Abstract
Foxi2 and Foxi3 are members of the Foxi class of Forkhead transcription factors. The Foxi transcription factor family has been shown to play roles in the development of the inner ear and pharyngeal arch derivatives in zebrafish. We describe the expression of Foxi2 and Foxi3 in chicken embryos during the first three days of embryonic development. Foxi3 is initially expressed broadly in the pre-placodal ectoderm surrounding the neural plate, which will give rise to all craniofacial sensory organs. It then becomes restricted to a region immediately anterior to the first pair of somites that will give rise to the otic and epibranchial placodes, before becoming down-regulated from this region and restricted to the ectoderm and endoderm of the pharyngeal arches. In contrast, Foxi2 is initially expressed broadly in cranial ectoderm with the striking exception of the otic placode, and ultimately becomes restricted to pharyngeal arch ectoderm. These expression patterns provide an insight into the roles of these transcriptional regulators during the development of the inner ear and pharyngeal arch derivatives.
Keywords: Otic placode, Pharyngeal arch, Craniofacial development, Pre-placodal domain
1. INTRODUCTION
The Forkhead box (Fox), family of winged helix domain-containing transcriptional regulators is named after the Drosophila melanogaster fork head mutant (fkh). More than a hundred Fox genes have been identified in species ranging from yeast to humans (Kaestner et al., 2000). The Forkhead gene family is divided into 16 sub-families on the basis of sequence similarity (Fetterman et al., 2008; Hannenhalli and Klaus, 2009). Fox genes have important biological functions in multiple species, from control of the cell cycle to differentiation of epithelia, placental development and formation of the inner ear (reviewed in Kaufmann and Knochel, 1996; Hedrick, 2009; Hannenhalli and Klaus, 2009; Hwang et al., 2009). The expression of different Foxi class genes has been analyzed in several species. Mouse Foxi1 is expressed in the developing endolymphatic duct of the inner ear (Ohyama and Groves, 2004), and mutation of Foxi1 in mice leads to a failure of fluid homeostasis in the inner ear and accompanying enlargement of the inner ear labyrinth similar to that seen in Pendred syndrome (Hulander et al., 2003). In zebrafish and amphibians, Foxi1 is expressed in early pre-placodal ectoderm and later in the pharyngeal arch region, and mutation or knockdown of Foxi1 in zebrafish causes inner ear and jaw defects (Solomon et al., 2003). Mouse Foxi2 is expressed initially in presumptive otic ectoderm adjacent to the rhombomeres 3 to 5, but is rapidly excluded from the otic region as inner ear differentiation proceeds and is later restricted to the pharyngeal arch region (Ohyama and Groves, 2004). A similarly restricted expression of Foxi2 in the chick otic-epibranchial precursor domain (OEPD) has been reported previously (Freter et al., 2008). Increasing or decreasing the size of the otic placode by manipulation of the Notch or Wnt signaling pathways leads to a concomitant decrease or expansion of the Foxi2 domain abutting the otic region (Ohyama et al., 2006; Jayasena et al., 2008). In zebrafish, Foxi2 is not expressed in the otic region and only appears in the pharyngeal arch region at later stages (Solomon et al., 2003). Mouse Foxi3 is expressed in pre-placodal ectoderm in a pattern reminiscent of zebrafish Foxi1 and is also later restricted to the pharyngeal pouches (Ohyama and Groves, 2004). The comparative expression of patterns of all three Foxi genes in mouse, chick and zebrafish is described in Table 1.
Table 1.
Comparison of Foxi family member expression mouse, chicken and zebrafish
| Gene | Species | Expression domain | References |
|---|---|---|---|
| Foxi1 | Mouse | Otic vesicle, endolymphatic duct and sac, embryonic and adult kidney | Overdier et al., 1997 Hulander et al., 1998, 2003 |
| Chicken | Not reported yet | ||
| Zebrafish | Pre-placodal ectoderm, early otic placode and branchial arches | Kwon HJ et al., 2010; Solomon et al., 2003b | |
| Foxi2 | Mouse | Cranial (non-otic) ectoderm, pharyngeal arches | Ohyama and Groves, 2004 |
| Chicken | Cranial (non-otic) ectoderm, pharyngeal arches | This study; Freter et al., 2008 | |
| Zebrafish | Chordamesoderm during early somitogenesis and the retina and branchial arches at later stages | Solomon et al., 2003b | |
| Foxi3 | Mouse | Pre-placodal ectoderm, otic placode, pharyngeal arches, dorsal optic cup, neural plate | Ohyama and Groves, 2004 |
| Chicken | Pre-placodal ectoderm, otic placode, pharyngeal arches, dorsal optic cup, neural plate | This study | |
| Zebrafish | Epidermal mucous cells throughout embryogenesis and early larval stages | Solomon et al., 2003b |
The chicken is an attractive developmental system due to the ease with which the embryo can be manipulated and genes introduced by electroporation. Here we provide a detailed expression of Foxi2 and Foxi3 in chicken embryos during the development of craniofacial placodes and the pharyngeal arches.
2. RESULTS and DISCUSSION
2.1. Foxi3 expression in the pre-placodal domain
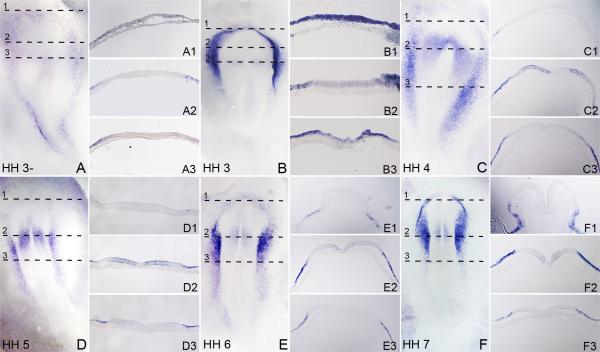
We analyzed the expression of Foxi3 by whole mount in situ hybridization in chick embryos. Foxi3 expression was first observed at the primitive streak stage (Hamburger and Hamilton stage 3). At this stage, Foxi3 expression surrounds the neural plate. Transverse sections through hybridized embryos show that the expression is limited exclusively to the epiblast and no expression is seen in the underlying hypoblast (Figure 1A and 1B). No Foxi3 expression is seen in the future neural plate except for a faint region anterior to the primitive streak (Figure 1A and 1B), which becomes stronger by HH stage 4 (Figure 1C). However, even at this stage, no expression is seen within the primitive streak region itself (Figure 1C). By HH stage 5-6, a number of genes such as members of the Six and Eya families are expressed in a region surrounding the neural plate, which will give rise to all craniofacial placodes. This region has been termed the pre-placodal domain (Streit, 2008; Baker and Bronner-Fraser, 2001; Schlosser, 2010). Foxi3 expression in the pre-placodal region becomes restricted to the lateral ectoderm on either side of the neural plate at HH stage 5 and 6, and is markedly reduced in the anterior ectoderm (Figure 1D-F). Foxi3 expression in the neural plate gradually disappears and is absent by HH stage 8 (Figure 2A). By the time the neural folds are fully formed and the first somites have appeared (HH stages 7), Foxi3 expression is completely lost from the anterior-most pre-placodal domain and is restricted to a region between the positions of the fusing neural folds down to the level of the first somite (Figure 1F). By HH stage 8-9 Foxi3 is restricted to the presumptive otic and epibranchial placode level and is not seen anywhere else in the embryo (Figure 2A and 2B). By stage 10, Foxi3 expression gradually disappears from the otic region and is confined to the pharyngeal arches (Figure 2D-E).
Figure 1.
Expression pattern of Foxi3 in early chick development between stages HH3-HH7. All whole-mount embryo images are visualized dorsally. The HH prefix in each large panel refer to the embryonic stage according to Hamburger and Hamilton (1992). Embryos were processed for whole mount in situ hybridization and then sectioned transversely at 18μm. The level of each section is indicated by dashed lines. (A and B): Foxi3 expression is first detected around the future neural plate in the epiblast but not the hypoblast region. (C and D): The rostral expression of Foxi3 gradually fades and becomes concentrated in the future trigeminal-otic-epibranchial region. Expression in a restricted region of the neural plate can be seen expanding out from the node towards the lateral sides of the embryo. No expression is seen in the primitive streak or node itself. (E and F): At the head-fold stage, rostral Foxi3 expression is more faint and more caudal expression concentrates further in the future trigeminal-otic-epibranchial region. Neural plate expression also fades and low expression is seen only at the level of the midbrain hindbrain boundary. Very faint expression can be seen in the neural fold region (E2 and F2).
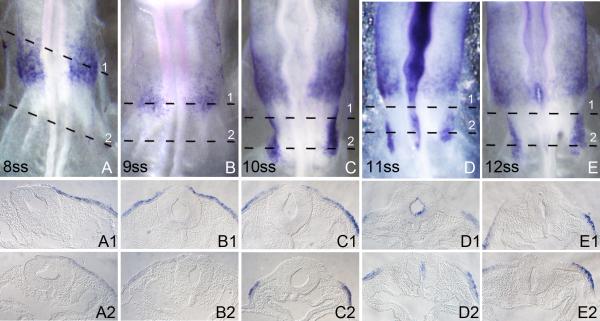
Figure 2.
The expression pattern of chick Foxi3 from stage 8ss to 12ss. Embryos were processed for whole mount in situ hybridization and then sectioned transversely at 18μm. All whole-mount embryo images are visualized dorsally. Numbers in each large panel refer to the embryonic age measured by the number of somites (ss). The level of each section is indicated by dashed lines. (A and B): Foxi3 expression is restricted to the future trigeminal region. Faint expression is seen in the surrounding presumptive otic/epibranchial region. This is the time when a second, more posterior patch of Foxi3 expression begins to appear. (C-E): Expression in the otic region is completely lost from the anterior otic placode region and restricted to the posterior placode. From this point, intense Foxi3 expression is seen in a second, more posterior region which becomes restricted to the second pharyngeal arch.
2.2. Foxi3 expression in the pharyngeal arch region
Pharyngeal arches are a vertebrate innovation. Each arch is comprised of outer ectoderm, inner endoderm and a mesenchymal core, together with contribution from neural crest. Between the arches the ectoderm and endoderm are in close apposition, forming the pharyngeal slits and pouches respectively. Development of pharyngeal arches is a complex process and different germ layers in each arch undergo a finely-orchestrated series of inductive interactions to generate the appropriate arch derivatives (Graham and Smith, 2001).
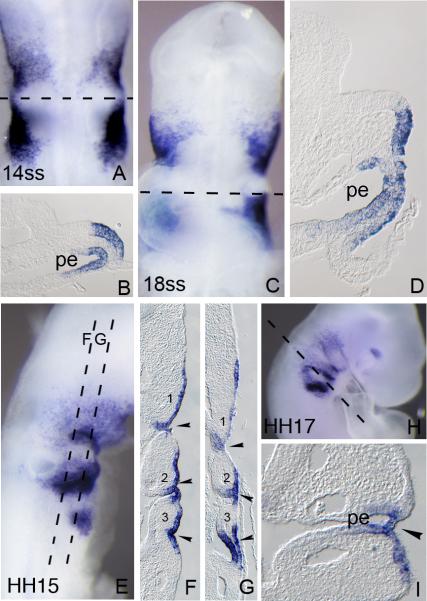
As described above, Foxi3 expression is down-regulated from the otic region at around HH stage 10. At this time, a second patch of Foxi3 expression appears lateral and posterior to the otic region (Figure 2D). Between HH stage 11-12 strong Foxi3 expression is seen in both the ectoderm and endoderm of the future pharyngeal arches (Figure 3A-D). A third, more posterior patch of Foxi3 expression is seen extending ventrally at HH stage 15. All three domains of expression extend well into the ventral regions of ectoderm corresponding to the pharyngeal arches (Figure 3E-G). After HH stage 15, Foxi3 starts to be down-regulated from the majority of the arch region in an anterior-posterior direction and becomes restricted to the pharyngeal pouch and cleft region between each arch. Faint Foxi3 expression is also seen in the future maxilla (Figure 3H) and strong expression is seen in a small portion of the dorsal optic cup.
Figure 3.
Foxi3 expression in the pharyngeal arches. Embryos were processed for whole mount in situ hybridization and then sectioned transversely or coronally at 18μm. Numbers in each large panel refer to the embryonic stage in number of somites (A-D) or the Hamburger and Hamilton stage (E-I). The level of each section is indicated by dashed lines. (A): Dorsal view of a 14ss embryo. (B) Transverse section of a 14ss embryo showing Foxi3 expression in the ectoderm and endoderm of the future second pharyngeal arch. No expression is seen in the otic cup. (C): Ventral view of an 18ss embryo. (D) Transverse section of an 18ss embryo showing Foxi3 expression in the ectoderm and endoderm corresponding to the future second pharyngeal arch. (E): Lateral view of an HH15 embryo, showing a third, more posterior region of Foxi3 expression in the arch region. (F and G): Coronal section of an HH15 embryo. Numbers indicate each pharyngeal arch. (H): Lateral view of HH17 embryo showing expression in all pharyngeal arches. Note that Foxi3 expression is down-regulated from the arches in a rostral-caudal direction. (I): Transverse section of an HH17 embryo showing Foxi3 expression in the second pharyngeal cleft. pe: pharyngeal endoderm.
2.3. Foxi2 expression in the developing otic and pharyngeal arch regions
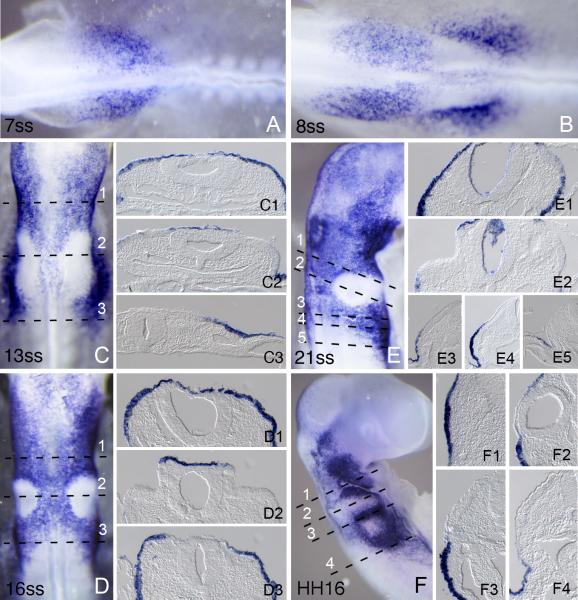
Unlike Foxi3, Foxi2 is not expressed in the early gastrulating chicken embryo. The earliest expression of Foxi2 can be seen at HH stage 9, where it occurs anterior to the future otic placode (Figure 4A), extending into the ectoderm at the future midbrain/trigeminal level. Shortly thereafter, a second domain of Foxi2 expression appears lateral to the otic placode and extends posteriorly into the ectoderm lateral to the first few pairs of somites (Figure 4B). Between stages 12-15, Foxi2 is expressed strongly in much of the cranial ectoderm including ectoderm overlying the neural tube, with the striking exception of the otic placode and vesicle, from which it is clearly excluded (Figure 4C-E). After stage 15, Foxi2 is down-regulated from most cranial ectoderm with the exception of the pharyngeal arch region. Like Foxi3, Foxi2 becomes restricted to the pharyngeal slits, although unlike Foxi3, Foxi2 is never expressed in pharyngeal endoderm.
Figure 4.
Foxi2 expression in cranial ectoderm at different developmental stages. Numbers in each large panel refer to the embryonic stage in number of somites or by by Hamburger and Hamilton stage designated by HH. Embryos were processed for whole mount in situ hybridization and then sectioned transversely at 18μm. The level of each section is indicated by dashed lines. (A) Expression at 7ss when Foxi2 expression is first seen anterior to the otic placode and extending into the presumptive trigeminal region. (B) Expression at 8ss when second patch of Foxi2 expression is seen lateral to the otic placode and extending caudally to the first few pairs of somites. (C) Expression at 13ss. Foxi2 extends more anteriorly and posteriorly in cranial ectoderm with the striking exception of the thickened otic region. (D) Expression at 16ss, where the exclusion of Foxi2 from the otic placode is even more pronounced. (E) Expression at 21ss where Foxi2 expression extends more anteriorly and is still expressed strongly in the cranial ectoderm with the exception of the otic cup. (F) Expression at HH16 (equivalent to 26-28 pairs of somites). Expression is down regulated from the most of the cranial ectoderm. Expression is now restricted to the pharyngeal arches.
3. EXPERIMENTAL PROCEDURES
3.1. Chicken Embryos
Fertilized chicken eggs were purchased from Ideal Poultry Breeding Farms (Cameron, TX), and stored at 13°C. Eggs were placed in a humidified incubator at 37.8°C to develop. Embryos were staged according to Hamburger and Hamilton (1992) or by counting the number of pairs of somites.
3.2. Whole mount in situ hybridization
Species-specific cDNA probes for the 3’ untranslated regions of chicken Foxi2 and Foxi3 were obtained from the UMIST Chick EST Repository (clone IDs chEST912M14 and chEST50h20). Chick embryos were fixed in 4% paraformaldehyde in PBS, pH7.2 overnight at 4°C or for two hours at room temperature. Embryos were then washed in PBS and stored in methanol at -20°C for no more than 5 days. Embryos were rehydrated in a series from methanol to phosphate-buffered saline (PBS, pH 7.4) containing 0.1% Tween-20 (PBST). Embryos were treated with 10μg/ml proteinase K for 25–30 min (15–17 min for embryos less than HH stage 8), washed gently and re-fixed in 4% paraformaldehyde/0.1% glutaraldehyde. After further washing in PBST, the embryos were pre-hybridized at 65°C in 50% formamide containing 1.3xSSC (buffered to pH 4.5 with citric acid), 50μg/ml yeast tRNA, 100 μg/ml heparin, 0.2% Tween-20, 0.5% CHAPS and 5 mM EDTA. After 1 hour, probe was added to the embryos to a final concentration of 1 μg/ml and incubated overnight. The embryos were washed three times with hybridization buffer for 1 h each at 65°C and then washed three times for 1 hour each at room temperature in MABT buffer (100 mM maleic acid pH 7.5, 150 mM NaCl, 0.1% Tween-20). The embryos were then incubated for 1 hour in MABT containing 20% sheep serum and 2% Roche Blocking Reagent. Sheep anti-digoxygenin antibody coupled to alkaline phosphatase (Roche) was added at a concentration of 1:2000 and the embryos incubated overnight. After washing, color development was carried out in alkaline phosphatase buffer (100mM Trip pH9.5, 50mM MgCl2, 100mM NaCl, 0.1% Tween-20) with NBT (338μg/ml) and BCIP (175μg/ml). After color development, stained embryos were re-fixed, washed for 10 minutes in methanol, 30 minutes in PBS + 0.1% Tween-20, embedded in 7.5% gelatin (300 Bloom) and 15% sucrose in PBS, and sectioned at 18μm.
HIGHLIGHTS.
Foxi3 transcription factor is expressed early in non-neural ectoderm
Foxi3 then becomes localized to the pre-placodal domain
Foxi3 is later restricted to the pharyngeal arch region
Foxi3 ultimately marks pharyngeal slits and clefts
Foxi2 is expressed in cranial ectoderm from stage 9 onwards
ACKNOWLEDGEMENTS
We thank Huiling Li and Hongyuan Zhang for excellent technical support. This work was supported by RO1 DC004675 (A.K.G.), F32 DC011672 (S.B.K.) and T32 HD055200 (S.B.K.).
Abbreviations
- HH
Embryonic stage according to the series of Hamburger and Hamilton (1992)
- ss
number of pairs of somites
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Baker CV, Bronner-Fraser M. Vertebrate cranial placodes I. Embryonic induction. Dev Biol. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- Fetterman CD, Rannala B, Walter MA. Identification and analysis of evolutionary selection pressures acting at the molecular level in five forkhead subfamilies. BMC Evol. Biol. 2008;8:>261. doi: 10.1186/1471-2148-8-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter S, Muta Y, Mak S, Rinkwitz S, Ladher RK. Progressive restriction of otic fate: the role of FGF and Wnt in resolving inner ear potential. Development. 2008;135:3415–3424. doi: 10.1242/dev.026674. [DOI] [PubMed] [Google Scholar]
- Graham A, Smith A. Patterning the pharyngeal arches. Bioessays. 2001;23:54–61. doi: 10.1002/1521-1878(200101)23:1<54::AID-BIES1007>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Hannenhalli S, Kaestner KH. The evolution of Fox genes and their role in development and disease. Nat. Rev. Genet. 2009;10:233–240. doi: 10.1038/nrg2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick SM. The cunning little vixen: Foxo and the cycle of life and death. Nat. Immunol. 2009;10:1057–1063. doi: 10.1038/ni.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulander M, Kiernan AE, Blomqvist SR, Carlsson P, Samuelsson EJ, Johansson BR, Steel KP, Enerbäck S. Lack of pendrin expression leads to deafness and expansion of the endolymphatic compartment in inner ears of Foxi1 null mutant mice. Development. 2003;130:2013–25. doi: 10.1242/dev.00376. [DOI] [PubMed] [Google Scholar]
- Hulander M, Wurst W, Carlsson P, Enerback S. The winged helix transcription factor Fkh10 is required for normal development of the inner ear. Nat Genet. 1998;20:374–376. doi: 10.1038/3850. [DOI] [PubMed] [Google Scholar]
- Hwang CH, Simeone A, Lai E, Wu DK. Foxg1 is required for proper separation and formation of sensory cristae during inner ear development. Dev. Dyn. 2009;238:2725–34. doi: 10.1002/dvdy.22111. [DOI] [PubMed] [Google Scholar]
- Jayasena CS, Ohyama T, Segil N, Groves AK. Notch signaling augments the canonical Wnt pathway to specify the size of the otic placode. Development. 2008;135:2251–61. doi: 10.1242/dev.017905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;15:142–146. [PubMed] [Google Scholar]
- Kaufmann E, Knochel W. Five years on the wings of fork head. Mech. Dev. 1996;57:3–20. doi: 10.1016/0925-4773(96)00539-4. [DOI] [PubMed] [Google Scholar]
- Kil SH, Streit A, Brown ST, Agrawal N, Collazo A, Zile MH, Groves AK. Distinct roles for hindbrain and paraxial mesoderm in the induction and patterning of the inner ear revealed by a study of vitamin-A-deficient quail. Dev Biol. 2005;285:252–71. doi: 10.1016/j.ydbio.2005.05.044. [DOI] [PubMed] [Google Scholar]
- Kwon HJ, Bhat N, Sweet EM, Cornell RA, Riley BB. Identification of early requirements for preplacodal ectoderm and sensory organ development. PLoS Genet. 2010;6:>e1001133. doi: 10.1371/journal.pgen.1001133. doi:10.1371/journal.pgen.1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Groves AK. Expression of mouse Foxi class genes in early craniofacial development. Dev. Dyn. 2004;231:640–6. doi: 10.1002/dvdy.20160. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Mohamed OA, Taketo MM, Dufort D, Groves AK. Wnt signals mediate a fate decision between otic placode and epidermis. Development. 2006;133:865–75. doi: 10.1242/dev.02271. [DOI] [PubMed] [Google Scholar]
- Overdier DG, Ye H, Peterson RS, Clevidence DE, Costa RH. The winged helix transcriptional activator HFH-3 is expressed in the distal tubules of embryonic and adult mouse kidney. J. Biol. Chem. 1997;272:13725–30. doi: 10.1074/jbc.272.21.13725. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Making senses development of vertebrate cranial placodes. Int. Rev. Cell Mol. Biol. 2010;283:129–234. doi: 10.1016/S1937-6448(10)83004-7. [DOI] [PubMed] [Google Scholar]
- Solomon KS, Kudoh T, Dawid IB, Fritz A. Zebrafish foxi1 mediates otic placode formation and jaw development. Development. 2003a;130:929–40. doi: 10.1242/dev.00308. [DOI] [PubMed] [Google Scholar]
- Solomon KS, Logsdon JM, Fritz A. Expression and phylogenetic analyses of three zebrafish FoxI class genes. Dev Dyn. 2003b;228:301–7. doi: 10.1002/dvdy.10373. [DOI] [PubMed] [Google Scholar]
- Streit A. StemBook [Internet] Harvard Stem Cell Institute; Cambridge (MA): 2008. The cranial sensory nervous system: specification of sensory progenitors and placodes. [PubMed] [Google Scholar]