Abstract
Estradiol is known to impact cognitive function including spatial learning and memory, with studies focused largely on rodent models. Estrogens can be produced peripherally or centrally as neuroestrogens, and the specific role for neuroestrogens in memory processes remains unresolved. Many songbirds possess remarkable spatial memory capabilities and also express the estrogen synthetic enzyme aromatase abundantly in the hippocampus, suggesting that locally-produced estrogens may promote the acquisition or retrieval of spatial memories in these birds. We examined the effect of estradiol on spatial memory in three contexts in the zebra finch: retrieval after discrimination training, retrieval after familiarization but without discrimination training, and memory acquisition, using a combination of estradiol implants and oral dosing with the aromatase inhibitor fadrozole (FAD). Retrieval of spatial memory in both contexts was impaired when estradiol production was blocked. However, spatial memory acquisition was enhanced when estradiol production was inhibited whereas estradiol replacement impaired acquisition. These results provide evidence for a context-specific role of estradiol in songbird spatial memory, results that finds accord with some mammalian studies but have not yet been observed in birds.
Keywords: Estradiol, spatial memory, learning, songbird
1. Introduction
Steroid hormones regulate numerous physiological and behavioral systems, including the neural circuits associated with spatial learning and memory. Spatial memory is reliant on the hippocampus (HP), and both testosterone and estradiol can influence the acquisition, retention, and retrieval of spatial memories (Luine, 1997; Luine, 2008; Barha & Galea, 2010; Leonard & Winsauer, 2011). In mammals, these sex steroids stimulate memory acquisition and retrieval in part by increasing dendritic spine density (Luine, 1997; Phan et al., 2012) and synapse formation (Mendez, Garcia-Segura, & Muller, 2011). In songbirds, estradiol or testosterone implants can also stimulate spatial memory acquisition and increase the size of hippocampal cells, whereas dihydrotestosterone (DHT) has no effect (Oberlander et al., 2004). Presumably, testosterone is locally aromatized into estradiol to promote spatial memory acquisition as aromatase is expressed abundantly in the songbird HP (Shen et al., 1995; Saldanha et al., 1998, 2000). Overall, however, studies examining the effect of estradiol on spatial memory acquisition and retrieval are often contradictory: some identify beneficial effects of estradiol on all aspects of spatial memory, while others indicate that estradiol impairs spatial memory acquisition (e.g., Daniel et al., 1997; Gibbs, 2000; Snihur, Hampson, & Cain, 2008).
Many species of birds exhibit exceptional spatial memory capabilities, especially some food-storing oscine songbirds (e.g., Paridae and Corvidae; Shettleworth, 1990; Bednekoff et al., 1997; Salwiczek, Watanabe, & Clayton, 2010; Gould, Ort, & Kamil, 2012; Roth, LaDage, & Pravosudov, 2012), yet relatively few studies have investigated how sex steroids regulate avian spatial memory and cognition. Although the HP differs in structure and position between mammals and birds, it is analogous in function (Colombo & Broadbent, 2000). For example, lesioning of the songbird HP produces deficits in spatial memory (Hampton & Shettleworth, 1996; Bischof, Lieshoff, & Watanabe, 2006) which are reversed with fetal hippocampal implants (Patel, Clayton, & Krebs, 1997). In addition, expression of immediate early genes (c-fos and ZENK) is up-regulated in the zebra finch HP during spatial task learning and recall (Mayer, Watanabe, & Bischof, 2010). Problematically, of the few studies that have examined the effect of sex steroids on spatial memory in birds, testing methodologies, methods of hormone delivery, and subject sex have differed, making definitive conclusions hard to establish. For example, Oberlander et al. (2004) used subcutaneous implants that produced long-term changes in circulating hormone levels and assessed the effects on learning and memory for hidden food in male zebra finches (Taeniopygia guttata). In contrast, Hodgson et al. (2008) examined effects of orally delivered steroid hormones on spatial memory in a delayed non-matching to sample task in male and female great tits (Parus major). This allowed for manipulation of hormone levels on a finer scale, i.e., a day-to-day basis. Interestingly, while Oberlander found a strong effect of estradiol on spatial memory acquisition, the effect of estradiol on memory was less clear in Hodgson et al. Clearly additional studies are warranted to further our understanding of the complex relationship between steroid hormones and spatial memory.
The goal of the present study was to assess the role of estrogens in promoting spatial memory acquisition and retrieval in zebra finches, with an emphasis on understanding the context-specific effects of estradiol. To this end, birds were tested in a four-arm maze based on the similar food-finding task used by Bischof, Lieshoff, & Watanabe (2006) that confirmed the role of the HP in spatial memory. We adapted the task to more closely resemble the radial-arm maze paradigm commonly used to test spatial memory in rodents (e.g., Olton, Collison, & Werz, 1977). A similar maze was previously used successfully to test zebra finch learning of a spatial memory task (Spence et al., 2009).
Three experiments were conducted using a combination of estradiol implants and oral dosing with fadrozole (FAD), an effective inhibitor of the estrogen synthetic enzyme aromatase in songbirds (Wade et al., 1994). In the first experiment, we allowed birds to learn the location of a food source (familiarization) and complete discrimination training for three days, then blocked estradiol synthesis and assessed the impact on memory retrieval. In experiment two, we examined the effect of aromatase inhibition with or without estradiol replacement on spatial memory retrieval after learning the location of the food source during the familiarization period but without discrimination training. Discrimination training was excluded in this experiment to limit the reinforcement created by multiple days of practice. In the final experiment, we examined the effects of aromatase inhibition with or without estradiol replacement on memory acquisition.
2. Materials and Methods
2.1 Subjects
This study used non-breeding female zebra finches of breeding age (>100 days of age) that were communally housed in our zebra finch colony at the University of California, Los Angeles. Prior to being used in experiments, all birds were maintained on a 14:10 light: dark cycle and provided with ad libitum water, grit, cuttlebone, and seed, supplemented with egg mix, spinach, and bird vitamins. All experimental procedures were approved by the University Chancellor’s Animal Research Committee.
2.2 Behavioral Test
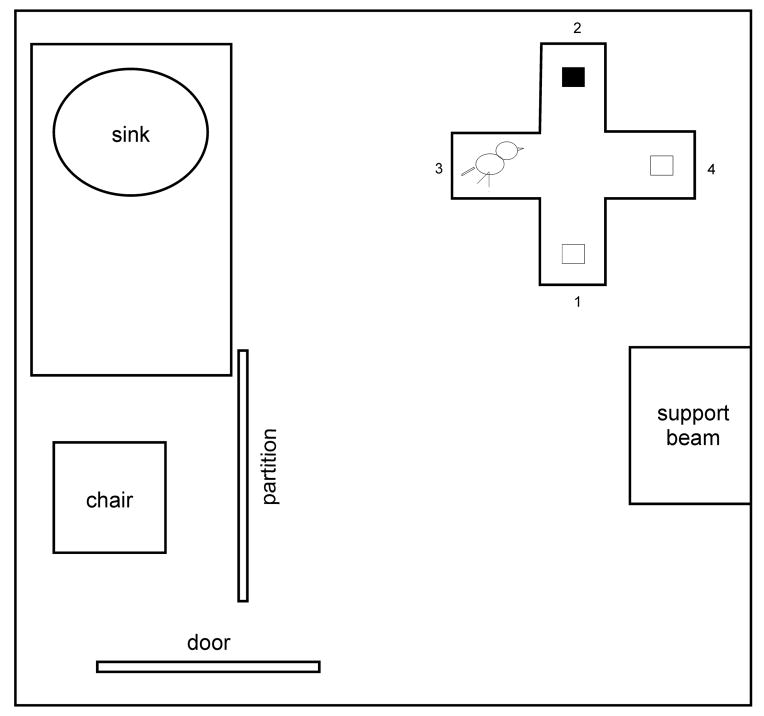
The apparatus was a four-arm maze similar to that used in Spence et al., 2009 (length = 44.8cm; width of each arm = 13.0cm; height = 45.7cm). The maze sat on the floor of an 125 by 134 inch testing room, and was constructed with Plexiglas or wooden supports surrounded by screening (1mm × 1mm mesh) on all sides, giving the birds free visual exposure to the complex features of the testing room. Each arm provided a different view of the room: arm 1 provided a view of the testing chair, partition, and support beam, arm 2 a view of the back wall and side view of the counter and sink, arm 3 a straight-on view of the counter and sink, and arm 4 a view of a wall and side view of the support beam (Fig. 1). For each bird, one arm of the maze was randomly selected as the reward arm. The reward arm contained a red food cup from which the bird could feed. Identical red plastic food cups were placed in two of the remaining three arms, but the food in these cups was covered with a thin sheet of clear plastic to prevent access (‘dummy cups’). On the morning of each testing day, the bird was food deprived for 3–4 hours while water remained in the maze. Testing consisted of three 30-min sessions per day. The bird was allowed to complete as many trials as possible over the course of each session. Sessions were separated by approximately 1–2 hours to enable synaptic consolidation, the first step in long-term memory formation (Dudai, 2004). During this period the bird remained in the maze with water, all food cups were removed, and the observer was absent from the room. To initiate testing, the bird was placed in the fourth arm of the maze in the dark; the lights were turned on, and the bird was allowed to search for the rewarded food cup and feed for several seconds (equal to one trial; max feeding time of 10 seconds). Afterwards, the lights were turned off and the bird’s position was randomly changed to one of the other three arms; a dummy cup was placed in the newly emptied arm. In this way, the bird’s starting location changed for each trial, while the location of the rewarded food cup remained constant. If a bird failed to complete a trial within 10-min, the light was turned off and a new trial was initiated. We recorded the number of errors made in each trial, with an error being defined as entry into an incorrect arm, even if the bird did not reach the food cup at the end of the arm. In addition, we recorded the amount of time each bird took to find the correct food cup per trial in experiments 2 and 3 (see below). Different birds were used for each of the three experiments described below.
Figure 1.
Layout of the experimental testing room. The maze sat in one corner of the room opposite of the observer’s chair and partition. External cues include a chair, partition and sink, as well as other cues such as a garbage can and light fixtures. In this example, arm 2 is the rewarded arm (black rectangle represents food cup), while arms 1 and 4 contain dummy food cups. The bird therefore starts the trial in arm 3. After completion of the trial, the bird is moved to arm 1, 3, or 4 (randomly determined), while the rewarded arm stays constant.
2.2.1 Experiment 1: Estradiol and Spatial Memory Retrieval after Discrimination Training
Each zebra finch was moved into the maze on the afternoon prior to the first training day and allowed to become familiarized with food in the reward arm overnight (allowing learning that the food cup provides food as well as providing spatial location information). Training on the behavioral task commenced the next morning and continued for three days. On the morning of day four, each bird received one of the following treatments: an oral dose of FAD (20μl of 1mg/ml dissolved in water; n = 6) or vehicle (water; n = 6). This dose of FAD has been previously shown to inhibit aromatase (Wade et al., 1994; Saldanha et al., 2000). To ensure that FAD had sufficient time to maximally inhibit aromatase, no testing was performed on day four. On days 5, 6, and 7, testing proceeded as described above, with an additional oral dose of FAD or vehicle given each morning of testing (3–4 hours prior to behavioral testing).
2.2.2 Experiment 2: Estradiol and Spatial Memory Retrieval without Discrimination Training
Prior to the start of experiment 2, each bird was implanted subcutaneously with a 5mm estradiol (Sigma; n = 5) or blank implant (n = 10). The bird was placed in the maze on the following afternoon and allowed to become familiarized with one food cup in the rewarded arm until the following morning. The next morning, each bird received an oral dose of FAD (20μl of 1mg/ml dissolved in water) or water vehicle (20μl). Behavioral testing took place starting the next day and lasted for two days, with a dose given on the morning of each day. The experimental treatments were as follows: blank implant and vehicle dose (n = 5), blank implant and FAD dose (n = 5), and estradiol implant and FAD dose (n = 5).
2.2.3 Experiment 3: Estradiol and Memory Acquisition
Prior to the start of experiment 3, each bird was implanted subcutaneously with a 5mm estradiol (n = 5) or blank implant (n = 5). On the following morning, the bird was moved into the maze with one accessible food cup located in the center and given an oral dose of FAD (20μl of 1mg/ml dissolved in water) at 8AM. With the cup placed centrally during the familiarization period, no spatial learning with regard to the reward arm could occur until testing commenced the next day. Testing commenced the next day and continued for a total of two days, with a FAD dose given on the morning of both testing days.
2.3 Statistical Analysis
We used generalized linear mixed models (GLMM) to assess the relationship between memory retrieval (experiments 1 and 2), and memory acquisition (experiment 3). To assess the effect of FAD on spatial memory retrieval after discrimination training (experiment 1), we used a GLMM with a Poisson distribution of errors and a log link function. Only trials in which the bird successfully found the food cup were included in the analysis. This allowed us to generate a conservative measure of performance in the maze that eliminated the possibility of assigning a bird a score of zero errors simply because the bird did not actually search for the food. Fixed factors were the following: group (FAD or vehicle), time (either before or after the treatment started on day 4, coded as an ordinal variable), and day before or after (1–3 for first three days and 1–3 for second three days after treatment, coded as an ordinal variable). This allowed us to test for a difference between groups both before and after treatment, as well as to test for a specific day effect relative to treatment (i.e., whether learning occurred over the course of the first three days, then after treatment). We included trial number nested within session nested within bird identity as a random variable in the analysis. To examine whether differences between treatment groups were due to differences in motivation and/or activity between the two groups, we used a GLMM to assess both the number of trials attempted (including those that were not completed within the allotted time) and the number of trials completed by each bird within a session. The fixed factors were identical to the previous analysis, and session nested within bird identity was included as a random factor. We were unable to investigate differences in search time between treatment groups in experiment 1 due to an oversight.
For experiments 2 and 3, we assessed the number of errors made in the maze using a GLMM with day (1–2) and group (blank implant + FAD, estradiol implant + FAD, and control) as fixed factors. Number of errors was coded with a Poisson distribution (log link function), and only trials in which the bird successfully found the food cup were included in the analysis. Trial was nested within session within bird identity as a random factor. To examine whether the experimental treatment influenced motivation and/or activity levels in the maze, we analyzed both the number of trials attempted and completed (log transformed if necessary) by each bird. We also assessed whether the treatment groups differed in the amount of time it took to find the food in each trial. In this analysis, the time spent searching in the maze was the independent variable (calculated as the time of success minus the time the bird started moving in the maze) with a Poisson distribution of errors. Day and group were included as fixed factors, with trial nested within session within bird identity as a random variable.
In all analyses, the initial model included all two and three-way interactions, which were then removed sequentially when found to be non-significant (i.e., three-way interaction removed before any two-way interactions). Significant main effects were analyzed with a least significant difference post-hoc test where appropriate. All figures show raw data values ± 1 SE, and statistical analyses were completed using SPSS 20 (IBM Statistics, 2012).
3. Results
3.1 Experiment 1: Spatial Memory Retrieval after Discrimination Training
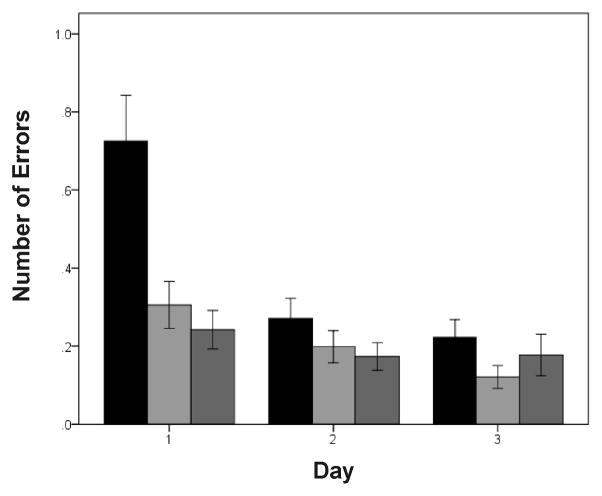
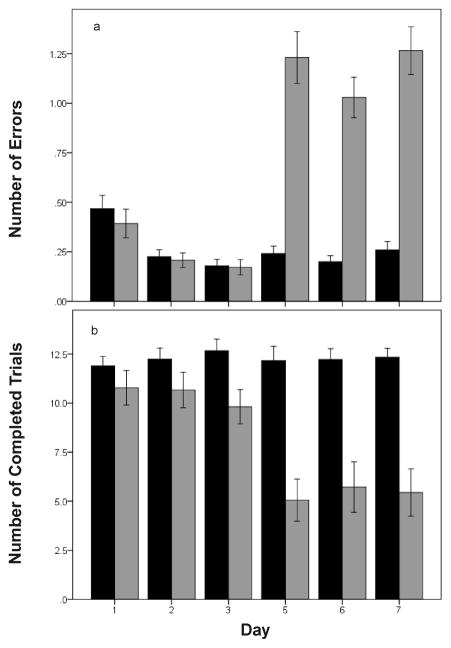
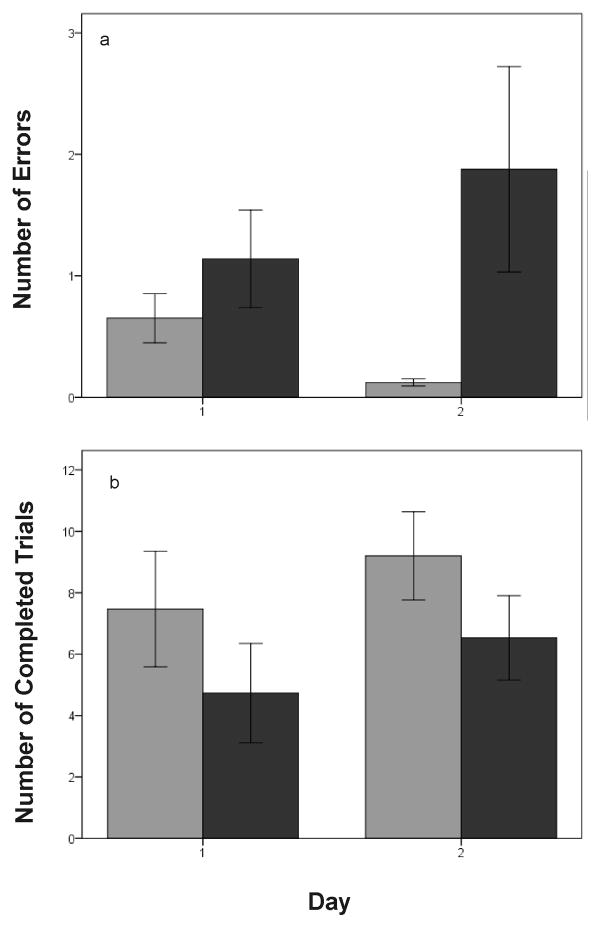
Zebra finches successfully learned the behavioral task within the first several days of testing (see summary figure of performance by session number; Fig. 2). The ability of birds to remember the location of the food (indicated by the number of errors made during each trial) was strikingly affected by the experimental treatment (summary of results by day; Fig. 3a). The number of errors was significantly influenced by day before or after treatment (days 1–3; F2,2068=14.68; p < 0.0001), group (F1,2068=50.18; p < 0.0001), and time (before or after treatment; F1,2068 = 76.60; p < 0.0001). In addition, there was a significant interaction between time and day (F 2,2068 = 16.35; p < 0.0001). Before beginning the experimental treatment, there was a significant effect of day on the number of errors made in the maze (F1,1156 = 37.58; p < 0.0001). Birds made more errors on day 1 than on days 2 or 3 (p < 0.01 in both cases) but did not differ between days 2 and 3 (p = 0.634). After the experimental treatment with FAD or vehicle began, the number of errors was no longer dependent on day (F2,914 = 2.51; p = 0.082). We also found a significant interaction between time and experimental group (F1,2068 = 122.93; p < 0.0001). Before experimental treatment began, the vehicle and FAD groups did not differ in the number of errors made in the maze (F1,1157 = 0.02; p = 0.891). After the treatment began, however, birds in the FAD group made significantly more errors than those in the vehicle group (F1,915 = 129.00; p < 0.0001). The interaction between day and group was non-significant (F2,2066 = 0.19; p = 0.825), as was the interaction between day, time, and group (F2,2064 = 0.01; p = 0.992).
Figure 2.
Learning in a spatial memory task (Experiment 1) over the first three days (prior to the start of experimental treatment). There was a significant decrease in number of errors between days 1 and 2. Session 1 = black bars; Session 2 = light gray bars; Session 3 = dark gray bars. Data shown are means ± SE.
Figure 3.
a) Performance in the spatial memory task in experiment 1 over the course of one week and according to treatment group (black = control; light gray = treatment with the aromatase inhibitor fadrozole; FAD). Before experimental treatment began (days 1–3), FAD and control groups did not differ in number of errors made. In addition, performance significantly improved between days 1 and 2. After treatment began, there was no effect of day on performance, and FAD birds made significantly more errors than control birds. b) Number of completed trials in experiment 1. Dosing with FAD or water did not begin until day 4. FAD-treated birds completed significantly fewer trials than control birds after treatment began. See text for details of the statistical analysis. Data shown are means ± SE.
We also tested whether the number of trials attempted within a session differed between the treatment groups. There was no effect of day on the number of trials attempted (F2,201 = 0.33; p = 0.722), while both time and group were significant (F1,201 = 63.40; p < 0.0001and F1,201 = 15.58; p < 0.0001). The interaction between time and group was also significant (F1,201 = 63.82; p < 0.0001). Before the experimental treatment began, the vehicle and FAD treatment groups attempted similar numbers of trials (F1,100 = 3.71; p = 0.057). After treatment began, however, the FAD-treated birds attempted fewer trials than vehicle-treated birds (F1,103 = 30.23; p < 0.0001). When we analyzed the number of trials completed by each bird, there was again no effect of day (F2,201 = 0.29; p = 0.747) and significant effects of time (F1,201= 87.48; p < 0.0001) and group (F1,201= 16.39; p < 0.0001). The interaction between time and group was again significant (F1,201 = 86.79; p < 0.0001), with FAD-treated birds completing fewer trials than vehicle treated birds after treatment began (F1,103 = 28.25; p < 0.0001) but not before (although this effect was marginally non-significant: F1,100 = 3.82; P = 0.053; Fig. 3b). For both attempted and completed trials, the interactions between day and time and day and group were non-significant, as well as the interaction between day, time, and group (results not shown).
3.2 Experiment 2: Spatial Memory Retrieval without Discrimination Training
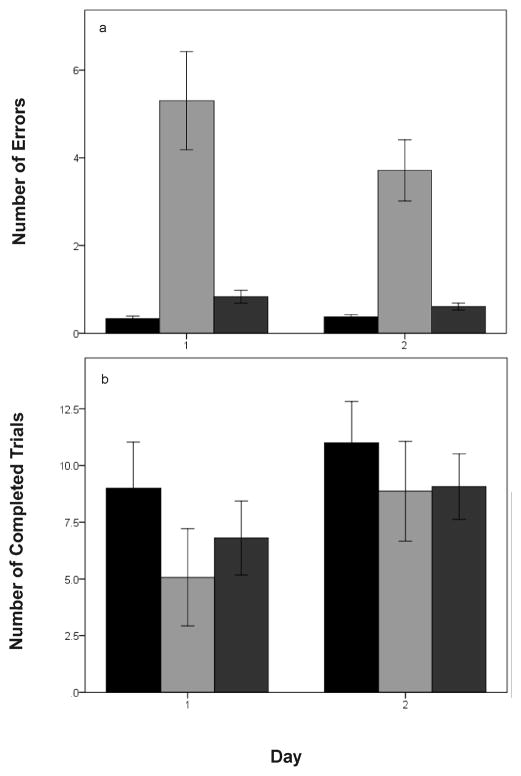
The number of errors made significantly decreased from day 1 to day 2 (F1,743 = 5.50; p = 0.019). There was a significant effect of treatment group as well (F2,743 = 60.68; p = < 0.0001). Birds with a blank implant and a water dose made the fewest errors, followed by birds given an estradiol dose and a FAD dose, followed by birds with a blank implant and a FAD dose (all p < 0.003; Fig. 4a). The interaction between day and treatment group was non-significant (F2,741 = 0.63; p = 0.535).
Figure 4.
a) Performance in the spatial memory task in experiment 2 according to treatment group and day and b) Number of completed trials. While there was no significant effect of day on number of errors, all treatment groups were significantly different from one another. Number of completed trials significantly increased from day 1 to day 2. Blank implant + control dose = black bars; blank implant + FAD dose = light gray bars; estradiol implant + FAD dose = dark gray bars. Data shown are means ± SE.
The number of trials attempted by birds in the maze was significantly higher on day 2 than day 1 (F1,86 = 27.12; p < 0.0001). There was no effect of treatment (F2,86 = 0.93; p = 0.40) or interaction between treatment and day (F2,84 = 0.63; p = 0.533). Similarly, the number of trials successfully completed was greater on day 2 than day 1 (F1,86 = 24.48; p < 0.0001) and did not differ between treatment groups (F2,86 = 1.55; p = 0.218) or as a function of the treatment by day interaction (F2,84 = 1.22; p = 0.301; Fig. 4b).
We also examined the impact of experimental treatment on the amount of time that birds spent searching for the food cup in the maze (defined as the time from when the bird started moving after the start of a trial until it found the food). There were significant effects of day (F1,735 = 68.27; p < 0.0001), treatment group (F2,735 = 12.64; p < 0.0001), and an interaction between day and treatment (F2,735 = 64.66; p < 0.0001). On day 1 of testing, there was a significant effect of treatment group (F2,308 = 11.50; p < 0.0001). Individuals in the blank group did not differ from those in the blank + FAD group (p = 0.115); however, those in the estradiol + FAD group spent significantly more time searching in the maze than blank birds and blank + FAD birds (both p < 0.02). On day two, there was again a significant effect of treatment group (F 2,427 = 7.96; p = 0.0004). In this case, however, estradiol + FAD birds spent more time searching than blank birds (p < 0.01) but not blank + FAD birds (p = 0.194). Interestingly, blank + FAD birds spent more time searching than blank birds (p = 0.018).
3.3 Experiment 3: Spatial Memory Acquisition
Surprisingly, performance did not increase over the course of the two days of testing (F 1,416 = 2.74; P = 0.098). Birds with an estradiol implant and FAD dose made significantly more errors than birds with a blank implant and FAD dose, however (F1,416 = 11.75; p = 0.001). The interaction between treatment group and day was also significant (F1,416 = 39.91; p < 0.0001). Within the group treated with a blank implant and FAD dose, the number of errors significantly decreased from day 1 to day 2 (F1,249 = 20.09; p < 0.0001). Within the group treated with a estradiol implant and FAD dose, the number of errors significantly increased from day 1 to day 2 (F 1,167 = 28.62; p < 0.0001; Fig. 5a).
Figure 5.
a) Performance in the spatial memory task in experiment 3 in birds with a blank implant and FAD dose (light gray bars) and birds with an estradiol implant and FAD dose (dark gray bars) and b) Number of completed trials. Estradiol + FAD birds made more errors on day 2 than day 1, while blank + FAD birds made fewer errors on day 2 than day 1. Data shown are means ± SE.
The number of trials attempted within a given session did not differ between days (F1,57 = 2.81; p = 0.099), treatment groups (F1,57 = 1.27; p = 0.265) or by the interaction between day and group (F1,56 = 0.02; p = 0.881). Similarly, there were no effects of day, treatment, or the interaction term on the number of successfully completed trials (day: F1,57 = 3.55; p = 0.065; treatment: F1,57 = 1.76; p = 0.19; treatment*day: F1,56 = 0.001; p = 0.972; Fig. 5b).
The amount of time spent searching for the food in the maze was not influenced by day (F1,416 = 0.001; p = 0.973) or treatment group (F1,416 = 0.31; p = 0.577), but there was a significant interaction between day and treatment group (F1,416 = 38.79; p < 0.0001). On day one of testing, there was a non-significant trend towards longer search times in the blank implant plus FAD group (F1,181 = 2.74; p = 0.099). On day two, the treatment groups did not differ in search time (F1,235 = 0.03; p = 0.857).
3.4 Results Summary
Reducing or eliminating circulating estradiol significantly increased the numbers of errors made by birds in two retrieval contexts: after discrimination training within the maze (i.e., practice finding the correct cup; Exp. 1), and without discrimination training within the maze (i.e., learning had occurred but was not reinforced by practice; Exp. 2). Replacement with estradiol partially restored spatial memory function in Exp. 2. In contrast, spatial memory acquisition was not impacted by reducing or eliminating estradiol, but was impaired by estradiol replacement (Exp. 3).
4. Discussion
These studies provide support for the hypothesis that, in songbirds, estrogens impact acquisition and retrieval of spatial memory differently depending on context. In mammals, estrogens are thought to influence spatial memory through the promotion of hippocampal neurogenesis, synapse formation, and dendrite formation, (McEwen & Woolley, 1994; Ormerod, Lee, & Galea, 2003; Li et al., 2004; Wallace et al., 2006) and by rapidly modulating metabotropic glutamate receptors via membrane-bound estrogen receptors (Meitzen & Mermelstein, 2011). Nevertheless, the effects of estrogens on spatial memory are far from clear. For example, the concentration of estradiol appears crucial as dose-dependent effects of estrogens have been reported. Small doses of estradiol benzoate enhanced rat performance in a radial arm maze but performance was impaired by large doses (Holmes, Wide, & Galea, 2002). Large doses of estradiol benzoate also impaired multiple forms of rat learning and memory, including spatial memory (Galea et al., 2001). Sex differences in learning and memory (Jonasson, 2005), as well as in hippocampal steroid action, are also described (Meitzen et al., 2012). Moreover, neuroactive estrogens can be produced peripherally or locally in brain, including at some hippocampal synapses (Saldanha, Remage-Healey, & Schlinger, 2011), making it exceedingly difficult to ascertain the natural concentrations of estradiol at HP synapses encoding spatial memories.
In accord with mammalian studies (reviewed in Luine, 1997), we found that estradiol enhanced spatial memory retrieval (experiments 1 and 2) independent of the degree of discrimination training or lack thereof. In experiment 1, when birds had already learned and reached their maximal performance in the spatial task (by 2–3 days after first performing the task), blockage of further estradiol production with FAD led to a dramatic increase in the number of errors made in the maze (Fig. 3a). In experiment 2, provisioning of FAD after birds had learned the location of the food (i.e., rehearsal and location discrimination) but not yet been subjected to the behavioral test had a similar effect: blockage of estradiol led to increased error rates in the maze (Fig. 4a). In this case, replacement with estradiol significantly reduced the error rates of FAD-treated birds, although not to the level seen in control birds. This lack of complete recovery may have been due to incomplete restoration of estradiol levels. These studies add to a growing literature on estradiol effects on spatial memory in birds. Haggis (2010) provided male and female zebra finches with oral testosterone, estradiol, or testosterone in conjunction with FAD and measured performance on a task in which the hidden location of a food source had to be recalled (small flaps had to be lifted off of wells to locate the food). Both testosterone and estradiol improved memory retrieval on this test, while combining FAD with testosterone increased error rates. Using silastic implants, Oberlander et al. (2004) found that treatment with estradiol or testosterone, but not DHT, promoted spatial memory in castrated male zebra finches. Similarly, oral treatment of male and female great tits with estradiol or testosterone produced a trend towards improved memory recall in a delayed-matching-to-sample test (Hodgson et al., 2008). Our observations that spatial memory retrieval is enhanced in the presence of estradiol is in accord with these studies, although unlike Oberlander et al. (2004), we found that estradiol interfered with memory acquisition per se. Differences in experimental design (i.e., testing apparatus, duration of treatment, etc.) may account for these differences and should be further explored.
In addition to an effect on the number of errors made in the maze in experiment 1, treatment with FAD decreased the number of attempted and completed trials (Fig. 3b). This suggests that FAD led to a decrease in motivation or activity level, although we found no such effect in experiments 2 or 3. In addition, in experiment 2, birds treated with FAD as well as FAD + estradiol spent more time searching in the maze than control birds (but only on day 2). These results suggest that FAD may have created some confusion in searching for food (some birds appeared to redundantly run back-and-forth between 2 arms); the effect was not reduced by estradiol replacement. Although effective at restoring estradiol concentrations and some estrogen-dependent functions, it is possible that the peripheral implants did not fully recreate brain levels needed for complete restoration of behavior. Regardless, effects of FAD on trial completion and on search time were inconsistent between experiments, suggesting that a lack of estradiol has differential effects depending on the timing of the spatial memory task at which it is delivered.
While most research suggests that estradiol enhances spatial memory function, there is a substantial body of evidence showing that estrogens can impair this capacity, particularly within the acquisition phase. For example, estradiol or estradiol plus progesterone injections impair spatial memory formation in the Morris water maze in rats (Chesler & Juraska, 2000; Snihur, Hampson, & Cain, 2008). Conversely, estradiol implants in rats improved spatial memory acquisition in the radial arm maze (Daniel et al., 1997). In the present study (experiment 3), there was no overall learning observed over the course of the two days, an effect which was driven by an increased error rate in estradiol and FAD-treated birds over the course of two days (Fig. 5a). Interestingly, birds that received FAD alone learned the task over the course of the two days. This suggests that unlike our results showing estradiol enhancement of memory retrieval, memory acquisition was impaired in the presence of estradiol. Thus the role of estradiol in songbird spatial memory is dynamic, a process that might be predicted if estradiol is produced in a punctuated, context-dependent fashion, as has been previously found within the auditory system of the zebra finch (Remage-Healey et al., 2008, 2012a). The zebra finch HP expresses high levels of aromatase (Saldanha et al., 1998, 2004), including within synaptic terminals (Peterson et al., 2005). In the song bird auditory system, rapid, post-translational control of aromatase activity in synaptic terminals appears to control local concentrations of estradiol (Remage-Healey et al., 2008, 2011, 2012a), influencing the strength of firing of auditory neurons in response to appropriate auditory stimuli (Remage-Healey et al., 2010; Pinaud and Tremere, 2012). We are currently investigating whether estradiol concentrations within the zebra finch HP itself fluctuate down- and up- along with the estrogenic demands of spatial memory acquisition and retrieval.
The mechanisms by which estradiol both impairs acquisition and enhances spatial memory retrieval were not examined in this study, although it is well known that multiple aspects of spatial memory are controlled by the HP (Broadbent, Squire, & Clark, 2004). Behaviorally-important manipulations of estradiol that occurred over the course of hours or days might have produced transcriptional changes as intranuclear ERα and ERβ have been detected in the songbird HP (Hodgson et al., 2008). Alternatively, estradiol may have activated membrane receptors producing rapid, non-genomic actions (Meitzen & Mermelstein, 2011; Meitzen, Grove, & Mermelstein, 2012). Evidence for such membrane-dependent actions of estradiol have recently been demonstrated in the songbird auditory system (Remage-Healey et al., 2012a,b) and are well described in the mammalian HP (Metzen & Mermelstein, 2011). Moreover, aromatase-positive neurons in the songbird HP colocalize with NMDA-type glutamate receptors, suggesting pre- and/or post-synaptic actions of estrogens may influence synaptic plasticity (Saldanha et al., 2004).
The results of the present study provide significant insight into spatial learning and memory in songbirds. We provide evidence that estradiol enhances retrieval but impairs acquisition. Future studies will address whether concentrations of locally produced estrogens vary in accord with these disparate roles in the avian HP as well as the mechanisms whereby estradiol exerts its effects. Hippocampal estrogen synthesis and action may be crucial for bird species whose survival is dependent on exquisite spatial memory capabilities, such as in birds that store large quantities of food needed to survive long winters.
We examined the effect of estradiol on spatial memory function in an avian model
Estradiol enhanced spatial memory retrieval
The beneficial effect of estradiol was independent of discrimination training
Estradiol impaired spatial memory acquisition
Estradiol has different effects on spatial memory function depending on context
Acknowledgments
We wish to thank the animal care staff at the University of California Los Angeles for their assistance in maintaining the zebra finch colony, as well as John Hoang and Nicole Gomez. We would also like to thank Matthew Fuxjager and Kristy Longpre for advice on experimental design and analysis. Fränzi Korner and Mihai Valcu provided assistance with statistical analysis. This work was funded by NIH grant MH061994 to B. Schlinger.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Barha CK, Galea LAM. Influence of different estrogens on neuroplasticity and cognition in the hippocampus. Biochimica et biophysica acta. 2010;1800:1056–1067. doi: 10.1016/j.bbagen.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Bednekoff P, Balda R, Kamil A, Hile A. Long-term spatial memory in four seed-caching corvid species. Animal Behaviour. 1997;53:335–341. [Google Scholar]
- Bischof H, Lieshoff C, Watanabe S. Spatial Memory and hippocampal function in a non foodstoring songbird, the zebra finch (Taeniopygia guttata) Reviews in the Neurosciences. 2006;17:43–52. doi: 10.1515/revneuro.2006.17.1-2.43. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proceedings of the National Academy of Sciences. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler EJ, Juraska JM. Acute administration of estrogen and progesterone impairs the acquisition of the spatial morris water maze in ovariectomized rats. Hormones and Behavior. 2000;38:234–242. doi: 10.1006/hbeh.2000.1626. [DOI] [PubMed] [Google Scholar]
- Colombo M, Broadbent N. Is the avian hippocampus a functional homologue of the mammalian hippocampus? Neuroscience and Biobehavioral Reviews. 2000;24:465–484. doi: 10.1016/s0149-7634(00)00016-6. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Hormones and Behavior. 1997;32:217–225. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annual Review of Psychology. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- Galea LA, Wide JK, Paine TA, Holmes MM, Ormerod BK, Floresco SB. High levels of estradiol disrupt conditioned place preference learning, stimulus response learning and reference memory but have limited effects on working memory. Behavioural Brain Research. 2001;126:115–126. doi: 10.1016/s0166-4328(01)00255-8. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiology of Aging. 2000;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Gould KL, Ort AJ, Kamil AC. Do Clark’s nutcrackers demonstrate what-where-when memory on a cache-recovery task? Animal Cognition. 2012;15:37–44. doi: 10.1007/s10071-011-0429-y. [DOI] [PubMed] [Google Scholar]
- Haggis O. Doctoral Thesis. University of Edinburgh; 2010. The effects of sex steroids on spatial cognition in the zebra finch (Taeniopygia guttata) [Google Scholar]
- Hampton RR, Shettleworth SJ. Hippocampal lesions impair memory for location but not color in passerine birds. Behavioral Neuroscience. 1996;110:831–835. doi: 10.1037//0735-7044.110.4.831. [DOI] [PubMed] [Google Scholar]
- Hodgson ZG, Meddle SL, Christians JK, Sperry TS, Healy SD. Influence of sex steroid hormones on spatial memory in a songbird. Journal of Comparative Physiology A. 2008;194:963–969. doi: 10.1007/s00359-008-0369-4. [DOI] [PubMed] [Google Scholar]
- Holmes MM, Wide JK, Galea LAM. Low levels of estradiol facilitate, whereas high levels of estradiol impair, working memory performance on the radial arm maze. Behavioral Neuroscience. 2002;116:928–934. doi: 10.1037//0735-7044.116.5.928. [DOI] [PubMed] [Google Scholar]
- Jonasson Z. Meta-analysis of sex differences in rodent models of learning and memory: a review of behavioral and biological data. Neuroscience & Biobehavioral Reviews. 2005;28:811–825. doi: 10.1016/j.neubiorev.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Leonard S, Winsauer P. The effects of gonadal hormones on learning and memory in male mammals: A review. Current Zoology. 2011;57:543–559. [Google Scholar]
- Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos AM, et al. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proceedings of the National Academy of Sciences. 2004;101:2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London S, Remage-Healey L, Schlinger B. Neurosteroid production in the songbird brain: A re-evaluation of core principles. Frontiers in Neuroendocrinology. 2009;30:302–314. doi: 10.1016/j.yfrne.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V. Steroid Hormone Modulation of Hippocampal Dependent Spatial Memory. Stress. 1997;2:21–36. doi: 10.3109/10253899709014735. [DOI] [PubMed] [Google Scholar]
- Luine VN. Sex steroids and cognitive function. Journal of Neuroendocrinology. 2008;20:866–872. doi: 10.1111/j.1365-2826.2008.01710.x. [DOI] [PubMed] [Google Scholar]
- Mayer U, Watanabe S, Bischof H-J. Hippocampal activation of immediate early genes Zenk and c-Fos in zebra finches (Taeniopygia guttata) during learning and recall of a spatial memory task. Neurobiology of Learning and Memory. 2010;93:322–329. doi: 10.1016/j.nlm.2009.11.006. [DOI] [PubMed] [Google Scholar]
- McEwen B, Woolley C. Estradiol and progesterone regulate neuronal structure and synaptic connectivity in adult as well as developing brain. Experimental Gerontology. 1994;29:431–436. doi: 10.1016/0531-5565(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Meitzen J, Mermelstein PG. Estrogen receptors stimulate brain region specific metabotropic glutamate receptors to rapidly initiate signal transduction pathways. Journal of Chemical Neuroanatomy. 2011;42:236–41. doi: 10.1016/j.jchemneu.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Grove DD, Mermelstein PG. The organizational and aromatization hypotheses apply to rapid, nonclassical hormone action: neonatal masculinization eliminates rapid estradiol action in female hippocampal neurons. Endocrinology. 2012;153:4616–21. doi: 10.1210/en.2012-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez P, Garcia-Segura LM, Muller D. Estradiol promotes spine growth and synapse formation without affecting pre-established networks. Hippocampus. 2011;21:1263–1267. doi: 10.1002/hipo.20875. [DOI] [PubMed] [Google Scholar]
- Oberlander JG, Schlinger BA, Clayton NS, Saldanha CJ. Neural aromatization accelerates the acquisition of spatial memory via an influence on the songbird hippocampus. Hormones and Behavior. 2004;45:250–258. doi: 10.1016/j.yhbeh.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Olton DS, Collison C, Werz MA. Spatial memory and radial arm maze performance of rats. Learning and Motivation. 1977;8:289–314. [Google Scholar]
- Ormerod BK, Lee TTY, Galea LAM. Estradiol initially enhances but subsequently suppresses (via adrenal steroids) granule cell proliferation in the dentate gyrus of adult female rats. Journal of Neurobiology. 2003;55:247–260. doi: 10.1002/neu.10181. [DOI] [PubMed] [Google Scholar]
- Patel SN, Clayton NS, Krebs JR. Hippocampal tissue transplants reverse lesion-induced spatial memory deficits in zebra finches (Taeniopygia guttata) The Journal of Neuroscience. 1997;17:3861–3869. doi: 10.1523/JNEUROSCI.17-10-03861.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proceedings of the Royal Society of London B. 2005;272:2089–2096. doi: 10.1098/rspb.2005.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan A, Gabor CS, Favaro KJ, Kaschack S, Armstrong JN, Maclusky NJ, Choleris E. Low doses of 17β-estradiol rapidly improve learning and increase hippocampal dendritic spines. Neuropsychopharmacology. 2012;37:2299–2309. doi: 10.1038/npp.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinaud R, Tremere L. Control of central auditory processing by a brain-generated oestrogen. Nature Reviews Neuroscience. 2012;13:521–527. doi: 10.1038/nrn3291. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment N, Schlinger B. Forebrain steroid levels fluctuate rapidly during social interactions. Nature Neuroscience. 2008;11:1327–1334. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Coleman MJ, Oyama RK, Schlinger BA. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:3852–3857. doi: 10.1073/pnas.0906572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong S, Maidment NT, Schlinger BA. Presynaptic control of rapid estrogen fluctuations in the songbird auditory forebrain. The Journal of Neuroscience. 2011;31:10034–10038. doi: 10.1523/JNEUROSCI.0566-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong SM, Chao a, Schlinger Ba. Sex-specific, rapid neuroestrogen fluctuations and neurophysiological actions in the songbird auditory forebrain. Journal of Neurophysiology. 2012a;107:1621–1631. doi: 10.1152/jn.00749.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Joshi NR. Changing neuroestrogens within the auditory forebrain rapidly transform stimulus selectivity in a downstream sensorimotor nucleus. The Journal of Neuroscience. 2012b;32:8231–8241. doi: 10.1523/JNEUROSCI.1114-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TC, LaDage LD, Pravosudov VV. Evidence for long-term spatial memory in a parid. Animal Cognition. 2012;15:149–154. doi: 10.1007/s10071-011-0440-3. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Popper P, Micevych P, Schlinger BA. The songbird hippocampus is a site of high aromatase: inter- and intra-species comparisons. Hormones and Behavior. 1998;34:85–97. doi: 10.1006/hbeh.1998.1447. [DOI] [PubMed] [Google Scholar]
- Saldanha C, Tuerk M, Kim Y-H, Fernandes AO, Arnold AP, Schlinger BA. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. The Journal of Comparative Neurology. 2000;423:619–630. doi: 10.1002/1096-9861(20000807)423:4<619::aid-cne7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Schlinger BA, Micevych PE, Horvath TL. Presynaptic N-methyl-D-aspartate receptor expression is increased by estrogen in an aromatase-rich area of the songbird hippocampus. The Journal of Comparative Neurology. 2004;469:522–534. doi: 10.1002/cne.11035. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Remage-Healey L, Schlinger BA. Synaptocrine signaling: steroid synthesis and action at the synapse. Endocrine Reviews. 2011;32:532–49. doi: 10.1210/er.2011-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salwiczek LH, Watanabe A, Clayton NS. Ten years of research into avian models of episodic-like memory and its implications for developmental and comparative cognition. Behavioural Brain Research. 2010;215:221–234. doi: 10.1016/j.bbr.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Shen P, Schlinger BA, Campagnoni AT, Arnold AP. An atlas of aromatase mRNA expression in the zebra finch brain. The Journal of Comparative Neurology. 1995;360:172–184. doi: 10.1002/cne.903600113. [DOI] [PubMed] [Google Scholar]
- Shettleworth SJ. Spatial memory in food-storing birds. Philosophical Transactions of the Royal Society of London B. 1990;329:143–151. [Google Scholar]
- Snihur AWK, Hampson E, Cain DP. Estradiol and corticosterone independently impair spatial navigation in the Morris water maze in adult female rats. Behavioural Brain Research. 2008;187:56–66. doi: 10.1016/j.bbr.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Spence RD, Zhen Y, White S, Schlinger BA, Day LB. Recovery of motor and cognitive function after cerebellar lesions in a songbird: role of estrogens. The European Journal of Neuroscience. 2009;29:1225–1234. doi: 10.1111/j.1460-9568.2009.06685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade J, Schlinger B, Hodges L, Arnold A. Fadrozole: a potent and specific inhibitor of aromatase in the zebra finch brain. General and Comparative Endocrinology. 1994;94:53–61. doi: 10.1006/gcen.1994.1059. [DOI] [PubMed] [Google Scholar]
- Wallace M, Luine V, Arellanos A, Frankfurt M. Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Research. 2006;1126:176–182. doi: 10.1016/j.brainres.2006.07.064. [DOI] [PubMed] [Google Scholar]