Abstract
Evidence from human and animal research indicates that choline metabolic pathways may be activated during a variety of diseases, including cancer. We report results of a case-control study of 2821 lung cancer cases and 2923 controls that assessed associations of choline and betaine dietary intakes with lung cancer. Using multivariable logistic regression analyses, we report a significant association between higher betaine intake and lower lung cancer risk that varied by smoking status. Specifically, no significant association was observed between betaine intake and lung cancer among never-smokers. However, higher betaine intake was significantly associated with reduced lung cancer risk among smokers, and the protective effect was more evident among current than former smokers: for former and current smokers, the ORs (95% CI) of lung cancer for individuals with highest as compared to lowest quartiles of intake were 0.70(0.55–0.88) and 0.51(0.39–0.66) respectively. Significant linear trend of higher betaine intake and lower lung cancer risk was observed among both former (ptrend = 0.002) and current (ptrend<0.0001) smokers. A similar protective effect was also observed with choline intake both in overall analysis as well as among current smokers, with p-values for chi-square tests being 0.001 and 0.004 respectively, but the effect was less evident, as no linear trend was observed. Our results suggest that choline and betaine intake, especially higher betaine intake, may be protective against lung cancer through mitigating the adverse effect of smoking.
Introduction
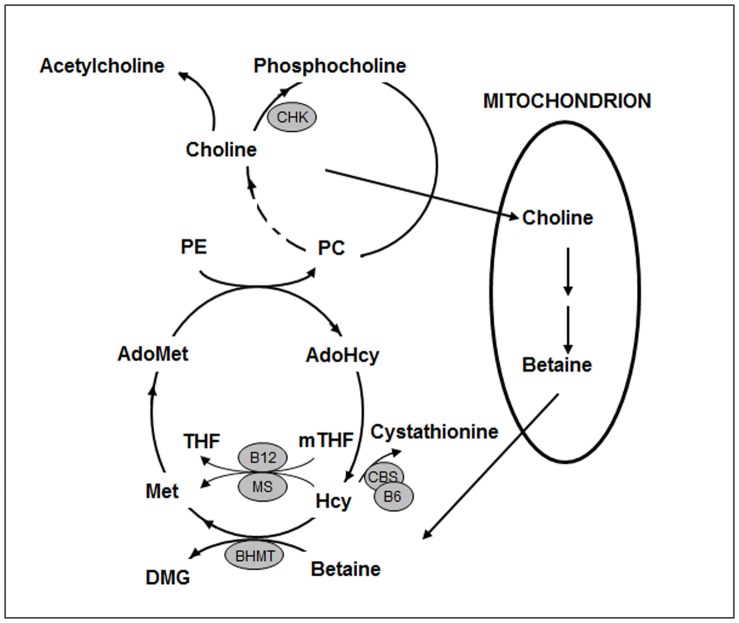
A locus on chr15q25 that includes a cluster of nicotinic acetylcholine receptor subunit genes CHRNA3, CHRNB4 and CHRNA5 was identified to be robustly associated with lung cancer risk in genome wide association studies, suggesting the potential relevance of choline and its metabolites in lung cancer risk [1], [2]. Choline and betaine are nutrients that can be either obtained from the diet or synthesized metabolically. Choline, the precursor of betaine, is abundantly present in egg yolks, beef, chicken, liver and soybeans [3], and is converted to betaine through a two-step oxidation process that occurs in the mitochondria of liver and kidney. Most of the remaining choline is phosphorylated to phosphocholine by choline kinase (CHK) and is further converted to phosphatidylcholine (PC) (Figure 1). Choline is important for cell membrane structure as well as neurotransmission; is associated with neurodevelopment and hippocampus cell proliferation, differentiation, and apoptosis in animals; and may affect brain development and cognitive functions in humans [4].
Figure 1. Choline and Betaine Metabolism.
Abbreviations: PC – phosphatidylcholine; PE – phosphatidylethanolamine; AdoMet – S-adenosylmethionine; AdoHcy – S-adenosylhomocysteine; THF – tetrahydrofolate; mTHF –5-methyltetrahydrofolate; Met – methionine; Hcy – homocysteine; DMG – dimethylglycine; MS – methionine synthase; BHMT – betaine-homocysteine S-methyltransferase; CBS – cystathionine β-synthase; B6– vitamin B6; B12– vitamin B12.
Betaine is a nutrient abundant in animal foods, especially seafood, and plant foods such as wheat bran and spinach [3], [5]. It is a methyl donor for the betaine-homocysteine methyltransferase (BHMT)-mediated reaction that converts homocysteine (Hcy) to methionine (Met) [6]. Betaine serves as an osmolyte that regulates cell volume and protects cells and proteins from environmental stresses such as ionic stress and elevated temperature [7]. It is involved in liver metabolism [8], [9].
Choline, betaine, methionine, and folate are major methyl donors in humans. Deprivation of methyl donors causes DNA hypomethylation. Global DNA hypomethylation, regional hypermethylation of tumor suppressor genes and regional hypomethylation of oncogenes and prometastatic genes are associated with nearly all types of cancers [10]–[15]. Many studies have revealed associations between choline/betaine metabolism and cancer [16]–[19], while others have examined the role of dietary intakes of choline and betaine and risk for different cancers, such as breast, colorectal, epithelial ovarian and liver cancers [20]–[24]. To our knowledge, no study has reported on the association between betaine or choline intake and lung cancer. Lung cancer is the second most common cancer, and the leading cause of cancer death, in both men and women in the United States, and there is an urgent need for promising dietary preventive strategies to reduce lung cancer risk, especially amongst smokers. In the current study, we explore the association between lung cancer risk and dietary intake of choline and betaine.
Materials and Methods
Study Subjects
This study is part of a previously described molecular epidemiological case-control study carried out by The University of Texas MD Anderson Cancer Center (UTMDACC) that aims to assess susceptibility markers for lung cancer [25], [26]. Briefly, patients with newly diagnosed, histologically confirmed lung cancer were recruited prior to initiation of radiotherapy or chemotherapy at UTMDACC between July 1995 and February 2009. Controls without a previous diagnosis of cancer (except for non-melanoma skin cancer) were recruited from the Kelsey-Seybold Clinics, Houston’s largest private multidisciplinary physician group. There were no age, sex, race/ethnicity or cancer stage restrictions, but the study attempted to match numbers of cases and controls within each smoking status, and age within 5 years of difference between cases and controls. A total of 5942 individuals were included in the analysis. The study was approved by the UTMDACC Institutional Review Board.
Data Collection
A 45-minute personal interview was conducted by a trained UTMDACC research interviewer for each newly recruited subject, using a structured questionnaire. Information on socio-demographic characteristics, smoking and second-hand smoke (SHS) exposure history, prior respiratory conditions and exposures, medical history, and family history of cancer was obtained during the interview. Never-smokers were defined as individuals who smoked less than 100 cigarettes in their lifetime. Former smokers were defined as individuals who had quit smoking at least one year prior to diagnosis (cases) or the interview (controls). Individuals who reported currently smoking or had quit smoking less than one year prior to enrollment were classified as current smokers. Individuals with daily cigarette consumption of at least one pack were considered heavy smokers. Family cancer history was based on a cancer diagnosis in at least one first-degree family member. Dust exposure represents the presence of any dust-related exposure including exposure to sawdust, coal dust, sand, metal and cotton. SHS exposure was defined as exposure to any type of second-hand-smoke (at work, at home, or during leisure time away from home or work). A nicotine addiction score (0–10) was calculated using a modified Fagerstrom Test of Nicotine Dependence (FTND) and used as a measure of nicotine dependency [25].
Dietary data were collected using a modified version of the Health Habits and History Questionnaire Food Frequency Questionnaire (FFQ) developed by the National Cancer Institute. The validity and reliability of this questionnaire has been documented [27], [28]. The FFQ includes a semi-quantitative list of 214 food and beverage items including ethnic foods popular in the Houston community, as well as questions about vitamin and mineral supplement use, eating at restaurants, and food preparation methods. The questionnaire assessed usual food intake during the year prior to enrollment in the study for controls and during the year prior to diagnosis for cases. Nutrient intake was calculated using the DIETSYS+Plus version 5.9 dietary analysis program (Block Dietary Data Systems, Berkeley, CA) to calculate grams of intake per day.
The USDA Database for the Choline Content of Common Foods, Release 2 [29] was used to obtain the choline and betaine composition of individual foods. Source of total calories and alcohol values for food and beverage items were Standard Reference, Release 21 [30]. We calculated average daily intake of choline and betaine by multiplying the frequency of consumption of each food item by its choline and betaine content, and a summary measure for all foods was generated for choline and betaine. Total choline intake was calculated as the sum of choline intake from free choline, phosphatidylcholine, phosphocholine, glycerophosphocholine and sphingomyelin.
Statistical Analysis
The initial dataset included 2925 lung cancer cases and 3017 controls. Total caloric intake ranged from 5 to 10965 kcal/day. Individuals with total caloric intake below the 1st and above the 99th percentiles for each sex were considered as outliers and were excluded from the analysis. The total caloric intake in the remaining sample ranged 749–5939 kcal/day for males, and 593–5522 kcal/day for females, with 181 individuals being excluded. Nineteen individuals (0.3%) were excluded due to missing data on race/ethnicity or for being other than African American, Hispanic, or non-Hispanic White.
Dietary intake of choline and betaine was adjusted by total calories using the residual method proposed by Willett et al. [31]. Energy-adjusted choline and betaine intake were categorized into quartiles based on their distributions in controls. Logistic regression was used to evaluate associations between choline and betaine intake and lung cancer. Based on previous studies [26], [32]–[35], age, sex, race/ethnicity, smoking status, dust exposure, previously diagnosed respiratory diseases (hay fever, asthma, and emphysema), family cancer history, SHS in never-smokers, nicotine dependency (addiction index) and cumulative tobacco exposure (smoking pack-year) among smokers, time since smoking cessation for former smokers, as well as alcohol consumption and total energy intake were regarded as potential confounders for the association between choline and betaine intake and lung cancer. All potential relevant confounders were tested for association with choline and betaine intake as well as with lung cancer risk. Variables associated with both choline or betaine intake and lung cancer risk at significance level of p<0.25, that also changed the odds ratio for crude association between choline or betaine intake and lung cancer by ≥10%, were considered as confounders [23]. Age, sex, race/ethnicity and smoking status are known confounders and were included in multivariable regression models along with total caloric intake, for analyses of the whole dataset, and age, sex, race/ethnicity and total caloric intake were included in regression models stratified by smoking status. Tests for linear trends were assessed by applying Wald tests to ordinal values of corresponding variables, entered as continuous variables.
Interactions between each covariate (other than smoking status) and choline or betaine intake were tested individually using likelihood ratio (LR) tests. Variables that were neither confounders nor effect modifiers were excluded from the model.
To ensure that no confounders were eliminated due to our stringent model selection criteria, complete multivariable regression models contained all individually evaluated potential confounders and risk factors as listed in previous text were tested and compared with above reduced models.
For all binary variables, a third category was added to denote missing observations. Among all continuous variables, only three variables contained missing data, and the maximal missingness was 2%. Therefore, missing data were ignored during analyses. Zero was assigned to smoking related variables pack-year of smoking and addition index, for never smokers. Current age was assigned to variable time since smoking cessation for never smokers, and 0 was assigned to current smokers for the variable.
All data were analyzed using SAS Version 9.2 (SAS Inc., Cary, NC).
Results
After data cleaning and processing, 5744 of 5942 individuals remained for analysis, which included 2821 cases and 2923 controls. Demographic characteristics of the sample and summary statistics for potential confounders are shown in Table 1. There were significant differences (p<0.05) between cases and controls for all variables listed except total calories and alcohol consumption. All variables in Table 1 were significantly (p<0.05) associated with betaine intake, while race/ethnicity, sex, smoking status, dust exposure, addiction index and alcohol intake were significantly associated with choline intake (data not shown).
Table 1. Comparison of demographic and other characteristics between cases and controls (n = 5744).
| Variable | Cases (n = 2821) | Controls (n = 2923) | P* | |||
| No. | % | No. | % | |||
| Sex | Male | 1507 | 53.42 | 1448 | 49.54 | 0.003 |
| Female | 1314 | 46.58 | 1475 | 50.46 | ||
| Race | Black | 389 | 13.79 | 383 | 13.10 | 0.028 |
| Hispanic | 154 | 5.46 | 209 | 7.15 | ||
| White | 2278 | 80.75 | 2331 | 79.74 | ||
| Smoking status | Never | 453 | 16.06 | 693 | 23.71 | <0.0001 |
| Former | 1225 | 43.42 | 1168 | 39.96 | ||
| Current | 1143 | 40.52 | 1062 | 36.33 | ||
| Emphysema | Yes | 524 | 18.57 | 169 | 5.78 | <0.0001 |
| No | 2050 | 72.67 | 2571 | 87.98 | ||
| Asthma | Yes | 386 | 13.68 | 351 | 12.01 | <0.0001 |
| No | 2176 | 77.14 | 2407 | 82.35 | ||
| Hay fever | Yes | 421 | 14.92 | 621 | 21.25 | <0.0001 |
| No | 2127 | 75.40 | 2142 | 73.28 | ||
| Family historya | Yes | 1637 | 58.03 | 1545 | 52.86 | <0.0001 |
| No | 757 | 26.83 | 1062 | 36.33 | ||
| Dust exposureb | Yes | 818 | 29.00 | 662 | 22.65 | <0.0001 |
| No | 1569 | 55.62 | 1948 | 66.40 | ||
| SHSc | Yes | 1597 | 56.61 | 1746 | 59.73 | <0.0001 |
| No | 168 | 5.96 | 348 | 11.91 | ||
| No. | Mean(SD) | No. | Mean(SD) | p** | ||
| Age (year) | 2821 | 62.49 (10.86) | 2923 | 58.75 (11.47) | <0.0001 | |
| Addction index (0–10)d | 2294 | 4.56 (2.41) | 2200 | 4.00 (2.59) | <0.0001 | |
| Smoking pack-yeard | 2329 | 49.80 (37.41) | 2205 | 38.78 (29.92) | <0.0001 | |
| Time since smoking cessation(year)e | 1214 | 14.86 (11.11) | 1155 | 15.95 (11.89) | 0.021 | |
| Calories (kcal/day) | 2821 | 2204 (907) | 2923 | 2206 (900) | 0.936 | |
| Alcohol (g/day) | 2821 | 8.24 (18.00) | 2923 | 7.71 (17.88) | 0.261 | |
Total 12.9% missing. b Total 13% missing. c Total 32.8% missing. d Smokers only. e Former smokers only.
p-value, two-sided chi-square test. ** p-value, Student’s t-test.
We found significant linear associations between smoking status and total caloric intake, as well as choline and betaine intake (Table 2). Higher total calorie intake was observed with elevated smoking status, where the mean caloric intake increased from 2032 kcal/day among never smokers, to 2337 kcal/day among current smokers (ptrend <0.0001). Similarly, energy-adjusted choline intake was the lowest among never smokers (mean = 298.3 mg/day) and highest among current smokers (mean = 305.7 mg/day, ptrend = 0.038). Conversely, a significant decreasing trend was observed for betaine intake with increased smoking status, where never-smokers had the highest mean betaine intake (55.7 mg/day), and current smokers had the lowest mean intake (42.7 mg/day, ptrend <0.0001).
Table 2. Summary Statistics for Energy-adjusted intake by smoking status.
| Variable | Statistics | Smoking Status | p* trend | ||
| Never | Former | Current | |||
| Total Calories | Mean | 2031.5 | 2166.4 | 2337.4 | <0.0001 |
| (kcal/day) | Median | 1856.4 | 2013.9 | 2168.4 | |
| SD | 818.9 | 869.2 | 961.2 | ||
| Choline | Mean | 298.3 | 305.7 | 305.7 | 0.038 |
| (mg/day) | Median | 290.5 | 295 | 298.2 | |
| SD | 72.4 | 87.4 | 91.6 | ||
| Betaine | Mean | 55.7 | 49.8 | 42.7 | <0.0001 |
| (mg/day) | Median | 45.3 | 39.6 | 35.8 | |
| SD | 35.4 | 36.4 | 30.8 | ||
p-value, test for linear trend.
Pack-years of smoking was identified as a confounder of the association between betaine intake and lung cancer. Our reduced model for betaine intake therefore adjusted for age, sex, race/ethnicity, total caloric intake, pack-years of smoking and smoking status. Under this model, a significant inverse association was observed between betaine intake and lung cancer in the overall analysis, where individuals of different smoking status were combined (poverall <0.0001, Table 3). Relative to those in the lowest quartile of betaine intake, individuals in the highest quartile of betaine intake were 33% less likely to be lung cancer cases (OR = 0.67, 95% CI: 0.58–0.79). Odds ratios for those in second and third quartiles of adjusted betaine intake, relative to those in the lowest quartile, were 0.83 (95% CI: 0.71–0.96) and 0.78 (95% CI: 0.67–0.90), respectively. Trend test suggested a significant linear trend between increased betaine intake and decreased lung cancer risk (ptrend <0.0001). No effect modification was identified for the association between betaine intake and lung cancer. The complete model did not change the association (poverall = 0.0006, ptrend <0.0001). Analyses using reduced models show that, when stratified by smoking status, there was no significant association between betaine intake and lung cancer risk among never-smokers in either individual quartiles or overall statistics (poverall = 0.635). Nor was a linear trend identified (ptrend = 0.702). However, significant associations were observed among both former (poverall = 0.023) and current smokers (poverall <0.0001), with an inverse trend by betaine intake. Among former smokers, the association was only significant in the highest quartile of betaine intake, where the odds ratio was 0.71 (95% CI: 0.56–0.89). Trend test identified a significant linear trend between increased intake and reduced OR (ptrend = 0.002). The association between betaine intake and lung cancer was most evident among current smokers, where significant associations were observed in all upper quartiles relative to the lowest quartile. For the 2nd quartile, OR = 0.70, 95% CI: 0.55–0.88; for the 3rd quartile, OR = 0.75, 95% CI: 0.59–0.95; and for the highest quartile, OR = 0.51, 95% CI: 0.39–0.66, with a significant linear trend, ptrend <0.0001. Similar results were observed when analyses were performed using complete models.
Table 3. Association between quartiles of betaine intake and lung cancer risk.
| Models | Quartiles of betaine intake | P** trend | ||||
| 1 | 2 | 3 | 4 | |||
| Case n (%) | 911 (55.51) | 713 (49.38) | 650 (47.07) | 547 (42.80) | ||
| Control n(%) | 730 (44.49) | 731 (50.62) | 731 (52.93) | 731 (57.20) | ||
| Overall | ||||||
| Reduceda | OR (95% CI) | 1.00 | 0.83 (0.71–0.96) | 0.78 (0.67–0.90) | 0.67 (0.58–0.79) | <0.0001 |
| Poverall | <0.0001 | |||||
| Completeb | OR (95% CI) | 1.00 | 0.85 (0.73–1.00) | 0.81 (0.69–0.95) | 0.71 (0.61–0.84) | <0.0001 |
| P* overall | 0.0006 | |||||
| Stratified by smoking status | ||||||
| Never Smokers | ||||||
| Reducedc | OR (95% CI) | 1.00 | 1.07 (0.73–1.57) | 0.87 (0.59–1.27) | 1.00 (0.69–1.45) | 0.702 |
| P* overall | 0.635 | |||||
| Completed | OR (95% CI) | 1.00 | 1.06 (0.72–1.57) | 0.86 (0.58–1.28) | 1.03 (0.70–1.52) | 0.869 |
| P* overall | 0.614 | |||||
| Former Smokers | ||||||
| Reducedc | OR (95% CI) | 1.00 | 0.92 (0.73–1.16) | 0.83 (0.66–1.05) | 0.71 (0.56–0.89) | 0.002 |
| P* overall | 0.023 | |||||
| Completed | OR (95% CI) | 1.00 | 0.95 (0.74–1.21) | 0.86 (0.67–1.11) | 0.75 (0.59–0.96) | 0.017 |
| P* overall | 0.112 | |||||
| Current Smokers | ||||||
| Reducedc | OR (95% CI) | 1.00 | 0.70 (0.55–0.88) | 0.75 (0.59–0.95) | 0.51 (0.39–0.66) | <0.0001 |
| P* overall | <0.0001 | |||||
| Completed | OR (95% CI) | 1.00 | 0.73 (0.57–0.93) | 0.80 (0.62–1.02) | 0.55 (0.42–0.73) | 0.0001 |
| P* overall | 0.0003 | |||||
Adjusted for sex, race/ethnicity, age, pack-years, total caloric intake, smoking status.
Adjusted for sex, race/ethnicity, age, pack-years, total caloric intake, family cancer history, dust exposure, second-hand smoke, emphysema, hay fever, asthma, addiction index, alcohol, time since smoking cessation, smking status.
Adjusted for sex, race/ethnicity, age, pack-years, total caloric intake.
Adjusted for sex, race/ethnicity, age, pack-years, total caloric intake, family cancer history, dust exposure, second-hand smoke, emphysema, hay fever, asthma, addiction index, alcohol, time since smoking cessation.
p-value, chi-square test using quartiles of betaine intake as categorical variable.
p-value, test for linear trend using quartiles of betaine intake as continuous variable.
No confounders apart from sex, race/ethnicity, total caloric intake, smoking status, and age were identified for the association between choline intake and lung cancer, and therefore, only these covariates were included in our final reduced multivariable models for choline. Similar to betaine, no variable was identified as effect modifiers for choline intake. A significant negative association between choline intake and lung cancer was observed for both overall analysis (poverall = 0.001, reduced model; poverall = 0.017, complete model; Table 4) and among current smokers (poverall = 0.004, reduced model; poverall = 0.047, complete model), where choline intake was associated with reduced lung cancer odds. However, no significant association was observed among never- and former smokers. Although significant associations were observed in some individual quartiles, the global test for linear trend was not significant in either the overall analysis (ptrend = 0.118, reduced model; poverall = 0.334, complete model) or any of the smoking categories. The Wald test p-values for trend tests were 0.070, 0.843 and 0.113 respectively for never, former and current smokers with reduced models, and 0.058, 0.299 and 0.260 respectively in complete models. No statistically significant interaction was observed between choline and betaine intake (data not shown).
Table 4. Association between quartiles of choline intake and lung cancer risk.
| Models | Quartiles of choline intake | p** trend | ||||
| 1 | 2 | 3 | 4 | |||
| Case n (%) | 809 (52.57) | 640 (46.68) | 638 (46.60) | 734 (50.10) | ||
| Control n (%) | 730 (47.43) | 731 (53.32) | 731 (53.40) | 731 (49.90) | ||
| Overall | ||||||
| Reduceda | OR (95% CI) | 1.00 | 0.77 (0.66–0.90) | 0.76 (0.66–0.89) | 0.89 (0.77–1.03) | 0.118 |
| P* overall | 0.001 | |||||
| Completeb | OR (95% CI) | 1.00 | 0.79 (0.68–0.93) | 0.82 (0.70–0.96) | 0.92 (0.78–1.07) | 0.334 |
| P* overall | 0.017 | |||||
| Stratified by smoking status | ||||||
| Never Smokers | ||||||
| Reducedc | OR (95% CI) | 1.00 | 0.72 (0.51–1.01) | 0.72 (0.51–1.02) | 0.71 (0.50–1.01) | 0.070 |
| P* overall | 0.150 | |||||
| Completed | OR (95% CI) | 1.00 | 0.70 (0.49–0.99) | 0.73 (0.51–1.04) | 0.68 (0.47–0.98) | 0.058 |
| P* overall | 0.118 | |||||
| Former Smokers | ||||||
| Reducedc | OR (95% CI) | 1.00 | 0.86 (0.68–1.08) | 0.88 (0.70–1.11) | 1.02 (0.81–1.28) | 0.843 |
| P* overall | 0.376 | |||||
| Completed | OR (95% CI) | 1.00 | 0.93 (0.72–1.19) | 0.98 (0.76–1.26) | 1.13 (0.88–1.44) | 0.299 |
| P* overall | 0.471 | |||||
| Current Smokers | ||||||
| Reducedc | OR (95% CI) | 1.00 | 0.71 (0.55–0.91) | 0.66 (0.52–0.84) | 0.84 (0.67–1.06) | 0.113 |
| P* overall | 0.004 | |||||
| Completed | OR (95% CI) | 1.00 | 0.72 (0.55–0.94) | 0.73 (0.56–0.95) | 0.86 (0.67–1.11) | 0.260 |
| P* overall | 0.047 | |||||
Adjusted for sex, race/ethnicity, age (continuous), smoking status, total caloric intake.
Adjusted for sex, race/ethnicity, age, pack-years, total caloric intake, family cancer history, dust exposure, second-hand smoke, emphysema, hay fever, smoking status, asthma, addiction index, alcohol, time since smoking cessation.
Adjusted for sex, race/ethnicity, age (continuous), total caloric intake.
Adjusted for sex, race/ethnicity, age, pack-years, total caloric intake, family cancer history, dust exposure, second-hand smoke, emphysema, hay fever, asthma, addiction index, alcohol, time since smoking cessation.
p-value, chi-square test using quartiles of choline intake as categorical variable.
p-value, test for linear trend using quartiles of choline intake as continuous variable.
Discussion
In the present study, we report for the first time the association between choline and betaine dietary intake and lung cancer risk. Our study showed that both choline and betaine dietary intake were significantly associated with lung cancer risk, but evidence for the effect of betaine was stronger. Specifically, compared to those in the lowest quartile of intake, individuals with higher betaine intake had lower odds for lung cancer. A statistically significant trend was observed with increased betaine intake and decreased lung cancer odds, suggesting that higher intake of betaine may be protective against lung cancer. Likewise, a significant protective effect was observed for choline intake, but no linear trend was noted.
Multiple studies have examined the association between choline and betaine dietary intake and various types of cancer, and different effects of the two nutrition factors have been reported. Higher choline intake had been observed to be associated with elevated risk of colorectal adenoma, while increasing betaine intake was associated with reduced risk of the disease [20]. Xu et al. reported in a population-based case-control study that higher intake of free choline was associated with lower risk of breast cancer. Higher intakes of betaine, free choline and phosphocholine were associated with reduced all-cause as well as breast cancer-specific mortality, while no association was observed for total choline intake on either the risk or mortality of breast cancer [23], [24]. Reports from existing studies showed more variation in the effect of choline intake compared to betaine intake. This is probably because various sources contribute to total choline intake (free choline, phosphocholine, phosphatidylcholine, etc.), while the intake source for betaine is relatively simple. As a result, it might be more difficult to obtain accurate measurement of total choline intake, which therefore, leads to high variation in the observed associations. It is also possible that, although choline and betaine are in the same metabolic pathway, different mechanisms are involved in the downstream effects in different diseases. Changes in choline metabolism have been reported in many types of cancers. Increased rate of choline transport and elevated phosphorylation of choline to phosphocholine and oxidation to betaine were observed in breast cancer [16]. CHK and PC-specific phospholipase C, enzymes that contribute to PC biosynthesis and degradation, were activated in both epithelial ovarian cancer cell line as well as cancerous tissue [17]. Similarly, elevated levels of CHK, phosphocholine and PC were detected in colon cancer tissue, suggesting that activation of choline metabolic pathways may play a role in carcinogenesis [18], [19].
Cigarette smoking has been observed to increase energy expenditure [36], but the mechanisms remain unknown. Smokers have different dietary preferences than non-smokers. In general, smokers tend to eat less fruits and vegetables than non-smokers, but consume more meat products, saturated fat, coffee, and alcohol, and have higher total energy intake [34], [37]. Consistent with previous studies, we observed a significant increase of total caloric intake with smoking. In addition, a significant increase of choline intake, along with a reduction of betaine intake, was observed from never- to former to current smokers. Since meat, egg yolk, liver, and bacon are major sources of choline, and whole wheat products and green vegetables are high in betaine, our finding that smoking is associated with lower betaine but higher choline intake is reasonable.
We observed a strong influence of smoking on the inverse association between betaine intake and lung cancer. Analyses stratified by smoking status revealed that a higher intake of betaine had no statistically significant association with lung cancer risk among never-smokers. However, higher betaine intake was significantly associated with lower lung cancer risk among both current and former smokers, with a stronger protective effect in current smokers. It is well known that smoking is the strongest risk factor for lung cancer. Our findings suggest that betaine intake may mitigate the adverse effect of smoking, while it may not be relevant as a protective factor among never smokers. Further studies are needed to elucidate the mechanisms of this association.
The effect of smoking on the association between choline intake and lung cancer was less evident compared to betaine. Pack-years of smoking was not significantly associated with choline intake, and no confounding effect of the variable was observed for the association between choline intake and lung cancer. The influence of smoking status on the association between choline intake and lung cancer was also not as obvious as that of betaine intake. A significant association was only present among current smokers, and no linear trend was observed with increased intake. Our results suggest that choline intake may also mitigate the adverse effect of smoking on lung cancer, but the effect was milder, and was independent of the amount of intake.
We observed that both choline and betaine intake were negatively associated with lung cancer risk. Although to our knowledge, no study has been reported on the effect of dietary choline and betaine intake on smoking or lung cancer, multiple studies have revealed strong associations between major nutritional factors in one-carbon metabolism and lung cancer and/or smoking. The results were largely consistent with our findings. Plasma choline and betaine concentrations were reported to be inversely associated with smoking [5]. It has been reported that dietary folate intake was associated with reduced risk of lung cancer among both former [32], [38] and current smokers [38]. A significant inverse association between lung cancer risk and serum concentrations of folate, methionine, and vitamin B6 in former and current smokers was also reported [39]. Choline, methionine and folate interact where homocysteine is converted into methionine [40]. During choline deprivation, the consumption of folate increased in rats [41], while folate deficiency resulted in reduced choline concentrations in liver of rats [42]. Because of the inter-relationships among nutrients involved in one-carbon metabolism, we believe that our findings of a potential protective effect of choline and betaine intake against lung cancer are consistent with previous studies.
One-carbon metabolism includes a complex network of biochemical pathways, which involve interactions between choline, betaine, several group B vitamins, as well as homocysteine and methionine (Figure 1) [40]. Epigenetic variations of DNA, particularly the CpG sites methylation, are important for genome regulation [43]–[45]. Disruption of DNA methylation and impaired DNA repair due to deficiency of methyl donors (folate, choline, betaine or methionine) in one-carbon metabolism were thought to be the underlying mechanism for carcinogenesis [46]–[48]. Given these hypotheses, high intake of choline and betaine may help prevent the adverse effect resulted from hypomethylation of DNA or restore DNA repair mechanism, and therefore, lead to reduced cancer risk.
Alterations in DNA methylation by smoking may contribute to the differential effect of smoking on the association between choline/betaine intake and lung cancer. Smoking has been associated with both altered global methylation [49], [50] and differential methylation in cancer-related genes [51]–[53]. Breitling et al. reported a genome-wide study that identified a CpG locus with significant lowered methylation in heavy smokers as compared to never smokers [54]. One can hypothesize that the variation of the effect of betaine by smoking status might due to difference in methylation patterns in smokers and non-smokers. However, our study is not designed to make any mechanistic conclusions, and a separate study should be undertaken to further explore this possibility.
The current study covers a broad range of ages (from 21 to 94) and race/ethnic groups (African Americans, Hispanics, and non-Hispanic Whites). Compared to many other case-control studies, our study included a large number of individuals and sufficient numbers of cases and controls for all smoking and dietary intake categories. Therefore, it allowed stratified analyses with relatively large sample size in each stratum. In addition, since the survey was conducted on newly diagnosed cancer patients, and the patients were asked to report their pre-diagnostic diet (one year prior to diagnosis), any post-diagnostic changes in diet should not affect the results.
Limitations in this case-control study include possible selection bias. Controls in the current study were recruited from clinics. Compared to healthy controls from the general population, these controls were more likely to have other types of diseases, and therefore, the distribution of choline and betaine consumption in our controls may not completely reflect that of controls recruited from general population. However, since MDACC is a major referral center for cancer cases in Houston area, and that Kelsey-Seybold Clinics is the largest private medical group that includes more than 20 clinics throughout Houston, cases and controls are representative of individuals of the same area. Furthermore, unrestrained sample collection by age, sex and ethnicity ensured that conclusions were drawn on a wider and more general population base, which may help alleviate the problem of selection bias. Secondly, due to the nature of survey studies, inaccurate recall may be a problem, and FFQ may introduce measurement errors that lead to biased estimates, likely towards the direction that attenuates the true association [55]. Although it is more likely that the nutritional intake affects the risk of cancer than the reverse, it is also possible that dietary intake is not the direct cause of the disease, but rather confounds the effect of other factors. A cohort study with long-term nutrition intake data or a Mendelian randomization analysis of genetic markers involved in choline and betaine metabolism may help us better understand the relationship between choline and betaine intake and risk of the disease. Moreover, we cannot rule out the possibility of potential residual confounding or unidentified confounders during our analysis, which may lead to biased results.
In conclusion, we observed that both choline and betaine dietary intake were protective against lung cancer, but the protective effect was more evident with betaine intake, and was strongly influenced by smoking. We propose that dietary intake of choline and betaine, especially higher betaine intake, may reduce lung cancer risk by mitigating the adverse effect of smoking.
Funding Statement
The study was supported in part by the NIH RO1 CA149462 and FAMRI Young Clinical Scientist Award (YCSA) to OYG and RO1 CA55769 and CA127219 to MRS, as well as by the NIH Centers for Translational Science Award (NIH CTSA) grant (UL1 RR024148), awarded to the University of Texas Health Science Center at Houston in 2006 by the National Center for Research Resources (NCRR). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Amos CI, Wu X, Broderick P, Gorlov IP, Gu J, et al. (2008) Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. NatGenet 40: 616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schwartz AG, Cote ML, Wenzlaff AS, Land S, Amos CI (2009) Racial differences in the association between SNPs on 15q25.1, smoking behavior, and risk of non-small cell lung cancer. JThoracOncol 4: 1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howe JC, Williams JR, Holden JM (2004) USDA Database for the Choline Content of Common Foods.
- 4. Ueland PM (2011) Choline and betaine in health and disease. J InheritMetab Dis 34: 3–15. [DOI] [PubMed] [Google Scholar]
- 5. Konstantinova SV, Tell GS, Vollset SE, Nygard O, Bleie O, et al. (2008) Divergent associations of plasma choline and betaine with components of metabolic syndrome in middle age and elderly men and women. JNutr 138: 914–920. [DOI] [PubMed] [Google Scholar]
- 6. Craig SA (2004) Betaine in human nutrition. AmJClinNutr 80: 539–549. [DOI] [PubMed] [Google Scholar]
- 7. Teixido N, Canamas TP, Usall J, Torres R, Magan N, et al. (2005) Accumulation of the compatible solutes, glycine-betaine and ectoine, in osmotic stress adaptation and heat shock cross-protection in the biocontrol agent Pantoea agglomerans CPA-2. LettApplMicrobiol 41: 248–252. [DOI] [PubMed] [Google Scholar]
- 8. Barak AJ, Beckenhauer HC, Junnila M, Tuma DJ (1993) Dietary betaine promotes generation of hepatic S-adenosylmethionine and protects the liver from ethanol-induced fatty infiltration. Alcohol ClinExpRes 17: 552–555. [DOI] [PubMed] [Google Scholar]
- 9. Barak AJ, Beckenhauer HC, Tuma DJ (1996) Betaine effects on hepatic methionine metabolism elicited by short-term ethanol feeding. Alcohol 13: 483–486. [DOI] [PubMed] [Google Scholar]
- 10. Esteller M, Sanchez-Cespedes M, Rosell R, Sidransky D, Baylin SB, et al. (1999) Detection of aberrant promoter hypermethylation of tumor suppressor genes in serum DNA from non-small cell lung cancer patients. Cancer Res 59: 67–70. [PubMed] [Google Scholar]
- 11.Kondo Y, Issa JPJ (2010) DNA methylation profiling in cancer. Expert Reviews in Molecular Medicine 12. [DOI] [PubMed]
- 12. Kondo Y, Kanai Y, Sakamoto M, Mizokami M, Ueda R, et al. (2000) Genetic instability and aberrant DNA methylation in chronic hepatitis and cirrhosis–A comprehensive study of loss of heterozygosity and microsatellite instability at 39 loci and DNA hypermethylation on 8 CpG islands in microdissected specimens from patients with hepatocellular carcinoma. Hepatology 32: 970–979. [DOI] [PubMed] [Google Scholar]
- 13. Pieretti M, Powell DE, Gallion HH, Conway PS, Case EA, et al. (1995) Hypermethylation at a chromosome 17 “hot spot” is a common event in ovarian cancer. HumPathol 26: 398–401. [DOI] [PubMed] [Google Scholar]
- 14. Stefanska B, Huang J, Bhattacharyya B, Suderman M, Hallett M, et al. (2011) Definition of the landscape of promoter DNA hypomethylation in liver cancer. Cancer Res 71: 5891–5903. [DOI] [PubMed] [Google Scholar]
- 15. Shao C, Sun W, Tan M, Glazer CA, Bhan S, et al. (2011) Integrated, genome-wide screening for hypomethylated oncogenes in salivary gland adenoid cystic carcinoma. Clin Cancer Res 17: 4320–4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Katz-Brull R, Seger D, Rivenson-Segal D, Rushkin E, Degani H (2002) Metabolic markers of breast cancer: enhanced choline metabolism and reduced choline-ether-phospholipid synthesis. Cancer Res 62: 1966–1970. [PubMed] [Google Scholar]
- 17. Iorio E, Ricci A, Bagnoli M, Pisanu ME, Castellano G, et al. (2010) Activation of phosphatidylcholine cycle enzymes in human epithelial ovarian cancer cells. Cancer Res 70: 2126–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dueck DA, Chan M, Tran K, Wong JT, Jay FT, et al. (1996) The modulation of choline phosphoglyceride metabolism in human colon cancer. MolCell Biochem 162: 97–103. [DOI] [PubMed] [Google Scholar]
- 19. Nakagami K, Uchida T, Ohwada S, Koibuchi Y, Suda Y, et al. (1999) Increased choline kinase activity and elevated phosphocholine levels in human colon cancer. JpnJCancer Res 90: 419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cho E, Willett WC, Colditz GA, Fuchs CS, Wu K, et al. (2007) Dietary choline and betaine and the risk of distal colorectal adenoma in women. JNatlCancer Inst 99: 1224–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Henning SM, Swendseid ME (1996) The role of folate, choline, and methionine in carcinogenesis induced by methyl-deficient diets. AdvExpMedBiol 399: 143–155. [DOI] [PubMed] [Google Scholar]
- 22. Kotsopoulos J, Hankinson SE, Tworoger SS (2010) Dietary betaine and choline intake are not associated with risk of epithelial ovarian cancer. EurJClinNutr 64: 111–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu X, Gammon MD, Zeisel SH, Bradshaw PT, Wetmur JG, et al. (2009) High intakes of choline and betaine reduce breast cancer mortality in a population-based study. FASEB J 23: 4022–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu X, Gammon MD, Zeisel SH, Lee YL, Wetmur JG, et al. (2008) Choline metabolism and risk of breast cancer in a population-based study. FASEB J 22: 2045–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hudmon KS, Honn SE, Jiang H, Chamberlain RM, Xiang W, et al. (1997) Identifying and recruiting healthy control subjects from a managed care organization: a methodology for molecular epidemiological case-control studies of cancer. Cancer EpidemiolBiomarkers Prev 6: 565–571. [PubMed] [Google Scholar]
- 26. Spitz MR, Hong WK, Amos CI, Wu X, Schabath MB, et al. (2007) A risk model for prediction of lung cancer. J NatlCancer Inst 99: 715–726. [DOI] [PubMed] [Google Scholar]
- 27. Block G, Coyle LM, Hartman AM, Scoppa SM (1994) Revision of dietary analysis software for the Health Habits and History Questionnaire. AmJEpidemiol 139: 1190–1196. [DOI] [PubMed] [Google Scholar]
- 28. Block G, Thompson FE, Hartman AM, Larkin FA, Guire KE (1992) Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. JAmDietAssoc 92: 686–693. [PubMed] [Google Scholar]
- 29.Patterson KY, Bhagwat SA, Williams JR, Howe JC, Holden JM (2008) USDA Database for the Choline Content of Common Foods, Release 2.
- 30.U.S. Department of Agriculture, Agricultural Research Service, USDA Nutrient Data Laboratory (2008). Composition of Foods Raw, Processed, Prepared, USDA National Nutrient Database for Standard Reference, Release 21.
- 31. Willett WC, Howe GR, Kushi LH (1997) Adjustment for total energy intake in epidemiologic studies. Am J ClinNutr 65: 1220S–1228S. [DOI] [PubMed] [Google Scholar]
- 32. Shen H, Wei Q, Pillow PC, Amos CI, Hong WK, et al. (2003) Dietary folate intake and lung cancer risk in former smokers: a case-control analysis. Cancer EpidemiolBiomarkers Prev 12: 980–986. [PubMed] [Google Scholar]
- 33. Schabath MB, Delclos GL, Martynowicz MM, Greisinger AJ, Lu C, et al. (2005) Opposing effects of emphysema, hay fever, and select genetic variants on lung cancer risk. AmJEpidemiol 161: 412–422. [DOI] [PubMed] [Google Scholar]
- 34. Osler M (1998) The food intake of smokers and nonsmokers: the role of partner’s smoking behavior. PrevMed 27: 438–443. [DOI] [PubMed] [Google Scholar]
- 35. Thornton A, Lee P, Fry J (1994) Differences between smokers, ex-smokers, passive smokers and non-smokers. J ClinEpidemiol 47: 1143–1162. [DOI] [PubMed] [Google Scholar]
- 36. Hofstetter A, Schutz Y, Jequier E, Wahren J (1986) Increased 24-hour energy expenditure in cigarette smokers. NEnglJ Med 314: 79–82. [DOI] [PubMed] [Google Scholar]
- 37. Palaniappan U, Jacobs SL, O’Loughlin J, Gray-Donald K (2001) Fruit and vegetable consumption is lower and saturated fat intake is higher among Canadians reporting smoking. J Nutr 131: 1952–1958. [DOI] [PubMed] [Google Scholar]
- 38. Voorrips LE, Goldbohm RA, Brants HA, van Poppel GA, Sturmans F, et al. (2000) A prospective cohort study on antioxidant and folate intake and male lung cancer risk. Cancer EpidemiolBiomarkers Prev 9: 357–365. [PubMed] [Google Scholar]
- 39. Johansson M, Relton C, Ueland PM, Vollset SE, Midttun O, et al. (2010) Serum B vitamin levels and risk of lung cancer. JAMA 303: 2377–2385. [DOI] [PubMed] [Google Scholar]
- 40. Niculescu MD, Zeisel SH (2002) Diet, methyl donors and DNA methylation: Interactions between dietary folate, methionine and choline. Journal of Nutrition 132: 2333S–2335S. [DOI] [PubMed] [Google Scholar]
- 41. Varela-Moreiras G, Ragel C, Perez de MJ (1995) Choline deficiency and methotrexate treatment induces marked but reversible changes in hepatic folate concentrations, serum homocysteine and DNA methylation rates in rats. JAmCollNutr 14: 480–485. [DOI] [PubMed] [Google Scholar]
- 42. Kim YI, Miller JW, da Costa KA, Nadeau M, Smith D, et al. (1994) Severe folate deficiency causes secondary depletion of choline and phosphocholine in rat liver. JNutr 124: 2197–2203. [DOI] [PubMed] [Google Scholar]
- 43. Feinberg AP (2010) Genome-scale approaches to the epigenetics of common human disease. Virchows Arch 456: 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, et al. (2009) Human DNA methylomes at base resolution show widespread epigenomic differences. Nature 462: 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van der Maarel SM (2008) Epigenetic mechanisms in health and disease. Ann Rheum Dis 67 Suppl 3iii97–100. [DOI] [PubMed] [Google Scholar]
- 46. Futscher BW, O’Meara MM, Kim CJ, Rennels MA, Lu D, et al. (2004) Aberrant methylation of the maspin promoter is an early event in human breast cancer. Neoplasia 6: 380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. de VS, Wouters KA, Gottschalk RW, van Schooten FJ, de Goeij AF, et al. (2011) Dietary methyl donors, methyl metabolizing enzymes, and epigenetic regulators: diet-gene interactions and promoter CpG island hypermethylation in colorectal cancer. Cancer Causes Control 22: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wei Q, Shen H, Wang LE, Duphorne CM, Pillow PC, et al. (2003) Association between low dietary folate intake and suboptimal cellular DNA repair capacity. Cancer Epidemiol Biomarkers Prev 12: 963–969. [PubMed] [Google Scholar]
- 49. Furniss CS, Marsit CJ, Houseman EA, Eddy K, Kelsey KT (2008) Line region hypomethylation is associated with lifestyle and differs by human papillomavirus status in head and neck squamous cell carcinomas. Cancer Epidemiol Biomarkers Prev 17: 966–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Smith IM, Mydlarz WK, Mithani SK, Califano JA (2007) DNA global hypomethylation in squamous cell head and neck cancer associated with smoking, alcohol consumption and stage. Int J Cancer 121: 1724–1728. [DOI] [PubMed] [Google Scholar]
- 51. Enokida H, Shiina H, Urakami S, Terashima M, Ogishima T, et al. (2006) Smoking influences aberrant CpG hypermethylation of multiple genes in human prostate carcinoma. Cancer 106: 79–86. [DOI] [PubMed] [Google Scholar]
- 52. Marsit CJ, Houseman EA, Schned AR, Karagas MR, Kelsey KT (2007) Promoter hypermethylation is associated with current smoking, age, gender and survival in bladder cancer. Carcinogenesis 28: 1745–1751. [DOI] [PubMed] [Google Scholar]
- 53. Wu JY, Wang J, Lai JC, Cheng YW, Yeh KT, et al. (2008) Association of O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation with p53 mutation occurrence in non-small cell lung cancer with different histology, gender, and smoking status. Ann Surg Oncol 15: 3272–3277. [DOI] [PubMed] [Google Scholar]
- 54. Breitling LP, Yang R, Korn B, Burwinkel B, Brenner H (2011) Tobacco-smoking-related differential DNA methylation: 27K discovery and replication. Am J Hum Genet 88: 450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schatzkin A, Kipnis V, Carroll RJ, Midthune D, Subar AF, et al. (2003) A comparison of a food frequency questionnaire with a 24-hour recall for use in an epidemiological cohort study: results from the biomarker-based Observing Protein and Energy Nutrition (OPEN) study. International Journal of Epidemiology 32: 1054–1062. [DOI] [PubMed] [Google Scholar]