Abstract
Cdh1 is a regulatory subunit of the Anaphase Promoting Complex/Cyclosome (APC/C), a ubiquitin E3 ligase known to be involved in regulating cell cycle progression. Recent studies have demonstrated a role for Cdh1 in neurons during developmental and adult synaptic plasticity, as well as memory. In order to better characterize the contribution of Cdh1 in synaptic plasticity and memory, we generated conditional knockout mice using a neuron-specific enolase (Nse) promoter where Cdh1 was eliminated in neurons from the onset of differentiation. Although we detected impaired long-term potentiation (LTP) in hippocampal slices from the Nse-Cdh1 knockout (KO) mice, performance on several hippocampus-dependent memory tasks remained intact. However, the Nse-Cdh1 KO mice exhibited impaired behavioral flexibility and extinction of previously consolidated memories. These findings suggest a role for Cdh1 in regulating the updating of consolidated memories.
1. Introduction
The ubiquitin proteasome system (UPS) is an evolutionarily conserved cellular mechanism used to degrade proteins and has been implicated in a variety of cellular processes including synaptic plasticity and memory (Karpova 2006; Fonseca et al. 2006; Lee et al. 2008; Lopez-Salon et al. 2001). Individual E3 ligases, the enzymes that confer specificity to the UPS, have also been shown to mediate synaptic plasticity and memory (Li et al. 2008; Yao et al. 2011; Jiang et al. 1998; Kuczera et al. 2011). The Anaphase Promoting Complex/Cyclosome (APC/C) is a multi-subunit RING finger E3 ligase that has been well characterized for its role in cell cycle regulation, but is also emerging as an important component of the molecular mechanisms underlying long-lasting synaptic plasticity and long-term memory (Harper et al. 2002).
Cdh1 is one of the adaptor subunits that regulates APC/C activity. It has been detected in post-mitotic neurons (Gieffers et al. 1999), leading to observations that it plays a role in developmental plasticity, adult synaptic plasticity, and memory. Loss of function studies in cultured cerebellar cells, Drosophila, and C. elegans have demonstrated that Cdh1 plays a role in the constitutive, developmental growth of axons and synapses (Konishi et al. 2004; van Roessel et al. 2004; Juo and Kaplan 2004). Cdh1 also has been implicated in activity-dependent changes in neuronal function, including homeostatic plasticity (Fu et al. 2011), long-term potentiation (LTP), and associative fear conditioning (Li et al. 2008). Previous studies to explore the crosstalk between the developmental and adult contribution of Cdh1 to synaptic plasticity and memory were conducted using constitutive Cdh1 heterozygous knockout (KO) mice, which exhibited impairments in LTP and associative fear memory in adult mice (Li et al. 2008). In order to focus on the contribution of l neuronal Cdh1 on LTP and memory during development, we developed a conditional KO (cKO) strain of mice using the cre-lox system to determine how restricting neuronal Cdh1 expression from the beginning of development using a neuron-specific enolase (Nse) promoter impacts synaptic plasticity and memory.
Consistent with the previous studies of constitutive Cdh1 heterozygous KO mice, we found that the Nse-Cdh1 cKO mice exhibited impaired late-phase long-term potentiation (L-LTP). Curiously, the Nse-Cdh1 cKO mice did not display learning and memory impairments on either an associative fear memory task or spatial memory tasks. However, the Nse-Cdh1 cKO mice exhibited impaired behavioral flexibility as measured by choice arm reversal in a water-based Y maze and impaired extinction of associative fear memory. Taken together, our findings suggest that developmental expression of neuronal Cdh1 is important for LTP, behavioral flexibility, and extinction.
2. Materials and Methods
2.1 Conditional knockout mice
Mice with floxed Fzr1 (Fzr1fl) alleles were generated as described previously (García-Higuera et al. 2008). The mouse Fzr1 locus encodes the Cdh1 gene. Mice expressing cre recombinase under the Nse promoter were purchased from Jackson Laboratories (Tg(Eno2-cre)39Jme/J strain). Mice were genotyped using cre-specific primers and primers that identify floxed alleles of the Fzr1 locus. Mice for these studies were generated using the following breeding strategy, male cre(−/−) Fzr1fl(+/−) Fzr1(+/−) were crossed to cre(+/+) Fzr1fl(+/−) Fzr1(+/−) females. The wild-type mice in this study were cre(+/−) Fzr1fl(−/−) Fzr1(+/+), whereas the knockout mice were cre(+/−) Fzr1fl(+/+) Fzr1(−/−).
2.2 Western blots
Total protein levels were quantified using a BCA assay (Bio-Rad Protein Assay) and 15 μg of protein per lane was loaded on a 10% SDS-polyacrylamide gel and separated by electrophoresis. Protein bands were transferred onto a nitrocellulose membrane and blots were blocked in 5% non-fat dry milk + 0.1% Tween-20 for 60 min. Membranes were incubated with antibodies to either Cdh1 (MBL, 1:500) or GAPDH (Cell Signaling, 1:10,000), overnight at 4°C. Excess antibody was removed by three washes in T-TBS. Membranes then were incubated in secondary HRP-conjugated anti-mouse or anti-rabbit IgG (Promega, 1:5000) for 60 min and excess antibody was again removed by three washes in T-TBS. Blots were visualized using a Kodak imager. Protein bands were quantified using the ImageJ software.
2.3 Morris water maze
A hidden platform was placed in a pool of opaque water. Mice were placed into the pool and permitted to swim for 60 seconds. If they found the platform, they were immediately removed and returned to their home cage. If they did not find the platform after 60 seconds, they were guided to the platform and were repeatedly placed on the platform until they remained on the platform, unassisted, for a total of 15 seconds. Mice were trained with four trials each day with at least a 10-minute intertrial interval. On the final day, the platform was removed and a probe test was performed. The escape latency was measured on each training trial. During the probe test, the number of times the mice crossed the location of the hidden platform, along with the amount of time they spent in the target quadrant was recorded. The day after the probe test, the reversal task was started. The hidden platform was placed in the opposite quadrant and the latency to find the new location of the platform was recorded. The reversal task continued for three days exactly as the initial training protocol. A two-day visible platform task ensued after the reversal task where a visible platform and flag were placed in the water and moved to a different zone for each of the four trials on both days. Latency of the mice to climb onto the visible platform was recorded. For analysis of escape latencies, a repeated-measures analysis of variance (ANOVA) was used to analyze the escape latencies, while a one-way ANOVA was used for analyzing the probe test with p < 0.05 as significance criteria.
2.4 Water-based Y maze
A submerged Y maze was placed in a pool of opaque water. On day one, the mice were habituated to the maze and allowed to swim in the maze for 60 seconds. On day two, a hidden platform was placed into one of the two arms and mice were positioned in the starting arm and swam into one of the two arms. If the mice selected the correct arm and climbed onto the hidden platform, they immediately were removed and returned to their cage. If the mice selected the wrong arm, they were trapped in the arm for 20 seconds before being removed and returned to the cage. If the mice selected the wrong arm on the first trial, after being trapped for 20 seconds they were shown the hidden platform and were placed repeatedly on it until it remained on the platform, unassisted, for a total of 15 seconds. Mice were tested with an intertrial interval of at least 10 minutes until they correctly selected the arm with the hidden platform on 10 out of 20 trials. On day three, the platform was removed and the memory of the mice was assessed by observing whether they swam into the arm containing the hidden platform. Mice that remembered the location of the hidden platform on at least four of five trials were subsequently used for the reversal task where the hidden platform was moved to the other arm. In the reversal task, mice were repeatedly tested until they found the new location of the hidden platform on nine of ten trials. A one-way ANOVA was used for analyzing the average number of trials required to achieve criteria with p < 0.05 as significance criteria.
2.5 Fear conditioning
Mice were introduced to a novel plexiglass cage (white overhead light, metallic grid floor, peppermint odor) and were allowed to explore for 150 seconds before exposure to two tone-footshock pairings separated by one minute (tone, 85 db white noise, 30 s duration; foot shock intensity, 0.6 mA, 2 s duration). Contextual memory tests were carried out 24 hours later in the same environment where the mice were allowed to explore for 5 minutes. Cued memory tests were carried out 25 hours after training in an altered environment (red overhead light, plastic floor, vanilla odor) where mice were allowed to explore for 150 seconds before re-exposure to the same auditory cues. During the extinction training tests, mice were re-exposed to the auditory cue fifteen times with random intertrial intervals over the course of two days in the altered environment. Extinction consolidation was examined 24 hours after the end of the extinction training. Behavior was videotaped and scored blind. One-way ANOVA was used for statistical analysis with p < 0.05 as significance criteria.
2.6 Electrophysiological recordings
8 to 12 week-old mice were killed by cervical dislocation and the brains were sectioned with a vibratome into 400 μm thick slices in an oxygenated sucrose solution. Brains were sectioned horizontally. Hippocampal slices were equilibrated with oxygenated ACSF at 32°C for at least 1 hour before recording. Extracellular recordings of field EPSPs (fEPSPs) were obtained from the stratum radiatum using microelectrodes filled with ACSF and a bipolar Teflon-coated platinum electrode was placed in stratum radiatum to stimulate Schaffer collateral-commissural afferents. The stimulation strength was set to elicit a response equivalent to 50% of the maximal fEPSPs. The slope of the fEPSP was expressed as a percentage of the baseline average prior to stimulation.
3. Results
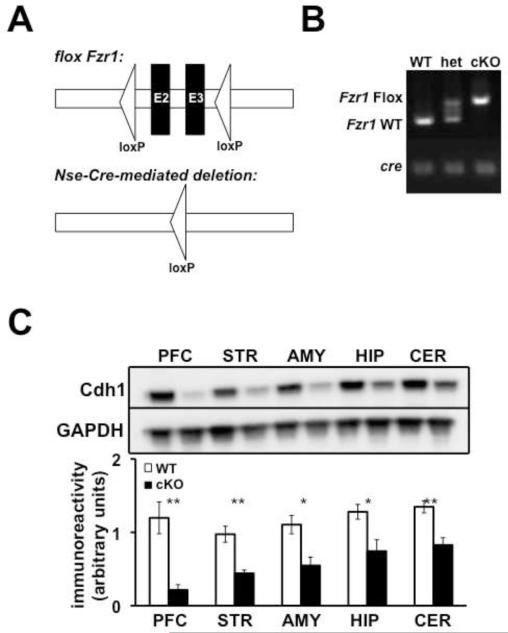
Previous studies of constitutive Cdh1 heterozygous KO mice suggested that deleting one copy of the Fzr1 allele (the gene that encodes the Cdh1 protein) from birth led to synaptic plasticity and memory impairments in the hippocampus (Li et al. 2008). In order to address the question as to whether this impairment was due to a developmental contribution of neuronal Cdh1, we used the cre-lox system to generate conditional KO (cKO) mice where Cdh1 expression was removed from neurons from the beginning of development. We crossed mice expressing loxP sites flanking exons 2 and 3 (E2 and E3) in the Fzr1 allele with mice expressing cre recombinase under a neuron-specific enolase (Nse) promoter (Figure 1A). Neuronal expression of Nse starts at the beginning of development during the onset of neuronal differentiation and functional synaptic activity (Marangos and Schmechel 1987). Specific PCR primers were used to identify expression of the cre allele and the floxed Fzr1 gene (Figure 1B). We confirmed knockdown of Cdh1 protein levels in all regions of the brain examined (Figure 1C).
Figure 1. Neuronal expression of Cdh1.
A, Schematic of the conditional Fzr1 allele. (Top) Exons 2 and 3 (E2 - E3) are flanked by two loxP sites in the Fzr1 (lox) mice. (Bottom) After expression of cre recombinase, E2 and E3 are excised. B, PCR identification of alleles of Fzr1 and Nse-Cre. Wild-type (WT) mice were identified by a band indicating the absence of any floxed Fzr1 alleles and the presence of a band indicating the expression of the Nse-Cre allele. Heterozygous mice (het) were identified by the presence of a double band, indicating the expression oa floxed and non-floxed Fzr1 alleles, as well as the presence of the Nse-Cre band. Cdh1 conditional knockout mice (cKO) were identified by the presence of a single band, indicating the expression of floxed Fzr1 alleles as well as the presence of the Nse-Cre band. C, (Top) Representative Western blot expressing Cdh1 and GAPDH (loading control) in Nse-Cdh1 cKO mice (cKO) and wild-type (WT) littermates. (Bottom) Quantification of Cdh1 expression in Nse-Cdh1 cKO mice and WT littermates in the prefrontal cortex (PFC), striatum (STR), amygdala (AMY), hippocampus (HIP), and cerebellum (CER). WT, n = 4 mice; cKO, n = 4 mice. *p < .05, Student’s t-test; **p ≤ .01 Student’s t-test.
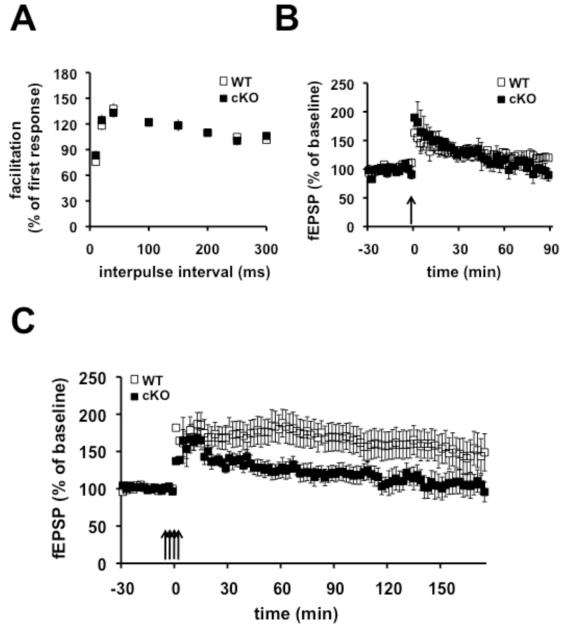
We proceeded to determine whether the Nse-Cdh1 cKO mice displayed impaired LTP similar to the constitutive Cdh1 heterozygous KO mice. We first confirmed that the paired-pulse facilitation was normal in the Nse-Cdh1 cKO mice compared with their wild-type littermates, to establish that they displayed normal short-term presynaptic plasticity (Figure 2A). We then examined two forms of LTP, early phase LTP (E-LTP) and late phase LTP (L-LTP). One train of high-frequency stimulation (HFS) was used to induced E-LTP that normally lasts one to two hours, whereas four trains of HFS was used to induce L-LTP (L-LTP) where synaptic transmission remains potentiated for over three hours. Similar to previous findings with the constitutive Cdh1 heterozygous KO mice, E-LTP was intact in the Nse-Cdh1 cKO mice (Figure 2B). In contrast, L-LTP induced in the Nse-Cdh1 cKO mice was impaired compared to their wild-type littermates (Figure 2C). This suggests that neuron-specific deletion of Cdh1 in the hippocampus from the onset of development results in impaired L-LTP.
Figure 2. E-LTP is normal but L-LTP is impaired in Cdh1 cKO mice.
A, Paired-pulse facilitation was not different between Nse-Cdh1 cKO (cKO) mice and wild-type (WT) littermates. WT, n = 15 slices; cKO, n = 12 slices. B, E-LTP induced with one train of HFS was similar in Nse-Cdh1 cKO mice and their wild-type littermates. WT, n = 6 slices; cKO, n = 5 slices. p > .05 ANOVA. C, L-LTP induced with four trains of HFS was impaired in Nse-Cdh1 cKO mice compared with their wild-type littermates. WT, n = 8 slices; cKO, n = 9 slices. *p < .05, ANOVA.
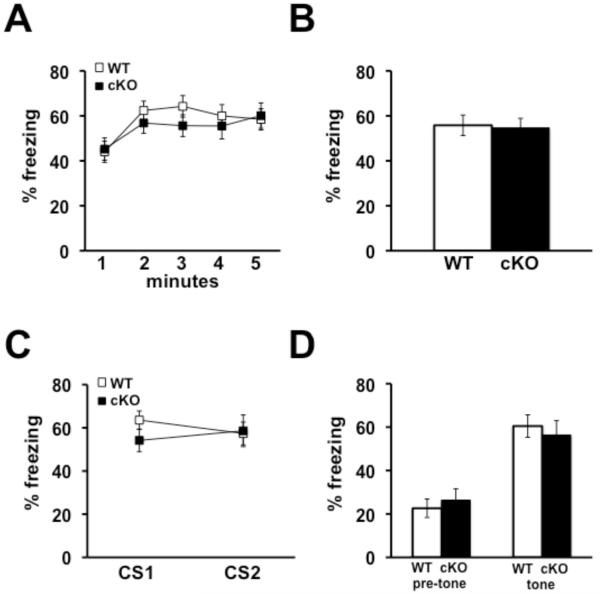
We next tested the Nse-Cdh1 cKO mice in several memory tasks in order to determine whether the impairment in L-LTP was correlated with aberrant memory. We first tested the Nse-Cdh1 cKO mice in two different paradigms of associative fear memory: contextual and cued fear conditioning. Although both forms of fear conditioning are known to require the amygdala, contextual fear conditioning also requires the hippocampus (Phillips and LeDoux 1992), and a previous study showed that constitutive Cdh1 heterozygous KO mice have impaired contextual, but not cued, associative fear memory (Li et al. 2008). We used a similar protocol to train the mice to associate the conditioning box and a neutral tone (CS) with a noxious footshock (US) and examined the amount of freezing in response to the CS 24 hours after training. We found that the Nse-Cdh1 cKO mice did not exhibit impairments in either contextual (Figures 3A & 3B) or cued fear memory (Figures 3C & 3D).
Figure 3. Associative fear memory is normal in Nse-Cdh1 cKO mice 24 hours after training.
A, The average percentage of time that Nse-Cdh1 cKO (cKO) mice spent freezing over each minute of a five-minute re-exposure to the training chamber was similar to wild-type (WT) littermates 24 hours after training. B, The average percentage of time that Nse-Cdh1 cKO mice spent freezing during the whole five minute re-exposure to the training chamber was similar to wild-type littermates, 24 hours after training. WT, n = 18; cKO, n = 17. p > .05, ANOVA. C, The average percentage of time that Nse-Cdh1 cKO mice spent freezing during each presentation of the conditioned stimulus (CS1 and CS2) was similar to wild-type littermates, 25 hours after training. D, The average percentage of time that Nse-Cdh1 cKO mice spent freezing prior to the CS presentation (pre-tone), as well as during the CS presentation (tone), was similar to their wild-type littermates, 25 hours after training. WT, n = 14; cKO, n = 12. p > .05, ANOVA.
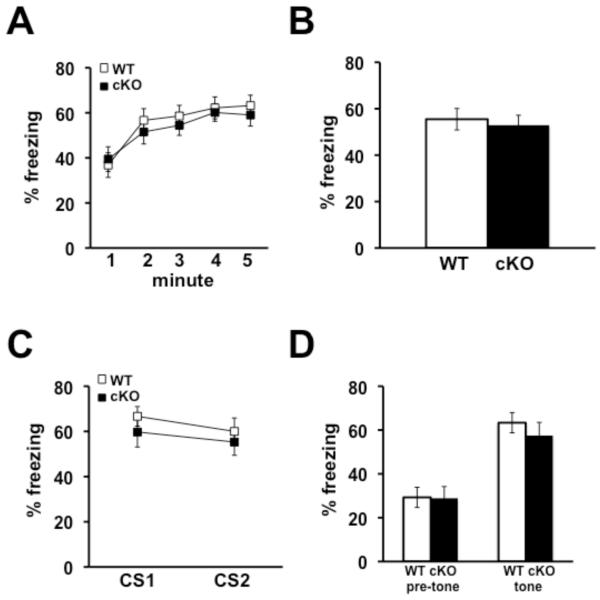
Although associative fear memory was normal in the Nse-Cdh1 cKO mice 24 hours after training, we reasoned that perhaps testing the mice at later time point after training would draw out more subtle differences in associative fear memory. We examined fear memory eight days after training and found that both contextual (Figures 4A & 4B) and cued (Figures 4C & 4D) fear memory was intact in the Nse-Cdh1 cKO mice. Thus, reducing Cdh1 expression in neurons from the onset of development does not affect associative fear memories either one or eight days after training.
Figure 4. Associative fear memory is normal in Nse-Cdh1 cKO mice eight days after training.
A, The average percentage of time that Nse-Cdh1 cKO (cKO) mice and their wild-type (WT) littermates spent freezing was similar over each minute during re-exposure to the training context, eight days after training. B, The average percentage of time that the Nse-Cdh1 cKO mice and their wild-type littermates spent freezing was similar when re-exposed to the training context eight days after training. WT, n = 14; cKO, n = 12. p > .05, ANOVA. C, The average percentage of time that the Nse-Cdh1 cKO mice and their wild-type littermates spent freezing was similar during each presentation of the conditioned stimulus (CS1 and CS2) in a novel context eight days after training. D, The average percentage of time that Nse-Cdh1 cKO mice and their wild-type littermates spent freezing was similar during the CS presentation in a novel context eight days after training. WT, n = 14; cKO, n = 12. p > .05, ANOVA.
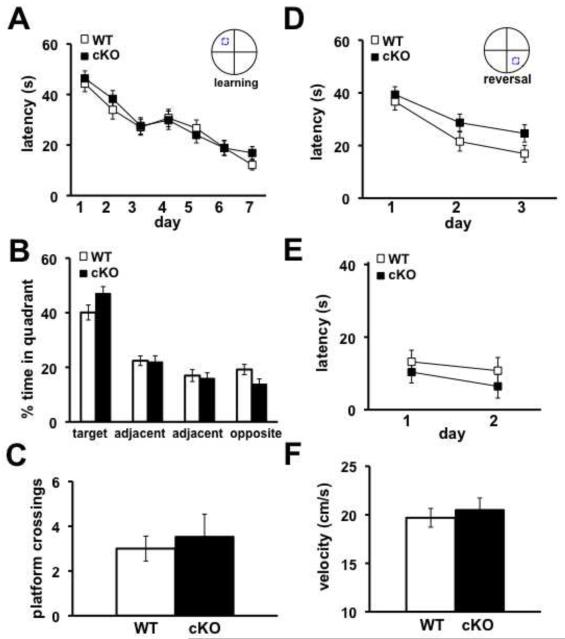
We subsequently examined the Nse-Cdh1 cKO mice for spatial learning and memory phenotypes on two hippocampus-dependent tasks, the Morris water maze (MWM) and a water-based Y maze. In the MWM, mice were trained to find the location of a hidden platform in an opaque pool of water over the course of seven days. Mice were trained for four trials each day and had 60 seconds to find the hidden platform. The latency of the mice to find the platform was recorded over each of the training days. After the final training trial, the hidden platform was removed and a probe test was administered. The amount of time the mice spent in the quadrant that had formerly contained the hidden platform as well as the number of times the mice crossed the previous location of the hidden platform were recorded. There was no difference in the latency of the Nse-Cdh1 cKO mice to find the hidden platform (Figure 5A) compared with their wild-type littermates. In addition, the amount of time that the Nse-Cdh1 cKO mice spent in the target quadrant (Figure 5B) as well as the number of platform crossings (Figure 5C) were comparable to their wild-type littermates. We confirmed that the visual capabilities (Figure 5E) and velocity that the Nse-Cdh1 cKO mice moved (Figure 5F) were normal compared with their wild-type littermates. Similarly, in a water-based Y maze where mice were trained to select the arm containing a hidden platform, there was no difference in the number of trials it took the Nse-Cdh1 cKO mice to either learn the location of the hidden platform or remember its location 24 hours later (Figures 6A and 6B). Taken together, these data suggest that the Nse-Cdh1 cKO mice do not have impairments in hippocampus-dependent spatial learning and memory tasks.
Figure 5. Nse-Cdh1 cKO mice display normal hippocampus-dependent memory as measured with the MWM.
A, Average latency of Nse-Cdh1 cKO (cKO) mice to find the hidden platform in the MWM was similar to wild-type (WT) littermates over seven days of training. WT, n = 10; cKO, n = 10. p > .05, ANOVA. B, Average percentage of time Nse-Cdh1 cKO mice spent in each of the four quadrants was similar to wild-type littermates during the probe test after the final day of training. WT, n = 10; cKO, n = 10. p > .05, Student’s t-test. C, Average number of times that the Nse-Cdh1 cKO mice crossed the previous location of the hidden platform during the probe test was similar to wild-type littermates. WT, n = 10; cKO, n = 10. p > .05, Student’s t-test. D, Latency of Nse-Cdh1 cKO mice and wild-type littermates to find the new location of a hidden platform over three days of reversal training in the MWM. WT, n = 10; cKO, n = 10. p > .05, ANOVA. E, Average latency of Nse-Cdh1 cKO mice to climb onto the visible platform was similar to wild-type littermates over two days of visual platform testing. WT, n = 10; cKO, n = 10. p > .05, ANOVA. F, Average velocity of Nse-Cdh1 cKO mice was similar to wild-type littermates during the probe test. WT, n = 10; cKO, n = 10. p > .05, Student’s t-test.
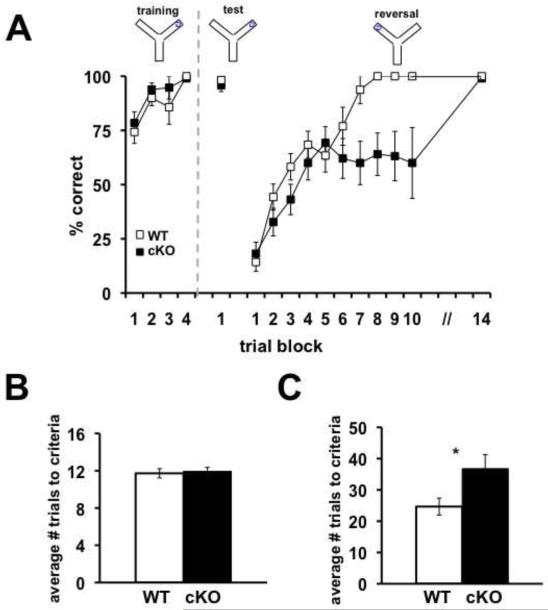
Figure 6. Nse-Cdh1 cKO mice display normal hippocampus-dependent memory, but impaired behavioral flexibility as measured with the water-based Y maze.
A, Average percentage of correct trials of Nse-Cdh1 cKO mice and their wild-type (WT) littermates during learning and reversal on a water-based Y maze task. Each trial block is the average percentage correct of five trials. A dashed grey line indicates the end of training on day one and the beginning of the tests the following day. WT, n = 13; cKO, n = 11. **p < .01; F(12,312) = 6.82, ANOVA. B, The average number of trials for the Nse-Cdh1 cKO mice to reach criterion was similar to wild-type littermates during training on a water-based Y maze. WT, n = 18; cKO, n = 16. p > .05, Student’s t-test. C, Average number of trials for Nse-Cdh1 cKO mice and wild-type littermates to reach criterion after reversing the location of the hidden platform on a water-based Y maze task. WT, n = 13; cKO, n = 11. *p < .05, Student’s t-test.
It previously was shown that impairing UPS activity in the hippocampus has no effects on memory consolidation but does affect memory adjustment, specifically extinction (Lee et al. 2008). Therefore, we reasoned that even though eliminating Cdh1 in neurons in the hippocampus had no effect on memory consolidation, perhaps it would affect the ability to modify the memory. In order to test this notion, we manipulated the conditions of the three memory tasks and examined the behavior of the Nse-Cdh1 cKO mice.
In the MWM, on the day following the probe test the hidden platform was moved to a different location and the latency of the mice to find the new location was recorded over the course of three days. The Nse-Cdh1 cKO mice displayed a slight impairment in reversal memory compared with their wild-type littermates (Figure 5D). When we moved the hidden platform to the opposite arm in the water-based Y maze, the Nse-Cdh1 cKO mice were significantly impaired when compared with their wild-type littermates in learning the new platform location (Figure 6A & 6C). Thus, although the hippocampus-dependent spatial memory of the Cdh1 cKO mice was intact, their ability to update the memory was impaired after moving the hidden platform to a new location.
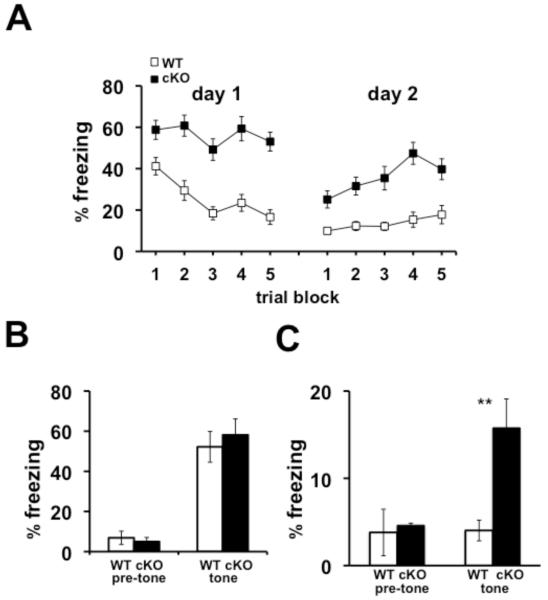
In light of the impaired behavioral flexibility displayed by the Nse-Cdh1 cKO mice, we examined the ability of the Nse-Cdh1 cKO mice to extinguish a previously learned associative fear memory. Mice were trained to associate a neutral tone (CS) with a noxious footshock (US) and were re-exposed to the CS 15 times in a novel environment over two subsequent days while their levels of freezing were recorded. The Nse-Cdh1 cKO mice were impaired in their ability to extinguish the fear memory compared with wild-type littermates (Figure 7A). Importantly, the initial freezing of the Nse-Cdh1 cKO mice during the first trial of extinction was not different from their wild-type littermates (Figure 7B). Furthermore, when the consolidation of the extinction of the associative fear memory was examined 24 hours after the end of the extinction training, the Nse-Cdh1 cKO mice were impaired compared with wild-type littermates (Figure 7C). Together with the reversal data from the hippocampus-dependent spatial memory tasks, these data suggest that although the spatial and associative memory consolidation of the Nse-Cdh1 cKO mice was intact, their ability to modify their memory, either after moving the hidden platform or with extinction of an associative fear memory, was impaired compared to wild-type littermates.
Figure 7. Extinction of associative auditory fear memory is impaired in Nse-Cdh1 cKO mice.
A, Average percentage of freezing of Nse-Cdh1 cKO (cKO) mice during extinction of an associative auditory CS over two consecutive days was significantly impaired compared with wild-type (WT) littermates. Each trial block was the average of three trials. WT, n = 9; cKO, n = 7. **p < .01; F(1,12) = 16.36, ANOVA. B, Average percentage of freezing of Nse-Cdh1 cKO mice was not different from wild-type littermates during the pre-tone and the first trial of extinction 24 hours after the initial training session. WT, n = 9; cKO, n = 7. p > .05 Student’s t-test. C, Average percentage of freezing of Nse-Cdh1 cKO mice 24 hours after the final extinction session was significantly impaired compared with wild-type littermates. WT, n = 9; cKO, n = 7. **p < .01, Student’s t-test.
4. Discussion
In recent years, recognition of the role of the APC/C, an E3 ligase that functions as part of the UPS, has expanded from its original characterization as a regulator of cell cycle progression to include an important role in synaptic plasticity and memory. In order to characterize the developmental contribution of neuronal Cdh1 to synaptic plasticity and memory,, we generated Cdh1 cKO mice using the cre-lox system, where Cdh1 was removed from neurons at the onset of development by breeding them with a mouse line expressing cre recombinase driven by the Nse promoter. The Nse-Cdh1 cKO mice exhibited normal E-LTP, but impaired L-LTP in hippocampal slices. Curiously, the Nse-Cdh1 cKO mice exhibited normal hippocampus-dependent learning and memory consolidation as measured with the MWM, a water-based Y maze, and an associative fear memory task. However, using additional tests that probed the Nse-Cdh1 cKO mice on their ability to modify memory, we detected a slight impairment in reversal learning in the MWM and a significant impairment in both choice reversal in a water-based Y maze, and extinction of an associative fear memory. Thus, our results suggest that reducing Cdh1 expression in neurons in the brain early in development has adverse effects on L-LTP, behavioral flexibility, and extinction in adulthood.
Our findings with the Nse-Cdh1 cKO mice differ somewhat from a previous study examining constitutive Cdh1 heterozygous KO mice. Although both the constitutive Cdh1 heterozygous KO mice and the Nse-Cdh1 cKO mice exhibited deficits in L-LTP, the constitutive Cdh1 heterozygous KO mice displayed impaired contextual fear memory, whereas the Nse-Cdh1 cKO mice exhibited normal contextual fear memory (Figures 3A and 3B). One possible explanation to account for this difference in contextual fear memory between these different Cdh1 mutant mice could be due to the types of cells that Cdh1 was eliminated from. For example, in the constitutive Cdh1 heterozygous KO mice Cdh1 was reduced in all cells, including glial cells, whereas Nse is not expressed in glial cells (Marangos and Schmechel 1987), suggesting that glial Cdh1 was intact in the Nse-Cdh1 cKO mice. In addition, it was shown that Cdh1 affects the number of glial cells in the Drosophila brain during development such that eliminating Cdh1 leads to an increase in gliogenesis, but a decrease in neurogenesis (Kaplow et al. 2008). Moreover, glial cells in the hippocampus have been shown to be important in LTP and hippocampus-dependent memory tasks (Yang et al. 2003; Carmona et al. 2009; Eroglu and Barres 2010). Thus, in contrast to the Nse-Cdh1 cKO mice, reduced expression of Cdh1 in glial cells in the constitutive Cdh1 heterozygous KO mice may contribute to deficits in contextual fear memory.
Although we detected impairments in hippocampal L-LTP in the Nse-Cdh1 cKO mice, the inability to detect changes in hippocampus-dependent memories in these mice can be understood in a larger context. A previous study examining the effect of proteasome inhibitors on contextual fear memory demonstrated that although the inhibitors do not affect consolidation of fear memories in the hippocampus, they do affect fear extinction (Lee et al. 2008). These authors suggested that during updating of a memory, the UPS may be required to degrade proteins necessary to destabilize a previously consolidated memory, but not to consolidate the initial memory. Although extinction classically has been described as new learning, a recent study suggests that there may be an element of erasure during extinction as spines are both eliminated and formed in the same region of dendrite during fear conditioning and extinction (Lai et al. 2012). It is possible that Cdh1 contributes to the destabilization process and thus, the Nse-Cdh1 cKO mice exhibit impairments in behavioral flexibility and extinction, but not in memory consolidation.
In addition, It is known that the medial PFC (mPFC) is involved in behavioral flexibility and extinction, specifically with either emotional or motivational tasks (Quirk et al. 2006; Sotres-Bayon et al. 2006). Consistent with our finding of impaired expression of Cdh1 in the PFC in the Nse-Cdh1 cKO mice, it is possible that Cdh1 plays a role in extinction by targeting proteins in mPFC neurons.
We have conducted studies on a different line of Cdh1 cKO mice where Cdh1 was removed from excitatory neurons in the forebrain post-developmentally using an αCaMKII promoter. In contrast to the Nse-Cdh1 cKO mice that exhibited impaired behavioral flexibility, the αCaMKII-Cdh1 cKO mice demonstrated enhanced behavioral flexibility in the MWM and the water-based Y maze (Pick et al., in press). One possibility for these different behavioral phenotypes is that Cdh1 has a range of targets that impact these behaviors in different ways. Thus, it is possible that variable expression of Cdh1 targets early in development and post-development can result in differences in behavioral flexibility.
An alternative explanation for the differences in behavioral flexibility between the two lines of Cdh1 cKO mice is that the enhanced behavioral flexibility in αCaMKII-Cdh1 cKO mice is due to impaired memory rather than enhanced behavioral flexibility. Although the hippocampus-dependent memory in the αCaMKII-Cdh1 cKO mice was intact as measured by various parameters on the MWM and the water-based Y maze, it is possible that these mice lack confidence in the memory, and changing the location of the hidden platform does not reflect enhanced behavioral flexibility as much as impaired confidence. In addition, although hippocampus-dependent memories were intact in the αCaMKII-Cdh1 cKO mice, amygdala-dependent memories were impaired, suggesting that their memory may be easily disturbed under certain conditions, which could be manifested as enhanced flexibility.
Cdh1 is known to play a role in neuronal development as well as in adult synaptic plasticity and memory. In this study we explored the developmental role of neuronal Cdh1 in synaptic plasticity and memory by eliminating it at the time that it transitions from regulating cell cycle progression to its more recently discovered role in post-mitotic neurons. Our findings demonstrate that removing neuronal Cdh1 early in development results in impaired L-LTP and deficits in behavioral flexibility during the updating of memories.
Neuron-specific deletion of Cdh1 causes impaired L-LTP
Neuron-specific deletion of Cdh1 causes impaired reversal learning
Neuron-specific deletion of Cdh1 causes impaired extinction of fear memory
Acknowledgments
This work was supported by National Institutes of Health grants NS034007, NS047384, and NS078708 (EK) and an NRSA Predoctoral Award AG034005 (JEP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Carmona MA, Murai KK, Wang L, Roberts AJ, Pasquale EB. Glial ephrin-A3 regulates hippocampal dendritic spine morphology and glutamate transport. Proceedings of the National Academy of Sciences. 2009;106:12524–12529. doi: 10.1073/pnas.0903328106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu C, Barres BA. Regulation of synaptic connectivity by glia. Nature. 2010;468:223–231. doi: 10.1038/nature09612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca R, Vabulas RM, Hartl FU, Bonhoeffer T, Nagerl UV. A Balance of Protein Synthesis and Proteasome-Dependent Degradation Determines the Maintenance of LTP. Neuron. 2006;52:239–245. doi: 10.1016/j.neuron.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Fu AKY, Hung K-W, Fu W-Y, Shen C, Chen Yu, Xia J, Lai K-O, Ip NY. APC(Cdh1) mediates EphA4-dependent downregulation of AMPA receptors in homeostatic plasticity. Nat Neurosci. 2011;14:181–189. doi: 10.1038/nn.2715. [DOI] [PubMed] [Google Scholar]
- García-Higuera I, Manchado E, Dubus P, Cañamero M, Méndez J, Moreno S, Malumbres M. Genomic stability and tumour suppression by the APC/C cofactor Cdh1. Nat. Cell Biol. 2008;10:802–811. doi: 10.1038/ncb1742. [DOI] [PubMed] [Google Scholar]
- Gieffers C, Peters BH, Kramer ER, Dotti CG, Peters JM. Expression of the CDH1-associated form of the anaphase-promoting complex in postmitotic neurons. Proc Natl Acad Sci USA. 1999;96:11317–11322. doi: 10.1073/pnas.96.20.11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JW, Burton JL, Solomon MJ. The anaphase-promoting complex: it’s not just for mitosis any more. Genes & Development. 2002;16:2179–2206. doi: 10.1101/gad.1013102. [DOI] [PubMed] [Google Scholar]
- Jiang YH, Armstrong D, Albrecht U, Atkins CM, Noebels JL, Eichele G, Sweatt JD, Beaudet AL. Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron. 1998;21:799–811. doi: 10.1016/s0896-6273(00)80596-6. [DOI] [PubMed] [Google Scholar]
- Juo P, Kaplan JM. The anaphase-promoting complex regulates the abundance of GLR-1 glutamate receptors in the ventral nerve cord of C. elegans. Curr Biol. 2004;14:2057–2062. doi: 10.1016/j.cub.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Kaplow ME, Korayem AH, Venkatesh TR. Regulation of glia number in Drosophila by Rap/Fzr, an activator of the anaphase-promoting complex, and Loco, an RGS protein. Genetics. 2008;178:2003–2016. doi: 10.1534/genetics.107.086397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova A. Involvement of Protein Synthesis and Degradation in Long-Term Potentiation of Schaffer Collateral CA1 Synapses. Journal of Neuroscience. 2006;26:4949–4955. doi: 10.1523/JNEUROSCI.4573-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi Y, Stegmüller J, Matsuda T, Bonni S, Bonni A. Cdh1-APC controls axonal growth and patterning in the mammalian brain. Science. 2004;303:1026–1030. doi: 10.1126/science.1093712. [DOI] [PubMed] [Google Scholar]
- Kuczera T, Stilling RM, Hsia H-E, Bahari-Javan S, Irniger S, Nasmyth K, Sananbenesi F, Fischer A. The anaphase promoting complex is required for memory function in mice. Learn Mem. 2011;18:49–57. doi: 10.1101/lm.1998411. [DOI] [PubMed] [Google Scholar]
- Lai CSW, Franke TF, Gan W-B. Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature. 2012 doi: 10.1038/nature10792. [DOI] [PubMed] [Google Scholar]
- Lee S-H, Choi J-H,, Lee N, Lee H-R,, Kim J-I,, Yu N-K,, Choi S-L,, Lee S-H,, Kim H, Kaang B-K. Synaptic protein degradation underlies destabilization of retrieved fear memory. Science. 2008;319:1253–1256. doi: 10.1126/science.1150541. [DOI] [PubMed] [Google Scholar]
- Li M, Shin Y-H,, Hou L, Huang X, Wei Z, Klann E, Zhang P. The adaptor protein of the anaphase promoting complex Cdh1 is essential in maintaining replicative lifespan and in learning and memory. Nat. Cell Biol. 2008;10:1083–1089. doi: 10.1038/ncb1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Salon M, Alonso M, Vianna MR, Viola H, Mello e, Souza T, Izquierdo I, Pasquini JM, Medina JH. The ubiquitin-proteasome cascade is required for mammalian long-term memory formation. Eur J Neurosci. 2001;14:1820–1826. doi: 10.1046/j.0953-816x.2001.01806.x. [DOI] [PubMed] [Google Scholar]
- Marangos PJ, Schmechel DE. Neuron specific enolase, a clinically useful marker for neurons and neuroendocrine cells. Annu Rev Neurosci. 1987;10:269–295. doi: 10.1146/annurev.ne.10.030187.001413. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, González-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Cain CK, Ledoux JE. Brain mechanisms of fear extinction: historical perspectives on the contribution of prefrontal cortex. Biol Psychiatry. 2006;60:329–336. doi: 10.1016/j.biopsych.2005.10.012. [DOI] [PubMed] [Google Scholar]
- van Roessel P, Elliott DA, Robinson IM, Prokop A, Brand AH. Independent regulation of synaptic size and activity by the anaphase-promoting complex. Cell. 2004;119:707–718. doi: 10.1016/j.cell.2004.11.028. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ge W, Chen Yiren, Zhang Z, Shen W, Wu C, Poo M, Duan S. Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proc Natl Acad Sci USA. 2003;100:15194–15199. doi: 10.1073/pnas.2431073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao I, Takao K, Miyakawa T, Ito S, Setou M. Synaptic E3 ligase SCRAPPER in contextual fear conditioning: extensive behavioral phenotyping of Scrapper heterozygote and overexpressing mutant mice. PLoS ONE. 2011;6:e17317. doi: 10.1371/journal.pone.0017317. [DOI] [PMC free article] [PubMed] [Google Scholar]