Abstract
Rationale
In the failing heart, persistent β-adrenergic receptor (βAR) activation is thought to induce myocyte death by protein kinase A (PKA)-dependent and PKA-independent activation of calcium/calmodulin-dependent kinase II (CaMKII). β-Adrenergic signaling pathways are also capable of activating cardioprotective mechanisms.
Objective
This study used a novel PKA inhibitor peptide (PKI) to inhibit PKA activity to test the hypothesis that βAR signaling causes cell death through PKA-dependent pathways and cardioprotection through PKA-independent pathways.
Methods and Results
In PKI transgenic mice, chronic isoproterenol (ISO) failed to induce cardiac hypertrophy, fibrosis, myocyte apoptosis and depressed cardiac function. In cultured adult feline ventricular myocytes (AFVMs), PKA inhibition protected myocytes from death induced by β1-AR agonists by preventing cytosolic and SR Ca2+ overload and CaMKII activation. PKA inhibition revealed a cardioprotective role of β-adrenergic signaling via cAMP/EPAC /Rap1/Rac/ERK pathway. Selective PKA inhibition causes protection in the heart after myocardial infarction (MI) that was superior to β-blocker therapy.
Conclusion
These results suggest that selective block of PKA could be a novel heart failure therapy.
Keywords: Apoptosis, Ca2+/dependent protein kinase II, ERK2, EPAC
INTRODUCTION
Congestive heart failure (CHF) affects 5 million people in the US with high morbidity and mortality1. The poor pump function of the failing heart induces chronic activation of neuroendocrine systems that supports cardiac performance but can also activate death signaling to cause CHF progression.
Persistent activation of the sympathoadrenergic system (SAS) in CHF can cause adverse cardiac remodeling, cardiac myocyte death and fibrosis replacement2. Reducing myocyte death has been proposed as one of the mechanisms responsible for the beneficial effects of β-blockers in heart failure patients3. The mechanisms of β-adrenergic mediated myocyte death are still not clearly defined and are the focus of this study. Some studies suggest that PKA is the mediator of β-adrenergic induced myocyte apoptosis by altering Ca2+ regulation4, 5, while others have suggested that Ca2+/camodulin-dependent kinase II (CaMKII) can mediate β-adrenergic induced myocyte death through a PKA-independent process6–10. A cAMP sensor, exchange protein directly activated by cAMP (EPAC), is expressed in the heart and has been suggested to activate CaMKII independent of PKA6–8.
The hypothesis of this study is that β-adrenergic mediated myocyte death requires PKA activation and subsequently enhanced Ca2+ signaling, but is independent of EPAC. To test this idea, we designed a PKA-specific inhibition gene (a fusion gene containing the nucleotide sequence coding the amino acids 1–25 of PKIα and GFP (PKI-GFP)). PKI-GFP was expressed in the mouse heart or in cultured adult feline ventricular myocytes (AFVMs). Our major findings are that: (1) β-agonists activated both PKA and EPAC, and PKI-GFP inhibited only PKA activation; (2) β-adrenergic agonists induced myocyte death is blocked by PKI; (3) PKA inhibition prevented myocyte death induced by β-adrenergic agonists by abolishing β-adrenergic effects on myocyte Ca2+ handling; (4) β-AR induced CaMKII activation was dependent on PKA activation; (5) EPAC did not promote myocyte apoptosis but instead protected myocytes from apoptosis by activating the pro-survival signal ERK; (6) PKA inhibition was superior to a β-blocker, metoprolol, to improve cardiac function after myocardial infarction (MI); (7) Metoprolol eliminated the beneficial effects of PKI after MI. Our results suggest that selective inhibition of PKA would be an effective therapy in heart failure patients.
METHODS
A DNA oligo corresponding to the coding sequence for amino acids 1–25 of mouse protein kinase inhibitor α (PKIα) (mouse Entrez gene ID 18767) was synthesized and subcloned into a plasmid to make a PKI-GFP fusion gene. Amino acids 1–25 of PKIα have the PKA inhibitory domain11 but not the nuclear export signal12. Then an adenovirus containing the fusion gene and a transgenic mouse line overexpressing this fusion gene were established13. Doxycycline-containing (625ppm) chow was offered to breeding animals and preweaned pups. Transgenic and littermate control animals were used at the age of 4 months. To test β-adrenergic overstimulation on cardiac myocyte death, acute isoproterenol (ISO, 60mg/kg) or chronic ISO (60mg/kg/day, 3 weeks) were applied14. Echocardiography (ECHO), cardiac morphology, gravimetric measurements, tissue histology and TUNEL staining were done at the end of 3 weeks14. To explore PKA-dependent and –independent mechanisms of myocyte apoptosis induced by β-AR signaling, adult feline ventricular myocytes (AFVMs) were isolated, cultured and infected with AdGFP (control) or AdPKI-GFP15. The inhibition of PKA by PKI-GFP was determined with a nonradioactive PKA activity kit (Assay Design, Ann Arbor, MI) and cAMP production upon ISO stimulation was determined with [3H]-adenine and radioactivity incorporation into newly synthesized cAMP. Myocyte death was determined by trypan blue staining, rod/ball ratio counting, TUNEL and FLICA staining. Myocyte contractions and intracellular Ca2+ transients, Ca2+ currents and SR Ca2+ content were measured as previously described16. To determine the activity of PKA and CaMKII, phospholamban phosphorylation at Ser16 and Thr17, and CaMKII phosphorylation at Thr286 were determined with Western blot. Western blot was also used to determine activated Rap1 that bound to GTP (Rap1GTP), ERK and pERK. To determine the effect of β-blockade on the protection of PKI in post-myocardial infarction (MI) mice, littermate control and PKI DTG mice were injected with saline or metoprolol (20mg/kg BW/day) daily17 for 4 weeks. Cardiac function was followed weekly with ECHO.
An expanded “Materials and Methods” can be found in the Supplemental Data.
RESULTS
PKI-GFP is evenly expressed in the mouse heart to suppress PKA activity
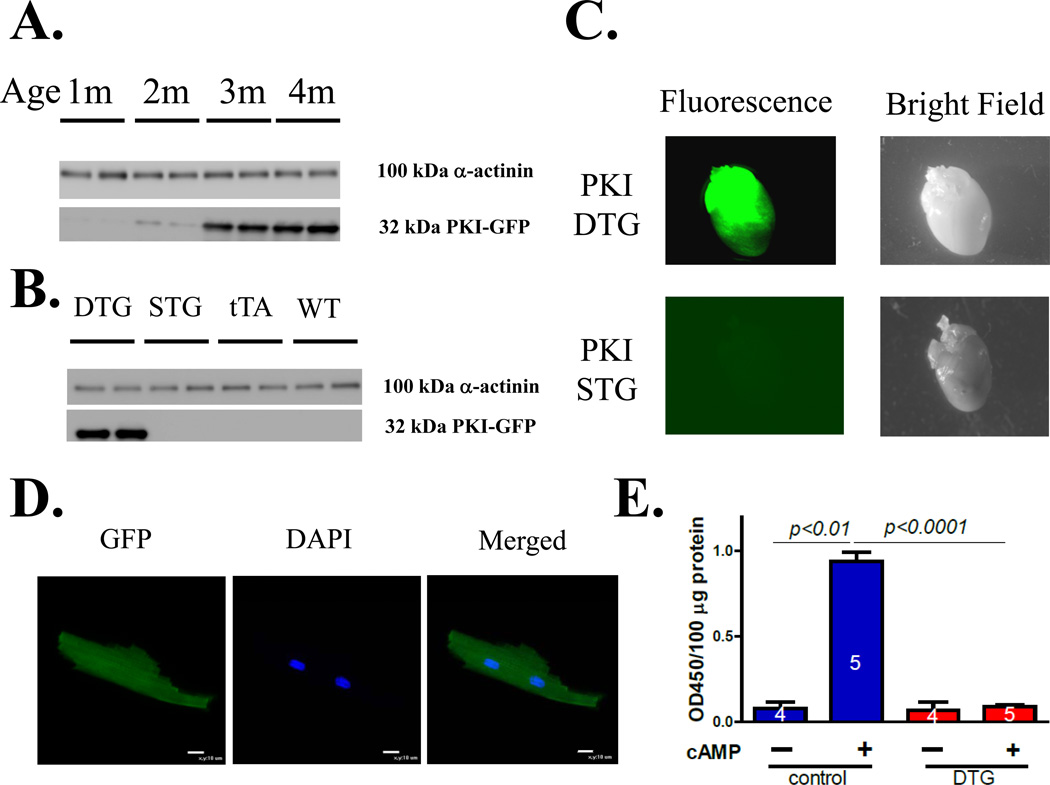
The PKI-GFP double transgenic (DTG) mouse began to express PKI-GFP at 2 months and had stable expression by 4 months of age (Figure 1A). PKI-GFP was expressed in DTG mice but not in PKI-GFP single transgenic (STG), tTA only and wild –type (WT) mice (Figure 1B). Homogenous GFP fluorescence was seen in DTG hearts but no GFP fluorescence was observed in PKI-GFP STG hearts (Figure 1C). In isolated ventricular myocytes, the GFP fluorescence was evenly distributed in the whole cell (Figure 1D). There was little PKA activity at baseline in both control and DTG hearts. However, when PKA activity in crude cardiac tissue extract was measured in the presence of 1µM cAMP, PKA activity was almost completely inhibitedin PKI transgenic samples (Figure 1E).
Figure 1. PKI –GFP overexpression in mouse hearts inhibits PKA.
A. The expression of PKI-GFP in double-transgenic (DTG) mice was stable at the age of 4 months; B. PKI-GFP was not expressed in ventricles of 4-month old single transgenic mice with PKI-GFP (no tTA) or tTA only, or wild type mice. C. GFP expression is homogenous in the DTG hearts but not expressed in the PKI STG hearts, agreeing with the Western blot results. D. Confocal images of a live DTG ventricular myocyte. PKI-GFP is evenly distributed in isolated PKI DTG myocytes. E. PKA activity without or with cAMP. PKA activity is inhibited by more than 90% in the crude extract from DTG hearts compared to control hearts.
PKI-GFP prevents cardiac dysfunction and myocyte death induced by isoproterenol
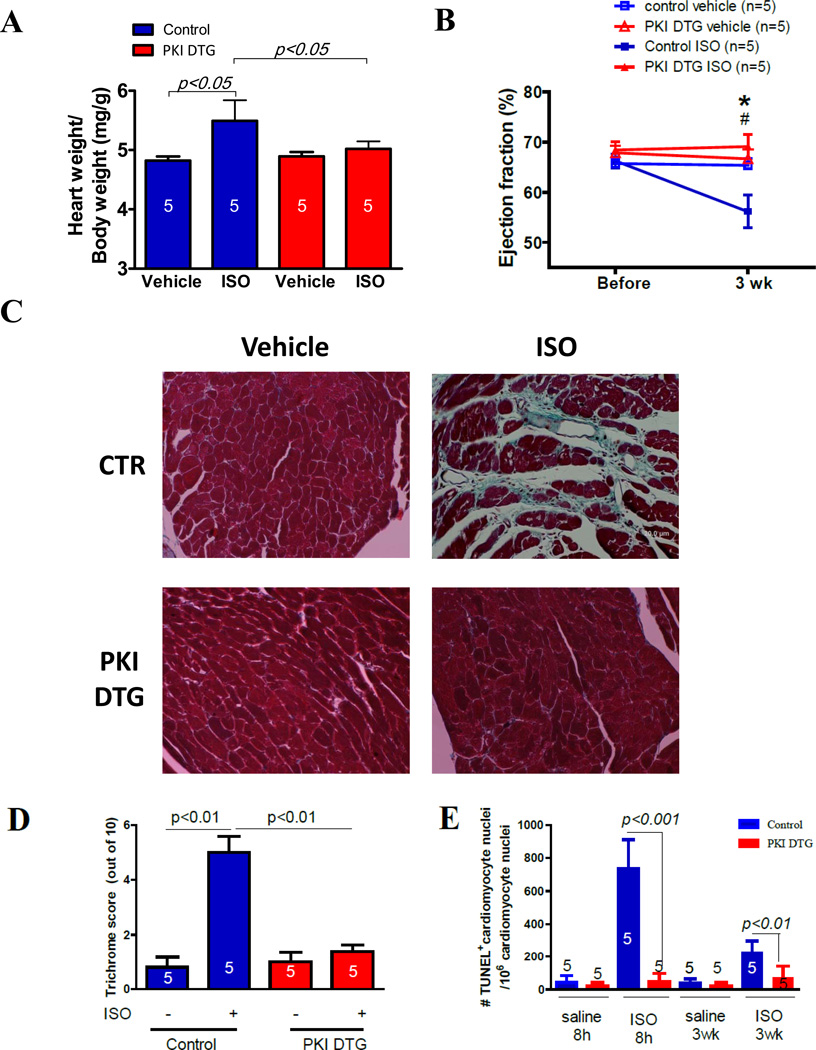
We first examined if β-adrenergic induced myocyte death was PKA dependent (in vivo) by measuring the effect of ISO on myocyte apoptosis in the PKI DTG mice. Chronic ISO infusion into PKI transgenic and control mice for 3 weeks induced cardiac hypertrophy and depressed cardiac function in control animals but not in PKI transgenic mice (Figure 2A and B). Chronic ISO infusion caused significant fibrosis in control cardiac tissue but not in PKI DTG tissue (Figure 2C and D). There were significant increases in TUNEL+ cardiac myocyte nuclei in control cardiac tissues but not in PKI DTG tissue (Figure 2E). A single injection of ISO into PKI DTG and control mice induced a higher percentage of TUNEL+ cardiac myocytes in control myocardium than in PKI DTG myocardium (Figure 2F).
Figure 2. PKA inhibition abolishes myocardial remodeling induced by isoproterenol infusion (3 weeks) in PKI DTG mice.
PKI prevents cardiac hypertrophy (A, Heart weight /body weight ratio), cardiac function depression (ejection fraction, B), fibrosis (C and D) and myocyte apoptosis (E). In E, some hearts were treated with ISO injection and some hearts were treated with ISO minipump for 3 weeks. In B, *: p<0.05, control ISO 3 weeks vs. control before ISO; #: p<0.05, control ISO 3 week vs. PKI ISO 3 weeks. The numbers in the bars of A, D, E are numbers of animals.
PKI-GFP overexpression in cultured adult feline ventricular myocytes (AFVMs) prevents PKA activation but does not affect ISO-induced cAMP production
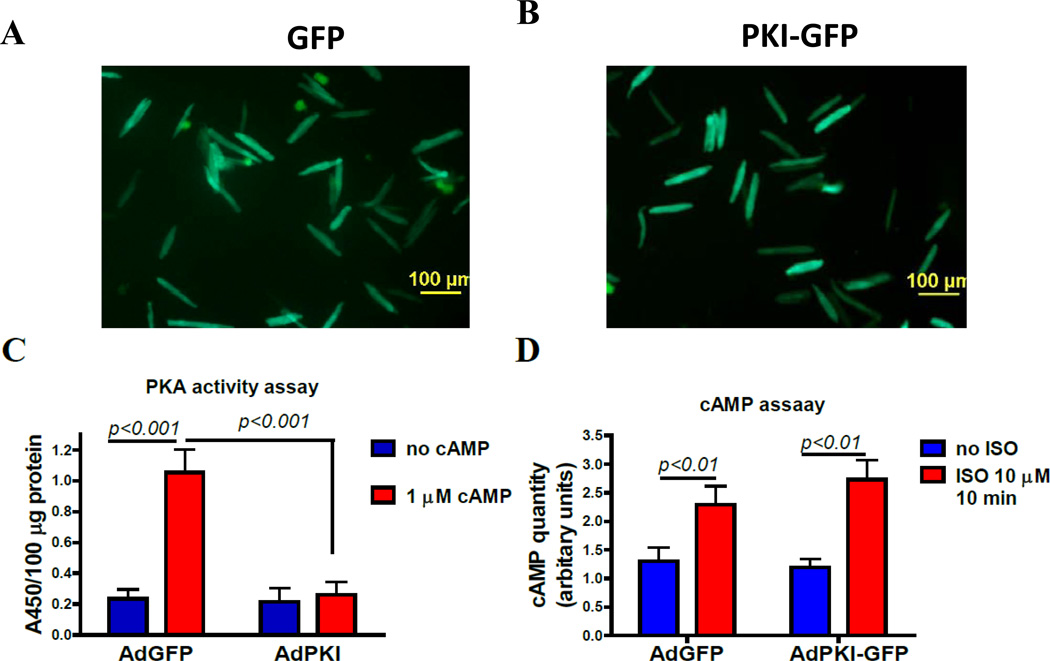
PKI-GFP should blunt β-agonist induced activation of PKA without altering cAMP production. To test this idea, AFVMs were infected with AdPKI-GFP or AdGFP. At 48 hours post infection, GFP and PKI-GFP were expressed (Figure 3A & B). Both PKI-GFP and GFP were evenly distributed through the cytoplasm of infected cells. The crude protein extract contained a low activity of PKA which was not different between the groups. 1µM cAMP increased the PKA activity in the crude extract from AdGFP infected cells but not in the crude extract from AdPKI infected cells (Figure 3C). When these two groups of myocytes were exposed to 10µM ISO for 10 minutes in the presence of a PDE inhibitor, IBMX, the total amount of cAMP produced was not different between groups (Figure 3D), showing no change of β-adrenergic signaling upstream of PKA.
Figure 3. PKI-GFP expression in cultured adult feline ventricular myocytes (AFVMs) inhibits PKA activity but not cAMP production.
AFVMs were infected with AdPKI-GFP or AdGFP (control) at MOI 100. A. and B. Fluorescent imaging of cultured AFVMs infected with AdPKI-GFP or AdGFP. More than 98% AFVMs were green and GFP fluorescence is evenly distributed in the cytoplasm. C. PKA activity in crude extract from GFP-VMs or PKI-VMs, without or with 1µM cAMP. D. cAMP production in AdGFP and AdPKI infected cells is not different.
PKI prevents myocyte apoptosis induced by β-adrenergic agonists in AFVMs
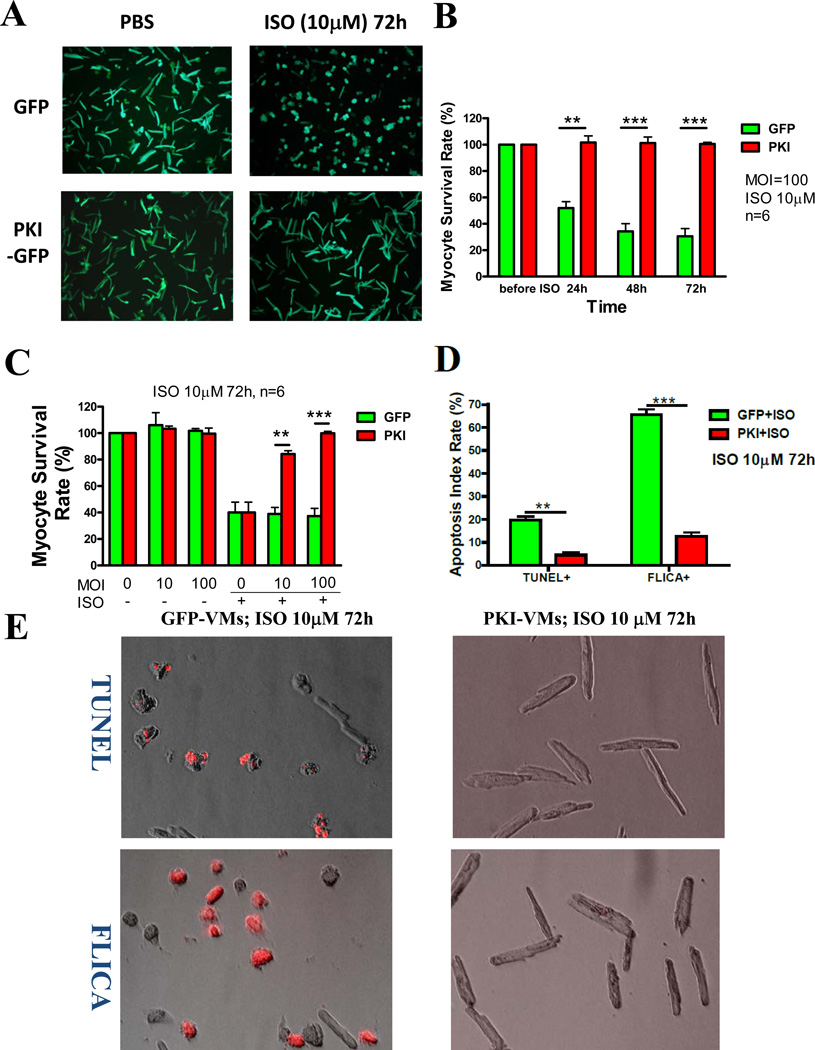
Excessive β-AR stimulation can lead to myocyte death via apoptosis 18. AdPKI-GFP infected AFVMs (PKI-AFVMs) were exposed to β-AR agonists to induce myocyte death. PKI-AFVMs were completely protected from cell death induced by ISO (a nonselective β1-AR and β2-AR agonist, 10µM) (Figure 4A &B). The survival of VMs exposed to 10µM ISO for 72 hours was increased when AdPKI-GFP MOI was increased from 10 to 100 (Figure 4C). This protection effect was associated with reduced myocyte apoptosis measured by TUNEL and FLICA (an index of caspase activity) (Figure 4D). These results do not agree with a previous study showing that exogenous PKA inhibitors could not rescue rodent myocyte death induced by β-AR activation 19. This could be due to the fact that our studies were performed with AFVMs which possess physiological properties similar to human ventricular myocytes and do not accumulate Ca2+ under unpaced culture condition20. Rodent myocytes accumulate Ca2+, have SR Ca2+ overload in culture and have a high rate of apoptosis without β-adrenergic agonists20. Any residual unblocked PKA effect would enhance SR Ca2+ overload in rodents while these effects are absent in AFVMs.
Figure 4. PKA inhibition protects AFVMs from death induced by β-adrenergic agonists.
A. ISO (10µM) treatment for 72 hours induced significant cell death (hypercontracted and ball-shaped) in GFP-VMs but not in PKI-VMs (remained rod-shaped). B. Average data showing ISO decreased cell survival in a time-dependent manner in GFP-VMs but had no effect on PKI-VMs. C. AFVMs were infected with AdGFP and AdPKI-GFP at MOI 10 or 100. VMs infected with AdPKI-GFP at MOI 100 had higher survival rate than VMs infected with AdPKI-GFP at MOI 10. D. Apoptotic rates of GFP-VMs and PKI-VMs challenged with ISO for 72h were evaluated with TUNEL and FLICA (Fluorochrome Inhibitor of Caspases) staining. TUNEL+ and FLICA+ myocytes were stained red. E. Example images of TUNEL and FLICA stained GFP-VMs and PKI-VMs. F. PKI protected myocytes from death induced by β1-adrenergic (ISO+ICI or dobutamine), β2-adrenergic (ISO+CGP or fenoterol) agonists and forskolin. *: p<0.05; **: p<0.01; ***: p<0.001.
β-adrenergic toxicity is mediated by β1-adrenergic receptor and linked to cAMP/PKA activation
Dobutamine (a β1-AR agonist), ISO+ICI 118551 (a β2-AR antagonist, 2µM), ISO+CGP 20712A (a β1-AR antagonist, 6µM), fenoterol (a β2-AR agonist, 10µM), and forskolin (an AC activator) were used to determine the effect of β1-AR vs. β2-AR activation on myocyte death (Figure 4F). Dobutamine, ISO+ICI, and forskolin induced myocyte death that was rescued by PKI-GFP. These results suggest that β1-AR activation and subsequent adenylyl cyclase (AC) and PKA activation are primarily responsible for myocyte death induced by ISO. In contrast, when myocytes were stimulated via β2-AR (ISO+CGP or fenoterol), only a small fraction of myocytes died. PKI also blocked this proapoptotic effect. These results agree with those studies showing that the β1-AR is the major mediator of β-adrenergic toxicity while β2-AR activation leads to much less myocyte death21. The new finding here is that the AC/cAMP/PKA signaling pathway is the exclusive pathway mediating myocyte death, at least in cultured AFVMs.
PKI prevents myocyte apoptosis induced by β-AR stimulation by reducing PKA-mediated SR Ca2+ overload
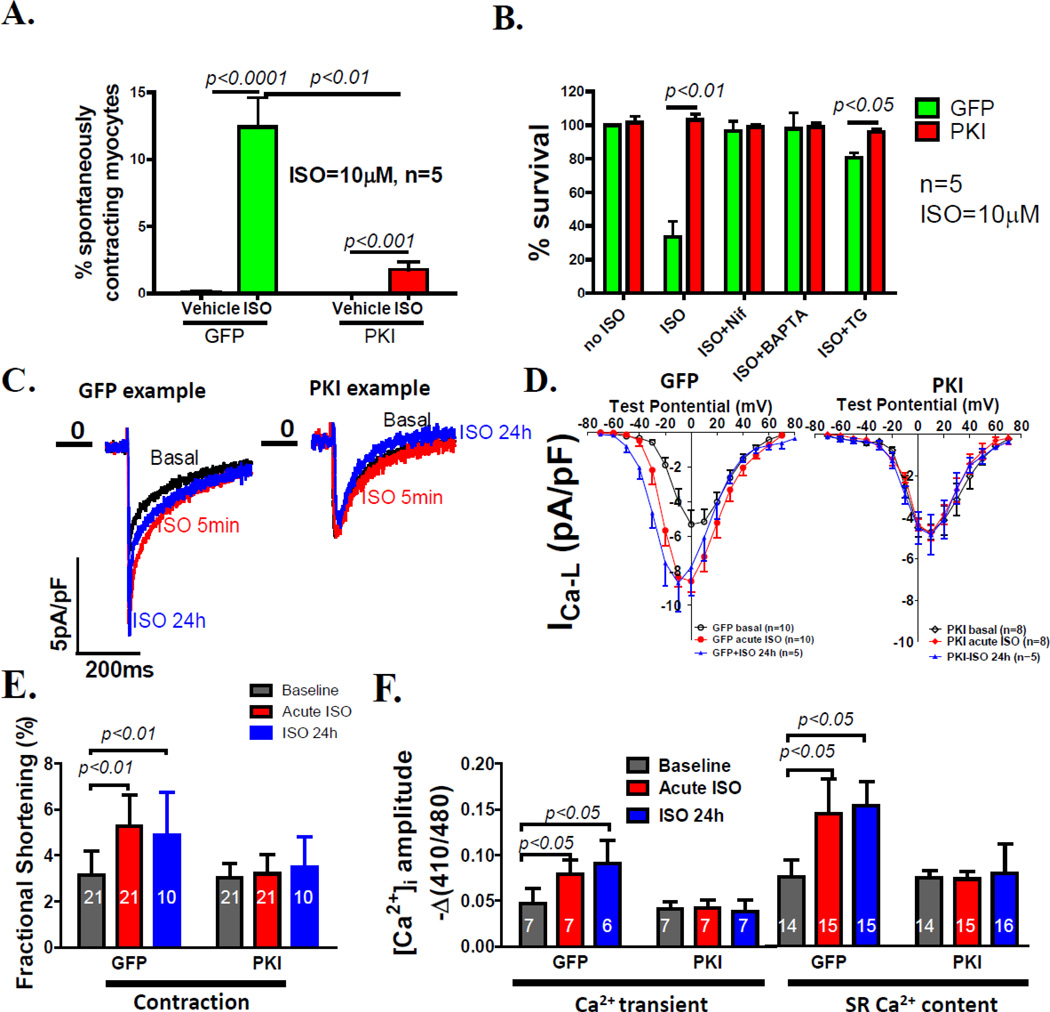
β-adrenergic agonists enhance SR Ca2+ by augmenting Ca2+ influx through the LTCC (ICa-L) and stimulating SR Ca2+ uptake through phospholamban phosphorylation22. We14, 15 and others 19 have found that this can lead to SR Ca2+ overload, which can cause myocyte death both in vitro 15 and in vivo 14. Exposure of AFVMs to ISO (10µM) for 1 hour increased the percentage of spontaneously contracting myocytes (SCMs) in GFP-VMs, indicating SR Ca2+ overload15. PKI significantly decreased the percentage of SCMs under this condition (Figure 5A). The LTCC blocker, nifedipine (13µM), decreased myocyte death induced by ISO and offered no further protection than PKI, indicating that PKI protects myocytes by reducing the increases in LTCC activity caused by β-adrenergic stimulation. Intracellular Ca2+ buffering (BAPTA-AM, 10µM) completely blocked GFP-VM death. A specific SERCA inhibitor, thapsigargin (TG, 10nM)15 also largely blocked ISO induced myocyte death and had no detrimental effects in PKI-VMs (Figure 5B).
Figure 5. PKA inhibition prevents myocyte Ca2+ overload to protect AFVMs from death induced by β-adrenergic agonists.
A. ISO (10µM) induced GFP-VMs to spontaneously contract, indicating Ca2+ overload. PKA inhibition significantly reduced the percentage of spontaneously contracting myocytes. In VMs not stimulated, there was almost no spontaneous contraction. B. Preventing ISO-induced Ca2+ overloading in GFP-VMs by nifedipine (an L-type Ca2+ channel blocker), BAPTA-AM (a Ca2+chelator), and thapsgargin (TG, an irreversible SERCA inhibitor) almost completely prevented myocyte death. C. Examples of maximum ICa-L without ISO (black curve), after acute (5 min ISO, red) and chronic ISO (blue, 24h) exposure. D. I–V relationships of the L-type Ca2+ currents (ICa-L) in GFP-VMs and PKI-VMs without treatment, treated with ISO acutely and chronically. PKA inhibition abolished both acute and chronic ISO effect on the ICa-L although both chronic and acute ISO increased ICa-L in GFP-VMs. E. Both acute and chronic ISO enhanced myocyte contractions (fractional shortenings) in GFP-VMs but not in PKI-VMs. F. Both acute and chronic ISO increased the amplitudes of Ca2+ transients and caffeine-induced Ca2+ transients (SR load) in GFP-VMs but not in PKI-VMs.
The effect of PKI on ISO effects on ICa-L, contraction, Ca2+ transients and SR Ca2+ content was measured. PKI abolished the acute stimulatory effects of ISO on myocyte ICa-L, fractional shortening, Ca2+ transients, and SR Ca2+ load (Figure 5C–F).
A previous report suggests that chronic β-adrenergic stimulation is able to enhance Ca2+ handling in cultured myocytes independent of PKA but dependent on Ca2+/CaMKII10. In our study, chronic ISO (10µM) exposure for 24 hours significantly increased ICa-L, fractional shortening, Ca2+ transient and SR Ca2+ content in GFP-VMs but not in PKI-VMs (Figure 5C–F). The blunted response to ISO in PKI-VMs was not due to changes in the expression of Ca2+ handling proteins (α1c, RyR2, calsequestrin, total PLB, NCX1 and SERCA; Figure 6 & Supplemental Figure II). These results indicate that in AFVMs the positive inotropic effect of chronic ISO stimulation are mediated by PKA and the protective effect of PKI against ISO induced myocyte death is largely mediated by preventing cytosolic and SR Ca2+ overload.
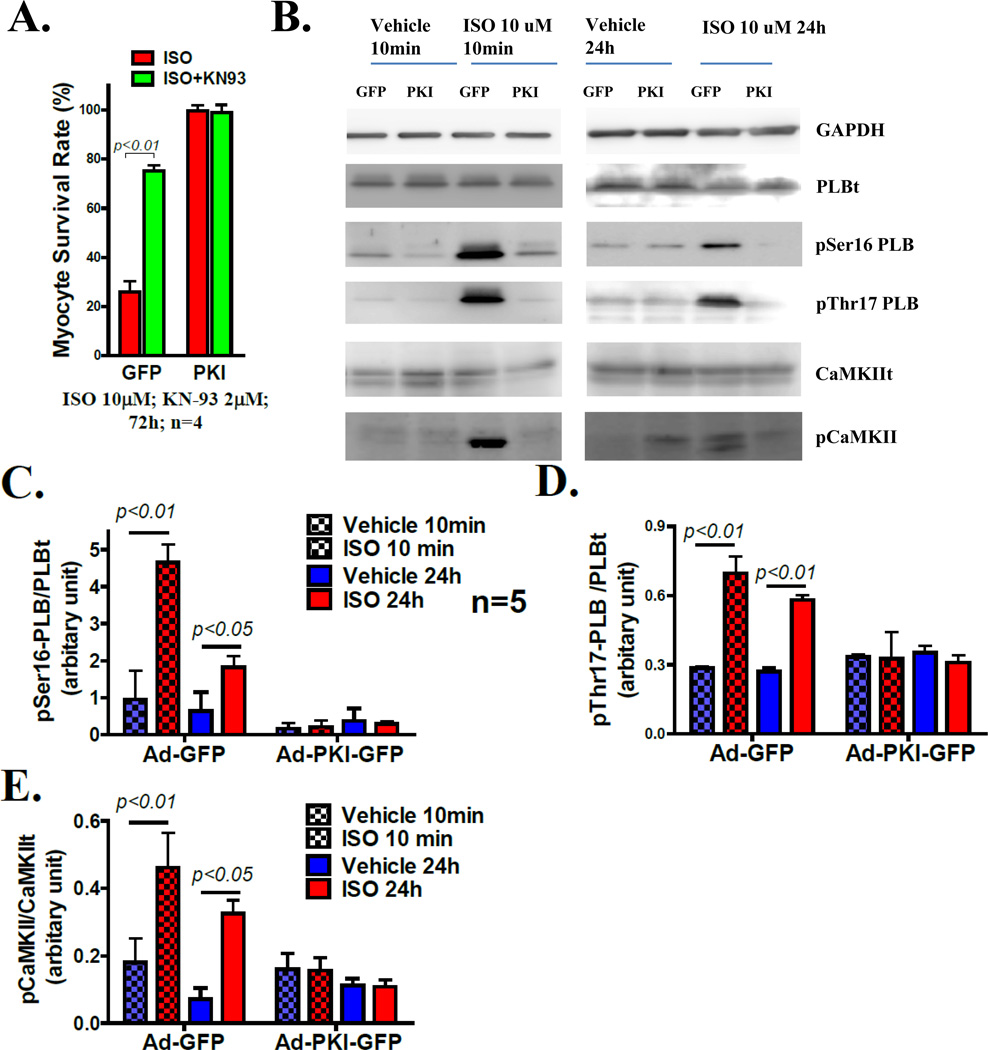
Figure 6. PKA inhibition prevents ISO induced CaMKII activation.
A. CaMKII inhibition by KN93 rescues most GFP-VMs from death induced by ISO (10µM). B. Western blots of total PLB, PLB phosphorylated at Ser16 (PKA) site and Thr17 (CaMKII) site in GFP-VMs and PKI-VMs upon acute and chronic ISO stimulation. C. Quantitation of PLB Ser16 (PKA site) phosphorylation in GFP- and PKI-VMs treated with acute and chronic ISO. D. Quantitation of PLB Thr17 (CaMKII site) phosphorylation in GFP- and PKI-VMs treated with acute and chronic ISO. E. Quantitaion of of Thr286 autophosphorylation of CaMKII upon acute and chronic ISO.
ISO induced CaMKII activation is downstream of PKA
It has been shown that CaMKII plays a critical role in adrenergic19, 23 and Ca2+ toxicity15 in cardiac myocytes. Previous studies have suggested that CaMKII can be activated independently of PKA during chronic β-adrenergic stimulation6–8, 10, 19. A central role for CaMKII as the mediator of chronic ISO-induced myocyte death was found in our system, since KN93, a CaMKII inhibitor, rescued most GFP-VMs from death induced by ISO (Figure 6A). KN93 did not improve PKI-VM survival after ISO exposure.
It is clear that β-AR stimulation can activate CaMKII via PKA dependent increases in ICa-L and SR Ca2+ 24. However, others suggest that CaMKII activation seen in myocytes chronically exposed to β-agonists is independent of PKA7, 8, 10, 19. We explored this issue by measuring PLB phosphorylation at Thr17 (a CaMKII specific phosphorylation site) and CaMKII autophosphorylation at Thr286 in GFP-AFVMs and PKI-AFVMs. Myocytes were exposed to ISO for either 10 minutes or 24 hours. PKI inhibited phosphorylation of PLB at both Ser16 (PKA site) and Thr17 (CaMKII site) and Thr286 phosphorylation of CaMKII after acute and chronic ISO treatment (Figure 6B–E). These results show that in our system CaMKII activation after β-AR stimulation is PKA-dependent.
To rule out that the possibility that PKI directly inhibits CaMKII, GFP-AFVMs and PKI-AFVMs were paced or co-infected with AdCavβ2a (adenovirus containing L-type calcium channel β2a subunit), or treated with an LTCC agonist (FPL 64176, 1µM) to activate CaMKII via directly increased cellular [Ca2+], without PKA activation. All these treatments induced PLB Thr17 phosphorylation in both GFP-VMs and PKI-VMs. FPL also enhanced CaMKII phosphorylation at Thr286 to the same extend in PKI and GFP-VMs. These data document that PKI does not inhibit CaMKII directly (Supplemental Figure III A–D).
Previous studies6 suggest that increased intracellular cAMP activates EPAC to activate CaMKII independent of PKA. These studies used 8-cp-TOME, a so-called “EPAC-specific” activator, to activate EPAC. There is evidence that 8-cp-TOME may inhibit phosphodieseterase25, leading to increased intracellular cAMP and thus PKA activation. 8-cp-TOME induced phosphorylation of PLB Ser16 (PKA site) and Thr17 (CaMKII site) in GFP-VMs in a dose-dependent manner. 8-cp-TOME could not activate PKA nor CaMKII in PKI-VMs (Supplemental Figure IV).
Collectively these results suggest that CaMKII activation induced by β-adrenergic stimulation is mediated by PKA in AFVMs. To determine if the increase in Ca2+ influx promoted by PKA activation is responsible for CaMKII activation, myocytes were treated with nifedipine (13µM) and ISO (10µM). Nifedipine significantly reduced PLB Thr17 phosphorylation in GFP-AFVMs treated with ISO (Supplemental Figure III E & F), consistent with our conclusion that increased Ca2+ influx caused by β-adrenergic stimulation activates CaMKII.
β-agonists induce cardioprotection in PKI treated myocytes by activating EPAC
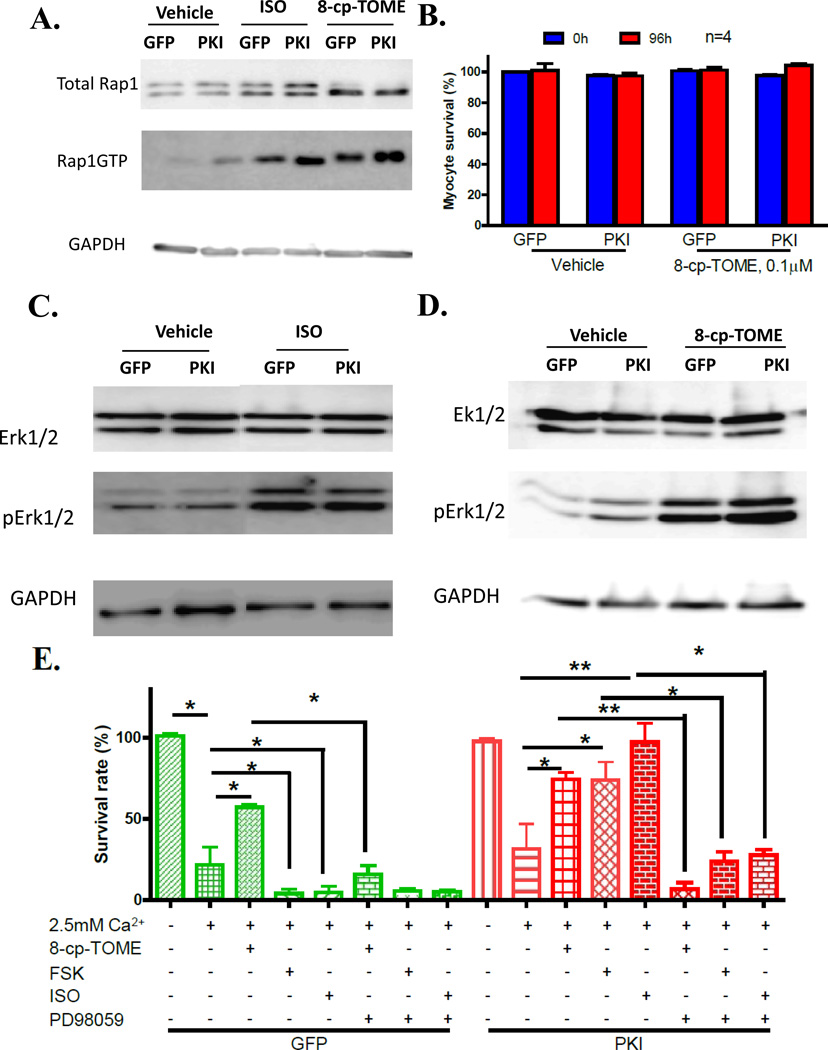
β-agonists increase cAMP that is able to activate EPAC. However, EPAC specific effects on myocytes are difficult to evaluate because of parallel PKA activation. In our PKI-VMs, PKA activity is inhibited and this provides a system to evaluate if EPAC regulates cell death signaling. EPAC, as a GTP exchange factor, activates Rap1-GTPase by catalyzing the formation of Rap1-GTP which activates ERK. As predicted, both ISO and 8-cp-TOME exposure increased the amount of Rap1-GTP (Figure 7A), indicating EPAC activation. High extracellular Ca2+ ([Ca2+]o =2.5mM) induced myocyte death at an equal rate in VMs infected with AdGFP and AdPKI, indicating high extracellular Ca2+ induced VM death is PKA independent. The EPAC activator 8-cp-TOME (0.1µM, a concentration not activating PKA, Supplemental Figure IV) did not have any effect on myocyte survival in both GFP-VMs and PKI-VMs in normal [Ca2+]o (Figure 7B). 8-cp-TOME protected GFP-VMs and PKI-VMs from death induced by high [Ca2+]o, indicating EPAC offers protection on VMs. In GFP-VMs, forskolin and ISO increased Ca2+-mediated myocyte death. In PKI-VMs, forskolin and ISO protected myocytes from Ca2+-mediated death and the effect was equivalent to that of 8-Cp-TOME in GFP-VMs (Figure 7 E). These data show that β-adrenergic agonists activate proapoptotic signaling via PKA/CaMKII signaling and in parallel activate cardioprotective signaling via EPAC.
Figure 7. EPAC activation protects myocytes from apoptosis induced by high extracellular Ca2+ through the ERK signaling pathway.
A. ISO activates EPACs in both GFP-VMs and PKI-VMs, as indicated by an increase in Rap1-GTP after ISO or 8-cp-TOME (0.1µM) treatment. B. EPAC activation by 8-cp-TOME did not induce myocyte death. (C) and (D). Western blots of total ERK1/2, pERK1/2 in GFP- and PKI-VMs stimulated with ISO (C) or 8-cp-TOME (D) showing ISO and 8-cp-TOME enhanced ERK1/2 phosphorylation independent of PKA. E. GFP-VMs and PKI-VMs challenged with 2.5mM Ca2+. PKI did not protect myocytes from death induced by high extracellular Ca2+. 8-cp-TOME protected myocytes from Ca2+ mediated death. FSK and ISO further promoted myocyte death in GFP-VMs treated with 2.5mM Ca2+. In PKI-VMs challenged with high extracellular Ca2+, 8-cp-TOME, ISO and FSK all reduced myocyte death. ERK1/2 inhibition by PD98059 (10µM) abolished the protective effect of 8-cp-TOME, FSK and ISO in PKI-VMs and also the protection of 8-cp-TOME in GFP-VMs.*: p<0.05; **: p<0.01.
EPAC activation induces ERK phosphorylation and phosphorylated ERK protects various cells from death19, 26–29. ERK phosphorylation was increased in both GFP-VM and PKI-VMs exposed to ISO and 8-cp-TOME (0.1µM, an EPAC specific activator at low concentrations, Supplemental Figure IV) and Figure 7C and D), indicating that this process is PKA-independent (Figure 7B & C). To confirm that activated ERK is the cardioprotective mediator, an ERK inhibitor, PD 98059, was used to pre-treat AFVMs before the exposure to ISO or 8-cp-TOME and high extracellular Ca2+. PD98059 almost completely abolished the protective effects of 8-cp-TOME in both GFP-VMs and PKI-VMs. The protective effect of ISO in PKI-VMs was also abolished by PD98059 (Figure 7E). These results strongly support the idea that ERK phosphorylation, downstream of EPAC activation, mediates the protective effects of adrenergic activation.
β-blockade eliminates the protective effect of β-adrenergic activation
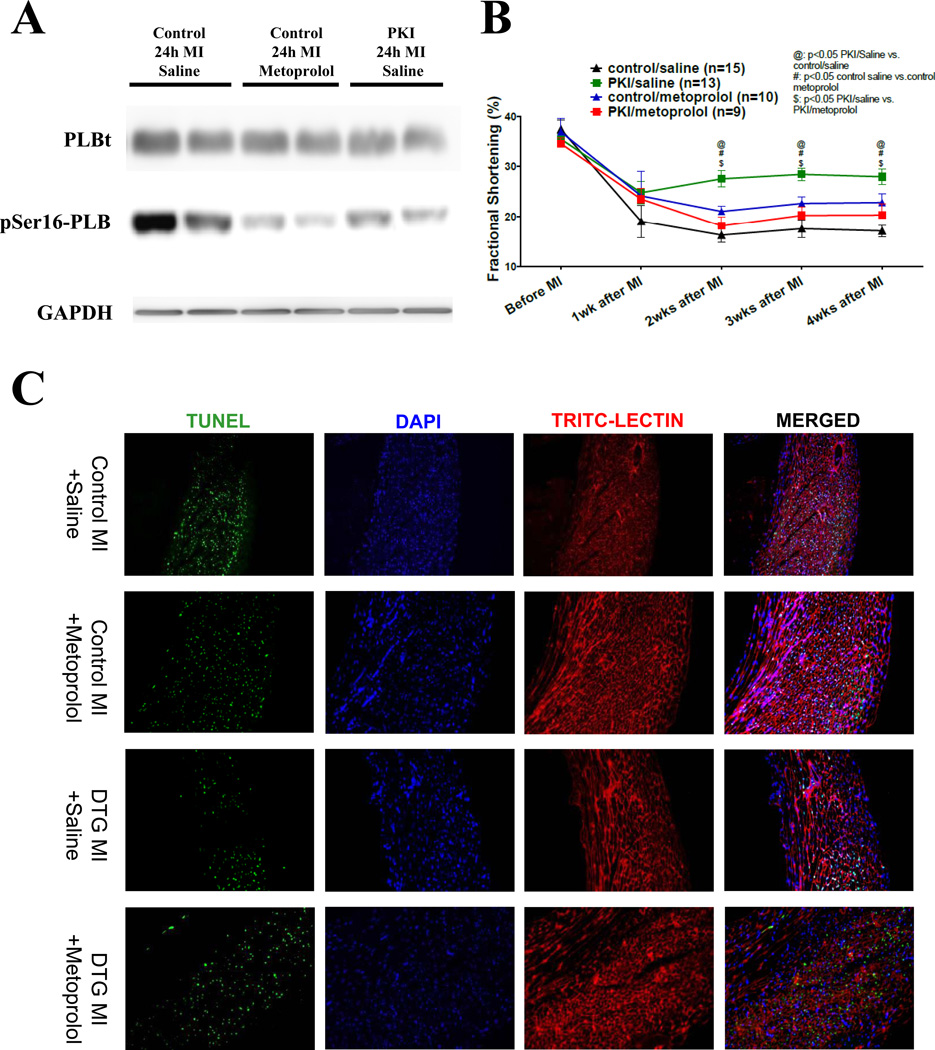
The present results suggest that β1-AR agonists can induce cell death signaling through PKA and cardioprotective signaling through EPAC/ERK. β1-AR antagonist therapies could abolish both cardiotoxic and cardioprotective features of β1-AR activation. Selective block of PKA with PKI in cardiovascular disease could preserve cardioprotective aspects of β1-AR signaling to provide benefit beyond β-AR antagonists. To test this idea myocardial infarction (MI) was induced in WT and PKI-DTG mice, with and without concomitant β1-AR antagonist therapy. We used a β1-AR blocker, metoprolol, to induce similar reductions of PKA activity as our PKI-GFP gene does after MI (Figure 8A). MI caused a reduction in cardiac function in all animals and both β-blocker and PKI improved function. However, PKI-DTG animals had better cardiac function than β-blocker treated animals. It seems that metoprolol at the dose used (20mg/kg BW/day) decreased some but did not abolish the beneficial effects of PKI, suggesting that it blocked some but not all cardioprotective adrenergic signaling (Figure 8B).
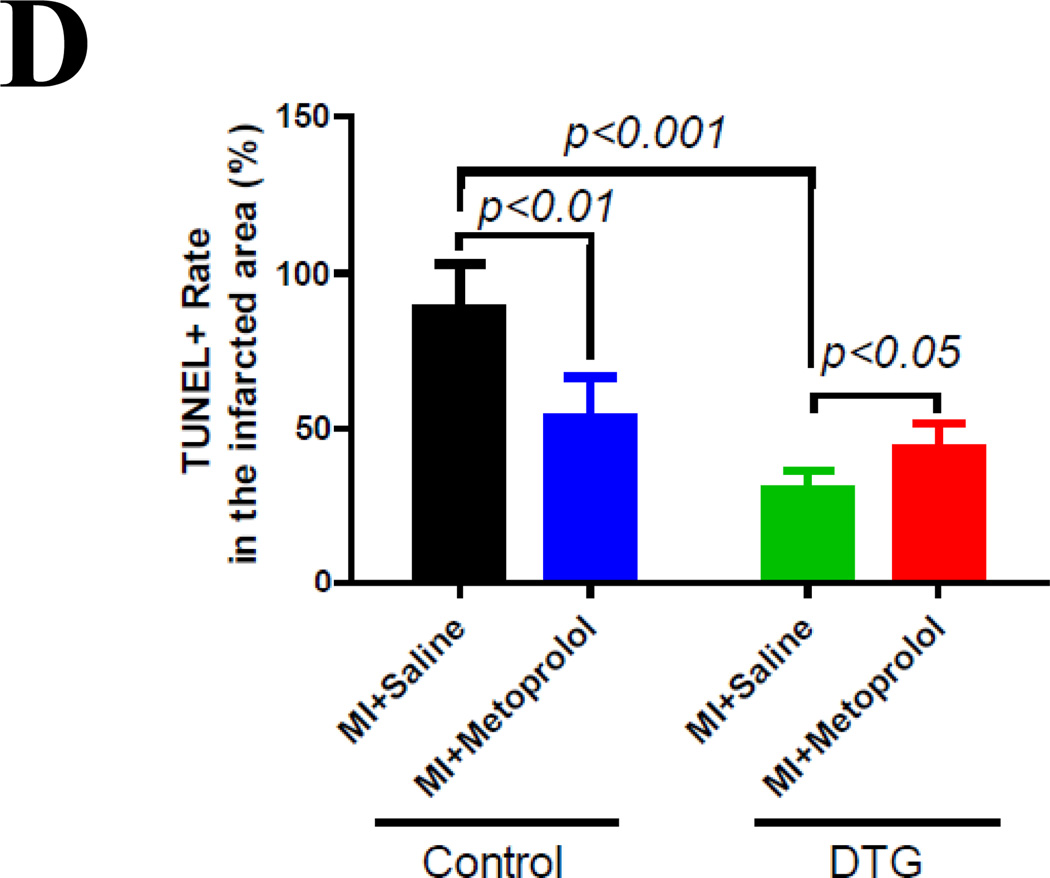
Figure 8. Metoprolol, a β1-blocker, reduces the protection of PKI in mice with myocardial infarction (MI).
(A). PLB phosphorylation at the PKA site (Ser16) at 24 hours post MI in mice. A dose of metoprolol that induced similar PKA inhibition (revealed by similar PLB phosphorylation at the Ser16 site) as PKI was used. (B). Cardiac function (fractional shortening) in control and PKI DTG MI mice receiving saline or metoprolol treatment. (C). Immunostaining of apoptotic nuclei (TUNEL, green), nuclei (DAPI, blue) and cell membrane (TRIC-LECTIN, red) in infarcted zone at 2 days after coronary artery ligation. (D). TUNEL rates in the infarcted zone in control and PKI DTG mice receiving saline or metoprolol treatments. @: p<0.05 PKI/saline vs. control saline; #: p<0.05 control saline vs. control metoprolol; $: PKI saline vs. PKI metoprolol.
DISCUSSION
Common cardiovascular diseases, such as hypertension and ischemic heart disease, increase the contractile demand of the heart and the SAS is activated to maintain basal cardiac output. Persistent SAS activation is associated with myocyte death and cardiac decompensation, culminating in heart failure. How persistent activation of β-ARs induces myocyte death is not clearly defined4, 5, 19 and was the topic of this study. The specific roles of downstream β-AR signaling effectors (PKA, CaMKII and EPAC) were examined. A novel PKA inhibition gene (PKI-GFP) was developed to inhibit PKA in vivo (transgenic mouse) and in vitro (cultured AFVMs infected with AdPKI-GFP). The new findings in this study are: (1) PKI-GFP can inhibit β-AR mediated activation of PKA; (2) PKA inhibition with PKI reduces β-adrenergic agonist induced myocyte death by preventing myocyte Ca2+ overload; (3) Inhibition of PKA eliminates β-AR induced CaMKII activation; (4) β-AR mediated increases in cAMP activates EPAC, and this exerts an ERK-dependent protective effect on myocyte death; (5) PKI protects the heart after MI and a β1-AR antagonist, metoprolol, reduced the protection exerted by PKI. Collectively, these results show that β–AR signaling can induce both cell death (through PKA, CaMKII and SR Ca2+ overload) and cardioprotection (through EPAC).
PKA-dependent activation of CaMKII is the mediator of myocyte apoptosis induced by β-adrenergic agonists
Previous studies support the conflicting ideas that CaMKII can be activated by either PKA-dependent30 or PKA-independent6–8, 10, 30, 31 mechanisms following β-adrenergic stimulation30. PKA-dependent activation of CaMKII is brought about by an increase in Ca2+ influx through the L-type Ca2+ channel after PKA-dependent phosphorylation30. Recently, it has been reported that EPAC, a cAMP sensor, can activate CaMKII independently of PKA activation in cardiac myocytes6–9. This is an important topic since CaMKII activation is required for myocyte apoptosis induced by β-adrenergic stimulation19, 32, 33. Inhibition of CaMKII in vivo significantly reduced myocyte apoptosis after isoproterenol stimulation23, suggesting that CaMKII would be a useful therapeutic target in CHF. Our results show that β-adrenergic stimulation enhances the phosphorylation of the CaMKII site (Thr17) on phospholamban in normal cardiac myocytes. In the presence of PKA inhibition, β-adrenergic stimulation had almost no effect on CaMKII autophosphorylation and PLB Thr17 phosphorylation. The present experiments show that when the β-adrenergic induced increase in Ca2+ influx through the L-type Ca2+ channel is prevented by PKI or blocked by nifedipine, CaMKII activity was eliminated. These results suggest that at least in PKI mice and PKI-infected AFVMs, CaMKII activation is dependent on PKA-mediated increase in LTCC activity and subsequent increase in [Ca2+]i. We also show that in the presence of PKA inhibition, β-AR agonists activate Rap1 downstream of EPAC activation, but CaMKII is not activated. These results show that EPAC does not cause CaMKII activation under our experimental conditions.
The present results contrast with those studies suggesting a PKA-independent, EPAC-mediated CaMKII activation during β-adrenergic stimulation6–10. The bases for these disparate results are not clear but might be due to differences in species, experimental conditions (e.g., concentrations of isoproterenol and 8-cp-TOME) and methods to inhibit PKA. Studies suggesting EPAC mediated CaMKII activation have used rodent myocytes in long-term culture. These preparations can be problematic because rodent myocytes accumulate Ca2+ when not paced. This results from their high intracellular Na+ which promotes Ca2+ entry via reverse mode of Na+/Ca2+ exchange20. Therefore, cultured rodent myocytes have SR Ca2+ overload, as evidenced by the spontaneous SR Ca2+ sparks that are exhibited by these preparations. The persistently high intracellular Ca2+ in these cultured rodent myocytes could activate CaMKII. Our study used AFVMs which like humans have lower intracellular Na+ 20, 34 and there is no Ca2+ accumulation or spontaneous SR Ca2+ release in long-term culture. The low basal Ca2+ state of AFVMs allowed us to dissect the role of PKA in mediating β-adrenergic induced CaMKII activation. We also found that 8-cp-TOME can activate PKA possibly by indirectly inhibiting phosphodiesterase as suggested by one previous study25. The Ki values for PDE1B, PDE2 and PDE6 of 8-cp-TOME are 8.6µM, 15µM and 3.5µM, respectively. Therefore, the interpretation of the results obtained with 10µM 8-cp-TOME should be cautious and adequate PKA inhibition should be ensured when evaluating EPAC effects with 8-cp-TOME. Our results clearly showed that in AFVMs when PKA activity is adequately inhibited, 8-cp-TOME cannot activate PKA and CaMKII (supplement Figure IV).
EPAC is a cardioprotective feature of β-adrenergic agonists
EPAC1 and EPAC2 are highly expressed in the heart but their functions are not well known. They are the guanodine exchange factor for the small GTPases, Rap1 and Rap2. EPAC is thought to exert both pro- and anti-apoptotic effects on cells depending on the cell types and conditions35. The role of EPACs in cardiac myoycte apoptosis has not been reported to date. As discussed, EPACs have been suggested to be involved in myocyte apoptosis by activating the proapoptotic multifunctional CaMKII in cardiac myocytes exposed to β-adrenergic agonists36. Our studies clearly indicate that prolonged treatment of cultured feline myocytes with 0.1µM 8-cp-TOME (a concentration sufficient to activate EPAC but not PKA, see Figure 7A) does not induce myocyte death (Figure 7B). Our results show that EPAC protects myocytes from death induced by high extracellular Ca2+. EPAC mediated ERK1/2 activation is linked to this protective effects. ERK1/2 activation downstream to EPAC activation has been reported in other cell types29.
PKI inhibition of PKA protects the heart after MI
Excessive adrenergic activation in cardiac disease is linked to heart failure progression, and β-AR antagonists improve outcome in CHF patients3. Part of the beneficial effect offered by PKA inhibition may be related to both the reduction of Ca2+ influx and the inhibition of SR Ca2+ uptake to prevent SR Ca2+ overload. In contrast, the effectiveness of LTCC blockers in treating failing hearts are controversial37. This discrepancy could be due to the fact that LTCC blockers exerts more potent effects on the vascular system than on the heart at the clinical doses37. LTCC blockers also have negative inotropic effect that has to be avoided when treating diseased heart. These effects cause a reactive excitation of the SAS to increase serum catecholamines and even cardiac attack. PKA inhibition effectively and specifically reduces Ca2+ influxes in cardiac myocytes. Therefore, clinically used doses of LTCC blockers cannot be as effective as cardiac specific PKA inhibition to reduce Ca2+ influx and SR Ca2+ content to protect stressed hearts.
PKA inhibition could also protect post-MI hearts by decreasing the heart rate to reduce the energy consumption38. Although at baseline, we did not observe difference in heart rates between control and DTG mice (basal HR: control 560±16bpm, n=8; DTG: 544±15bpm, n=8), DTG hearts had reduced response to ISO stimulation (HR after ISO (2mg/kg BW, i.p.): control 752±24bpm, n=8; DTG: 603±14bpm, n=8). Our study suggests that the detrimental aspects of β-AR signaling in HF are mediated by PKA signaling, while parallel PKA-independent signaling is cardioprotective. Our data suggest that beneficial effects of β-adrenergic activation would be reduced with the use of β-adrenergic antagonists (Figure 8). These beneficial effects could result from cAMP-mediated effects via EPAC or from “biased ligand” effects shown by others39.
CONCLUSION
The present study shows that chronic exposure of the heart to β-adrenergic agonists, as occurs in heart failure, causes myocyte death. The mechanism involves cAMP mediated activation of PKA and resultant increase in Ca2+ influx and SR Ca2+ load. Ca2+ mediated activation of CaMKII is dependent on PKA activation. β-adrenergic mediated increase in cAMP also activates EPAC to induce a cardioprotective effect. These results suggest that selective inhibition of excessive PKA activation could be an effective CHF therapy.
Supplementary Material
Novelty and Significance.
What Is Known?
The β-adrenergic/sympathetic system is constantly activated when the heart is under stress.
Persistently activated β-adrenergic system induces chronic loss of contractile heart cells (cardiomyocytes) via apoptosis.
PKA, CaMKII and EPAC are activated during chronic β-adrenergic stimulation and CaMKII is required for β-adrenergic agonist induced cardiac myocyte apoptosis but the roles of PKA and EPAC are still controversial.
What New Information Does This Article Contribute?
PKA inhibition prevents myocyte death induced by β-adrenergic stimulation and myocardial infarction in vivo and in vitro.
CaMKII is activated by increased cytosolic Ca2+ that is brought about by PKA to mediate myocyte apoptosis.
EPAC activation in the presence of PKA inhibition protects cardiomyocytes from death through the prosurvival ERK signaling pathway.
Chronic activation of the β-adrenergic system after cardiac stress leads to the loss of contractile heart cells (cardiomyocytes), an important contributor to the progression of heart disease. Though PKA, CaMKII and EPAC are three effectors activated by the β–adrenergic system, the roles of these molecules in β-adrenergic induced myocyte death are controversial. In the study, we used a genetic tool to suppress PKA activity in cardiomyocyte in a transgenic mouse model and in cultured myocytes to explore their roles. We have proven that PKA is an important mediator of myocyte death and myocardial remodeling after β-adrenergic agonist challenge and myocardial infarction. The proapoptotic mediator CaMKII during β-adrenergic stimulation is activated by increased cellular Ca2+ caused by PKA. Furthermore, for the first time, we show that EPAC activation protects myocytes from death through the prosurvival ERK signaling and PKA inhibition provides better protection than metoprolol at the dose offering similar PKA activity reduction. Collectively, our data show that the β-adrenergic system carries both detrimental and protective effects when activated, which is a novel finding. Therefore, PKA inhibition as a strategy to abolish the harmful signaling but preserves the protective signaling could be a novel approach for heart disease treatment.
Acknowledgments
SOURCE OF FUNDING
This study was supported by NIH HL088243 and AHA 0730347N to XC.
Non-standard Abbreviations
- AC
adenylyl cyclase
- AdCavβ2a
adenovirus containing L-type calcium channel β2a subunit
- AdPKI
adenovirus containing PKI-GFP fusion gene
- AdGFP
adenovirus containing GFP gene
- AFVMs
adult feline ventricular myocytes
- β–AR
β-adrenergic receptor
- β–ARS
β–adrenergic system
- CaMKII
Ca2+/calmodulin-dependent kinase II
- cAMP
cyclic AMP
- CHF
congestive heart failure
- EPAC
exchange protein directly activated by cAMP
- FLICA
fluorochrome inhibitor of caspases
- GFP-AFVMs
adult feline ventricule myocytes infected with AdGFP
- ISO
isoproterenol
- LTCC
L-type calcium channel
- MI
myocardial infarction
- PKA
protein kinase A
- PKI
PKA inhibitor peptide
- PKI-AFVMs
adult feline ventricular myocytes infected with AdPKI
- PLBt
total phospholamban
- pS16-PLB
phospholamban phosphoryated at Serine 16 site
- pT17-PLB
phospholamban phosphorylated at Threonine 17 site
- RAAS
renin-angiotensin-adolsterone system
- RyR
ryanodine receptor
- SAS
sympathoadrenergic system
- SCM
spontaneously contracting myocytes
- TG
thapsigargin
- VM
ventricular myocytes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None.
REFERENCES
- 1.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. Acc/aha 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: A report of the american college of cardiology/american heart association task force on practice guidelines (writing committee to update the 2001 guidelines for the evaluation and management of heart failure): Developed in collaboration with the american college of chest physicians and the international society for heart and lung transplantation: Endorsed by the heart rhythm society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 2.Francis GS. Pathophysiology of chronic heart failure. The American journal of medicine. 2001;110(Suppl 7A):37S–46S. doi: 10.1016/s0002-9343(98)00385-4. [DOI] [PubMed] [Google Scholar]
- 3.Sabbah HN. Biologic rationale for the use of beta-blockers in the treatment of heart failure. Heart failure reviews. 2004;9:91–97. doi: 10.1023/B:HREV.0000046363.59374.23. [DOI] [PubMed] [Google Scholar]
- 4.Communal C, Singh K, Pimentel DR, Colucci WS. Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the beta-adrenergic pathway. Circulation. 1998;98:1329–1334. doi: 10.1161/01.cir.98.13.1329. [DOI] [PubMed] [Google Scholar]
- 5.Iwai-Kanai E, Hasegawa K, Araki M, Kakita T, Morimoto T, Sasayama S. Alpha- and beta-adrenergic pathways differentially regulate cell type-specific apoptosis in rat cardiac myocytes. Circulation. 1999;100:305–311. doi: 10.1161/01.cir.100.3.305. [DOI] [PubMed] [Google Scholar]
- 6.Mangmool S, Shukla AK, Rockman HA. Beta-arrestin-dependent activation of ca(2+)/calmodulin kinase ii after beta(1)-adrenergic receptor stimulation. The Journal of cell biology. 2010;189:573–587. doi: 10.1083/jcb.200911047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oestreich EA, Malik S, Goonasekera SA, Blaxall BC, Kelley GG, Dirksen RT, Smrcka AV. Epac and phospholipase cepsilon regulate ca2+ release in the heart by activation of protein kinase cepsilon and calcium-calmodulin kinase ii. The Journal of biological chemistry. 2009;284:1514–1522. doi: 10.1074/jbc.M806994200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pereira L, Metrich M, Fernandez-Velasco M, Lucas A, Leroy J, Perrier R, Morel E, Fischmeister R, Richard S, Benitah JP, Lezoualc'h F, Gomez AM. The camp binding protein epac modulates ca2+ sparks by a ca2+/calmodulin kinase signalling pathway in rat cardiac myocytes. The Journal of physiology. 2007;583:685–694. doi: 10.1113/jphysiol.2007.133066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smrcka AV, Oestreich EA, Blaxall BC, Dirksen RT. Epac regulation of cardiac ec coupling. The Journal of physiology. 2007;584:1029–1031. doi: 10.1113/jphysiol.2007.145037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W, Zhu W, Wang S, Yang D, Crow MT, Xiao RP, Cheng H. Sustained beta1-adrenergic stimulation modulates cardiac contractility by ca2+/calmodulin kinase signaling pathway. Circ Res. 2004;95:798–806. doi: 10.1161/01.RES.0000145361.50017.aa. [DOI] [PubMed] [Google Scholar]
- 11.Dalton GD, Dewey WL. Protein kinase inhibitor peptide (pki): A family of endogenous neuropeptides that modulate neuronal camp-dependent protein kinase function. Neuropeptides. 2006;40:23–34. doi: 10.1016/j.npep.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Wen W, Meinkoth JL, Tsien RY, Taylor SS. Identification of a signal for rapid export of proteins from the nucleus. Cell. 1995;82:463–473. doi: 10.1016/0092-8674(95)90435-2. [DOI] [PubMed] [Google Scholar]
- 13.Sanbe A, Gulick J, Hanks MC, Liang Q, Osinska H, Robbins J. Reengineering inducible cardiac-specific transgenesis with an attenuated myosin heavy chain promoter. Circ Res. 2003;92:609–616. doi: 10.1161/01.RES.0000065442.64694.9F. [DOI] [PubMed] [Google Scholar]
- 14.Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, Jaleel N, Chua BH, Hewett TE, Robbins J, Houser SR, Molkentin JD. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. The Journal of clinical investigation. 2007;117:2431–2444. doi: 10.1172/JCI31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Zhang X, Kubo H, Harris DM, Mills GD, Moyer J, Berretta R, Potts ST, Marsh JD, Houser SR. Ca2+ influx-induced sarcoplasmic reticulum ca2+ overload causes mitochondrial-dependent apoptosis in ventricular myocytes. Circ Res. 2005;97:1009–1017. doi: 10.1161/01.RES.0000189270.72915.D1. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Nakayama H, Zhang X, Ai X, Harris DM, Tang M, Zhang H, Szeto C, Stockbower K, Berretta RM, Eckhart AD, Koch WJ, Molkentin JD, Houser SR. Calcium influx through cav1.2 is a proximal signal for pathological cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2011;50:460–470. doi: 10.1016/j.yjmcc.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao E, Lei YH, Shang X, Huang ZM, Zuo L, Boucher M, Fan Q, Chuprun JK, Ma XL, Koch WJ. A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circ Res. 2010;107:1445–1453. doi: 10.1161/CIRCRESAHA.110.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Communal C, Colucci WS. The control of cardiomyocyte apoptosis via the beta-adrenergic signaling pathways. Archives des maladies du coeur et des vaisseaux. 2005;98:236–241. [PubMed] [Google Scholar]
- 19.Zhu WZ, Wang SQ, Chakir K, Yang D, Zhang T, Brown JH, Devic E, Kobilka BK, Cheng H, Xiao RP. Linkage of beta1-adrenergic stimulation to apoptotic heart cell death through protein kinase a-independent activation of ca2+/calmodulin kinase ii. The Journal of clinical investigation. 2003;111:617–625. doi: 10.1172/JCI16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bers DM. Cardiac na/ca exchange function in rabbit, mouse and man: What's the difference? J Mol Cell Cardiol. 2002;34:369–373. doi: 10.1006/jmcc.2002.1530. [DOI] [PubMed] [Google Scholar]
- 21.Xiao RP, Zhu W, Zheng M, Cao C, Zhang Y, Lakatta EG, Han Q. Subtype-specific alpha1- and beta-adrenoceptor signaling in the heart. Trends in pharmacological sciences. 2006;27:330–337. doi: 10.1016/j.tips.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y, Zhu WZ, Joiner ML, Zhang R, Oddis CV, Hou Y, Yang J, Price EE, Gleaves L, Eren M, Ni G, Vaughan DE, Xiao RP, Anderson ME. Calmodulin kinase ii inhibition protects against myocardial cell apoptosis in vivo. American journal of physiology. Heart and circulatory physiology. 2006;291:H3065–H3075. doi: 10.1152/ajpheart.00353.2006. [DOI] [PubMed] [Google Scholar]
- 24.Maier LS, Bers DM. Calcium, calmodulin, and calcium-calmodulin kinase ii: Heartbeat to heartbeat and beyond. J Mol Cell Cardiol. 2002;34:919–939. doi: 10.1006/jmcc.2002.2038. [DOI] [PubMed] [Google Scholar]
- 25.Poppe H, Rybalkin SD, Rehmann H, Hinds TR, Tang XB, Christensen AE, Schwede F, Genieser HG, Bos JL, Doskeland SO, Beavo JA, Butt E. Cyclic nucleotide analogs as probes of signaling pathways. Nat Methods. 2008;5:277–278. doi: 10.1038/nmeth0408-277. [DOI] [PubMed] [Google Scholar]
- 26.Eijkelkamp N, Wang H, Garza-Carbajal A, Willemen HL, Zwartkruis FJ, Wood JN, Dantzer R, Kelley KW, Heijnen CJ, Kavelaars A. Low nociceptor grk2 prolongs prostaglandin e2 hyperalgesia via biased camp signaling to epac/rap1, protein kinase cepsilon, and mek/erk. J Neurosci. 30:12806–12815. doi: 10.1523/JNEUROSCI.3142-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang Y, Olah ME. Cyclic amp-dependent, protein kinase a-independent activation of extracellular signal-regulated kinase 1/2 following adenosine receptor stimulation in human umbilical vein endothelial cells: Role of exchange protein activated by camp 1 (epac1) J Pharmacol Exp Ther. 2007;322:1189–1200. doi: 10.1124/jpet.107.119933. [DOI] [PubMed] [Google Scholar]
- 28.Lotfi S, Li Z, Sun J, Zuo Y, Lam PP, Kang Y, Rahimi M, Islam D, Wang P, Gaisano HY, Jin T. Role of the exchange protein directly activated by cyclic adenosine 5'-monophosphate (epac) pathway in regulating proglucagon gene expression in intestinal endocrine l cells. Endocrinology. 2006;147:3727–3736. doi: 10.1210/en.2006-0056. [DOI] [PubMed] [Google Scholar]
- 29.Misra UK, Pizzo SV. Epac1-induced cellular proliferation in prostate cancer cells is mediated by b-raf/erk and mtor signaling cascades. J Cell Biochem. 2009;108:998–1011. doi: 10.1002/jcb.22333. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Grimm M, Ling H, Brown JH. Crossing signals: Relationships between beta-adrenergic stimulation and camkii activation. Heart Rhythm. 2011;8:1296–1298. doi: 10.1016/j.hrthm.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson ME, Brown JH, Bers DM. Camkii in myocardial hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51:468–473. doi: 10.1016/j.yjmcc.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu W, Woo AY, Yang D, Cheng H, Crow MT, Xiao RP. Activation of camkiideltac is a common intermediate of diverse death stimuli-induced heart muscle cell apoptosis. The Journal of biological chemistry. 2007;282:10833–10839. doi: 10.1074/jbc.M611507200. [DOI] [PubMed] [Google Scholar]
- 33.Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, Price EE, Jr, Thiel W, Guatimosim S, Song LS, Madu EC, Shah AN, Vishnivetskaya TA, Atkinson JB, Gurevich VV, Salama G, Lederer WJ, Colbran RJ, Anderson ME. Calmodulin kinase ii inhibition protects against structural heart disease. Nature medicine. 2005;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 34.Pieske B, Houser SR, Hasenfuss G, Bers DM. Sodium and the heart: A hidden key factor in cardiac regulation. Cardiovascular research. 2003;57:871–872. doi: 10.1016/s0008-6363(02)00849-0. [DOI] [PubMed] [Google Scholar]
- 35.Insel PA, Zhang L, Murray F, Yokouchi H, Zambon AC. Cyclic amp is both a pro-apoptotic and anti-apoptotic second messenger. Acta Physiol (Oxf) 2012;204:277–287. doi: 10.1111/j.1748-1716.2011.02273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grimm M, Brown JH. Beta-adrenergic receptor signaling in the heart: Role of camkii. J Mol Cell Cardiol. 2010;48:322–330. doi: 10.1016/j.yjmcc.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cleophas TJ, van Marum R. Meta-analysis of efficacy and safety of second-generation dihydropyridine calcium channel blockers in heart failure. The American journal of cardiology. 2001;87:487–490. A487–A488. doi: 10.1016/s0002-9149(00)01413-2. [DOI] [PubMed] [Google Scholar]
- 38.Cucherat M. Quantitative relationship between resting heart rate reduction and magnitude of clinical benefits in post-myocardial infarction: A meta-regression of randomized clinical trials. European heart journal. 2007;28:3012–3019. doi: 10.1093/eurheartj/ehm489. [DOI] [PubMed] [Google Scholar]
- 39.Kim IM, Tilley DG, Chen J, Salazar NC, Whalen EJ, Violin JD, Rockman HA. Beta-blockers alprenolol and carvedilol stimulate beta-arrestin-mediated egfr transactivation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:14555–14560. doi: 10.1073/pnas.0804745105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.