Abstract
Context
Studies of smoking in relation to prostate cancer mortality or recurrence in prostate cancer patients are limited, with few prostate cancer-specific outcomes.
Objective
To assess the relation of cigarette smoking and smoking cessation with overall, prostate cancer-specific, and CVD mortality and biochemical recurrence among men with prostate cancer.
Design, Setting, and Participants
Prospective observational study of 5 366 men diagnosed with prostate cancer between 1986–2006 in the Health Professionals Follow-Up Study.
Main Outcome Measures
Hazard ratios (HRs) for overall, prostate cancer-specific, and CVD mortality, and biochemical recurrence, defined by PSA rise.
Results
We documented 1,630 deaths, 524 (32%) due to prostate cancer and 416 (26%) due to CVD, and 878 biochemical recurrences. The absolute crude rates for prostate cancer-specific death for never smokers vs. current smokers were 9.6 vs. 15.3 per 1,000 person-years; for all-cause mortality the corresponding rates were 27.3 and 53.0 per 1,000 person-years. In multivariable analysis, compared with never smokers, current smokers had an increased risk of prostate cancer mortality (HR, 1.61; 95% confidence interval [CI], 1.11–2.32 and among men with clinical stage T1–T3: HR, 1.80; 95% CI, 1.04–3.12), biochemical recurrence (HR, 1.61; 95% CI, 1.16–2.22), total mortality (HR, 2.28; 95% CI, 1.87–2.80), and CVD mortality (HR, 2.13; 95% CI, 1.39–3.26). After adjusting for clinical stage and grade which are likely intermediates of the relation of smoking with prostate cancer recurrence and survival, the estimates for current smoking were as follows: prostate cancer mortality (HR, 1.38; 95% CI, 0.94–2.03 and HR, 1.41; 95% CI, 0.80–2.49); biochemical recurrence (HR, 1.47; 95% CI, 1.06–2.04). A greater number of pack-years was associated with a significantly increased risk of prostate cancer mortality but not biochemical recurrence: for current smokers of 40+ pack-years compared to never smokers: prostate cancer mortality (HR, 1.82; 95% CI, 1.03–3.20; biochemical recurrence (HR, 1.48; 95% CI, 0.88–2.48). Compared to current smokers, those who had quit smoking for 10 or more years, or who had quit for less than 10 years but smoked less than 20 pack-years, had prostate cancer mortality risks similar to never smokers: former smoker, quit 10+ years (HR, 0.60; 95% CI, 0.42–0.87); quit <10 years and <20 pack-years (HR, 0.64; 95% CI, 0.28–1.45); never smoker (HR, 0.61; 95% CI, 0.42–0.88).
Conclusions
Smoking at the time of prostate cancer diagnosis is associated with increased overall and CVD mortality and prostate cancer-specific mortality and recurrence. 10-year quitters have prostate cancer-specific mortality risks similar to never smokers.
INTRODUCTION
Accumulating evidence suggests that smoking may increase risk of aggressive prostate cancer and prostate cancer mortality. The latest review by the US Surgeon General found the evidence ‘probable’ that smoking contributes to a higher prostate cancer mortality rate,1 in agreement with our findings from a review of the literature where we reported a ~30% increase in risk of fatal prostate cancer when comparing current smokers to never smokers.2 Several studies reported that smoking is associated with more aggressive disease at diagnosis, defined as a higher stage or tumor grade3–5 and the relation between smoking and progression of the disease after diagnosis, defined as biochemical recurrence,5–7 metastasis,8 and hormone refractory prostate cancer9 is suggestively positive. However, these studies had few prostate cancer-specific deaths and either observed no clear association with prostate cancer mortality5, 8 or did not examine this outcome.6, 9 Three studies reported a positive association between smoking and prostate cancer mortality but were based on very few prostate cancer deaths (N=57 and N=54)3,10 or an unspecified number of deaths in a single institution study of 214 patients.11 Moreover, concern remains that some or all of the observed associations may be due to delayed diagnosis and treatment among smokers. With 8 years of follow-up in the HPFS, we previously provided preliminary data on smoking status at baseline and prostate cancer mortality.12 With 22 years of follow-up and a large number of outcomes (524 prostate cancer-specific deaths and 878 biochemical recurrences), we now can examine in detail the relation of current and former smoking to overall and prostate-specific mortality and recurrence in a nationwide cohort of prostate cancer patients.
METHODS
Study population
The Health Professionals Follow-Up Study (HPFS) is a prospective cohort study of 51,529 US male health professionals who enrolled in 1986 by completing a mailed questionnaire. Participants provided information about medical history and risk factors for chronic diseases, including cancer. Participants complete biennial follow-up questionnaires to collect information on new medical diagnoses and to update information on lifestyle factors (response rate 96%). This study was approved by the Institutional Review Board of the Harvard School of Public Health; participants provided implied consent by virtue of returning their questionnaires, and written informed consent for review of medical records.
Assessment of smoking
Current smoking status (cigarettes smoked/day) was assessed every two years, beginning in 1986. At baseline, we also inquired about past smoking and time since quitting, and the average number of cigarettes smoked per day before age 15 years, ages 15–19, 20–29, 30–39, 40–49, 50–59, and 60 and older. Pack-years was calculated as years of smoking multiplied by the average number of packs smoked/day. A pack contains 20 cigarettes. All smoking variables were updated every 2 years until the questionnaire just before the report of prostate cancer diagnosis. We focused on smoking prior to prostate cancer diagnosis as the majority of ever-smokers at diagnosis were former smokers, with only 5.2% of men reporting current smoking.
Ascertainment of prostate cancer diagnosis, recurrence, and death
After a participant reported a diagnosis of prostate cancer, medical records and pathology reports were sought from treating physicians and hospitals to confirm the diagnosis and obtain information on pathology, treatments, prostate specific antigen (PSA) values at diagnosis, rises in PSA after treatment (PSA or biochemical recurrence), and metastasis. Participants completed biennial follow-up questionnaires to update data on treatments, PSA, and clinical progression. The primary outcomes for this analysis are prostate cancer mortality and biochemical recurrence. Other outcomes of interest are total mortality and cardiovascular (CVD) mortality (ICD 9th revision codes 350–459). Biochemical recurrence was defined from medical records and physician questionnaires based on the primary treatment, using standard definitions: for radical prostatectomy,13, 14 PSA above 0.2 ng/ml post-surgery, sustained over 2 measures; for radiation,15 a rise of 2 or more ng/ml above the nadir PSA; for brachytherapy,16 hormones or other treatments, a rise of 1 or more ng/ml above nadir, sustained over 2 measures; for watchful waiting, a post-diagnosis PSA increase of 1 or more ng/ml, sustained over 2 measures. We also used patient-reported PSA rise from participants’ questionnaires that comprised 37% of all PSA rises. Date of failure was the date of first rise. Men for whom we could not ascertain a PSA recurrence but who reported metastasis or died of prostate cancer were assigned a date of biochemical recurrence as the earliest date for any of these events. Using reports of deaths from families and the National Death Index for non-respondents, we ascertained >98% of deaths.17 Causes of death were centrally adjudicated by study physicians who reviewed medical records and death certificates, without knowledge of their smoking status.
Population for Analysis
We included in our analyses men who initially were free of a cancer diagnosis (except non-melanoma skin cancer) in 1986, and had provided information on their smoking status before diagnosis (N=5,366). For the mortality analyses, we included all participants regardless of their stage at diagnosis. We also performed an additional analysis among men with clinical stage T1–T3. For the analyses of prostate cancer recurrence, we excluded men with metastatic disease at diagnosis (N=220) and those who reported either a PSA rise or metastasis after diagnosis but did not provide a date for this event (N=5). Additionally, there were 1,508 participants for whom we do not have data on recurrence or progression after diagnosis and therefore could not be included.
Statistical analysis
We used multinomial logistic regression models, adjusting for age at diagnosis in 5-year age categories, to test whether there were differences in clinical stage and clinical Gleason score by smoking status at diagnosis. We used Cox proportional hazards regression models to calculate hazard ratios (HR) of death from any cause, prostate cancer death, CVD death, and biochemical recurrence. In the main analysis for prostate cancer and CVD mortality, deaths from other causes were censored, and Cox survival analysis was used. Person-years were calculated beginning at diagnosis until death or end of follow-up (January 1, 2008), whichever came first, for mortality analyses, and until the earliest of the following events - progression or mortality or end of follow-up, for the recurrence analysis. We used the participant’s most recent smoking status reported prior to their diagnosis and classified current smokers by categories of pack-years (<40 and 40+), in addition to cigarettes smoked per day, and past smokers by a combination of pack-years (<20 and 20+ pack-years), and time since quitting (<10 and 10+ years).
Our final models for prostate cancer mortality and recurrence included age at diagnosis (years), previous PSA screening history (yes, no, unknown), pre-diagnosis vigorous activity (<1, 1–3, and 3+ h/wk), body mass index (BMI) (categories <25, 25 to <30, 30+ kg/m2), energy (quartiles) and coffee intake (quartiles). Coffee intake was added to the models based on recent findings from our study reporting a lower risk of advanced prostate cancer with increased coffee consumption.18 We used months since diagnosis as the time scale and stratified by calendar time in two-year intervals. We considered models adjusted for race, height, family history of prostate cancer, parental history of MI at or less than age 60, diabetes, elevated cholesterol, elevated blood pressure, and intakes of calcium, saturated fat, cholesterol, red meat, tomato sauce, fish, and α-linolenic acid, as most of these factors were previously associated with prostate cancer incidence or progression in our study19, 20 or could be potential confounders. We considered similar models for overall and CVD mortality. There was little evidence of confounding by these factors, so they were not included in our final models. We hypothesized that smoking may affect prostate cancer mortality risk by promoting more aggressive tumors, characterized by poorer differentiation (higher Gleason score) or more advanced stage at diagnosis. Because we considered stage and grade as likely intermediates of the relation of smoking with biochemical recurrence and prostate cancer-specific mortality, we did not initially adjust for these factors in our primary analysis. However, we considered these factors in a secondary analysis.
We conducted two sensitivity analyses to evaluate potential bias from any difference in screening behavior between smokers and nonsmokers. The first analysis included only men who reported having a PSA screening history in the cycle prior to diagnosis, starting with men diagnosed from 1988. The second analysis included only men diagnosed from 1994, after PSA screening had become well established. In that analysis, we further adjusted for screening intensity as reflected in the proportion of two-year periods in which a participant reported at least one PSA screen, dichotomizing at 50%.
We assessed the interaction between smoking and vitamin E supplement use and BMI by entering cross products of smoking status with those variables in models. We chose these two covariates a priori because previous studies have reported a potential interaction of smoking and vitamin E supplements for prostate cancer incidence21, 22 and higher pre-diagnostic BMI is related to increased prostate cancer mortality.23 We also evaluated the effect of smoking among men treated by radical prostatectomy and radiation as primary treatments. All P values were 2-sided; P<.05 was considered statistically significant. All analyses utilized SAS version 9.1 (SAS Institute Inc, Cary, NC).
RESULTS
Among the 5,366 men diagnosed with prostate cancer, we documented 1,630 deaths, 524 (32%) due to prostate cancer and 416 (26%) due to CVD. The other causes of death included infectious disease (0.5%), cancer (19.5%), metabolic/endocrine disorders (1%), neurologic disease (6%), respiratory disease (5%), digestive disease (1%), kidney failure (1%), other causes (5%), and unknown (2%). In the mortality analysis, which included all cases including those advanced at diagnosis, the median duration of follow-up time from diagnosis to censoring (either until death or the end of follow-up in January 2008) was 8.1 years (25th and 75th percentiles: 5.0, 12.2 years) for survivors and 6.5 years (25th and 75th percentiles: 3.5, 10.2 years) for men who died. In the recurrence analysis, which excluded men with metastatic disease at diagnosis, the median duration of follow-up time from diagnosis to censoring (recurrence, death, or end of follow-up) was 3.8 (25th and 75th percentiles: 2.0, 6.6 years) for those who recurred and 8.1 (25th and 75th percentiles: 4.8, 12.2 years) for those who did not. Age-standardized characteristics after diagnosis are presented in TABLE 1. Compared to never smokers, current smokers had higher clinical stage and grade (p <0.0001 for both). For example, 14.7% of current smoker had stage T3 or higher at diagnosis, compared to 8.3% of never smokers, and 16.0% of current smokers had a Gleason score of 7 or more, compared to 10.7% of never smokers. Current smokers exercised less, drank more coffee, and had a higher intake of saturated fat and lower intake of calcium compared to never and former smokers. Both former and current smokers consumed more alcohol but had similar BMIs compared with never smokers. Current smokers tended to have less intense PSA testing, but the rates among past smokers were similar to never smokers.
Table 1.
Age-Standardized Characteristics According to Prediagnosis Smoking Status Among 5366 Men With Prostate Cancera
| Smoking Status prior to Diagnosisb | ||||
|---|---|---|---|---|
|
| ||||
| Characteristic | Never (n = 2,449) |
Former: Quit10+ y (n = 2,063) |
Former: quit<10 y (n = 297) |
Current (n = 277) |
| Age at diagnosis, mean (SD), yc | 69.3 (7.6) | 70.8 (7.2) | 68.0 (7.1) | 68.1 (7.1) |
| Clinical stage, %d | ||||
| T1 | 54.2 | 56.0 | 47.2 | 39.0 |
| T2 | 37.5 | 35.4 | 38.0 | 46.3 |
| T3 or T4 | 3.6 | 4.4 | 5.0 | 8.2 |
| M1 and/or N1 | 4.7 | 4.3 | 9.8 | 6.5 |
| Gleason score, %d | ||||
| <7 | 65.3 | 62.7 | 58.8 | 57.3 |
| 7 | 24.0 | 27.7 | 26.9 | 26.8 |
| >7 | 10.7 | 9.6 | 14.3 | 16.0 |
| Primary treatment, %d | ||||
| Radical prostatectomy | 47.5 | 47.2 | 43.1 | 41.4 |
| EBRTc or brachytherapy | 34.8 | 36.1 | 39.5 | 34.3 |
| Hormones | 7.2 | 7.2 | 10.3 | 8.7 |
| Watchful Waiting | 8.5 | 8.0 | 4.5 | 8.7 |
| Other | 2.1 | 1.5 | 2.5 | 6.9 |
| Parental history of MI at age ≤ 60, % | 11.6 | 11.5 | 11.0 | 8.3 |
| Family history of prostate cancer, % | 10.5 | 10.0 | 8.0 | 10.6 |
| History of PSA tests prior to dx cycle among all prostate cancer cases, % | 64.2 | 61.8 | 46.8 | 43.7 |
| History of PSA tests prior to dx cycle among cases diagnosed after 1994, % | 85.7 | 86.0 | 81.3 | 75.1 |
| High PSA screening intensity among cases diagnosed after 1994, %e | 72.9 | 74.0 | 65.4 | 63.4 |
| PSA at diagnosis among cases diagnosed after 1994, median (25th and 75th percentile) | 6.6 (4.9, 9.9) | 6.9 (4.9, 10.2) | 7.2 (5.3, 10.0) | 7.6 (4.8, 12.4) |
| Diabetes, % | 6.5 | 8.0 | 7.4 | 10.0 |
| High blood pressure, % | 39.4 | 45.7 | 45.5 | 32.7 |
| Elevated cholesterol, % | 44.9 | 49.2 | 46.9 | 39.6 |
| BMI, mean (SD), kg/m2 | 25.6 (3.4) | 26.0 (3.5) | 26.4 (3.5) | 25.1 (3.2) |
| Total activity, mean (SD), MET-hr/wk | 37.5 (42.8) | 33.7 (37.2) | 29.7 (31.4) | 29.3 (40.3) |
| Intake, mean (SD) | ||||
| Calories/d | 1963.2 (613.2) | 1944.3 (595.0) | 1998.5 (676.6) | 2015.6 (586.7) |
| Saturated fat, g/d | 21.7 (6.1) | 21.8 (6.2) | 24.1 (6.3) | 25.2 (6.6) |
| Calcium, mg/d | 1048.7 (502.0) | 1024.9 (504.5) | 940.4 (380.0) | 935.6 (444.1) |
| Red meat, servings/d | 0.4 (0.4) | 0.4 (0.5) | 0.6 (0.5) | 0.5 (0.4) |
| Tomato sauce, servings/wk | 1.1 (1.1) | 1.0 (1.1) | 0.9 (1.0) | 0.9 (0.9) |
| Fish, servings/d | 0.3 (0.3) | 0.3 (0.4) | 0.3 (0.4) | 0.3 (0.2) |
| Coffee, servings/d | 1.1 (1.3) | 1.6 (1.6) | 1.9 (1.7) | 2.2 (2.0) |
| Alcohol | ||||
| Non-drinker, % | 30.9 | 19.3 | 18.7 | 19.9 |
| < 15g/day, % | 50.7 | 48.0 | 47.9 | 43.8 |
| ≥15 g/day, % | 18.3 | 32.7 | 33.4 | 36.4 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); EBRT, external beam radiation therapy; MET, metabolic equivalent task; MI, myocardial infarction; PSA, prostate-specific antigen.
Age-standardized to the age distribution of the study population at prostate cancer diagnosis. Lifestyle factors are from participant’s questionnaire prior to the diagnosis.
A total of 280 former smokers are missing data on years since quitting smoking and thus are not included in this table.
Age is not age standardized.
For interpretability, we excluded men missing stage (16.8%) when calculating distribution of stage, men missing Gleason score (25.6%) when calculating distribution of Gleason score, and men missing primary treatment data (16.0%) when calculating distribution of treatment.
High PSA screening intensity is defined as the participant reporting at least 1 PSA screen in at least 50% of 2-year periods, not including the cycle of diagnosis.
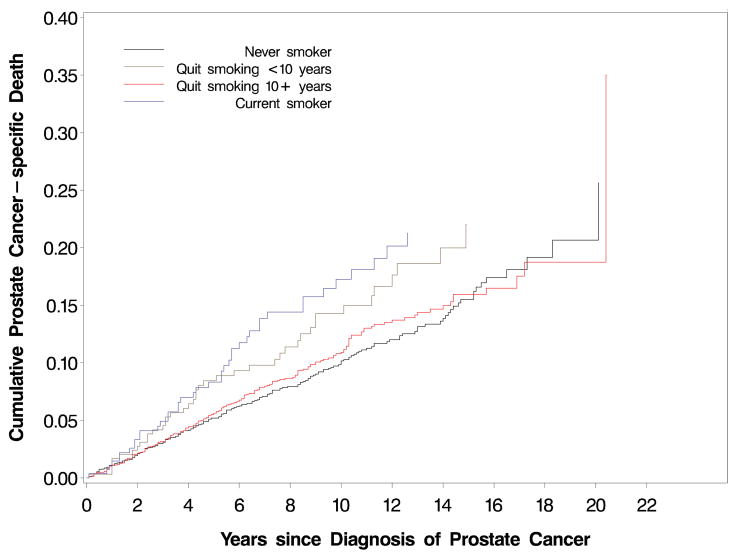
Survival curves and absolute rates by smoking status
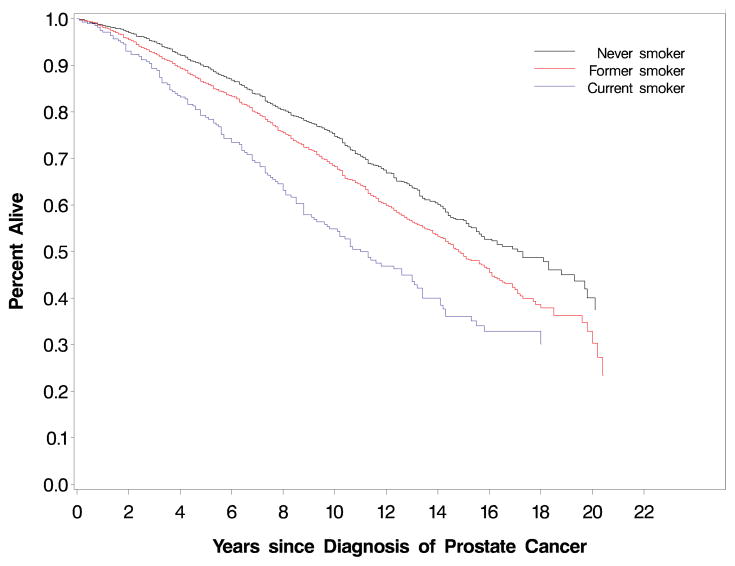
The proportions of men free of prostate cancer cancer-specific death at 5 years (FIGURE 1) were 94.8% for never smokers, 94.4% for former smokers quitting more than 10 years, 91.6% for former smokers quitting less than 10 years, and 91.7% for current smokers (P=.02, log-rank test); at 10 years, the proportions of men free of prostate cancer-specific death were 89.8%, 89.1%, 85.7%, and 82.7%, respectively. The absolute crude rates for prostate cancer-specific death for the categories of never smokers, former smokers quitting more than 10 years, former smokers quitting less than 10 years, and current smokers were 9.6, 10.3, 13.8, and 15.3 per 1,000 person-years, respectively (P≤.001, χ2 test). A similar pattern was observed for prostate cancer recurrence: 26.4, 28.1, 34.6, and 38.2 per 1,000 person-years, respectively (P=.006, χ2 test. We observed a statistically significant difference in overall survival across smoking status (P<.0001, FIGURE 2). The proportions of men alive at 5 years were 89.7% for never smokers, 86.2% for former smokers, and 78.8% for current smokers; at 10 years, the proportions of men alive were 74.8%, 68.2%, and 54.8%, respectively. The absolute crude rates for all-cause mortality for the categories of never smokers, former smokers, and current smokers were 27.3, 35.2, and 53.0 per 1,000 person-years, respectively (P≤.001, χ2 test).
FIGURE 1.
Cumulative incidence of prostate cancer-specific death (P=.02, log-rank test) by smoking status prior to diagnosis
Table that corresponds to Figures 1 and 2:
| Figure 1 and 2: Number at risk | 0 years | 5 years | 10 years | 15 years | 20 years |
|---|---|---|---|---|---|
| Never smoker | 2449 | 1723 | 816 | 256 | 16 |
| Former smoker | 2640 | 1870 | 904 | 264 | 12 |
| Quit <10 years* | 297 | 216 | 124 | 35 | 4 |
| Quit 10+ years* | 2063 | 1473 | 695 | 207 | 9 |
| Current smoker | 277 | 194 | 104 | 38 | 3 |
FIGURE 2.
Kaplan-Meier curves for overall mortality (P<.0001, log-rank test) by smoking status prior to diagnosis
Table that corresponds to Figures 1 and 2:
| Figure 1 and 2: Number at risk | 0 years | 5 years | 10 years | 15 years | 20 years |
|---|---|---|---|---|---|
| Never smoker | 2449 | 1723 | 816 | 256 | 16 |
| Former smoker | 2640 | 1870 | 904 | 264 | 12 |
| Quit <10 years* | 297 | 216 | 124 | 35 | 4 |
| Quit 10+ years* | 2063 | 1473 | 695 | 207 | 9 |
| Current smoker | 277 | 194 | 104 | 38 | 3 |
Current Smoking
Compared with never smokers, current smokers had an increased risk of dying from prostate cancer, CVD, and all-cause mortality, and an increased risk of biochemical recurrence. In multivariable models, the estimates for current smoking for these outcomes were the following: prostate cancer mortality (HR, 1.61; 95% confidence interval [CI], 1.11–2.32 and among men with clinical stage T1–T3: HR, 1.80; 95% CI, 1.04–3.12), biochemical recurrence (HR, 1.61; 95% CI, 1.16–2.22), total mortality (HR, 2.28; 95% CI, 1.87–2.80), and CVD mortality (HR, 2.13; 95% CI, 1.39–3.26) (Table 2). When limiting the biochemical recurrence analysis to men treated with radical prostatectomy, external beam radiation, or brachytherapy, current smokers had a HR for biochemical recurrence of 1.63 (95% CI, 1.15–2.31). As noted in the Methods, because smoking likely acts to increase prostate cancer mortality through changes in grade and stage, we did not adjust for those mediating factors in our primary analysis. In secondary analyses, we adjusted for stage and grade to address the impact of smoking apart from its effect on those mediating factors; the estimates for current smoking were as follows: prostate cancer mortality (HR, 1.38; 95% CI, 0.94–2.03 and among men with clinical stage T1–T3, HR, 1.41; 95% CI, 0.80–2.49); biochemical recurrence (HR, 1.47; 95% CI,1.06–2.04); total mortality (HR, 2.01; 95% CI,1.64–2.47); and CVD mortality (HR, 1.98; 95% CI, 1.29–3.04). Among men treated with radical prostatectomy, external beam radiation, or brachytherapy, current smokers had a HR for biochemical recurrence of 1.48 (95% CI, 1.04–2.10). A greater number of pack-years was associated with an increased risk of prostate cancer mortality, CVD mortality, and total mortality, but not biochemical recurrence (TABLE 2). Among current smokers, we found no association for smoking dose (cigarettes per day) for prostate cancer mortality (ptrend=0.85) and biochemical recurrence (ptrend=0.71): for prostate cancer mortality, 1–14 cigs/day (HR, 1.69; 95% CI, 0.99–2.91); 15–24 cigs/day (HR, 1.32; 95% CI, 0.73–2.37), 25+ cigs/day (HR, 1.67; 95% CI, 0.78–3.61); for biochemical recurrence, 1–14 cigs/day (HR, 1.73; 95% CI, 1.09–2.76); 15–24 cigs/day (HR, 1.78; 95% CI, 1.03–3.06); for 25+ cigs/day (HR, 1.48; 95% CI, 0.77–2.85).
Table 2.
Pre-diagnosis smoking status and mortality and recurrence among prostate cancer patients
| Total | Smoking Status prior to Diagnosis | Pack-years of Current Smoking prior to Diagnosisa | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Never | Former | Current | >0 to <40 pack-years | 40+ pack-years | ||
| Prostate cancer deaths | ||||||
| No. of Events | 524 | 219 | 264 | 41 | 17 | 17 |
| No. of Participants | 5366 | 2449 | 2640 | 277 | 99 | 129 |
| Age-adjusted HR (95% CI) | 1.00 | 1.02 (0.85, 1.23) | 1.58 (1.11, 2.26) | 1.56 (0.89, 2.75) | 1.86 (1.08, 3.20) | |
| Age-adjusted + PSA hx HR (95% CI) | 1.00 | 1.02 (0.85, 1.24) | 1.57 (1.10, 2.24) | 1.63 (0.92, 2.90) | 1.73 (1.00, 3.00) | |
| Multivariable-adjusted HR (95% CI)b | 1.00 | 1.05 (0.86, 1.27) | 1.61 (1.11, 2.32) | 1.72 (0.96, 3.08) | 1.82 (1.03, 3.20) | |
| Prostate cancer deaths among men with clinical stage T1–T3c | ||||||
| No. of Events | 241 | 165 | 121 | 19 | 9 | 7 |
| No. of Participants | 4244 | 1982 | 2062 | 200 | 71 | 95 |
| Age-adjusted HR (95% CI) | 1.00 | 0.97 (0.73, 1.28) | 1.73 (1.02, 2.94) | 1.82 (0.81, 4.09) | 1.57 (0.67, 3.67) | |
| Age-adjusted + PSA hx HR (95% CI) | 1.00 | 0.96 (0.73, 1.28) | 1.68 (0.98, 2.86) | 1.78 (0.79, 4.01) | 1.56 (0.67, 3.65) | |
| Multivariable-adjusted HR (95% CI)b | 1.00 | 1.00 (0.75, 1.34) | 1.80 (1.04, 3.12) | 1.86 (0.81, 4.28) | 1.75 (0.73, 4.19) | |
| Biochemical recurrence | ||||||
| No. of Events | 878 | 392 | 435 | 51 | 23 | 21 |
| No. of Participants | 3633 | 1746 | 1740 | 147 | 53 | 68 |
| Age-adjusted HR (95% CI) | 1.00 | 1.06 (0.92, 1.23) | 1.53 (1.11, 2.10) | 1.92 (1.14, 3.23) | 1.46 (0.89, 2.41) | |
| Age-adjusted + PSA hx HR (95% CI) | 1.00 | 1.06 (0.92, 1.22) | 1.49 (1.08, 2.05) | 1.90 (1.12, 3.21) | 1.40 (0.85, 2.32) | |
| Multivariable-adjusted HR (95% CI)b | 1.00 | 1.11 (0.96, 1.29) | 1.61 (1.16, 2.22) | 2.13 (1.24, 3.64) | 1.48 (0.88, 2.48) | |
| Total deaths | ||||||
| No. of Events | 1630 | 620 | 868 | 142 | 48 | 71 |
| No. of Participants | 5366 | 2449 | 2640 | 277 | 99 | 129 |
| Age-adjusted HR (95% CI) | 1.00 | 1.21 (1.09, 1.35) | 2.26 (1.86, 2.75) | 1.61 (1.14, 2.28) | 3.17 (2.37, 4.24) | |
| Multivariable-adjusted HR (95% CI)b | 1.00 | 1.23 (1.10, 1.37) | 2.28 (1.87, 2.80) | 1.64 (1.15, 2.32) | 3.26 (2.40, 4.42) | |
| Cardiovascular deaths | ||||||
| No. of Events | 416 | 160 | 223 | 33 | 9 | 19 |
| No. of Participants | 5366 | 2449 | 2640 | 277 | 99 | 129 |
| Age-adjusted HR (95% CI) | 1.00 | 1.15 (0.92, 1.43) | 2.20 (1.45, 3.32) | 0.91 (0.40, 2.05) | 3.38 (1.86, 6.14) | |
| Multivariable-adjusted HR (95% CI)b | 1.00 | 1.11 (0.88, 1.39) | 2.13 (1.39, 3.26) | 0.88 (0.38, 2.06) | 3.53 (1.85, 6.73) | |
Abbreviations: CI, confidence interval; HR, hazard ratio; PSA hx, prostate specific antigen screening history
The number of current smokers available for the analysis of pack-years of smoking is less than the total number of current smokers because some current smokers were missing data on pack-years.
Adjusted for age at diagnosis (years), previous PSA screening history (yes, no, unknown), body mass index (<25, 25 to <30, 30+ kg/m2), vigorous physical activity (<1, 1–3, and 3+ hours/wk), total calories (quartiles), and coffee intake (quartiles) from the pre-diagnostic questionnaire. We used months since diagnosis as the time scale and stratified by calendar time in two-year intervals.
These results exclude men with stage T4, stage N1, stage M1 or missing stage at diagnosis (N=1122).
Adjustment for PSA screening attenuated the estimates for current smoking and prostate cancer mortality and minimally attenuated prostate cancer recurrence (TABLE 2). In the first sensitivity analysis limited to those who had reported PSA screening in the cycle prior to being diagnosed with prostate cancer, the estimates for current smoking became even stronger, though the confidence intervals were wider due to a smaller number of outcomes: for prostate cancer mortality HR = 2.13 (95% CI, 0.97–4.68) and biochemical recurrence HR = 2.06 (95% CI, 1.25–3.42). In the second sensitivity analysis which included cases only diagnosis from 1994, with adjustment for PSA screening intensity, the hazard ratios were similar to the first sensitivity analysis: for prostate cancer mortality HR = 2.12 (95% CI, 1.18–3.79) and biochemical recurrence HR = 2.02 (95% CI,1.30–3.13).
We observed no statistically significant interaction between smoking status and vitamin E supplementation (p=0.16) or BMI (p=0.26) for prostate cancer mortality; nor for smoking status and vitamin E supplementation (p=0.53) or BMI (p=0.60) for biochemical recurrence. Finally, we also assessed the relation between current smoking and prostate cancer mortality and biochemical recurrence among those with RP or radiation as primary treatment. After adjusting for stage and grade, the HRs for prostate cancer mortality were 1.30 (95% CI, 0.55–3.06) for RP patients and 2.07 (95% CI, 0.60–7.08) for radiation patients. The HRs for recurrence were 1.68 (95% CI, 1.02–2.76) for RP patients and 1.33 (95% CI, 0.70–2.52) for radiation patients.
Former smoking
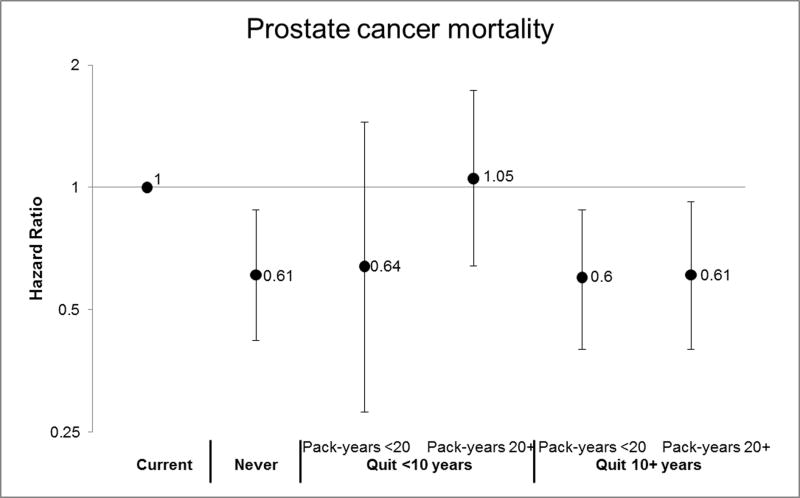
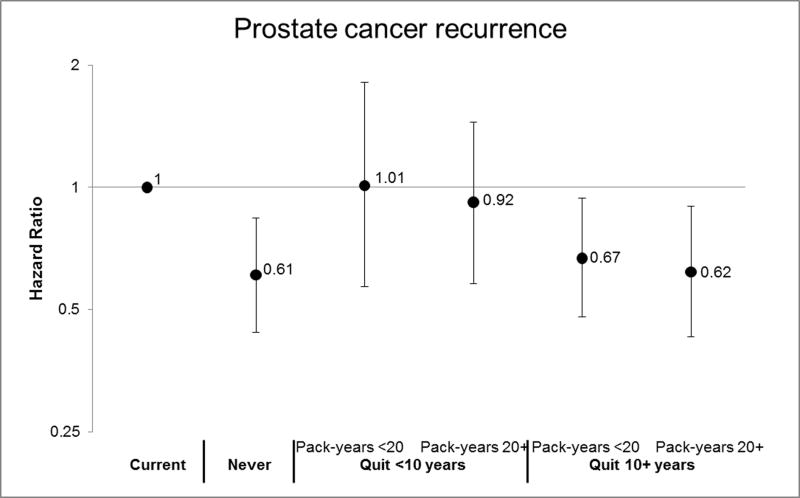
Former smokers overall, which includes past smokers quitting less than 10 years before diagnosis, had a HR for total mortality of 1.23 (95% CI, 1.10–1.37). Former smokers overall were not at significantly increased risk of the other endpoints (TABLE 2). Compared to current smokers, men who had quit smoking for 10 or more years, or who had quit for less than 10 years but smoked less than 20 pack-years, had prostate cancer mortality risks similar to never smokers (Figure 3): former smoker quit 10+ years (HR, 0.60; 95% CI, 0.42–0.87); former smoker, quit 10+ years and <20 pack-years (HR, 0.60; 95% CI, 0.40–0.88); quit 10+ years and 20+ pack-years (HR, 0.61; 95% CI, 0.40–0.92); quit <10 years and <20 pack-years (HR, 0.64; 95% CI, 0.28–1.45; never smoker (HR, 0.61; 95% CI, 0.42–0.88). Those who had quit for less than 10 years and had smoked 20 or more pack-years had risks similar to the reference group of current smokers (HR, 1.05; 95% CI, 0.64–1.73). The estimates for recurrence were similar for former smokers quitting more than 10 years before diagnosis, but we observed no reduction in risk among former smokers quitting less than 10 years before diagnosis (FIGURE 3).
FIGURE 3.
Risk of Prostate Cancer Mortality and Recurrence by Smoking Status & Intensity
Now in separate EXCEL file (see Tab 1 for Mortality and Tab 2 for Recurrence).
See footnotes in Table 2 for variables included in the multivariable model. Current smokers are the reference group. Please see the table below for the number of participants and events included in the analysis.
| Total | Smoking Status prior to Diagnosis
|
||||||
|---|---|---|---|---|---|---|---|
| Quit <10 years | Quit 10+ years | ||||||
|
| |||||||
| Current | Never | >0 to <20 pack-years | 20+ pack-years | >0 to <20 pack-years | 20+ pack-years | ||
| Prostate cancer deaths | |||||||
| No. of Events | 499 | 41 | 219 | 8 | 33 | 119 | 79 |
| No. of Participants | 5084 | 277 | 2449 | 87 | 210 | 1361 | 702 |
| Biochemical recurrence | |||||||
| No. of Events | 835 | 51 | 392 | 18 | 38 | 229 | 107 |
| No. of Participants | 3442 | 147 | 1746 | 52 | 119 | 951 | 427 |
DISCUSSION
We observed an elevated risk of prostate cancer mortality, CVD mortality, total mortality, and biochemical recurrence among men who were current smokers at the time of their prostate cancer diagnosis. Former smokers who had quit 10 or more years prior to diagnosis had risks similar to never smokers for prostate cancer mortality and recurrence. Those quitting less than 10 years prior to diagnosis and with less than 20 pack-years had risks similar to current smokers for prostate cancer recurrence (an early progression event) but risks similar to never smokers for prostate cancer mortality.
Since we considered stage and grade as intermediates of the relation of smoking with biochemical recurrence and prostate cancer-specific mortality, we did not initially control for these factors. In analyses that adjusted for these factors, to assess the impact of smoking beyond its effect on stage and grade, the associations with prostate cancer specific-mortality and recurrence remained elevated but were attenuated, as expected, providing evidence that the effect of smoking is mediated by these factors. Nonetheless, even after adjustment for stage and grade, the estimate for biochemical recurrence remained significant.
Smokers tend to have less PSA testing,24 and thus might be diagnosed at a more advanced stage, and hence be at increased risk of dying of prostate cancer or of having a biochemical recurrence due to the differences in screening behavior rather than the smoking per se. However, differential PSA screening across strata of smoking status was unlikely to fully account for our results, because the percentage of men who had at least one PSA test before the cycle of their diagnosis varied little among never smokers (86%), former smokers (85%), and current smokers (73%) when considering cases diagnosed after 1994, when screening became widely used (TABLE 1). We adjusted for PSA screening history in the multivariate analyses and this did not materially affect the estimates. We also conducted two sensitivity analyses, one limited to men who had reported PSA testing in a cycle prior to their diagnosis and the other adjusted for PSA screening intensity. If the association between smoking and fatal prostate cancer and recurrence resulted from delayed diagnosis, we would have expected to see an attenuation of the association. Instead, we observed stronger associations. These analyses were limited to cases diagnosed after introduction of PSA screening, so they included fewer cases and fewer endpoints, leading to wider confidence intervals. However, the relative risk estimates were larger than in the main analysis, suggesting that our primary analyses were not largely affected by this potential bias.
Several studies in specific treatment populations report that outcomes in men treated with external beam radiation therapy,5, 8 androgen deprivation therapy,9 and radical prostatectomy6, 7 are poorer among current smokers, in univariate5–9 as well as multivariate5, 7–9 models adjusted for other clinical factors. One other study in brachytherapy patients noted a non-significant trend for poorer outcome in current smokers vs. never or former smokers (p=0.13).25 We found that the association of smoking with prostate cancer recurrence and mortality may differ in men treated with radical prostatectomy or radiation, the two commonest treatments in our cohort, after adjustment for stage and grade, but the confidence intervals were wide so these findings should be interpreted cautiously.
A direct effect of smoking on prostate cancer progression is biologically plausible. The main hypotheses proposed include: 1) tumor promotion through carcinogens from tobacco smoke,26 with suggestive prostate cancer-specific data for nitrosamines27 and cadmium28–32 and one study reporting that gene variants involved in detoxification may influence risk of aggressive prostate cancer;33 2) increased plasma levels of total and free testosterone, an androgen involved in the development and progression of prostate cancer, in current smokers34–38, with some studies reporting a dose-dependent association36, 37; 3) epigenetic effects, including aberrant methylation profiles among current smokers, which correlate with aggressive disease39; and 4) nicotine-induced angiogenesis, capillary growth, and tumor growth and proliferation.40–42
In summary, smoking at the time of diagnosis was associated with substantially increased overall mortality and prostate cancer mortality and recurrence. 10-year quitters had risks similar to never smokers. These results provide further support that smoking may increase risk of death from prostate cancer.
Acknowledgments
Funding/Support: The project described was supported by grants CA055075 and CA141298 from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Role of the Sponsor: The funding source had no role in the design or conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval or the manuscript.
Footnotes
Author Contributions: Dr. Kenfield had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Study concept and design: Kenfield, Stampfer, Chan, and Giovannucci.
Acquisition of data: Kenfield, Stampfer, Chan, and Giovannucci.
Analysis and interpretation of data: Kenfield, Stampfer, Chan, and Giovannucci.
Drafting of the manuscript: Kenfield.
Critical revision of the manuscript for important intellectual content: Kenfield, Stampfer, Chan, and Giovannucci
Statistical analysis: Kenfield and Giovannucci.
Obtained funding: Stampfer and Giovannucci.
Administrative, technical, or material support: Stampfer, Chan, and Giovannucci.
Study supervision: Stampfer, Chan, and Giovannucci.
Financial Disclosures: None reported.
Additional Contributions: We thank the participants and staff of the Health Professionals Follow-Up Study for their valuable contributions. We thank the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY.
References
- 1.U.S. Department of Health and Human Services. The Health Consequences of Smoking. A Report of the Surgeon General. Washington, DC: U.S. Dept of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. [Google Scholar]
- 2.Zu K, Giovannucci E. Smoking and aggressive prostate cancer: a review of the epidemiologic evidence. Cancer Causes Control. 2009 Jun 27;27:27. doi: 10.1007/s10552-009-9387-y. [DOI] [PubMed] [Google Scholar]
- 3.Daniell HW. A worse prognosis for smokers with prostate cancer. J Urol. 1995 Jul;154(1):153–157. [PubMed] [Google Scholar]
- 4.Yu GP, Ostroff JS, Zhang ZF, Tang J, Schantz SP. Smoking history and cancer patient survival: a hospital cancer registry study. Cancer Detect Prev. 1997;21(6):497–509. [PubMed] [Google Scholar]
- 5.Pickles T, Liu M, Berthelet E, Kim-Sing C, Kwan W, Tyldesley S. The effect of smoking on outcome following external radiation for localized prostate cancer. J Urol. 2004 Apr;171(4):1543–1546. doi: 10.1097/01.ju.0000118292.25214.a4. [DOI] [PubMed] [Google Scholar]
- 6.Moreira DM, Antonelli JA, Presti JC, Jr, et al. Association of Cigarette Smoking With Interval to Biochemical Recurrence After Radical Prostatectomy: Results from the SEARCH Database. Urology. 2010 Nov;76(5):1218–1223. doi: 10.1016/j.urology.2010.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joshu CE, Mondul AM, Meinhold CL, et al. Cigarette Smoking and Prostate Cancer Recurrence After Prostatectomy. J Natl Cancer Inst. 2011 Apr 15;2011:15. doi: 10.1093/jnci/djr124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pantarotto J, Malone S, Dahrouge S, Gallant V, Eapen L. Smoking is associated with worse outcomes in patients with prostate cancer treated by radical radiotherapy. BJU Int. 2007 Mar;99(3):564–569. doi: 10.1111/j.1464-410X.2006.06656.x. [DOI] [PubMed] [Google Scholar]
- 9.Oefelein MG, Resnick MI. Association of tobacco use with hormone refractory disease and survival of patients with prostate cancer. J Urol. 2004 Jun;171(6 Pt 1):2281–2284. doi: 10.1097/01.ju.0000125123.46733.93. [DOI] [PubMed] [Google Scholar]
- 10.Gong Z, Agalliu I, Lin DW, Stanford JL, Kristal AR. Cigarette smoking and prostate cancer-specific mortality following diagnosis in middle-aged men. Cancer Causes Control. 2008 Feb;19(1):25–31. doi: 10.1007/s10552-007-9066-9. [DOI] [PubMed] [Google Scholar]
- 11.Jager T, Eisenhardt A, Rubben H, Lummen G. Does cigarette smoking influence the survival of patients with prostate cancer? Urologe A. 2007 Apr;46(4):397–400. doi: 10.1007/s00120-006-1252-y. [DOI] [PubMed] [Google Scholar]
- 12.Giovannucci E, Rimm EB, Ascherio A, et al. Smoking and risk of total and fatal prostate cancer in United States health professionals. Cancer Epidemiol Biomarkers Prev. 1999 Apr;8(4 Pt 1):277–282. [PubMed] [Google Scholar]
- 13.Kupelian PA, Elshaikh M, Reddy CA, Zippe C, Klein EA. Comparison of the efficacy of local therapies for localized prostate cancer in the prostate–specific antigen era: a large single-institution experience with radical prostatectomy and external-beam radiotherapy. J Clin Oncol. 2002 Aug 15;20(16):3376–3385. doi: 10.1200/JCO.2002.01.150. [DOI] [PubMed] [Google Scholar]
- 14.Freedland SJ, Sutter ME, Dorey F, Aronson WJ. Defining the ideal cutpoint for determining PSA recurrence after radical prostatectomy. Prostate-specific antigen. Urology. 2003 Feb;61(2):365–369. doi: 10.1016/s0090-4295(02)02268-9. [DOI] [PubMed] [Google Scholar]
- 15.Roach M, 3rd, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006 Jul 15;65(4):965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 16.Kuban DA, Levy LB, Potters L, et al. Comparison of biochemical failure definitions for permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2006 Aug 1;65(5):1487–1493. doi: 10.1016/j.ijrobp.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 17.Rich-Edwards J, Corsano K, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140:1016–1019. doi: 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]
- 18.Wilson KM, Kasperzyk JL, Stark JR, et al. Coffee consumption and prostate cancer risk and progression in the Health Professionals Follow-Up Study. JNCI. doi: 10.1093/jnci/djr151. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. Risk factors for prostate cancer incidence and progression in the Health Professionals Follow-up Study. Int J Cancer. 2007 Apr 20; doi: 10.1002/ijc.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan JM, Holick CN, Leitzmann MF, et al. Diet after diagnosis and the risk of prostate cancer progression, recurrence, and death (United States) Cancer Causes Control. 2006 Mar;17(2):199–208. doi: 10.1007/s10552-005-0413-4. [DOI] [PubMed] [Google Scholar]
- 21.Heinonen OP, Albanes D, Virtamo J, et al. Prostate cancer and supplementation with alpha-tocopherol and beta-carotene: incidence and mortality in a controlled trial. J Natl Cancer Inst. 1998 Mar 18;90(6):440–446. doi: 10.1093/jnci/90.6.440. [DOI] [PubMed] [Google Scholar]
- 22.Chan JM, Stampfer MJ, Ma J, Rimm EB, Willett WC, Giovannucci EL. Supplemental vitamin E intake and prostate cancer risk in a large cohort of men in the United States. Cancer Epidemiol Biomarkers Prev. 1999 Oct;8(10):893–899. [PubMed] [Google Scholar]
- 23.Ma J, Li H, Giovannucci E, et al. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol. 2008 Nov;9(11):1039–1047. doi: 10.1016/S1470-2045(08)70235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Byrne MM, Davila EP, Zhao W, et al. Cancer screening behaviors among smokers and non-smokers. Cancer Epidemiol. 2010 Oct;34(5):611–617. doi: 10.1016/j.canep.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 25.Merrick GS, Butler WM, Wallner KE, Galbreath RW, Lief JH, Adamovich E. Effect of cigarette smoking on biochemical outcome after permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2004 Mar 15;58(4):1056–1062. doi: 10.1016/j.ijrobp.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 26.Hecht SS. Cigarette smoking: cancer risks, carcinogens, and mechanisms. Langenbecks Arch Surg. 2006 Nov;391(6):603–613. doi: 10.1007/s00423-006-0111-z. [DOI] [PubMed] [Google Scholar]
- 27.Pour PM. Prostatic cancer induced in MRC rats by N-nitrosobis(2-oxopropyl)-amine and N-nitrosobis(2-hydroxypropyl)amine. Carcinogenesis. 1983;4(1):49–55. doi: 10.1093/carcin/4.1.49. [DOI] [PubMed] [Google Scholar]
- 28.Waalkes MP, Rehm S. Cadmium and prostate cancer. J Toxicol Environ Health. 1994 Nov;43(3):251–269. doi: 10.1080/15287399409531920. [DOI] [PubMed] [Google Scholar]
- 29.Achanzar WE, Diwan BA, Liu J, Quader ST, Webber MM, Waalkes MP. Cadmium-induced malignant transformation of human prostate epithelial cells. Cancer Res. 2001 Jan 15;61(2):455–458. [PubMed] [Google Scholar]
- 30.Zeng X, Jin T, Jiang X, Kong Q, Ye T, Nordberg GF. Effects on the prostate of environmental cadmium exposure--a cross-sectional population study in China. Biometals. 2004 Oct;17(5):559–565. doi: 10.1023/b:biom.0000045739.89653.67. [DOI] [PubMed] [Google Scholar]
- 31.Vinceti M, Venturelli M, Sighinolfi C, et al. Case-control study of toenail cadmium and prostate cancer risk in Italy. Sci Total Environ. 2007 Feb 1;373(1):77–81. doi: 10.1016/j.scitotenv.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Benbrahim-Tallaa L, Liu J, Webber MM, Waalkes MP. Estrogen signaling and disruption of androgen metabolism in acquired androgen-independence during cadmium carcinogenesis in human prostate epithelial cells. Prostate. 2007 Feb 1;67(2):135–145. doi: 10.1002/pros.20479. [DOI] [PubMed] [Google Scholar]
- 33.Nock NL, Liu X, Cicek MS, et al. Polymorphisms in polycyclic aromatic hydrocarbon metabolism and conjugation genes, interactions with smoking and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2006 Apr;15(4):756–761. doi: 10.1158/1055-9965.EPI-05-0826. [DOI] [PubMed] [Google Scholar]
- 34.Handa K, Ishii H, Kono S, et al. Behavioral correlates of plasma sex hormones and their relationships with plasma lipids and lipoproteins in Japanese men. Atherosclerosis. 1997 Apr;130(1–2):37–44. doi: 10.1016/s0021-9150(96)06041-8. [DOI] [PubMed] [Google Scholar]
- 35.Tamimi R, Mucci LA, Spanos E, Lagiou A, Benetou V, Trichopoulos D. Testosterone and oestradiol in relation to tobacco smoking, body mass index, energy consumption and nutrient intake among adult men. Eur J Cancer Prev. 2001 Jun;10(3):275–280. doi: 10.1097/00008469-200106000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Allen NE, Appleby PN, Davey GK, Key TJ. Lifestyle and nutritional determinants of bioavailable androgens and related hormones in British men. Cancer Causes Control. 2002 May;13(4):353–363. doi: 10.1023/a:1015238102830. [DOI] [PubMed] [Google Scholar]
- 37.Svartberg J, Jorde R. Endogenous testosterone levels and smoking in men. The fifth Tromso study. Int J Androl. 2007 Jun;30(3):137–143. doi: 10.1111/j.1365-2605.2006.00720.x. [DOI] [PubMed] [Google Scholar]
- 38.Shiels MS, Rohrmann S, Menke A, et al. Association of cigarette smoking, alcohol consumption, and physical activity with sex steroid hormone levels in US men. Cancer Causes Control. 2009 Aug;20(6):877–886. doi: 10.1007/s10552-009-9318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Enokida A, Shiina H, Urakami S, et al. Smoking influences aberrant CpG hypermethylation of multiple genes in human prostate carcinoma. Cancer. 2006;106(1):79–86. doi: 10.1002/cncr.21577. [DOI] [PubMed] [Google Scholar]
- 40.Villablanca AC. Nicotine stimulates DNA synthesis and proliferation in vascular endothelial cells in vitro. J Appl Physiol. 1998;84(6):2089–2098. doi: 10.1152/jappl.1998.84.6.2089. [DOI] [PubMed] [Google Scholar]
- 41.Heeschen C, Jang JJ, Weis M, et al. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat Med. 2001;7(7):833–839. doi: 10.1038/89961. [DOI] [PubMed] [Google Scholar]
- 42.Jain RK. Clearing the smoke on nicotine and angiogenesis. Nat Med. 2001;7(7):775–777. doi: 10.1038/89889. [DOI] [PubMed] [Google Scholar]