Abstract
The in vivo zebrafish models have recently attracted great attention in molecular oncology to investigate multiple genetic alterations associated with the development of human cancers and validate novel anticancer drug targets. Particularly, the transparent zebrafish models can be used as a xenotransplantation system to rapidly assess the tumorigenicity and metastatic behavior of cancer stem and/or progenitor cells and their progenies. Moreover, the zebrafish models have emerged as powerful tools for an in vivo testing of novel anticancer agents and nanomaterials for counteracting tumor formation and metastases and improving the efficacy of current radiation and chemotherapeutic treatments against aggressive, metastatic and lethal cancers.
Introduction
The zebrafish (Danio rerio) as an in vivo model organism offers great promise to investigate the molecular mechanisms of diverse human diseases, including cancers, because it constitutes a simple and cost effective animal model for performing some genetic alterations and large-scale experiments with high reproducibility [1–9]. Specifically, zebrafish possess a genetic background, hematopoietic, immune, vascular, musculoskeletal and central nervous systems, and organs and tissues, such as eyes, ear, heart, gastrointestinal tract, pancreas, liver and kidney with some phenotypic features resembling a human being [1,4,5,10–18]. In addition, some studies of mutagenesis combined large-scale genetic screens in zebrafish have led to the generation of many mutant zebrafish showing phenotypic features associated with particular human diseases or predisposing to cancer development that may be exploited as powerful models of human disorders to establish the functions of specific gene products [19–22]. Different transgenic zebrafish models have also been engineered that developed neoplasms with the morphological, histological and cellular alterations comparable to human cancers [4,5,11–13,15,16,18]. Hence, the use of mutant and transgenic zebrafish models has provided important information on molecular mechanisms and functions of specific gene products involved in the development of diverse cancers and treatment resistance [4,5,8,13,14,16,18,23–26]. These cancer types include germ cell tumors, embryonal rhabdomyosarcoma (ERMS), leukemias, lymphomas, melanomas, sarcomas and diverse epithelial cancers such as liver, gastrointestinal, colon, and pancreatic, prostate and breast cancers [4,5,8,13,14,16,18,23–27].
The use of zebrafish models as a xenotransplantation platform has multiple advantages over more conventional animal models such as the use of immunodeficient mice and rat for the implantation of cancer cells, including cancer stem and/or progenitor cells with stem cell-like properties, to rapidly delineate their tumorigenic and metastatic properties and validate novel anticancer agents [6,7,9,28–32]. Particularly, zebrafish are not expensive and can be easily maintained in an aquarium with a minimal requirement of equipment and propagated in a large number due to their high rate of fecundity (100–300 embryos per week/couple) [9,33]. Moreover, the rapid ex utero development, small size and optical transparency of zebrafish embryos and larvae, which have an immature immune system until 11 day postfertilization (dpf), make zebrafish an ideal organism model for the xenotransplantation of human cells without rejection [6,7,9,26,28–32,34,35]. Additionally, zebrafish models also require only a few cancer cells for xenotransplantation assays and the optical clarity of zebrafish embryos and adult zebrafish mutants can permit the real-time observation of the formation of tumor masses and migration and metastatic spread at distant tissues of fluorescently labeled cancer cells in addition to their interactions with the tumor vasculature [6,7,9,28–32]. Of therapeutic interest, the use of fluorescently labeled cancer cells and transparent zebrafish models engineered for overexpressing the fluorescent molecules in endothelial cells can also enable users to easily assign the anticarcinogenic and/or antiangiogenic effects and systemic toxicity of tested drugs and nanomaterials in a short lapse of time [7,9,30–32,35–46]. In this matter, we review recent advances on the potential applications of transparent zebrafish embryo and adult zebrafish models in molecular oncology to investigate the specific functions of gene products and molecular pathways altered in cancer cells during the disease progression in addition to a xenotransplantation system to validate novel anticarcinogenic drugs. The emphasis is on the studies carried out with zebrafish models to investigate the malignant transforming events associated with the development of leukemias, melanomas and prostate, breast and pancreatic cancers and test new therapeutic agents for eradicating cancer cells in these cancer types including cancer- and metastasis-initiating cells with the stem cell-like properties.
Potential applications of zebrafish models in molecular oncology
Zebrafish models of human cancers
Zebrafish system represents a powerful animal model to perform in vivo studies on the regulation of the expression and biological functions of multiple gene products that have been conserved during the vertebrate evolution in addition to establish their implications in the normal and pathological embryonic development and human diseases [4,5,14–17,43,47,48]. Although the partial genome duplication in zebrafish has occurred during the evolution of teleosts and resulted in the presence of duplicated chromosome segments corresponding to at least 20% of duplicated gene pairs, some zebrafish chromosomes are mosaically orthologous to human chromosomes [49–51]. Moreover, although zebrafish do not have anatomical structures such as mammary gland and prostate, they possess several tissues, organs and glands such as cardiovascular and musculoskeletal systems, eyes, brain, liver, heart, gastrointestinal tract and pancreas with the functions comparable to mammals and develop the diseases resembling human disorders [4,5,8,11–14,16–18,23–25,52]. Thus, the availability of fully characterized genome of the zebrafish combined with the presence of well-characterized anatomical structures can enable researchers to alter specific gene products in zebrafish to establish their functions in diverse diseases and cancer development.
Different genetic approaches used for generating mutant and transgenic zebrafish lines
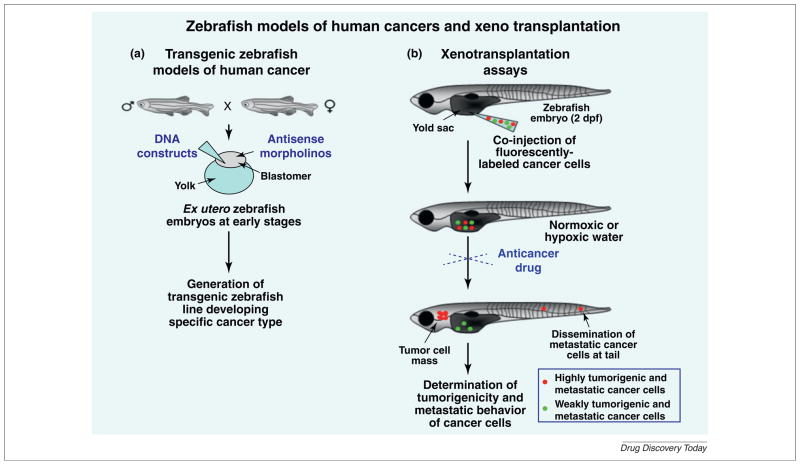
The use of distinct forward- and reverse-genetic approaches incorporating new DNA recombinant technologies, mutagenesis and genetic manipulations in whole zebrafish organism or specific organs, tissues or glands of zebrafish have led to the generation of different mutant and transgenic zebrafish models of human diseases and cancers [4,5,8,13,14,16,18,23–25,48,53]. Among reverse-genetic approaches, there is the microinjection of morpholino antisense oligonucleotides into the blastomers or yolks of early stage zebrafish embryos, which bind to endogenous mRNAs and prevent their translation through a steric interference or microRNAs (miRNA) (Fig. 1a) [40,44,54–56]. The morpholino antisense technique has been widely used to downregulate the expression of specific gene products or miRNA function during the embryonic development of zebrafish and establish their biological consequences (Fig. 1a) [40,44,54–56]. The disadvantages of morpholino technology are that it constitutes a transient method and the loss-of-function of target product is effective only in early stages of zebrafish development and depends of the dose of morpholinos used [56]. Moreover, the morpholinos may cause nonspecific side effects in zebrafish embryos and requires the use of appropriate control oligos [56].
FIGURE 1.
Different strategies used for generating transgenic zebrafish models of human cancers and performing xenotransplantation studies on the malignant behavior of cancer cells in zebrafish system. (a) Shows the strategies used to generate stable transgenic zebrafish lines by performing the microinjection of DNA construct in the early stage of zebrafish embryos followed by genotyping. The regulation of the transgene expression in a specific organ, tissue or cell type may also be performed by using different binary expression systems such as GAL:UAS or Cre/LoxP. In addition, the functions of specific gene products may also be established by injection of antisense morpholino oligonucleotides directed against RNAs encoding the products of interest at the early stage of zebrafish embryos. (b) Shows the potential strategy used for xenotransplantation assays in zebrafish embryos. The cancer cells are first stained with red- or green-fluorescent dye and injected, alone or in combination, with a glass micropipette in the yolk sac or perivitalline cavity of zebrafish embryos at 2-day postfertilization (dpf). The xenografted zebrafish embryos might be maintained at a specific temperature between 28 and 37°C under normoxic or hypoxic conditions. After two to seven days postinjection, the number of tumor cell masses derived from cancer cells and cancer cells disseminated at distant sites, including head and tail regions, can be determined by fluorescence microscopy.
Other genetic approaches to investigate the function of specific gene products in zebrafish also include the random mutagenesis and forward genetic screening or target-selected mutagenesis followed by a genetic screening and mapping for mutations in target genes of interest [20,21,57–61]. The target-selected mutagenesis in genome of zebrafish embryos has been performed with different technologies such as zing-finger nuclease-targeted mutagenesis or targeting induced local lesions in genomes (TILLING) using chemical mutagens such as N-ethyl-N-nitrosourea (ENU) [57,59,62,63]. These investigations have led to the generation of some stable mutant zebrafish lines harboring the mutations in specific tumor suppressor genes such as p53, adenomatous polyposis coli (APC), neurofibromatosis type 2 (NF2) and ribosomal protein (RP) genes [20–22,57–60]. For instance, it has been shown that a tp53M214K mutant zebrafish line harboring a mutation in the wild-type p53 DNA-binding domain spontaneously developed malignant peripheral nerve sheath tumors starting at 8.5 months with an incidence of 28% by 16.5 months [58]. In addition, several transgenic zebrafish models of cancer have also been developed by microinjection of DNA constructs in early stage zebrafish embryos and using different tissue-specific promoters and systems to achieve the spatial and temporal control of the transgene expression such as Tol2 transposon and LexPR, GAL4-UAS and Cre-LoxP binary systems (Fig. 1a) [5,8,15,17,18,26,64–66]. Hence, these mutant and transgenic zebrafish lines can be used to delineate the functions of multiple inherited or somatic genetic alterations occurring in cancer cells and their local tumor microenvironment that can cooperate to cancer initiation and progression in addition to validate novel anticancer drugs targets.
Xenotransplantation assays
Several xenotransplantation assays of well-established human cancer cell lines and primary or metastatic tumor cell suspensions from cancer patients into the zebrafish system have been carried out to establish their tumorigenicity and metastatic behavior (Fig. 1b) [6,29–32,35,40,43,55,67,68]. It has been reported that the injection of human cancer cells in zebrafish embryos at 2 dpf represents the ideal developmental stage where the adaptive immune response of zebrafish is not yet established [26,29,30,34,35]. As a result, the use of 2 dpf zebrafish embryos might promote the survival of cancer cells and tumor formation without rejection and requirement for a prior immunosuppressive treatment [26,29,30,34,35]. For instance, some studies have been carried out by microinjection of cancer cell lines, such as leukemic cells, melanoma cells and brain, breast, prostate, gastrointestinal, colon and pancreatic cancer cells into the yolk sac, abdominal perivitalline space or pericardium of 2 dpf zebrafish embryos [18,26,29,30,34,35,37,55,69]. It has been observed that the xenotransplantation of the invasive and metastatic cancer cells into 2 dpf zebrafish embryos led to the induction of angiogenesis, formation of tumor cell masses and dissemination of cancer cells at distant sites while low metastatic cancer cell lines remain at the injection site (Fig. 1b) [18,26,29,30,34,35,37,55,69]. Interestingly, the data of in vivo imaging have also indicated the potential implication of more differentiated and nonpropagating tumor cells, myogenin+ ERMS cells endowed with a highly migratory potential, in intravasation and formation of ‘pre-metastatic niches’ in fluorescent transgenic zebrafish [27]. In fact, it has been observed that poorly differentiated and tumor-propagating myf5+ ERMS cells populated newly formed tumors only after the migration of myogenin+ ERMS cells [27]. Moreover, the investigations of the functions of hypoxia for the tumor cell invasion, dissemination and metastases in zebrafish models have been performed through the microinjection of fluorescent cancer cells in the perivitelline cavity of 2 dpf Tg[fli1: enhanced green fluorescent protein (EGFP)] zebrafish embryos followed by an incubation of fishes in hypoxic water for three days [18,69]. The hypoxia-induced zebrafish tumor model has enabled users to visualize the promoting effect induced by a persistent exposure of living zebrafish embryos to hypoxia on the cancer cell invasion, metastases and tumor angiogenesis at the single-cell level as compared with normoxic conditions by fluorescent microscopy [18,69].
In addition, although zebrafish possess an immune system that matures at 4–6 wpf (week postfertilization), numerous studies have also indicated the ability of cancer cells to form tumor masses and metastasize when transplanted in immunosuppressed 21 dpf, older stage or adult zebrafish [6,29,34,40,43,68,70]. In this regard, several transparent adult zebrafish pigmentation mutants, including golden, albino, rose, panther and casper have been developed for overcoming the normal opacity of skin and presence of sub-dermal structures in adult zebrafish [6,71]. Among them, the roy−/−:nacre−/− double mutant zebrafish designated casper, which shows a complete loss of all melanocytes and iridophores in both embryogenesis and adulthood, may enable researchers to better visualize some internal organs and tissues such as heart, gastrointestinal tract, liver and gallbladder as compared to other transparent mutant zebrafishes [71]. For instance, it has been possible to visualize the engraftment at two to five hours post-transplantation and homing of green fluorescent protein (GFP)-labeled marrow population of hematopoietic stem and/or progenitor cells (HSCs and HPCs) in kidney marrow of adult casper zebrafish after irradiation with 25 Gy whereas the cancer cell engraftment was not detected in wild-type opaque zebrafish [71]. By contrast, homozygous diploid clonal zebrafish lines can also be used to perform a serial transplantation of tumor cells from one fish to another without prior sublethal γ-irradiation and rejection of the graft by the host zebrafish [3,28,72]. Interestingly, an optical platform has also been developed for a image-based visualization of the tissue microvasculature and noninvasive and dynamic tracking of fluorescent cells at stationary or circulating states in even casper zebrafish under physiological conditions overtime without the need of sacrificing animals [70].
Collectively, these observations have indicated that the transparent zebrafish embryos and adult casper zebrafish represent powerful xenotransplantation systems to rapidly establish the tumorigenicity and metastatic behavior of cancer cells. Consequently, these zebrafish models might also constitute excellent in vivo systems to establish the antitumoral, antiangiogenic and antimetastatic effects of drugs and nanomaterials, alone or in combination therapy, in a short lapse of time.
Potential applications of zebrafish models for in vivo validation of novel therapeutic agents
The transparent zebrafish embryos or adult mutant zebrafish lines are attractive models over cell culture systems and other animal models to rapidly test and validate the bioavailability, toxicity profiling and therapeutic effects including anticarcinogenic and antiangiogenic properties of new chemical bioactive agents and nanomaterials in vivo (Figs 2 and 3) [8,9,26,31,32,37,44,45,73–78]. Particularly, the small size of ex utero zebrafish embryos can enable researchers to use 96-well plates (2–5 embryos/well) or small dishes for a high-throughput screening of different bioactive substances that are soluble in water and which directly diffuse into embryos (Fig. 2) [8,9,31,32,37,38,46,74–76,79]. Importantly, the results from a comparative analysis of the drug toxicity induced by 18 different chemical compounds in zebrafish embryos and mammals have also indicated that the lethal concentration (LC50) of drugs was comparable between two in vivo systems [80,81]. Moreover, several investigations have revealed the possibility to determine the protective effect or toxicity of various drugs and nanomaterials on internal organs of zebrafish embryos during their development [45,80–82]. These data suggest that zebrafish embryos may constitute potential in vivo models to rapidly establish the pharmacological profile and toxicity of anticancer agents in preclinical studies. Moreover, several studies aiming to rapidly estimate the therapeutic efficacy of small chemical molecules have been made by performing an incubation of transparent or mutant zebrafish embryos in water containing drugs as well as by treating cancer cells with the drugs before their implantation into zebrafish embryos or adult casper zebrafish line (Fig. 2) [8,31,32,37–39,46,74–76,79,83]. These investigations have led to the identification of new pharmacological compounds acting as anticancer, radio sensitizing and antiangiogenic agents that were effective, alone or in combination therapy, at decreasing the number of fluorescent cancer cells transplanted in zebrafish embryos compared with vehicle-treated cancer cells or vehicle-treated zebrafish used as controls [26,31,32,37–39,42,46,55,74–76,79,83–86]. For instance, it has been reported that a treatment of mifepristone-inducible K-RasV12 transgenic zebrafish larva starting at 3–3.5 dpf with specific inhibitors of Raf/mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) and phosphatidylinosol 3′-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathways, including PD98059 plus LY294002 or rapamycin, was effective at inhibiting the hyperplastic growth of livertumors in 78–96% of larvae at 7 dpf as compared with untreated K-RasV12 transgenic zebrafish [8].
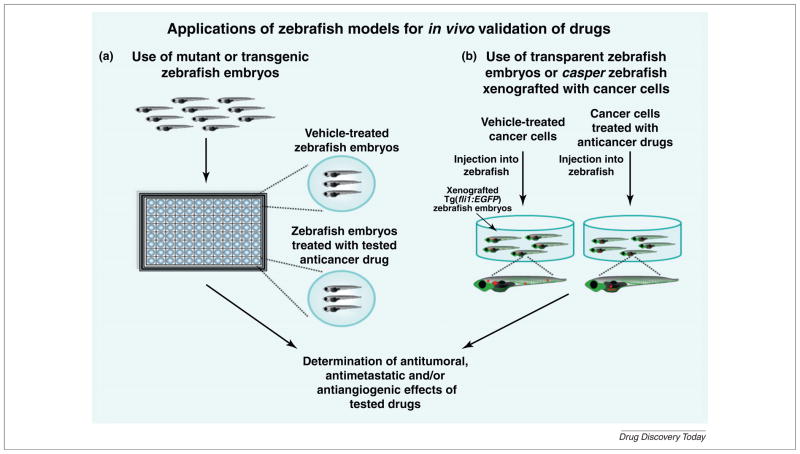
FIGURE 2.
Potential applications of in vivo zebrafish models for anticancer drug screening and validation. (a) Shows the experimental strategies for performing in vivo high-throughput screening of novel bioactive molecules by treating the mutant or transgenic zebrafish embryos with tested anticancer drugs versus vehicle used as a control. At the end of the incubation period, the visual analyses of zebrafish system can be performed to establish the therapeutic effects of tested drugs. (b) Shows the strategies for in vivo testing of anticarcinogenic effects of drugs or nanomaterials on cancer cells xenografted into wild-type or transgenic zebrafish embryos or adult casper zebrafish line. The cancer cells can be treated with tested agents before their injection into embryos or alternatively the zebrafish embryos can be treated after the microinjection of cancer cells with anticancer agents that directly diffuse into embryos or by injection of drugs into circulatory system of zebrafish. The zebrafish embryos can be incubated in water at a desired temperature between 28 and 37°C under normoxic or hypoxic conditions. At the end of the incubation period, the analyses of the antitumoral and antimetastatic effects induced by tested drugs or nanomaterials on the tumor formation and metastatic spread of fluorescent cancer cells in zebrafish models can be estimated by fluorescence microscopy. More specifically, the simultaneous analyses of the anticarcinogenic and antiangiogenic properties of tested agents on malignant behavior of cancer cells and intratumoral vessels induced by implanted cancer cells may be determined using Tg(fli1:EGFP) zebrafish embryos expressing enhanced green fluorescent protein (EGFP) in all vascular system of zebrafish body.
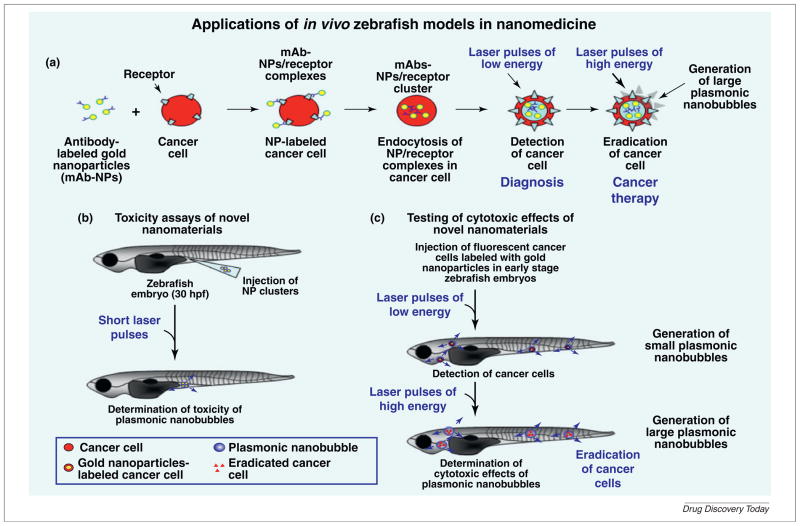
FIGURE 3.
Potential applications of in vivo zebrafish models for investigating toxicity and anticancer effects of novel nanomaterials. (a) Shows the specific interaction of monoclonal antibody (mAb)–gold nanoparticle (NP) complexes with the receptor molecules present at the cancer cell surface followed by their cellular internalization by endocytosis and formation of intracellular clusters between mAb–NPs. Moreover, the effects induced by laser pulses of low or high energy on mAb–NP clusters formed within cancer cells and which may result in the generation of small or large nanobubbles that are cytotoxic to cancer cells respectively are also illustrated. Hence, this nanotechnology which can be used to detect or eradicate cancer cells offers great promise for optimizing the diagnosis and therapeutic treatment of cancer patients. (b) Shows the potential strategies used to estimate the toxicity of nanomaterials including gold NP after their microinjection into the zebrafish embryos and irradiation with a short laser pulses and (c) theranostic effects induced by mAb–NP clusters within cancer cells xenografted into zebrafish embryos after their irradiation with laser pulses of low or high energy.
In addition, a novel compound designated as eIF4E inhibitor-1 (4Ei-1) has also been observed to impair the epithelial–mesenchymal transition (EMT) program in a dose-dependent manner in a zebrafish ectoderm explant model of the EMT process by negatively regulating the association of eIF4E with mRNA cap during the translation initiation step [87]. These data support the interest to use this zebrafish explant model of the EMT process to identify and validate new anticancer drugs that are able to inhibit the molecular events associated with the EMT process without major toxicity on normal cells. In this matter, it has been reported that the current clinical drug cisplatin induced the EMT program in human tumor cells from primary advanced stage ovarian carcinomas and ascites of ovarian cancer patients in addition to epithelial ovarian cancer OVCA 433 cell line [46]. The induction of the EMT program by cisplatin was associated with an increased expression of some stem cell-like markers such as CD44, CD133, CD117, epithelial cell adhesion molecule (EpCAM), 2α-integrin, Nanog and Oct-4 in these ovarian cancer cell types [46]. It has also been observed that cisplatin-treated OVCA 433 cells injected into the yolk sac of 2 dpf zebrafish embryos displayed an enhanced migration at the tail region as compared with untreated cancer cells which could be abrogated by a cotreatment of transplanted cells with a selective MEK inhibitor U0126 [46]. In the same way, a new radiosensitizer, 4′-bromo-3′-nitropropiophenone (NS-123), has also been shown to improve the antitumoral effects induced by ionizing radiation on tumor xenografts derived from human glioma U251 cells implanted into zebrafish embryos or mice [39]. It has also been noted that NS-123 had no adverse effect on the normal development or lethality of irradiated zebrafish embryos and irradiated mice [39]. Importantly, it has also been observed that the inhibitory effect induced by ionizing radiation on tumors formed by fluorescent U251 human glioma cells transplanted into early stage zebrafish embryos was potentiated in the presence of current chemotherapeutic drug temozolomide whereas temozolomide alone had no discernible adverse effects on the embryonic development [83].
Together these studies support the interest to use in vivo zebrafish models of human cancers to validate novel drug targets for improving the current radiation therapies and chemotherapeutic treatments against aggressive, metastatic and lethal cancers. In this context, we are reporting here in a more detailed manner the potential applications of zebrafish models to investigate leukemias, melanomas and distinct epithelial cancers such as prostate, breast, and pancreatic cancers.
Leukemias
In spite of the fact that zebrafish have no bone marrow, it has been shown the zebrafish kidney marrow constitutes the principal anatomical site of hematopoiesis where all of major blood cell types are produced during late stages of embryogenesis and in adult zebrafish while the maturation of some hematopoietic cells can also occur in the spleen and thymus [10]. Importantly, it has also been observed that the hematopoietic tissue in zebrafish kidney displays a high conservation of the spatio-temporal expression patterns of the genes involved in the regulation of self-renewal of HSCs and HPCs and their maturation into different lymphoid and myeloid cell lineages with human bone marrow [10]. Consequently, zebrafish constitutes an attractive model to study the events controlling normal hematopoiesis and genetic and cellular alterations occurring in immature hematopoietic cell lineages that are associated with leukemogenesis [12,13,54].
Some investigations have revealed that acute and chronic lymphoblastic and/or lymphoid leukemias (ALLs and CLLs) or myeloblastic and/or myeloid leukemias (AMLs and CMLs) may derive from different mutations and/or genetic rearrangements in specific genes leading to a clonal expansion of primitive HSCs and/or HPCs, also designated as long-lived preleukemic cells, into leukemic cells in zebrafish models as observed in human leukemias [12,41,88–90]. More specifically, diverse chromosomal translocations generating oncogenic fusion proteins, which frequently occur during human leukemogenesis in HSCs and HPCs, have been shown to cause important perturbations in the development of normal hematopoietic cell lineages and leukemogenesis in zebrafish [12,41,89,90]. For instance, both ETS transcription factor TEL (also known as ETV6) and acute myeloid leukemia 1 (AML1 also known as RUNX1), which encodes the AML1 transcription factor acting as a master regulator of hematopoietic cell differentiation, are commonly involved in chromosomal rearrangements observed in human leukemogenesis [88–91]. It has also been observed that the expression of TEL-AML1 fusion protein in all lineages, including noncommitted hematopoietic cells in the zebrafish led to a clonal expansion of HPCs endowed with a high self-renewal capacity that did not differentiate to mature and functional B cell lineage [12]. These molecular events culminated in an accumulation of lymphoblastic blasts in the bloodstream and B cell lineage ALL development in 3% of the transgenic zebrafish after a long latency of 8–12 months [12]. Of therapeutic interest, a high-throughput chemical screening of 2000 bioactive molecules acting as modifiers of AML1-ETO-mediated hematopoietic dysregulation has also been performed on 12–16 hpf Tg(hsp:AML1-ETO) zebrafish embryos by treating five embryos into each well of the 96-well plate with different drugs (Fig. 2a) [74]. This study has led to the identification of small molecules, including nimesulide, that antagonized the AML1-ETO fusion oncoprotein-induced dysregulated hematopoietic differentiation of HPCs through the induction of prostaglandin E2 and β-catenin [74].
By contrast, T cell-ALLs (T-ALLs) comprise distinct subtypes of malignancies principally occurring in childhood and rarely in adults that are associated with the accumulation of different genetic alterations in immature T cell precursors resulting in an arrest of their differentiation at a specific developmental stage and malignant transformation [92,93]. Importantly, the data from array comparative genomic hybridization analyses of genetic changes detected in human T-ALLs and those contributing to disease relapse relative to zebrafish T-ALL samples have revealed that zebrafish and human T-ALLs have several genetic similarities that govern their initiation and progression [13]. Different zebrafish models of T-ALLs have also been developed by overexpressing Myc in immature T cells of zebrafish driven by the zebrafish rag2 promoter [3,14,15,38,48,72]. For instance, the conditional lymphoid expression of EGFP-mMyc driven by the zebrafish rag2 promoter by using the Cre recombinase enzyme/loxP system has been reported to result in the development of T-ALLs in 100% of the injected double transgenic zebrafish that pathologically resembled to human T-ALLs [15]. Moreover, the expression of rag2:myr-mAkt2 transgene encoding a constitutively active form of Akt2 in Myc-ER-negative zebrafish has also been observed to result in the T-ALL development in 17% of zebrafish by 20 weeks of age and accelerate the onset of Myc-induced T-ALL in rag2:Myc-ER transgenic zebrafish [48]. Of particular interest, the serial and single-cell transplantation assays of fluorescent leukemic cells from Myc-induced T-ALLs in Tg(rag2-mMyc) zebrafish into syngeneic recipient zebrafish have also been performed without the need for an immune suppression of engrafted animals [3,72]. It has been shown that the population of self-renewing leukemia-initiating cells are abundant in Tg(rag2-mMyc) zebrafish model of T-ALLs and comprise 0.1–15.9% of the total leukemic cell mass [3]. Of therapeutic interest, it has also been observed that a treatment with cyclophosphamide or vincristine of T-ALL tumor-bearing zebrafish larvae induced by mosaic expression of zRag2-EGFP-mMyc transgene and began on day 5 after tumor engraftment improved the lifespan of zebrafish larvae as compared with untreated larvae [38].
In addition, the xenotransplantation assays of leukemic cells into zebrafish embryos have also been used to establish the anticarcinogenic effects of different drugs (Fig. 2b) [37]. For instance, it has been observed that the microinjection of human K562 or Jurkat leukemic cells in the circulatory system of 2 dpf zebrafish embryos had no toxicity on normal zebrafish embryo development [37]. Importantly, the treatment of the zebrafish embryos xenografted with K562 leukemic cells established from a CML patient in the blastic phase with the clinical drug, imatinib mesylate that acts as a specific inhibitor of oncogenic BCR–ABL fusion protein significantly reduced the number of fluorescent leukemic cells detected in the circulation of zebrafish embryos as compared with untreated animals [37]. In the same way, the treatment of the zebrafish embryos xenografted with Jurkat leukemic cells established from a T-ALL with the clinical drugs such as oxaphorines, mafosfamide or cyclophosphamide was also accompanied by a decrease of the number of fluorescent leukemic cells detected in zebrafish embryos relative to untreated animals [37]. It has however been noticed that all-trans retinoic acid (ATRA) and a small molecule inhibitor of the translation initiating complex eIF4F designated as 4EGI-1 induced the teratogenic effects at effective concentrations on zebrafish embryos which precluded the potential investigation of their antileukemic activity in this in vivo vertebrate model [37].
Together these data obtained with transgenic zebrafish models of leukemias have provided additional information on the implications of specific genetic alterations for the malignant transformation of immature hematopoietic cells and leukemogenesis. The similarity noticed between the leukemias developed by transgenic zebrafish relative to human diseases support the interest to further use these zebrafish models for identifying novel molecular targets and validating novel antileukemic drugs to optimize current antileukemic therapies.
Melanomas
Cutaneous melanoma is the most aggressive and lethal malignancy among skin cancers with a poor median survival of patients varying from 6 to 12 months and a five-year survival rate of less than 10% [94–96]. Although localized melanomas are usually curable by surgical tumor resection, the rapid progression to invasive and metastatic disease states that are resistant to current therapeutic treatments typically culminates in the death of the patients [94,96,97]. Current therapeutic options for patients diagnosed with locally advanced, invasive and metastatic melanomas such as radiation therapy, chemotherapy with alkylating agent, dacarbazine or its orally active analog temozolomide and/or stem cell-based therapies are only palliative and generally result in the disease relapse [98]. The inefficacy of currently available treatments for patients diagnosed with locally advanced melanomas underlines the urgent need to validate novel molecular targets for counteracting the rapid progression of skin-confined melanomas to metastatic and lethal disease states.
Several investigations using zebrafish models relevant to human melanogenesis have provided important information on the molecular transforming events occurring in the melanocytic lineage during disease progression and treatment resistance [4,29,71]. For instance, it has been observed that the microinjection of mitfa-B-RafV600E construct in which the expression of constitutively active B-RafV600E mutant is under the control of the melanocyte mitfa promoter into wild-type zebrafish embryos at the single-cell stage resulted in the formation of highly pigmented melanocyte patches termed fish (f)-nevi in the epidermis that did not progress to the melanoma development [4]. By contrast, the expression of B-RafV600E mutant in p53−/− mutant zebrafish line led to the formation of melanocytic lesions that rapidly progressed to invasive tumors resembling to human melanomas at 4–12 months of age and which could be serially transplanted [4,79]. Moreover, a comparative analysis of gene profiles of Tg(mitfa:B-RafV600E); p53−/− zebrafish embryos with Tg(mitfa:B-RafV600E);p53−/− melanomas has uncovered a signature of 123 overlapping genes enriched for markers of multipotent crestin+ neural crest progenitor cells [79]. These data suggest that immature melanocytic cells derived from neural crest lineages can be maintained through embryogenesis and expanded during melanogenesis in this zebrafish model. Hence, these observations indicate that the activated B-RafV600E mutant may cooperate with the downregulation of p53 tumor suppressor protein for the expansion of melanoma cells with neural crest stem and/or progenitor cell-like phenotypes in Tg(mitfa:B-RafV600E);p53−/− zebrafish model as observed in certain human melanoma subtypes. Of therapeutic interest, a chemical genetic screening for suppressors of neural crest progenitor expansion in Tg(mitfa:B-RafV600E);p53−/− zebrafish embryos has also led to the identification of small molecules, including inhibitors of dihydroorotate dehydrogenase such as leflunomide, which almost completely abrogate the neural crest development in Tg(mitfa:B-RafV600E);p53−/− zebrafish embryos [79]. Leflunomide, alone or in combination with a B-RafV600E inhibitor termed PLX4720, was also effective at inhibiting the melanoma growth in a mouse xenograft model [79].
The results from xenotransplantation assays of different metastatic melanoma cell lines such as human WM-266-4 and murine B16 and highly metastatic B16-F10 cells into the yolk sac or perivitelline space of zebrafish embryos have also indicated that the transplanted melanoma cells formed cancer cell masses and disseminated at distant sites including head and tail regions (Fig. 1b) [29,31,32]. For example, a study has been performed by the microinjection of 20–30 red fluorescence-labeled melanoma B16 and B16-F10 cells into 2 dpf Tg(flk1:EGFP) zebrafish embryos within the perivitelline space, which is enriched in vascular capillaries and blood supply [32]. The results have revealed that the melanoma cells firstly formed the aggregates within one day followed by the neoangiogenic sprouts within tumors from the pre-existent host-dilated vessels at day 3 post-injection and obvious tumor mass at day 6 after the cell injection [32]. It has also been shown that the blockade of angiogenic sprouts within tumors formed by B16 melanoma cells by an incubation of zebrafish embryos with a specific vascular endothelial growth factor receptor-2 (VEGFR-2) inhibitor, SU5416 significantly inhibited the tumor growth [31,32]. In the same way, the transplantation through the ventral peritoneum or intraventricular injection of melanoma cell suspensions obtained from tumors formed in stable Tg(mitf:B-Raf);p53−/− or Tg(mitf:N-Ras-GFP);p53−/− adult zebrafish into irradiated casper recipients has also enabled users to visualize the tumor formation, proliferation and dissemination at distant sites including skin of fluorescent melanoma cells in adulthood [71].
Altogether, these studies suggest the implication of the malignant transformation of immature cells of melanocytic cell lineage and the crucial function of vascular remodeling for the formation of tumor cell masses and metastases of melanoma cells in zebrafish models as observed in other animal models and human melanomas. Therefore, zebrafish models might constitute as useful tools to further investigate the molecular mechanisms of melanogenesis, angiogenesis, metastases and treatment resistance and validate novel combination therapies targeting the total mass of melanoma cells and tumor vasculature.
Prostate cancer
Although significant improvement in the early diagnosis had led to a higher rate of curative treatments of patients with organ-confined prostate cancers (PCs) by radical prostatectomy and/or radiation therapy, many patients still progress to locally advanced, androgen-independent and metastatic states [96,99–102]. The first-line standard chemotherapeutic treatment for the patients with metastatic castration-resistant PCs consisting to use docetaxel combined with the anti-inflammatory drug pre-dnisone, is only palliative and typically results in disease relapse and the death of patients within 9–12 months after treatment initiation [96,99,103,104]. In this regard, accumulating lines of evidence suggest that PC stem and/or progenitor cells, also designated as PC- and metastasis-initiating cells with a high self-renewal ability and tumorigenic potential, might provide crucial roles for PC progression, treatment resistance and disease relapse due to their intrinsic and/or acquired resistance to current antihormonal and chemotherapeutic treatments [105–107]. Consequently, this underlines the therapeutic interest to identify new therapeutic targets in PC- and metastasis-initiating cells for eradicating the total PC cell mass and improving current clinical treatments. Importantly, some recent studies have indicated that the polycomb group protein designated as B-cell-specific MMLV insertion site-1 (BMI-1), which is overexpressed in PC-initiating cells, might provide crucial functions for their high self-renewal potential, proliferation and survival [108,109]. In this matter, it has been reported that specific inhibitors of BMI-1, alone and in synergy with docetaxel, were effective at inhibiting the growth of PC-initiating cells from primary human prostate tumor or DU-145 cells xenografted into zebrafish embryos [109].
Among new methods that can be used for the diagnosis and therapy of cancers including PCs, the development of novel nanoparticle (NP)-based approaches that selectively target cancer cells has given promising results in animal models including zebrafish models (Fig. 3) [45,82]. For instance, the studies have been undertaken to establish the potential toxicity and theranostic applications of the plasmonic gold nanobubbles using in vivo zebrafish system (Fig. 3) [45,82]. These investigations have been performed using gold NPs consisting of gold spheres conjugated with C225 monoclonal antibody (mAb) molecules directed against epidermal growth factor receptor (EGFR) for the cellular up-take of gold NPs by endocytosis in cancer cells and metastatic and androgen-independent PC C4-2B cells labeled with Dil fluorescent dye for PC cell tracking (Fig. 3) [45,82]. It has been shown that the subcutaneous injection of gold NP clusters into the flank at the end of the yolk extension of zebrafish embryos at 30 hpf followed by their irradiation with a short laser pulses resulted in the generation of plasmonic nanobubbles without major detrimental effects on the viability of zebrafish embryos at 9 dpf (Fig. 3a) [45]. Moreover, it has been observed that metastatic PC C4-2B cells labeled with these gold NPs could be detected after their xenotransplantation into zebrafish embryos after a first red probe laser pulse of low energy generating small plasmonic nanobubbles (Fig. 3b) [82]. Importantly, the generation of large plasmonic gold nanobubbles through a second laser pulse of high energy under optical guidance in C4-2B cells xenotransplanted in zebrafish embryos was also effective at selectively eradicating these PC cells without detrimental effects on normal embryonic cells (Fig. 3b) [82]. These data support the potential use of zebrafish embryos as an in vivo animal model to rapidly test the systemic toxicity and theranostic property of novel nanomaterials to develop novel nanotechnologies for improving noninvasive diagnosis and effective treatment for cancer patients.
In addition, transgenic zebrafish models with fluorescent vasculature have also been used to first test the antiangiogenic effects of novel chemical compounds which have subsequently been shown to inhibit the tumor growth of PC cells implanted in mouse models [36,44,76]. For instance, a chemical genetic screen of some small molecules in developing Tg(flk1:EGFP) zebrafish embryos had led to the identification of different chemical compounds, including isorotenone, dihydromunduletone, aristolochic acid, simvastatin, mevastatin, lovastatin and rosuvastatin that inhibited the growth of zebrafish intersegmental vessels [76]. Among them, rosuvastatin has subsequently been shown to induce the antiangiogenic and growth inhibitory effects on human primary PCC-1 cell line xenografted in nonobese diabetic severe combined immunodeficiency (NOD-SCID) mice [76]. In the same way, it has also been reported that the downregulation of the expression level of a cytoskeletal protein designated as kindlin-2 by injection of morpholino antisense oligonucleotides in Tg(flk1:EGFP);Tg(gata-1:dsred) fluorescent zebrafish embryos at the one to two cell stage caused important defects in angiogenesis process during the embryonic development relative to control morpholino oligos [44]. The partial inactivation of kindlin-2 in kindlin-2+/− mice was also effective at inhibiting the angiogenesis and tumor growth derived from RM1 murine PC cells subcutaneously implanted in mouse models [44]. The antiangiogenic and growth inhibitory effects induced by downregulating kindlin-2 in mice was mediated in part through an inhibition of vascular endothelial growth factor (VEGF) signaling pathway and αVβ3-integrin-dependent migration and adhesion of endothelial cells that resulted in an impairment of the formation of new intratumoral blood vessel [44]. Furthermore, it has also been observed that the overexpression of insulin-like growth factor-binding protein-3 (IGFBP-3) or non-IGF-binding GGG-IGFBP-3 mutant in Tg(flk1:GFP) fluorescent zebrafish embryos at one-cell stage inhibited the vascular patterning [36]. A treatment of human PC 22RV-1 cells xenografted in SCID mice with IGFBP-3 was also effective at suppressing the angiogenesis and tumor growth [36]. These observations support the potential interest to use rosuvastatin, kindlin-2 inhibitor or IGFBP-3 as adjuvant antiangiogenic agents in the treatment of PC patients.
Collectively, these data have indicated that the zebrafish models might be useful to perform xenotransplantation of PC cells, including PC stem and/or progenitor cells, to establish their malignant behavior. Moreover, the transparent zebrafish models also offer the possibility to rapidly assess antitumor, antimetastatic and antiangiogenic effects of novel drugs and nanomaterials to develop novel targeted therapies for improving current treatments against androgen-independent, metastatic and lethal PCs.
Breast cancer
Breast cancer comprises a heterogeneous group of malignancies characterized by different cellular origins and accumulation of specific genetic alterations occurring in basal and/or luminal breast epithelial cells that led to their malignant transformation and development of distinct molecular subtypes of breast cancer associated with different outcomes of patients [110,111]. Consequently, the choice of clinical treatments of breast cancer patients may vary with the histological features, molecular gene signature and aggressive and metastatic propensity of diagnosed breast cancer subtypes [110–113]. Current therapeutic treatments such as tumor surgical resection, radiation therapy and chemotherapy, alone or in combination with hormone therapy and targeted therapy directed against ErbB2/Her2, might be effective for treating certain patients with localized breast cancers [112,113]. Nevertheless, the progression to locally invasive and metastatic states usually results in disease relapse and the death of breast cancer patients [96,112,113]. Therefore, additional studies are necessary to further delineate the molecular mechanisms associated with aggressive and metastatic behavior of breast cancer cells and optimize the therapeutic options for patients.
Some xenotransplantation studies of breast cancer cells into 2 dpf zebrafish embryos have led to the establishment of molecular events and gene products that may be associated with their tumorigenic and metastatic behavior [7,15,30,6]. It has been observed that highly aggressive breast cancer MDA-MB-435 and MDA-MB-231 cells injected into 2 dpf transparent zebrafish embryos displayed a higher rate of formation of tumor cell masses and vessel density and dissemination at the head and tail relative to breast cancer BT-474 cells endowed with a low metastatic potential (Fig. 1b) [6,7,30,114,115]. More specifically, the visualization of fluorescently labeled MDA-MB-435 cells after their microinjection into 2 dpf Tg(fli1:EGFP) zebrafish embryos has indicated that these cancer cells entered into the blood circulation, homed in small vessels in the head and tail regions including intersegmental vessels and extravasated into surrounding tissues [30]. It has also been reported that the active extravasation process of MDA-MB-435 cells in Tg(fli1:EGFP) zebrafish embryos involved a local vessel remodeling, β1-integrin-mediated adhesion of tumor cells to blood vessel walls and intravascular and transendothelial migration of cancer cells [30]. The extravasation of MDA-MB-435 cells was also accompanied by the formation of membrane protrusions which was promoted by overexpressing pro-metastatic genes, such as twist in xenografted tumor cells [30]. In the same way, the microinjection of MDA-MB-435 cells engineered to overexpress Ras homolog gene family member C (RhoC) and VEGF into the peritoneal cavity of a one-month-old transparent Tg(fli1:EGFP) zebrafish has also revealed that VEGF secreted by MDA-MB-435 cells induced a classic angiogenic response characterized by the formation of novel tortuous and disorganized vascular network that could be reversed by a treatment with VEGFR-2 inhibitor SU5416 [115]. Moreover, it has also been noted that RhoC might contribute to the intravasation of MDA-MB-435 cells by inducing the formation of membrane protrusions that enable cancer cells to penetrate the VEGF-induced vascular opening [115]. Interestingly, a study has also used zebrafish to establish the metastatic properties of fluorescent Ha-Ras-transformed EpH4 mammary epithelial cells (EpRas) stimulated by transforming growth factor-β (TGF-β) injected into the yolk sac of Tg(fli1:EGFP) zebrafish embryos at 2 dpf [6]. The results have indicated that the induction of the EMT program in EpRas cells by TGF-β was accompanied by their metastatic spread at distant tissues in zebrafish embryos whereas unstimulated EpRas cells used as control remained into the yolk sac [6]. These observations support the interest to target twist, β1-integrin, VEGF/VEGFR-2, RhoC and/or TGF-β signaling elements to prevent the metastatic spread of breast cancer cells.
In addition, the xenotransplantation assays using 2 dpf zebrafish embryos have also been performed to rapidly establish the tumorigenicity and metastatic potential of breast cancer stem and/or progenitor cells endowed with a high self-renewal ability as well as to test the efficacy of novel anticancer drugs [7,35,42]. It has been observed that the microinjection of fluorescent single-cell suspensions from BT-474 breast cancer cell-derived mammo-spheres into the yolk sac of 2 dpf zebrafish embryos resulted in the formation of cancer cell masses at 5 dpf and the migration of cancer cells at the tail of the embryos at 9 dpf with a significantly higher frequency than single cells from parental BT-474 breast cancer cell monolayer [7]. Moreover, a treatment of BT-474 cells with curcumin before their injection in zebrafish embryos has been observed to reduce their efficacy to form tumor masses and disseminate at the tail suggesting that this dietary agent can target breast cancer-initiating cells [7]. Also, the targeting of ATP-gated P2X7 receptors (P2X7R), which are plasma membrane ion channels overexpressed on MDA-MB-435s breast cancer cells, by a treatment with a specific P2X7R antagonist A-438079 significantly reduced the invasion of red fluorescent MDA-MB-435s cells from the yolk sac and their dissemination at the tail region of zebrafish embryos compared with vehicle-treated MDA-MB-435s cells [116]. In the same way, the targeted delivery of PI3K inhibitor-loaded biodegradable NPs targeting PI3K/Akt pathways was also effective at inhibiting MDA-MB-231 cell-induced endothelial cell proliferation and tumor formation in a zebrafish tumor xenotransplant model supporting the potential use of this nanomaterial as a novel antiangiogenic strategy [35].
On the basis of these observations, it appears that the zebrafish models can be used to rapidly establish the signaling elements that may cooperate to the neoangiogenesis, tumor formation and metastases of different breast cancer cells, including breast cancer stem and/or progenitor cells, and validate novel anticancer and antiangiogenic agents for treating breast cancer patients.
Pancreatic cancer
Pancreatic ductal adenocarcinoma (PDAC) is the fourth most common cause of cancer-related mortality among men and women with a poor one-year survival rate of only 24% and a five-year survival rate of less than 5–6% [96,117,118]. This poor prognosis of pancreatic cancer patients is due in part to the difficult to diagnose PDACs in its early stages, rapid regional spread and metastases at distant tissues and intrinsic and acquired resistance of pancreatic cancer cells to current radiotherapies and gemcitabine-based chemotherapies [118–121].
PDAC initiation and progression is generally accompanied by an accumulation of some genetic alterations in pancreatic cancer cells such as the mutations in the K-Ras oncogene in up to 75–90% cases of PDACs and changes in their local microenvironment including an intense fibrotic reaction or desmoplasia [118,122–124]. Importantly, it has been shown that the wild-type zebrafish possess a functional pancreas with an endocrine tissue and exocrine compartment containing pancreatic duct and exocrine cells that is formed at 52 hpf during embryogenesis [11]. Moreover, recent studies of genetic analyses of exocrine pancreatic development and targeted expression of transgenes in zebrafish models have also provided additional information about the crucial implication of specific developmental pathways and oncogenic products during pancreatic fibrosis and PDAC development [123,125–128]. For instance, the transgenic zebrafish lines have been engineered by usingGal4:UAS binary system for overexpressing either the zebrafish orthologous transgene of human Indian or Sonic hedgehog ligand (IHHa or SHH) under the control of pancreas transcription factor-1a (ptf1a) promoter, which drives the expression of transgene in exocrine pancreatic progenitor cells in developing pancreas of zebrafish embryos [17]. It has been observed that the expression of IHHa or SHH hedgehog ligand in transgenic zebrafish embryos detected with GFP induced a progressive pancreatic fibrosis concomitant with the formation of desmoplastic lesions and proliferating ductular structures that did not progress to tumor development but which was accompanied by a destruction of the acinar structures in older transgenic zebrafish lines [17]. Of therapeutic interest, it has also been reported that the effects of IHHa or SHH ligand on the embryonic development of transgenic zebrafish and pancreatic fibrosis were prevented by the injection of a hedgehog primary inhibitor-4 (HPI-4) that counteracted the ciliogenesis and hedgehog activation [17]. Additional studies are however required to establish the potential cooperative interactions between the activation of the hedgehog cascade and other oncogenic events in novel compound transgenic zebrafish models of PDAC. In this regard, it has also been shown that the targeted expression of oncogenic K-RasG12V mutant transgene under the control of zebrafish ptf1a promoter into one-cell stage zebrafish embryos resulted in a block of the differentiation of exocrine pancreatic progenitor cells [5]. The persistence of undifferentiated and proliferative pancreatic progenitor cells in adult Tg(ptf1a:eGFP-K-RasG12V) zebrafish pancreas subsequently culminated in the formation of detectable but heterogeneous pancreatic tumor subtypes in almost half of transgenic zebrafish at nine months old age [5]. Some pancreatic tumors in Tg(ptf1a:eGFP-K-RasG12V) zebrafish exhibited the evidence of tumor cell invasion of the surrounding organs such as liver and gut as well as spinal and ovarian metastases as compared with Tg(ptf1a:eGFP) zebrafish used as control [5]. The pancreatic tumor subtype with a predominant ductal differentiation detected in Tg(ptf1a:eGFP-K-RasG12V) zebrafish also showed several phenotypic features in common with human PDACs [5]. The phenotypic features of ductal pancreatic tumors formed in these transgenic zebrafish include a dramatic stromal expansion, expression of cytokeratins and intracytoplasmic and intraluminal mucins in invasive glandular structures and activation of ERK, Akt and hedgehog signaling elements in tumor epithelium as compared with a normal pancreas [5]. Moreover, it has also been observed that the tumors formed in Tg(ptf1a:eGFP-K-RasG12V) zebrafish model of PDAC exhibited a nearly complete loss of expression of miRNA-143 and miRNA-145 which act as the tumor suppressors relative to normal zebrafish pancreas [127].
In addition, the transplantation of primary gastrointestinal human tumors, including fluorescently labeled small tumor explants or dissociated tumor cells from pancreas, colon or stomach carcinoma into the yolk sac of zebrafish embryos followed by immunofluorescence microscopy analyses of live transparent zebrafish has also indicated that the tumor cells displayed an invasive and metastatic behavior [6]. Especially, the transplanted tumor cells displayed a tendency to invade, home and form the micro-metastases in organs of the gastrointestinal tract, including liver, gut and intestine, which was not observed after transplantation of nonmalignant tissue explants [6]. For instance, it has been shown that the coimplantation of green fluorescent PaTu 8988S and red fluorescent PaTu 8988T pancreatic cancer cells expressing high and low levels of E-cadherin, respectively in the yolk sac of 2 dpf zebrafish embryos resulted in the invasion and metastases of PaTu 8988T cells whereas PaTu 8988S cells remain in the yolk sac [6]. By contrast, PaTu 8988T cells did not metastasize when injected into homozygous cloche mutant zebrafish embryos, which lack a functional vasculature, suggesting the crucial function of vascular system for the metastatic spread of pancreatic cancer cells in the zebrafish model as noticed in human PDACs [6].
The analyses of the dissemination of fluorescent pancreatic cancer cells xenografted into the yolk sac of zebrafish embryos have also enabled researchers to perceive the implications of specific genes in the metastatic spread of cancer cells to near and distant tissues of the injection site [40,43,68]. For instance, it has been shown that the silencing of metastasis-associated protein anterior gradient 2 or lysosomal protease such as cathepsin B or cathepsin D in fluorescent PaTu 8988S pancreatic cancer cells significantly decreased the number of disseminated PaTu 8988S cells detected at the tail of the zebrafish embryos at 24 hours postinjection [68]. The downregulation of miRNA-10a expression in PaTu-8988T pancreatic cancer cells by using morpholino anti-sense oligonucleotides or retinoic acid receptor-α antagonist Ro-41-5253 followed by their injection into the yolk sac of 2 dpf zebrafish embryos was also effective at decreasing their invasion and dissemination relative to the control morpholino oligos and untreated PaTu-8988T cells [40]. In the same way, it has also been observed that the silencing of two LIM serine/threonine protein kinases (LIMKs), LIMK1 and LIMK2, which are involved in the cell cycle regulation, in pancreatic cancer cells by small interference RNAs also resulted in a complete inhibition of their invasion and formation of micrometastases after their injection into zebrafish embryos [43].
Together these studies support great interest to use transgenic zebrafish models to further explore multiple molecular events occurring in exocrine pancreatic cells during the development of chronic pancreatitis and PDACs. Also, zebrafish models may constitute powerful in vivo xenotransplantation systems to rapidly validate the anticarcinogenic effects versus systemic toxicity of different classes of drugs for improving the efficacy of current gemcitabine-based chemotherapies against highly aggressive and lethal PDACs.
Concluding remarks
Taken together, these recent innovative investigations in oncology by using different zebrafish models of human cancers have provided important and complementary information to other animal models on the transforming events occurring in cancer cells and their local microenvironment that might contribute to human carcinogenesis, metastases and treatment resistance. These observations support great interest to develop novel compound transgenic zebrafish models and xenotransplantation assays to further define multiple genetic alterations that accumulate cancer cells and which may cooperate for cancer progression to invasive and metastatic disease states. The therapy studies in transparent zebrafish models have also led to novel anticancer drugs and nanomaterials that offer great promise to optimize new multitargeted strategies to eradicate the total cancer cell mass. Future investigations are however required to further establish the antitumoral and antimetastatic effects versus systemic toxicity of different classes of agents, alone or in combination with the current radiation therapies and chemotherapeutic drugs, in different zebrafish models relevant to human carcinogenesis. Hence, these future studies with different zebrafish models of human cancers and metastases should lead to the development of novel combination therapies that could be exploited for improving the treatments of the patients diagnosed with aggressive, metastatic and lethal cancers.
Acknowledgments
The authors on this work are supported by the grants from the National Institutes of Health (RO1 CA138791, U54 CA163120 and EDRN UO1 CA111294) and the U.S. Department of Defense (PC074289).
Biographies
Murielle Mimeault, M.Sc, Ph.D., has over 16 years of the experience in molecular pharmacology, cellular biology, cancer biology and targeted therapy. She has contributed to numerous scientific articles on the validation of novel biomarkers and combination therapies targeting EGFR and hedgehog cascades in cancer cells. She is currently working at the Department of Biochemistry and Molecular Biology in the College of Medicine at the University of Nebraska Medical Center on the validation of novel drug targets in cancer stem/progenitor cells and their progenies for improving the efficacy of current clinical treatments against aggressive, metastatic and lethal cancers. Her recent research interests focus on altered metabolic pathways and hypoxia.
Surinder K. Batra, M.Sc, Ph.D., is currently a Helen Freytag Professor of Cancer Biology and Chairman of the Department of Biochemistry and Molecular Biology in the College of Medicine and Associate Director of Education and Training at the Eppley Cancer Institute at the University of Nebraska Medical Center, Omaha, Nebraska USA. He is working on the development of therapeutics and diagnostics for various cancers from his early training at Duke University Medical Center and North Carolina State University, NC, USA. He has authored over 275 publications and patented several targets for cancer diagnosis and therapy. Currently, he is working on understanding tumor microenvironment (TMEN) that is hostile for therapy against lethal pancreatic cancer. His program is developing new spontaneous cancer models for understanding of TMEN and evaluation of targeted radionuclide therapy and vaccine.
Footnotes
Conflicts of interest
The authors disclose no potential conflicts of interest.
References
- 1.Barros TP, et al. Zebrafish: an emerging technology for in vivo pharmacological assessment to identify potential safety liabilities in early drug discovery. Br J Pharmacol. 2008;154:1400–1413. doi: 10.1038/bjp.2008.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keller ET, Murtha JM. The use of mature zebrafish (Danio rerio) as a model for human aging and disease. Comp Biochem Physiol C: Toxicol Pharmacol. 2004;138:335–341. doi: 10.1016/j.cca.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Smith AC, et al. High-throughput cell transplantation establishes that tumor-initiating cells are abundant in zebrafish T-cell acute lymphoblastic leukemia. Blood. 2010;115:3296–3303. doi: 10.1182/blood-2009-10-246488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patton EE, et al. BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr Biol. 2005;15:249–254. doi: 10.1016/j.cub.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 5.Park SW, et al. Oncogenic KRAS induces progenitor cell expansion and malignant transformation in zebrafish exocrine pancreas. Gastroenterology. 2008;134:2080–2090. doi: 10.1053/j.gastro.2008.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marques IJ, et al. Metastatic behaviour of primary human tumours in a zebrafish xenotransplantation model. BMC Cancer. 2009;9:128. doi: 10.1186/1471-2407-9-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eguiara A, et al. Xenografts in zebrafish embryos as a rapid functional assay for breast cancer stem-like cell identification. Cell Cycle. 2011;10:3751–3757. doi: 10.4161/cc.10.21.17921. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen AT, et al. An inducible krasV12 transgenic zebrafish model for liver tumorigenesis and chemical drug screening. Dis Model Mech. 2012;5:63–72. doi: 10.1242/dmm.008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zon LI, Peterson RT. In vivo drug discovery in the zebrafish. Nat Rev Drug Discov. 2005;4:35–44. doi: 10.1038/nrd1606. [DOI] [PubMed] [Google Scholar]
- 10.Gerlach GF, et al. Dissection of the adult zebrafish kidney. J Vis Exp. 2011;29:2839. doi: 10.3791/2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ober EA, et al. From endoderm formation to liver and pancreas development in zebrafish. Mech Dev. 2003;120:5–18. doi: 10.1016/s0925-4773(02)00327-1. [DOI] [PubMed] [Google Scholar]
- 12.Sabaawy HE, et al. TEL-AML1 transgenic zebrafish model of precursor B cell acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 2006;103:15166–15171. doi: 10.1073/pnas.0603349103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudner LA, et al. Shared acquired genomic changes in zebrafish and human T-ALL. Oncogene. 2011;30:4289–4296. doi: 10.1038/onc.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langenau DM, et al. Myc-induced T cell leukemia in transgenic zebrafish. Science. 2003;299:887–890. doi: 10.1126/science.1080280. [DOI] [PubMed] [Google Scholar]
- 15.Langenau DM, et al. Cre/lox-regulated transgenic zebrafish model with conditional myc-induced T cell acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 2005;102:6068–6073. doi: 10.1073/pnas.0408708102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langenau DM, et al. Effects of RAS on the genesis of embryonal rhabdomyosarcoma. Genes Dev. 2007;21:1382–1395. doi: 10.1101/gad.1545007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung IH, et al. Aberrant hedgehog ligands induce progressive pancreatic fibrosis by paracrine activation of myofibroblasts and ductular cells in transgenic zebrafish. PLoS ONE. 2011;6:E27941. doi: 10.1371/journal.pone.0027941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SL, et al. Hypoxia-induced pathological angiogenesis mediates tumor cell dissemination, invasion, and metastasis in a zebrafish tumor model. Proc Natl Acad Sci U S A. 2009;106:19485–19490. doi: 10.1073/pnas.0909228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patton EE, Zon LI. The art and design of genetic screens: zebrafish. Nat Rev Genet. 2001;2:956–966. doi: 10.1038/35103567. [DOI] [PubMed] [Google Scholar]
- 20.Wiellette E, et al. Combined haploid and insertional mutation screen in the zebrafish. Genesis. 2004;40:231–240. doi: 10.1002/gene.20090. [DOI] [PubMed] [Google Scholar]
- 21.Amsterdam A, et al. Zebrafish Hagoromo mutants up-regulate fgf8 postembryonically and develop neuroblastoma. Mol Cancer Res. 2009;7:841–850. doi: 10.1158/1541-7786.MCR-08-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai K, et al. Many ribosomal protein mutations are associated with growth impairment and tumor predisposition in zebrafish. Dev Dyn. 2009;238:76–85. doi: 10.1002/dvdy.21815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z, et al. Inducible and repressable oncogene-addicted hepatocellular carcinoma in Tet-on xmrk transgenic zebrafish. J Hepatol. 2012;56:419–425. doi: 10.1016/j.jhep.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 24.Neumann JC, et al. Zebrafish models of germ cell tumor. Methods Cell Biol. 2011;105:3–24. doi: 10.1016/B978-0-12-381320-6.00001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gutierrez A, et al. Aberrant AKT activation drives well-differentiated liposarcoma. Proc Natl Acad Sci U S A. 2011;108:16386–16391. doi: 10.1073/pnas.1106127108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu NA, et al. Targeting zebrafish and murine pituitary corticotroph tumors with a cyclin-dependent kinase (CDK) inhibitor. Proc Natl Acad Sci U S A. 2011;108:8414–8419. doi: 10.1073/pnas.1018091108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ignatius MS, et al. In vivo imaging of tumor-propagating cells, regional tumor heterogeneity, and dynamic cell movements in embryonal rhabdomyosarcoma. Cancer Cell. 2012;21:680–693. doi: 10.1016/j.ccr.2012.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mizgireuv IV, Revskoy SY. Transplantable tumor lines generated in clonal zebrafish. Cancer Res. 2006;66:3120–3125. doi: 10.1158/0008-5472.CAN-05-3800. [DOI] [PubMed] [Google Scholar]
- 29.Haldi M, et al. Human melanoma cells transplanted into zebrafish proliferate, migrate, produce melanin, form masses and stimulate angiogenesis in zebrafish. Angiogenesis. 2006;9:139–151. doi: 10.1007/s10456-006-9040-2. [DOI] [PubMed] [Google Scholar]
- 30.Stoletov K, et al. Visualizing extravasation dynamics of metastatic tumor cells. J Cell Sci. 2010;123:2332–2341. doi: 10.1242/jcs.069443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao C, et al. A novel xenograft model in zebrafish for high-resolution investigating dynamics of neovascularization in tumors. PLoS ONE. 2011;6:E21768. doi: 10.1371/journal.pone.0021768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao C, et al. Distinct contributions of angiogenesis and vascular co-option during the initiation of primary microtumors and micrometastases. Carcinogenesis. 2011;32:1143–1150. doi: 10.1093/carcin/bgr076. [DOI] [PubMed] [Google Scholar]
- 33.Kim S, et al. Modular, easy-to-assemble, low-cost zebrafish facility. Zebrafish. 2009;6:269–274. doi: 10.1089/zeb.2009.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lam SH, et al. Development and maturation of the immune system in zebrafish, Danio rerio: a gene expression profiling, in situ hybridization and immunological study. Dev Comp Immunol. 2004;28:9–28. doi: 10.1016/s0145-305x(03)00103-4. [DOI] [PubMed] [Google Scholar]
- 35.Harfouche R, et al. Nanoparticle-mediated targeting of phosphatidylinositol-3-kinase signaling inhibits angiogenesis. Angiogenesis. 2009;12:325–338. doi: 10.1007/s10456-009-9154-4. [DOI] [PubMed] [Google Scholar]
- 36.Liu B, et al. Insulin-like growth factor-binding protein-3 inhibition of prostate cancer growth involves suppression of angiogenesis. Oncogene. 2007;26:1811–1819. doi: 10.1038/sj.onc.1209977. [DOI] [PubMed] [Google Scholar]
- 37.Pruvot B, et al. Leukemic cell xenograft in zebrafish embryo for investigating drug efficacy. Haematologica. 2011;96:612–616. doi: 10.3324/haematol.2010.031401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mizgirev IV, Revskoy S. A new zebrafish model for experimental leukemia therapy. Cancer Biol Ther. 2010;9:895–902. doi: 10.4161/cbt.9.11.11667. [DOI] [PubMed] [Google Scholar]
- 39.Lally BE, et al. Identification and biological evaluation of a novel and potent small molecule radiation sensitizer via an unbiased screen of a chemical library. Cancer Res. 2007;67:8791–8799. doi: 10.1158/0008-5472.CAN-07-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiss FU, et al. Retinoic acid receptor antagonists inhibit miR-10a expression and block metastatic behavior of pancreatic cancer. Gastroenterology. 2009;137:2136–2145. doi: 10.1053/j.gastro.2009.08.065. [DOI] [PubMed] [Google Scholar]
- 41.Forrester AM, et al. NUP98-HOXA9-transgenic zebrafish develop a myeloproliferative neoplasm and provide new insight into mechanisms of myeloid leukaemogenesis. Br J Haematol. 2011;155:167–181. doi: 10.1111/j.1365-2141.2011.08810.x. [DOI] [PubMed] [Google Scholar]
- 42.Vollmer LL, et al. A simplified synthesis of novel dictyostatin analogues with in vitro activity against epothilone B-resistant cells and antiangiogenic activity in zebrafish embryos. Mol Cancer Ther. 2011;10:994–1006. doi: 10.1158/1535-7163.MCT-10-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vlecken DH, Bagowski CP. LIMK1 and LIMK2 are important for metastatic behavior and tumor cell-induced angiogenesis of pancreatic cancer cells. Zebrafish. 2009;6:433–439. doi: 10.1089/zeb.2009.0602. [DOI] [PubMed] [Google Scholar]
- 44.Pluskota E, et al. The integrin coactivator kindlin-2 plays a critical role in angiogenesis in mice and zebrafish. Blood. 2011;117:4978–4987. doi: 10.1182/blood-2010-11-321182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lukianova-Hleb EY, et al. Generation and detection of plasmonic nanobubbles in zebrafish. Nanotechnology. 2010;21:225102. doi: 10.1088/0957-4484/21/22/225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Latifi A, et al. Cisplatin treatment of primary and metastatic epithelial ovarian carcinomas generates residual cells with mesenchymal stem cell-like profile. J Cell Biochem. 2011;112:2850–2864. doi: 10.1002/jcb.23199. [DOI] [PubMed] [Google Scholar]
- 47.Shepard JL, et al. A mutation in separase causes genome instability and increased susceptibility to epithelial cancer. Genes Dev. 2007;21:55–59. doi: 10.1101/gad.1470407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gutierrez A, et al. Pten mediates Myc oncogene dependence in a conditional zebrafish model of T cell acute lymphoblastic leukemia. J Exp Med. 2011;208:1595–1603. doi: 10.1084/jem.20101691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Postlethwait JH, et al. Zebrafish comparative genomics and the origins of vertebrate chromosomes. Genome Res. 2000;10:1890–1902. doi: 10.1101/gr.164800. [DOI] [PubMed] [Google Scholar]
- 50.Woods IG, et al. A comparative map of the zebrafish genome. Genome Res. 2000;10:1903–1914. doi: 10.1101/gr.10.12.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Menuet A, et al. Molecular characterization of three estrogen receptor forms in zebrafish: binding characteristics, transactivation properties, and tissue distributions. Biol Reprod. 2002;66:1881–1892. doi: 10.1095/biolreprod66.6.1881. [DOI] [PubMed] [Google Scholar]
- 52.Stuckenholz C, et al. FACS-assisted microarray profiling implicates novel genes and pathways in zebrafish gastrointestinal tract development. Gastroenterology. 2009;137:1321–1332. doi: 10.1053/j.gastro.2009.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lawson ND, Weinstein BM. Arteries and veins: making a difference with zebrafish. Nat Rev Genet. 2002;3:674–682. doi: 10.1038/nrg888. [DOI] [PubMed] [Google Scholar]
- 54.Fu CT, et al. An evolutionarily conserved PTEN-C/EBPalpha-CTNNA1 axis controls myeloid development and transformation. Blood. 2010;115:4715–4724. doi: 10.1182/blood-2009-11-255778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nicoli S, et al. Mammalian tumor xenografts induce neovascularization in zebrafish embryos. Cancer Res. 2007;67:2927–2931. doi: 10.1158/0008-5472.CAN-06-4268. [DOI] [PubMed] [Google Scholar]
- 56.Heasman J. Morpholino oligos: making sense of antisense? Dev Biol. 2002;243:209–214. doi: 10.1006/dbio.2001.0565. [DOI] [PubMed] [Google Scholar]
- 57.Feitsma H, Cuppen E. Zebrafish as a cancer model. Mol Cancer Res. 2008;6:685–694. doi: 10.1158/1541-7786.MCR-07-2167. [DOI] [PubMed] [Google Scholar]
- 58.Berghmans S, et al. tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc Natl Acad Sci U S A. 2005;102:407–412. doi: 10.1073/pnas.0406252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haramis AP, et al. Adenomatous polyposis coli-deficient zebrafish are susceptible to digestive tract neoplasia. EMB Rep. 2006;7:444–449. doi: 10.1038/sj.embor.7400638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amsterdam A, et al. Many ribosomal protein genes are cancer genes in zebrafish. PLoS Biol. 2004;2:E139. doi: 10.1371/journal.pbio.0020139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin J, et al. Progenitor expansion in apc mutants is mediated by Jak/Stat signaling. BMC Dev Biol. 2011;11:73. doi: 10.1186/1471-213X-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doyon Y, et al. Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat Biotechnol. 2008;26:702–708. doi: 10.1038/nbt1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meng X, et al. Targeted gene inactivation in zebrafish using engineered zinc-finger nucleases. Nat Biotechnol. 2008;26:695–701. doi: 10.1038/nbt1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thummel R, et al. Cre-mediated site-specific recombination in zebrafish embryos. Dev Dyn. 2005;233:1366–1377. doi: 10.1002/dvdy.20475. [DOI] [PubMed] [Google Scholar]
- 65.Le X, et al. Heat shock-inducible Cre/Lox approaches to induce diverse types of tumors and hyperplasia in transgenic zebrafish. Proc Natl Acad Sci U S A. 2007;104:9410–9415. doi: 10.1073/pnas.0611302104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feng H, et al. Heat-shock induction of T-cell lymphoma/leukaemia in conditional Cre/lox-regulated transgenic zebrafish. Br J Haematol. 2007;138:169–175. doi: 10.1111/j.1365-2141.2007.06625.x. [DOI] [PubMed] [Google Scholar]
- 67.Lee LM, et al. The fate of human malignant melanoma cells transplanted into zebrafish embryos: assessment of migration and cell division in the absence of tumor formation. Dev Dyn. 2005;233:1560–1570. doi: 10.1002/dvdy.20471. [DOI] [PubMed] [Google Scholar]
- 68.Dumartin L, et al. AGR2 is a novel surface antigen that promotes the dissemination of pancreatic cancer cells through regulation of cathepsins B and D. Cancer Res. 2011;71:7091–7102. doi: 10.1158/0008-5472.CAN-11-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rouhi P, et al. Hypoxia-induced metastasis model in embryonic zebrafish. Nat Protoc. 2010;5:1911–1918. doi: 10.1038/nprot.2010.150. [DOI] [PubMed] [Google Scholar]
- 70.Zhang L, et al. An optical platform for cell tracking in adult zebrafish. Cytometry A. 2012;81:176–182. doi: 10.1002/cyto.a.21167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.White RM, et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell. 2008;2:183–189. doi: 10.1016/j.stem.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blackburn JS, et al. Quantifying the frequency of tumor-propagating cells using limiting dilution cell transplantation in syngeneic zebrafish. J Vis Exp. 2011;53:E2790. doi: 10.3791/2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Benyumov AO, et al. Anti-proliferative and anti-leukemic activity of DDE46 (compound WHI-07), a novel bromomethoxylated arylphosphate derivative of zidovudine, and related compounds Studies using human acute lymphoblastic leukemia cells and the zebrafish model. Arzneimittelforschung. 2005;55:114–122. doi: 10.1055/s-0031-1296832. [DOI] [PubMed] [Google Scholar]
- 74.Yeh JR, et al. Discovering chemical modifiers of oncogene-regulated hematopoietic differentiation. Nat Chem Biol. 2009;5:236–243. doi: 10.1038/nchembio.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murphy EA, et al. Disruption of angiogenesis and tumor growth with an orally active drug that stabilizes the inactive state of PDGFRbeta/B-RAF. Proc Natl Acad Sci U S A. 2010;107:4299–4304. doi: 10.1073/pnas.0909299107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang C, et al. Rosuvastatin, identified from a zebrafish chemical genetic screen for antiangiogenic compounds, suppresses the growth of prostate cancer. Eur Urol. 2010;58:418–426. doi: 10.1016/j.eururo.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 77.Sachidanandan C, et al. Identification of a novel retinoid by small molecule screening with zebrafish embryos. PLoS ONE. 2008;3:E1947. doi: 10.1371/journal.pone.0001947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bar-Ilan O, et al. Toxicity assessments of multisized gold and silver nanoparticles in zebrafish embryos. Small. 2009;5:1897–1910. doi: 10.1002/smll.200801716. [DOI] [PubMed] [Google Scholar]
- 79.White RM, et al. DHODH modulates transcriptional elongation in the neural crest and melanoma. Nature. 2011;471:518–522. doi: 10.1038/nature09882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang C, et al. Current Protocols in Toxicology. 1.7. John Wiley & Sons; New York, NY, U.S.A: 2003. Zebrafish: an animal model for toxicological studies; pp. 1–18. [DOI] [PubMed] [Google Scholar]
- 81.Kari G, et al. Zebrafish: an emerging model system for human disease and drug discovery. Clin Pharmacol Ther. 2007;82:70–80. doi: 10.1038/sj.clpt.6100223. [DOI] [PubMed] [Google Scholar]
- 82.Wagner DS, et al. The in vivo performance of plasmonic nanobubbles as cell theranostic agents in zebrafish hosting prostate cancer xenografts. Biomaterials. 2010;31:7567–7574. doi: 10.1016/j.biomaterials.2010.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Geiger GA, et al. Temozolomide-mediated radiosensitization of human glioma cells in a zebrafish embryonic system. Cancer Res. 2008;68:3396–3404. doi: 10.1158/0008-5472.CAN-07-6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Daroczi B, et al. In vivo radioprotection by the fullerene nanoparticle DF-1 as assessed in a zebrafish model. Clin Cancer Res. 2006;12:7086–7091. doi: 10.1158/1078-0432.CCR-06-0514. [DOI] [PubMed] [Google Scholar]
- 85.Daroczi B, et al. Nuclear factor kappaB inhibitors alleviate and the proteasome inhibitor PS-341 exacerbates radiation toxicity in zebrafish embryos. Mol Cancer Ther. 2009;8:2625–2634. doi: 10.1158/1535-7163.MCT-09-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Head M, Jameson MB. The development of the tumor vascular-disrupting agent ASA404 (vadimezan DMXAA): current status and future opportunities. Expert Opin Investig Drugs. 2010;19:295–304. doi: 10.1517/13543780903540214. [DOI] [PubMed] [Google Scholar]
- 87.Ghosh B, et al. Nontoxic chemical interdiction of the epithelial-to-mesenchymal transition by targeting cap-dependent translation. ACS Chem Biol. 2009;4:367–377. doi: 10.1021/cb9000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pui CH, et al. Acute lymphoblastic leukemia. N Engl J Med. 2004;350:1535–1548. doi: 10.1056/NEJMra023001. [DOI] [PubMed] [Google Scholar]
- 89.Kalev-Zylinska ML, et al. Runx1 is required for zebrafish blood and vessel development and expression of a human RUNX1-CBF2T1 transgene advances a model for studies of leukemogenesis. Development. 2002;129:2015–2030. doi: 10.1242/dev.129.8.2015. [DOI] [PubMed] [Google Scholar]
- 90.Onnebo SM, et al. Hematopoietic perturbation in zebrafish expressing a tel-jak2a fusion. Exp Hematol. 2005;33:182–188. doi: 10.1016/j.exphem.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 91.Lutterbach B, Hiebert SW. Role of the transcription factor AML-1 in acute leukemia and hematopoietic differentiation. Gene. 2000;245:223–235. doi: 10.1016/s0378-1119(00)00014-7. [DOI] [PubMed] [Google Scholar]
- 92.Goldberg JM, et al. Childhood T-cell acute lymphoblastic leukemia: the Dana-Farber Cancer Institute acute lymphoblastic leukemia consortium experience. J Clin Oncol. 2003;21:3616–3622. doi: 10.1200/JCO.2003.10.116. [DOI] [PubMed] [Google Scholar]
- 93.Bassan R, et al. Adult acute lymphoblastic leukaemia. Crit Rev Oncol Hematol. 2004;50:223–261. doi: 10.1016/j.critrevonc.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 94.Tsao H, et al. Management of cutaneous melanoma. N Engl J Med. 2004;351:998–1012. doi: 10.1056/NEJMra041245. [DOI] [PubMed] [Google Scholar]
- 95.Garbe C, Leiter U. Melanoma epidemiology and trends. Clin Dermatol. 2009;27:3–9. doi: 10.1016/j.clindermatol.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 96.Siegel R, et al. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 97.Francken AB, et al. Follow-up in patients with localised primary cutaneous melanoma. Lancet Oncol. 2005;6:608–621. doi: 10.1016/S1470-2045(05)70283-7. [DOI] [PubMed] [Google Scholar]
- 98.Tawbi HA, Buch SC. Chemotherapy resistance abrogation in metastatic melanoma. Clin Adv Hematol Oncol. 2010;8:259–266. [PubMed] [Google Scholar]
- 99.Tannock IF, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 100.Petrylak DP, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 101.Mimeault M, Batra SK. Recent advances on multiple tumorigenic cascades involved in prostatic cancer progression and targeting therapies. Carcinogenesis. 2006;27:1–22. doi: 10.1093/carcin/bgi229. [DOI] [PubMed] [Google Scholar]
- 102.Haas GP, et al. The worldwide epidemiology of prostate cancer: perspectives from autopsy studies. Can J Urol. 2008;15:3866–3871. [PMC free article] [PubMed] [Google Scholar]
- 103.Mimeault M, Batra SK. Frequent gene products and molecular pathways altered in prostate cancer- and metastasis-initiating cells and novel promising multitargeted therapies. Mol Med. 2011;17:649–664. doi: 10.2119/molmed.2011.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Freedland SJ. Screening, risk assessment, and the approach to therapy in patients with prostate cancer. Cancer. 2010;117:1123–1135. doi: 10.1002/cncr.25477. [DOI] [PubMed] [Google Scholar]
- 105.Mimeault M, Batra SK. Animal models of prostate carcinogenesis underlining the critical implication of prostatic stem progenitor cells. Biochim Biophys Acta. 2011;1816:25–37. doi: 10.1016/j.bbcan.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mimeault M, et al. Pathobiological implications of the expression of EGFR, pAkt, NF-kB and MIC-1 in prostate cancer stem cells and their progenies. PLoS ONE. 2012;7:E31919. doi: 10.1371/journal.pone.0031919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mimeault M, et al. Cytotoxic effects induced by docetaxel, gefitinib, and cyclopamine on side population and non-side population cell fractions from human invasive prostate cancer cells. Mol Cancer Ther. 2010;9:617–630. doi: 10.1158/1535-7163.MCT-09-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lukacs RU, et al. Bmi-1 is a crucial regulator of prostate stem cell self-renewal and malignant transformation. Cell Stem Cell. 2010;7:682–693. doi: 10.1016/j.stem.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bansal N, et al. Targeting Bmi1 in human prostate tumor initiating cells. AACR Meeting Abstracts; 2010. p. 4279. [Google Scholar]
- 110.Sorlie T, et al. Distinct molecular mechanisms underlying clinically relevant subtypes of breast cancer: gene expression analyses across three different platforms. BMC Genomics. 2006;7:127. doi: 10.1186/1471-2164-7-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guedj M, et al. A refined molecular taxonomy of breast cancer. Oncogene. 2011;31:1196–1206. doi: 10.1038/onc.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Arteaga CL, et al. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol. 2012;9:16–32. doi: 10.1038/nrclinonc.2011.177. [DOI] [PubMed] [Google Scholar]
- 113.Punglia RS, et al. Local therapy and survival in breast cancer. N Engl J Med. 2007;356:2399–2405. doi: 10.1056/NEJMra065241. [DOI] [PubMed] [Google Scholar]
- 114.Stoletov K, et al. High-resolution imaging of the dynamic tumor cell vascular interface in transparent zebrafish. Proc Natl Acad Sci U S A. 2007;104:17406–17411. doi: 10.1073/pnas.0703446104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stoletov K, Klemke R. Catch of the day: zebrafish as a human cancer model. Oncogene. 2008;27:4509–4520. doi: 10.1038/onc.2008.95. [DOI] [PubMed] [Google Scholar]
- 116.Jelassi B, et al. P2X(7) receptor activation enhances SK3 channels- and cystein cathepsin-dependent cancer cells invasiveness. Oncogene. 2011;30:2108–2122. doi: 10.1038/onc.2010.593. [DOI] [PubMed] [Google Scholar]
- 117.Brand RE, et al. Advances in counselling and surveillance of patients at risk for pancreatic cancer. Gut. 2007;56:1460–1469. doi: 10.1136/gut.2006.108456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 119.Di Marco M, et al. Metastatic pancreatic cancer: is gemcitabine still the best standard treatment? Oncol Rep. 2010;23:1183–1192. doi: 10.3892/or_00000749. (review) [DOI] [PubMed] [Google Scholar]
- 120.Mimeault M, et al. Recent advances on the molecular mechanisms involved in pancreatic cancer progression and therapies. Pancreas. 2005;31:301–316. doi: 10.1097/01.mpa.0000175893.04660.1b. [DOI] [PubMed] [Google Scholar]
- 121.Mimeault M, Batra SK. Recent progress on normal and malignant pancreatic stem/progenitor cell research: therapeutic implications for the treatment of type 1 or 2 diabetes mellitus and aggressive pancreatic cancer. Gut. 2008;57:1456–1468. doi: 10.1136/gut.2008.150052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kamisawa T, et al. Long-term follow-up of chronic pancreatitis patients with K-ras mutation in the pancreatic juice. Hepatogastroenterology. 2011;58:174–176. [PubMed] [Google Scholar]
- 123.Ji Z, et al. Oncogenic KRAS activates hedgehog signaling pathway in pancreatic cancer cells. J Biol Chem. 2007;282:14048–14055. doi: 10.1074/jbc.M611089200. [DOI] [PubMed] [Google Scholar]
- 124.Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol. 2008;3:157–188. doi: 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yee NS, et al. Transient receptor potential ion channel Trpm7 regulates exocrine pancreatic epithelial proliferation by Mg2+-sensitive Socs3a signaling in development and cancer. Dis Model Mech. 2011;4:240–254. doi: 10.1242/dmm.004564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhou W, et al. Histone deacetylase 1 is required for exocrine pancreatic epithelial proliferation in development and cancer. Cancer Biol Ther. 2011;11:659–670. doi: 10.4161/cbt.11.7.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kent OA, et al. Repression of the miR-143/145 cluster by oncogenic Ras initiates a tumor-promoting feed-forward pathway. Genes Dev. 2010;24:2754–2759. doi: 10.1101/gad.1950610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Prasad NB, et al. Gene expression profiles in pancreatic intraepithelial neoplasia reflect the effects of Hedgehog signaling on pancreatic ductal epithelial cells. Cancer Res. 2005;65:1619–1626. doi: 10.1158/0008-5472.CAN-04-1413. [DOI] [PubMed] [Google Scholar]