DNA in productively infected and in transformed cells. Proc Natl Acad Sci U S A. 1968 Jun;60(2):525–532. doi: 10.1073/pnas.60.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piña M., Green M. Biochemical studies on adenovirus multiplication. XIV. Macromolecule and enzyme synthesis in cells replicating oncogenic and nononcogenic human adenovirus. Virology. 1969 Aug;38(4):573–586. doi: 10.1016/0042-6822(69)90178-0. [DOI] [PubMed] [Google Scholar]
- Russell W. C., Hayashi K., Sanderson P. J., Pereira H. G. Adenovirus antigens--a study of their properties and sequential development in infection. J Gen Virol. 1967 Oct;1(4):495–507. doi: 10.1099/0022-1317-1-4-495. [DOI] [PubMed] [Google Scholar]
- SCHERRER K., DARNELL J. E. Sedimentation characteristics of rapidly labelled RNA from HeLa cells. Biochem Biophys Res Commun. 1962 Jun 4;7:486–490. doi: 10.1016/0006-291x(62)90341-8. [DOI] [PubMed] [Google Scholar]
- Salser W., Bolle A., Epstein R. Transcription during bacteriophage T4 development: a demonstration that distinct subclasses of the "early" RNA appear at different times and that some are "turned off" at late times. J Mol Biol. 1970 Apr 28;49(2):271–295. doi: 10.1016/0022-2836(70)90246-9. [DOI] [PubMed] [Google Scholar]
- Soeiro R., Darnell J. E. Competition hybridization by "pre-saturation" of HeLa cell DNA. J Mol Biol. 1969 Sep 28;44(3):551–562. doi: 10.1016/0022-2836(69)90379-9. [DOI] [PubMed] [Google Scholar]
- Stubbs J. D., Hall B. D. Level of tryptophan messenger RNA in Escherichia coli. J Mol Biol. 1968 Oct 28;37(2):289–302. doi: 10.1016/0022-2836(68)90268-4. [DOI] [PubMed] [Google Scholar]
- Thomas D. C., Green M. Biochemical studies on adenovirus multiplication, xi. Evidence of a cytoplasmic site for the synthesis of viral-coded proteins. Proc Natl Acad Sci U S A. 1966 Jul;56(1):243–246. doi: 10.1073/pnas.56.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. C., Green M. Biochemical studies on adenovirus multiplication. XV. Transcription of the adenovirus type II genome during productive infection. Virology. 1969 Oct;39(2):205–210. doi: 10.1016/0042-6822(69)90040-3. [DOI] [PubMed] [Google Scholar]
- Velicer L. F., Ginsberg H. S. Cytoplasmic synthesis of type 5 adenovirus capsid proteins. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1264–1271. doi: 10.1073/pnas.61.4.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velicer L. F., Ginsberg H. S. Synthesis, transport, and morphogenesis of type adenovirus capsid proteins. J Virol. 1970 Mar;5(3):338–352. doi: 10.1128/jvi.5.3.338-352.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warocquier R., Samaille J., Green M. Biochemical studies on adenovirus multiplication. XVI. Transcription of the adenovirus genome during abortive infection of elevated temperatures. J Virol. 1969 Oct;4(4):423–428. doi: 10.1128/jvi.4.4.423-428.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


