Abstract
Caffeine induces locomotor activation by its ability to block adenosine receptors. Caffeine is metabolized to several methylxanthines, with paraxanthine being the main metabolite in humans. In this study we show that in rats paraxanthine has a stronger locomotor activating effect than caffeine or the two other main metabolites of caffeine, theophylline and theobromine. As previously described for caffeine, the locomotor activating doses of paraxanthine more efficiently counteract the locomotor depressant effects of an adenosine A1 than an adenosine A2A receptor agonist. In drug discrimination experiments in rats trained to discriminate a maximal locomotor activating dose of caffeine, paraxanthine, unlike theophylline, generalized poorly to caffeine suggesting the existence of additional mechanisms other than adenosine antagonism in the behavioral effects of paraxanthine. Pretreatment with the nitric oxide inhibitor N(G)-nitro-L-arginine methyl ester (L-NAME) reduced the locomotor activating effects of paraxanthine, but not caffeine. On the other hand, pretreatment with the selective cGMP-preferring phosphodiesterase PDE9 inhibitor BAY 73-6691, increased locomotor activity induced by caffeine, but not paraxanthine. Ex vivo experiments demonstrated that paraxanthine, but not caffeine, can induce cGMP accumulation in the rat striatum. Finally, in vivo microdialysis experiments showed that paraxanthine, but not caffeine, significantly increases extracellular levels of dopamine in the dorsolateral striatum, which was blocked by L-NAME. These findings indicate that inhibition of cGMP-preferring PDE is involved in the locomotor activating effects of the acute administration of paraxanthine. The present results demonstrate a unique psychostimulant profile of paraxanthine, which might contribute to the reinforcing effects of caffeine in humans.
Keywords: caffeine, paraxanthine, psychostimulant, dopamine release, phosphodiesterase inhibitor, rat striatum, drug discrimination
1. INTRODUCTION
Caffeine is the most widely consumed psychoactive substance in the world. It is generally believed that caffeine exerts psychostimulant effects acting as a nonselective adenosine A1 receptor (A1R) and A2A receptor (A2AR) antagonist (Fredholm and Persson 1982; Nehlig et al., 1992; Fredholm and Lindstrom, 1999; Fisone et al., 2004; Ferré, 2008). In rats, the trimethylxanthine caffeine is mainly demethylated to the dimethylxanthines paraxanthine, theophylline and theobromine in roughly similar amounts (Arnaud, 1985; Berthou et al., 1988). In humans, caffeine is also rapidly metabolized to the three dimethylxanthines, but with a very different metabolizing rate, with paraxanthine constituting by far the main metabolite (approximately 80% of the three dimethylxanthines) (Lelo et al., 1989; Berthou et al., 1992; for a recent review see Arnaud, 2011). The first studies which compared the pharmacological effects of caffeine and its main metabolites were reported almost 30 year ago when it was shown that caffeine, paraxanthine, theophylline, but not theobromine, were able to increase locomotor activation in mice (Seale et al., 1984). Also, in rats trained to discriminate caffeine from saline, both paraxanthine and theophylline, but not theobromine, were able to generalize to the caffeine-cue (Carney et al., 1985). Moreover, caffeine but not paraxanthine, was able to generalize to theophylline in rats trained to discriminate theophylline from saline (Carney et al., 1985). Therefore, those studies already suggested more similarities between caffeine and theophylline than with paraxanthine and even less with theobromine, which was consistently the least active methylxanthine. Paraxanthine has also less anxiogenic activity and toxicity in rodents than caffeine (Stavric, 1988; Benowitz et al., 1995; Okuro et al., 2010).
It is widely assumed that the main mechanism of action involved in the behavioral effects of caffeine and paraxanthine is their antagonism of adenosine receptors, but they have little differences in their affinities for both adenosine A1Rs and A2ARs (Snyder et al., 1981), which suggests the existence of additional mechanisms to explain their pharmacological differences. A dopaminergic component for paraxanthine was proposed based on results showing displacement for a low concentration of the labeled dopamine D1 receptor (D1R) antagonist [3H]SCH-23390 in the rat striatum (Ferré et al., 1990) and the ability of SCH-23390 to partially counteract the motor activating properties of paraxanthine in reserpinized mice (Ferre et al., 1991). However, the binding experiments with [3H]SCH-23390 could not be replicated by other authors (K.A. Jacobson, personal communication). It is well known that methylxanthines have also modest phosphodiesterase (PDE) inhibitory activity (Essayan, 2001; Francis et al., 2011). PDEs play an important role in intracellular signal transduction pathways (Bender and Beavo, 2006). Among all the different phosphodiesterase families present in the brain, PDE1, PDE2, PDE4, PDE5, PDE9 and PDE10 are the most prevalent (Domek-Lopacinska and Strosznajder, 2010). Some of them, like PDE1, PDE2 and PDE10 hydrolyze both cAMP and cGMP and regulate the duration and the amplitude of cyclic nucleotide signaling activity (Bender and Beavo, 2006), whereas PDE5 and PDE9 isoforms are very selective for cGMP (Domek-Lopacinska and Strosznajder, 2010). The synthesis of cGMP is regulated by a soluble guanylyl cyclase (Garthwaite, 2008) that in turn is regulated by different molecules such as hormones, bacterial toxins, or neurotransmitters like nitric oxide (NO) (Snyder, 1992). In the CNS the neuronal isoform (nNOS) is the main enzyme implicated in the synthesis of NO and it is present in a discrete way in different areas of the brain, and in particular in the striatum (Vincent and Kimura, 1992). Furthermore, NO seems to be also involved in the decrease of basal locomotor activation (Stewart et al., 1994) and in some of the mechanisms responsible for the psychostimulant effect of drugs of abuse (Itzhak, 1997; Przegalinski and Filip, 1997; Li et al., 2002; Zarrindast et al., 2002 and 2003; Kayir and Uzbay 2004).
In the present study we first demonstrate that paraxanthine has a significantly stronger locomotor activating effect than caffeine, theophylline and theobromine in rats. We then present behavioral and biochemical evidences for a selective inhibitory effect of paraxanthine on cGMP-preferring PDEs (most probably PDE9) as a main mechanism responsible for the difference in the locomotor activating effects, which is associated with a significant release of dopamine in the striatum.
2. MATERIALS AND METHODS
2.1 Animals and Drugs
Male Sprague-Dawley albino rats (Charles River Laboratories, Wilmington, MA), weighting 300–350 g, were used in all the experimental procedures. Animals were housed 2 per cage and kept on a 12/12-h dark/light cycle with food and water available ad libitum. All animals used in the study were maintained in accordance with the guidelines of the National Institutes of Health Animal Care and the animal research conducted to perform this study was approved by the NIDA IRP Animal Care and Use Committee (protocol #:09-BNRB-73). The methylxanthines caffeine, theophylline, theobromine and paraxanthine, the adenosine A1R agonist CPA (N6- cyclopentyladenosine), the adenosine A1R antagonist CPT (8-Cyclopentyl-1, 3-dimethylxanthine) and the PDE9 inhibitor BAY 73-6691 were purchased from Sigma-Aldrich (St. Louis, MO). The adenosine A2AR antagonist KW-6002 and the adenosine A2AR agonist CGS 21680 (2-p-(2-carboxyethyl) phenethylamino-5′-N-ethylcarboxamidoadenosine) were kindly provided by the CHDI Foundation Inc. (Los Angeles, CA, USA). The NO synthase inhibitor L- NAME (NG-Nitro-L-arginine methyl ester hydrochloride) and the PDE4 inhibitor rolipram were purchased from Tocris Bioscience (Ellisville, MI). MSX-3, a soluble phosphate pro-drug of MSX-2, was synthesized at the Pharmaceutical Institute, University of Bonn, Germany (Hockemeyer et al., 2004). The doses and the preparation of the adenosine receptor agonists and antagonists were selected based on previous experiments in which they elicited fully significant effects in the behavioral parameters tested, under our experimental conditions (Karcz-Kubicha et al., 2003; Orrú et al., 2011b). Paraxanthine, theophylline, theobromine, CPT, BAY 73-6691 and rolipram were suspended in a solution of 5% dimethyl-sulfoxide, 5% TWEEN80 and 90% ddH2O; KW-6002 was suspended in a solution of 8% TWEEN80 and 92% ddH2O. Caffeine was dissolved in saline solution. All drugs were administered i. p. in an injection volume of 2 ml/kg body weight.
2.2 Locomotor activity
Locomotor activity was measured by placing the animals individually in an open field motility soundproof chambers (50×50 cm) (Med Associates Inc., VT). Locomotion was measured by counting the number of breaks in the infrared beams of the chambers during consecutive periods of 10 min. Before each testing session, the animals were moved into the experimental room and allowed to habituate to the new environment for at least 2 hours before being introduced in the chamber. Recording of the locomotor activity started immediately after placing the animals in the boxes (without habituation) and lasted for 90 min. Data were analyzed as the average of all transformed values (square root) of averaged counts per 10 min during the first hour. This period corresponds to the highest exploratory activity during exposure to a new test environment. All animals were tested only once.
2.3 Surgical procedures and in vivo microdialysis
Rats were deeply anesthetized with 3 ml/kg of Equithesin (NIDA Pharmacy, Baltimore, MD), placed in a stereotaxic apparatus and implanted with concentric dialysis probes (Eicom Corp., Tokyo) in the lateral striatum: anterior = + 0.0 mm from bregma, lateral = ± 4.5 mm from bregma, vertical = −6.0 mm from dura (Paxinos and Watson, 2006). After surgery, rats were allowed to recover in hemispherical CMA-120 cages (CMA Microdialysis AB, Solna, Sweden) equipped with a swivel (Plastics One, Roanoke, VA). Twenty-four hours after implanting the probe, experiments were performed on freely moving rats in the same hemispherical home cages in which they recovered overnight from surgery. A Ringer solution containing (in mM): 147 NaCl, 4.0 KCl, 2.2 CaCl2 was pumped through the microdialysis probe at a constant rate of 1 μl/min. After a washout period of 90 min, the microdialysate samples of each animal were collected with 30 min of interval. In the first set of experiments, after 3 stable samples for baseline (in a range of 10% of variability), animals received either an injection of 30 mg/kg of L-NAME, caffeine, paraxanthine or vehicle. In a second set of experiments, rats were injected with 30 mg/kg of L-NAME or vehicle followed by a treatment of 30 mg/kg of paraxanthine. The dopamine microdialysate samples were collected for an additional 150 minutes, loaded in a refrigerated autosampler and analyzed by reverse high-performance liquid chromatography (HPLC) coupled with a coulometric detector (5200a Coulochem III, ESA, Chelmsford). Dopamine values were transformed as percentage of basal values (mean of the three values before vehicle or drug injection) and analyzed using a two-way ANOVA followed by Tukey’s multiple comparisons test (Statistica, Stat Soft, Tulsa, OK). At the end of the experiment, rats were given an overdose of equithesin, the brains were extracted, fixed in formaldehyde, and the probe placement was checked using cresyl violet staining.
2.4 Drug- discrimination procedure
Slightly food-restricted rats were trained as described previously (Solinas et al., 2005) under a discrete-trial schedule of food-pellet delivery to respond on one lever after an injection of a training dose of 30 mg/kg of caffeine (n = 10) and on the other lever after an injection of 1 ml/kg saline vehicle. Injections of caffeine or saline were given i.p. 30 min before the start of the session. At the start of the session, a white house light was turned on, and in its presence, the rats were required to make 10 consecutive responses (fixed-ratio 10 schedule of food delivery) on the lever appropriate to the pre-session treatment. The completion of 10 consecutive responses on the correct lever produced delivery of a 45-mg food pellet and initiated a 45-s timeout during which lever-press responses had no programmed consequences and the chamber was dark. Responses on the incorrect lever had no programmed consequences other than to reset the fixed-ratio requirement on the correct lever. After each timeout, the white house light was again turned on, and the next trial began. Each session ended after completion of 20 fixed-ratio trials or after 30 min elapsed, whichever occurred first. Discrimination-training sessions were conducted 5 days per week under a single alternation schedule (i.e., DSDSDSDSDS etc., where D = drug and S = saline). Training continued until there were eight consecutive sessions during which rats completed at least 80% of their responses during the session on the correct lever and no more than four responses occurred on the incorrect lever during the first trial. Test sessions with other doses and other drugs were then initiated. The caffeine dose-response curve (3, 10, 18, 30, and 56 mg/kg) was determined after the discrimination was acquired, before testing other drugs. Then, a range of doses of different methylxanthines – paraxanthine (10 – 56 mg/kg; n = 8), theophylline (3 – 56 mg/kg; n = 10), and theobromine (10 – 56 mg/kg; n = 10) – were administered instead of caffeine to examine generalization to training stimulus. All methylxanthines were injected i.p. 10 min before the start of the session. Test sessions were identical to training sessions, with the exception that 10 consecutive responses on either one of the two levers ended the trial. Switching responding from one lever to the other lever reset the ratio requirement. In a test phase, a single alternation schedule was introduced, and test sessions were usually conducted on Tuesdays and Fridays. Thus, a 2-week sequence starting on Monday was: DTDSTSTSDT (T = test). Test sessions occurred with equal probability after saline and drug sessions. Test sessions were conducted only if the criterion of 80% accuracy and not more than four incorrect responses during the first trial was maintained in the two preceding training sessions.
2.5 Radioligand-binding experiments
Membrane suspensions from sheep striatum were processed as described previously (Casado et al., 1990; Sarrio et al., 2000). Tissue was disrupted with a Polytron homogenizer (PTA 20 TS rotor, setting 3; Kinematica, Basel, Switzerland) for three 5 s-periods in 10 volumes of 50 mM Tris–HCl buffer, pH 7.4 containing a proteinase inhibitor cocktail (Sigma). Cell debris was eliminated by centrifugation at 1,000 g (10 min, 4°C) and membranes were obtained by centrifugation at 105,000 g (40 min, 4°C). The pellet was resuspended and recentrifuged under the same conditions and was stored at −80°C. Membranes were washed once more as described above and resuspended in 50 mM Tris–HCl buffer, pH 7.4, for immediate use. Protein was quantified by the bicinchoninic acid method (Pierce Chemical Co., Rockford, IL) using bovine serum albumin dilutions as standard. Binding experiments were performed with sheep striatal membrane suspensions at 25°C in 50 mM Tris-HCl buffer, pH 7.4, containing 10 mM MgCl2 and of 0.2 I.U./ml of ADA (EC 3.5.4.4; Roche, Basel, Switzerland). For competition experiments, we incubated membrane suspensions (0.1 mg of protein/ml) with a constant free concentration (0.8 nM) of the D1R antagonist [3H]SCH23390 (80.5 Ci/mmol; Perkin Elmer, Boston, MA), in the absence or in the presence of increasing free concentrations of paraxantine (10 nM to 3 mM) or caffeine (10 nM to 10 mM). In all cases, membranes were incubated with ligands providing enough time (2 h) to achieve stable equilibrium for the lower ligand concentrations. Nonspecific binding was determined in the presence of 10 μM SCH23390 (Tocris Bioscience, Ellisville, MO) and confirmed that the value was the same as calculated by extrapolation of the competition curves. Free and membrane bound ligand were separated by rapid filtration of 500 μl aliquots in a cell harvester (Brandel, Gaithersburg, MD) through Whatman GF/C filters embedded in 0.3% polyethylenimine that were subsequently washed for 5 s with 5 ml of ice-cold Tris–HCl buffer. The filters were incubated with 10 ml of Ecoscint H scintillation cocktail (National Diagnostics, Atlanta, GA) overnight at room temperature and radioactivity counts were determined using a Tri-Carb 2800TR scintillation counter (PerkinElmer, Boston, MO, USA) with an efficiency of 62%.
2.6 Measurement of cGMP and cAMP in ex vivo rat striatal tissue
Rats were injected i.p. with vehicle or either caffeine, paraxanthine or BAY73-6691 at different doses and killed by decapitation 30 minutes after the drug adminisration. The heads of the animals were immersed in liquid nitrogen for 6 s and the striata were rapidly dissected out on an ice-cold surface. The tissue, after the dissection, was frozen out in liquid nitrogen. The striata were homogenized in 0.1 N HCl followed by centrifugation and the supernatant was used to measure cGMP or cAMP concentrations using an enzyme immunoassay kit (Enzo Life Science, NY).
2.7 Statistical Analysis
Statistical analysis for locomotor activity, drug discrimination and ex vivo tissue studies were carried out using a one-way ANOVA followed by Dunnett’s or Newman’s post hoc tests or by a two-way ANOVA using the Bonferroni post-hoc as comparison test (GraphPAD Prism Software, San Diego, CA). For the microdialysis experiments, data were analyzed using a one-way ANOVA for repeated measures over time followed by Dunnett’s multiple comparison tests (Statistica, Stat Soft, Tulsa, OK). Statistical significance was accepted at a level of p<0.05. All data are presented as mean ± S.E.M. Radioligand binding experiments were analyzed by non-linear regression, using the commercial Grafit software (Erithacus Software).
3. RESULTS
3.1 Locomotor activation induced by caffeine, paraxanthine, theophylline and theobromine in non-habituated rats
Increasing doses of paraxanthine, theophylline, theobromine and caffeine were tested in these experiments (from 1 to 56 mg/kg). All four compounds but theobromine produced locomotor activity with inverted “U” shape dose-response curves with the order of potency being theophylline > caffeine = paraxanthine > theobromine (Figure 1). These results are in correspondence with their described affinities for both adenosine A1Rs and A2ARs (Shi and Daly, 1999). Paraxanthine was the most efficient compound among the methylxanthines that were tested and it produced the strongest locomotor activation (particularly at the dose of 30 mg/kg), with the order of efficacies being: paraxanthine > caffeine = theophylline > theobromine (Figure 1). At the peak dose of 30 mg/kg, paraxanthine produced a significantly stronger locomotor activating effect than caffeine (Figure 1).
Figure 1.
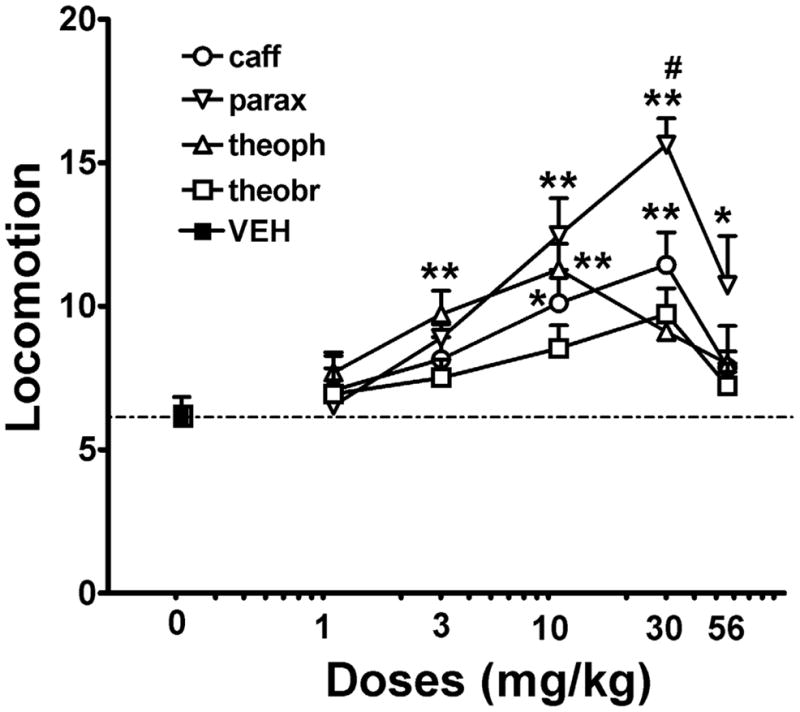
Locomotor activation induced by the methylxanthines caffeine (caff), theophylline (theoph), paraxanthine (parax) and theobromine (theobr) in non-habituated rats after 40 min following drug administration. Data represent means ± S.E.M. of transformed data (square root) of accumulated counts during 60 min period of observation (n = 6–8 per group). * and **: P <0.05 and P < 0.01 compared to vehicle-treated animals (VEH), respectively; one-way ANOVA with Dunnett’s post-hoc comparisons. #: P < 0.05 compared to caff 30 mg/kg (one-way ANOVA followed by Bonferroni’s post-hoc test).
3.2 Counteraction of adenosine A1R and A2AR agonist-induced locomotor depression by caffeine, paraxanthine, theophylline and theobromine
Based on a previous study from our laboratory (Karcz-Kubicha et al., 2003), we used equipotent locomotor depressant doses of the A1R agonist CPA (0.1 mg/kg) and the A2AR agonist CGS 21680 (0.5 mg/kg) to evaluate the A1R and A2AR antagonistic profile of caffeine, theophylline, paraxanthine and theobromine. In fact, the selective A1R antagonist CPT (1 mg/kg) significantly counteracted the locomotor depressant effect of CPA, but not CGS 21680, and the selective A2AR antagonist MSX-3 (1 mg/kg) significantly counteracted the locomotor depressant effect of CGS 21680, but not CPA (Figure 2). As previously published (Karcz-Kubicha et al., 2003), locomotor activating doses of caffeine significantly counteracted the locomotor depressant effect of CPA, but not of CGS 21680 (Figure 3a), indicating that the locomotor activating effects of an acute administration of caffeine depend mostly on A1R blockade. Similarly, locomotor activating doses of paraxanthine and theophylline but not theobromine were more potent at counteracting the effect of CPA than CGS 21680 (Figure 3b, 3c and 3d). Paraxanthine was by far the most efficient at counteracting the locomotor depression induced by either CPA or CGS 21680 when compared to theophylline and theobromine). These results indicate that A1R and not A2AR antagonism is primarily involved in the locomotor activating effects of acute administration of methylxanthines. However, the previously described similar affinity of A1R for caffeine, theophylline and paraxanthine (see Introduction) suggests that the stronger locomotor activating effect of paraxanthine must involve an additional mechanism of action.
Figure 2.
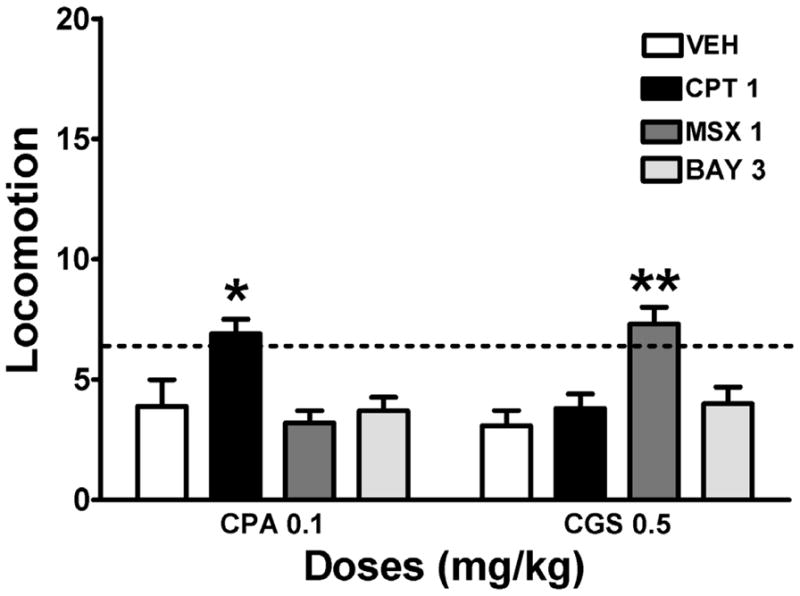
Counteraction of adenosine receptors agonist-induced motor depression by the selective A1R antagonist CPT and the selective A2AR antagonist MSX-3. The possible counteracting effect of the PDE9 inhibitor BAY-73-6691 was also tested. Data represent means ± S.E.M. of transformed data (square root) of total accumulated counts during a 60 min period of observation (n = 6–8 per group). The adenosine A1R agonist CPA (0.1 mg/kg) and the A2AR agonist CGS 21680 (CGS; 0.5 mg/kg) were administrated 10 min before the animals were placed in the activity cages. CPT (1 mg/kg), MSX-3 (MSX, 1 mg/kg) BAY 73-6691 (BAY, 3 mg/kg) or vehicle (VEH) were administered 30 min before CPA or CGS 21680. The dashed horizontal line represents the average of locomotion in animals not treated with adenosine agonists. * and **: P <0.05 and P <0.01, respectively, compared to the group that received the same dose of CPA or CGS 21680 alone (one-way ANOVA with Dunnett’s post hoc comparisons).
Figure 3.
Counteraction of adenosine receptors agonist-induced motor depression by caffeine, theophylline, paraxanthine and theobromine. Data represent means ± S.E.M. of transformed data (square root) of total accumulated counts during a 60 min period of observation (n = 6–8 per group). The adenosine A1R agonist CPA (0.1 mg/kg) and the A2AR agonist CGS 21680 (0.5 mg/kg) were administrated 10 min before the animals were placed in the activity cages. Caffeine (caff), theophylline (theoph), paraxanthine (parax) and theobromine (theobr), at 1, 3, 10 or 30 mg/kg, were administered 30 min before CPA or CGS 21680. * and **: P <0.05 and P <0.01, respectively, compared to the group that received the same dose of CPA or CGS 21680 alone (one-way ANOVA with Dunnett’s post hoc comparisons).
3.3 Generalization tests with caffeine, paraxanthine, theophylline and theobromine in rats discriminating caffeine (30 mg/kg) from vehicle
Caffeine produced a dose-dependent increase in drug-lever selection with maximal selection of the drug lever (96.22%) at the 30 mg/kg training dose of caffeine. Generalization to the training stimulus was significant at caffeine doses of 30 and 56 mg/kg (Figure 4). The 56 mg/kg dose of caffeine significantly decreased rates of responding. Theophylline produced partial generalization to the caffeine-training stimulus, which was significant at doses of 30 and 56 mg/kg (maximal drug-lever selection was 68.45% and 62.65%, respectively) (Figure 4). The 56 mg/kg dose of theophylline significantly decreased rates of responding. Paraxanthine produced a low level of partial generalization (maximal drug-lever selection 33.05%) to the training stimulus at a dose of 56 mg/kg (Figure 4). At the same dose (56 mg/kg), theobromine also produced similar level of partial generalization as paraxanthine (maximal drug-lever selection 33.13%), but statistical significance was not reached. In the tested range of doses, neither paraxanthine nor theobromine significantly decreased rates of responding. These results indicate that theophylline shares very much the same mechanism than caffeine and again suggest that paraxanthine must involve other mechanism of action, at least responsible for the stimulus discrimination effects.
Figure 4.

Effects of the adenosine receptor antagonists paraxanthine (parax), theophylline (theoph) and theobromine (theobr) in rats trained to discriminate 30 mg/kg caffeine (caff) from vehicle (VEH). Data represent mean ± S.E.M. (n=10). Ordinates percentage of responses on the lever associated with caffeine administration (left panel) and overall rate of lever pressing expressed as responses per second averaged over the session (right panel). Drug doses are expressed in mg/kg (log scale). Complete generalization to the caffeine-training stimulus (80% caffeine-lever selection) is represented with dashed horizontal line. * and **: P < 0.05 and P < 0.01 compared with VEH, respectively (one-ANOVA followed by Bonferroni’s post-hoc test).
3.4 Displacement of D1R antagonist binding from striatal membranes by paraxanthine and caffeine
In view of the existence of contradictory results about its previously proposed direct effects on D1R (see Introduction), we reevaluated the possible ability of paraxanthine to displace the selective D1R antagonist [3H]SCH-23390 from striatal membrane preparations (Ferré et al., 1990, 1991). Paraxanthine, and to a lesser extent caffeine, could displace the binding of [3H]SCH-23390, but only partially and at very high, non-pharmacological, concentrations. At 3 mM, paraxanthine displaced 27% and caffeine displaced 16% of the specific binding. This indicates that the effects of paraxanthine on locomotion in the doses used in the present study cannot be mediated by direct effects at D1R.
3.5 Role of NO-cGMP signaling on the locomotor activity induced by paraxanthine
In view of the possible role of NO-cGMP signaling in the behavioral effects of psychostimulants (see Introduction) we first evaluated the effect of the NO synthase inhibitor L-NAME. The dose of L-NAME used (30 mg/kg) was based on pilot experiments and on results obtained by other research groups (Kayir and Uzbay, 2004). L-NAME, when administered alone, did not produce any significant change in locomotor activity in rats, excluding then any sedative or muscles relaxant effect. However, pretreatment of rats with L-NAME (30mg/kg) significantly reduced the locomotor activating effect of paraxanthine (30 mg/kg) (Figure 5a and 5b) but not of caffeine (30 mg/kg) (Figure 5c and 5d). The degree of locomotor activation induced by paraxanthine after pretreatment with L-NAME was similar to the degree of locomotor activation induced by caffeine itself. Based on that, we then checked if the NO-dependent component of the locomotor activating effects of paraxanthine was due to a selective inhibition of cGMP-preferring PDEs. At a dose of 3 mg/kg, the PDE9 inhibitor BAY 73-6691 did not produce any locomotor activation per se (Figure 6) and did not counteract the motor depressant effects of CPA or CGS 21680 (Figure 1), indicating that at this dose it does not act as an A1R or A2AR antagonist. Nevertheless, at 3 mg/kg, BAY 73-6691 was able to increase the locomotor activity induced by caffeine (30 mg/kg), but not paraxanthine (30 mg/kg) (Figure 6a and 6b). BAY 73-6691 was then tested in combination with locomotor activating doses of the A1R and A2AR antagonists CPT or KW-6002, respectively. The doses and the preparation of KW-6002, CPT and the PDE 9 inhibitor BAY 73-6691 were selected based on previous experiments in which they elicited fully significant effects in the behavioral parameters tested and under our experimental conditions (Karcz-Kubicha et al., 2003; Orrú et al., 2011a; Van der Staay et al., 2008). Co-administration of BAY 73-6691 was able to increase the locomotor activation induced by CPT, but not by KW-6002 (Figure 6c and 6d). Altogether these results strongly suggest that the locomotor activating effects of an acute administration of paraxanthine depend on the combined A1 receptor blockade and PDE9 inhibition (see Discussion).
Figure 5.
Effect of the NO synthase inhibitor L-NAME treatment on locomotor activation induced by paraxanthine and caffeine. Non-habituated animals were placed in the motility cages 40 min after vehicle (VEH), paraxanthine (parax, 30 mg/kg) or caffeine (caff, 30 mg/kg) administration and 30 min after VEH or L-NAME (L-NAME, 30 mg/kg). (a) and (c) represent time course for locomotion of transformed data (square root) of accumulated counts per 10-min period (mean ± S.E.M.; n = 6–8 per group). (b) and (d) represent the average (mean ± S.E.M.) of the 10-min period transformed values during the first 60 min of recording (n = 6–8 per group). ** and ***: P < 0.01 and P <0.001, compared to VEH-treated animals, respectively; #: P < 0.05 compared to paraxanthine alone (one-way ANOVA followed by Bonferroni’s post-hoc test).
Figure 6.
Effect of the PDE9 inhibitor BAY 73-6691 treatment on locomotor activation induced by caffeine, paraxanthine, the A2AR antagonist KW-6002 and the A1R antagonist CPT. Animals were placed in the motility cages 40 min after vehicle (VEH), caffeine (caff, 30 mg/kg), paraxanthine (parax, 30 mg/kg), KW-6002 (KW, 0.1 mg/kg) or CPT (CPT, 4.8 mg/kg) administration and 30 min after VEH or BAY 73-6691 (BAY, 3 mg/kg). Data represent the average (mean ± S.E.M.) of the 10-min period transformed values during the first 60 min of recording (n = 6–8 per group). *, ** and ***: P < 0.05, P < 0.01 and P <0.001, compared to VEH-treated animals, respectively. #: P < 0.05 compared to caffeine alone (b) or to CPT alone (d) (one-way ANOVA with Bonferroni’s post-hoc comparisons).
3.6 Effects of paraxanthine on cGMP accumulation in the rat striatum
The selective inhibition of a cGMP-preferring PDE by paraxanthine was then confirmed in ex vivo experiments. As shown in Figure 7, the administration of the PDE9 inhibitor BAY 73-6691 (3 mg/kg) produced a significant cGMP accumulation in homogenates of rat striatum. The same quantitative effect was obtained with paraxanthine at the dose of 30 mg/kg, but not 10 mg/kg. On the other hand caffeine, at 10 and 30 mg/kg did not produce any significant effect and did not modify the effect of BAY 73-6691 (Figure 7). Neither caffeine nor paraxanthine, at the dose of 30 mg/kg, produced a significant increase in cAMP accumulation in the same striatal preparations, with values of 102 ± 9 and 111 ± 13, respectively, versus vehicle (100 ± 4). As a positive control, the selective PDE4 inhibitor rolipram produced a significant increase in cAMPc levels (125 ± 2; one-way ANOVA with Newman-Keuls’ post hoc comparisons: P < 0.05; n = 6–8 per group).
Figure 7.

cGMP accumulation in the rat striatum after the systemic administration of paraxanthine, caffeine or the PDE9 inhibitor BAY 73-6691. Data represent means ± S.E.M. of cGMP accumulation in striatal homogenates from animals sacrificed 30 min after the systemic administration of BAY 73-6691 (3 mg/kg; BAY 3), paraxanthine (10 or 30 mg/kg; parax 10 or parax 30) or caffeine (10 or 30 mg/kg; caff 10 or caff 30). **: P < 0.01 compared to vehicle-treated animals (VEH); no significant differences were observed between the groups treated with BAY, parax 30 and BAY 3 + caff 30 (one-way ANOVA with Newman-Keuls’ post hoc comparisons).
3.7 Paraxanthine-induced elevation in dopamine extracellular levels in the lateral striatum
In vivo microdialysis experiments showed that paraxanthine (30 mg/kg), but not caffeine (30 mg/kg) was able to significantly increase extracellular levels of dopamine in the lateral striatum for about 50% when compared with basal levels (Figure 8). The administration of the nitric oxide synthase inhibitor L-NAME (30 mg/kg) by itself did not alter dopamine levels as compared to basal levels. However, pretreatment with L-NAME (30 mg/kg) significantly reduced paraxanthine-induced extracellular dopamine levels.
Figure 8.

Effect of systemic administration of paraxanthine, caffeine and L-NAME on the increase of dopamine extracellular levels in the lateral striatum. Results express the mean ± S.E.M. in percentage of the average of the three stable values before the pretreatment (n = 6–7 per group). The arrows indicate the time of systemic administration of caffeine (30 mg/kg; caff 30), paraxanthine (30 mg/kg; parax 30) or L-NAME (30 mg/kg; L-NAME 30). * and **: P < 0.05, and P < 0.01, respectively, compared to the average of the three basal level values before drug administration (one-way ANOVA for repeated measurement with Dunnett’s post-hoc comparisons).
4. DISCUSSION
Previous reports have shown dose-dependent psychostimulant and locomotor activating effects of caffeine and its metabolites paraxanthine, theophylline and theobromine. Adenosine receptor antagonism has been shown to be a main mechanism of action responsible for the locomotor activating effects of caffeine, and the same mechanism has been suggested to be involved in the locomotor activating effects of its main metabolites, particularly theophylline and paraxanthine (Snyder et al., 1981; Fredholm and Persson, 1982). After some debate about the role of the adenosine receptor subtypes in the behavioral effects of caffeine (Snyder et al., 1981; Goldberg et al., 1985; Svenningsson et al., 1995; El Yacoubi et al., 2000; Lindskog et al., 2002), we reported evidence for a predominant role of A1R in the locomotor activating effects of an acute administration of caffeine, with a more predominant involvement of A2AR under conditions of chronic caffeine treatment (Karcz-Kubicha et al., 2003; Antoniou et al., 2005). Interestingly, A2AR seems to be preferentially involved in the caffeine-induced enhancement of operant responding in rats (Randall et al., 2011) and in the caffeine-induced reversal of the motor depressant effects of dopamine D2 receptor (D2R), but not D1R, antagonists (Collins et al., 2010). The latter results can be explained by the known role of A2AR in the depressant effects of D2R antagonists, recently suggested to depend on a subpopulation of striatal postsynaptic A2AR that do not form heteromers with D2R (Orrú et al., 2011b). On the other hand, the differential involvement of A1R and A2AR in the acute activating effects of caffeine on operant responding and locomotion (exploratory activity) suggest the existence of a differential tone of endogenous adenosine on different goal-directed behaviors. In the present study we also provide evidence for a predominant A1R antagonistic profile of acute locomotor activating doses of theophylline, paraxanthine and theobromine. An interesting and not previously reported finding is that paraxanthine is significantly the most efficient compound among methylxanthines and produced the strongest locomotor activation in rats, particularly at the dose of 30 mg/kg. At this dose paraxanthine could not generalize to the most efficient locomotor activating dose of caffeine (30 mg/kg) and, at an even higher dose (56 mg/kg), paraxanthine could only partially generalize to caffeine. Since the affinities of A1R (and also A2AR) for caffeine and paraxanthine are similar (Shi and Daly, 1999), these results indicated the existence of an additional mechanism other than adenosine receptor antagonism that controls the locomotor- and discriminative-stimulus effects of paraxanthine.
In the present study we found a role of the NO-cGMP signaling in the locomotor activating effects of paraxanthine. First, the inhibitor of NO synthase L-NAME was able to decrease the locomotor activating-effects of paraxanthine, but not the locomotor activating effects of caffeine. Interestingly, after L-NAME administration, the levels of locomotor activity produced by paraxanthine (30 mg/kg) were comparable to those of caffeine (30 mg/kg). These results appear to be in contradiction with published results regarding a counteracting effect of L-NAME on locomotor activity induced by caffeine in mice (Kayir and Uzbay, 2004). Apart from the difference in species (mice versus rats), these discrepancies might involve other paradigm variables, such as different doses used for instance. In any case, in the present study we observe a very clear differential effect of L-NAME, indicating at the least that nitric oxide has a more relevant role in the locomotor activating effects of paraxanthine than caffeine.
We then investigated if the NO-cGMP signaling-dependent mechanism involved in the locomotor activating effects of paraxanthine would be due to a selective inhibition of cGMP-preferring PDEs. Several studies had already shown that when comparing the psychostimulant effects of methylxanthines, relatively higher concentrations (100–1000 μM) of caffeine are required to inhibit cAMP-preferring PDE activity than those doses required for the interaction with adenosine receptors (10–100 μM) (Sutherland and Rall 1958; Butcher and Sutherland 1962; reviewed by Francis et al., 2011). However, although this property of caffeine has been known for decades, the ability of caffeine and its main metabolites to modify cGMP-preferring PDE activity had not been investigated. We found that the cGMP-preferring PDE (PDE9) inhibitor BAY 73-6691, potentiated caffeine- but not paraxanthine-induced locomotor activation. Based on our results, we speculated that the differences in the locomotor activating effects of paraxanthine and caffeine are related to differences in their ability to inhibit cGMP-preferring PDE, most probably PDE9. In fact, in ex vivo experiments, we were able to demonstrate that at the dose with maximal differences in the locomotor activating effects (30 mg/kg), paraxanthine, but not caffeine, induced a significant increase in the striatal concentration of cGMP. Importantly, the striatum contains one of the highest PDE9 levels in the rat brain (Van Staveren et al., 2005; Reyes-Irisarri et al., 2007).
The results seemed to indicate that simultaneous blockade of adenosine receptors and activation of NO-cGMP pathway underlies the behavioral effects paraxanthine. In fact, we found that BAY 73-6691 potentiated the locomotor activation induced by the A1R antagonist CPT, but not by the A2AR antagonist KW-6002. The relatively low level of activation induced by CPT (as compared for instance with that of KW-6002) is related to its short duration of action, which agrees with its reported pharmacokinetic properties (Baumgold et al., 1992), and to the fact that we were using animals not habituated to the recording environment. Thus, the locomotor activating effects of CPT coincided with the initial burst of exploratory activity and there was very little, albeit significant, locomotor activation compared with vehicle-treated animals. In habituated animals, CPT produces a very significant locomotor activation (Karcz-Kubicha et al., 2003; Antoniou et al., 2005), which we have previously demonstrated it is qualitatively very similar to that of caffeine (Antoniou et al., 2005). We also reported that, on the other hand, the locomotor activating effects of the A2AR antagonist MSX-3 are qualitatively dissimilar to that of caffeine (Antoniou et al., 2005), again underscoring a predominant role of A1R on the acute locomotor activating effects of the caffeine. Also, in our experience within the same rat strain, the effect of the dose of KW-6002 used in our experiments is far from the maximal effect (Orrú et al., 2011a), indicating that the lack of a potentiating effect of BAY 73-6691 on the locomotor activating effects of KW-6002 cannot be related to a ceiling effect. Altogether, our results strongly suggest the existence of an interaction of two main mechanisms, A1R antagonism and PDE9 inhibition, as being responsible for the locomotor activating effects of an acute administration of paraxanthine.
We have previously shown in experiments with in vivo microdialysis in rats that the locomotor activating effects of A1R antagonists (or for the A1R-mediated component of caffeine) correlate with their ability to induce dopamine release in the striatum, particularly in ventral compartments (Solinas et al., 2002, Quarta et al., 2004; Borycz et al., 2007). This effect, however, is milder compared to classical psychostimulants, which produce larger increases in the extracellular striatal concentrations of dopamine (Di Chiara et al., 2004). In the present study, also correlating with locomotor activity, we found that paraxanthine is more efficient that caffeine at increasing the extracellular striatal concentration of dopamine. In fact, paraxanthine increased dopamine in a lateral striatal compartment, where caffeine was ineffective. It is therefore possible that the interaction between A1R and NO-cGMP signaling takes place in striatal dopaminergic nerve terminals and that A1R antagonism plus cGMP-preferring phosphodiesterase inhibition is responsible for the effect of paraxanthine on striatal extracellular concentrations of dopamine. In fact, L-NAME, at a dose that was ineffective on its own, completely counteracted the effect of paraxanthine, demonstrating the involvement of NO-cGMP signaling in this classical psychostimulant-like effect of paraxanthine.
When considering the classical psychostimulants-like pharmacological profile of paraxanthine, a series of questions arise. Does paraxanthine have reinforcing properties? Does it contribute to the reinforcing properties of caffeine? To answer this questions we should fist know if after caffeine consumption, paraxanthine reaches the CNS at concentrations high enough to elicit its psychostimulants effects. At least two factors support this possibility. First, that paraxanthine is the main metabolite of caffeine in humans (see Introduction) and, second, that the plasma levels of caffeine metabolites are known to increase with its chronic consumption, due to induction of caffeine metabolization. In the experimental animal, during chronic caffeine consumption, the metabolites can even surpass caffeine plasma levels (see for instance Gasior et al., 2002). Definitively, more clinical research is needed to undoubtedly demonstrate a role of paraxanthine in the psychostimulant effects of caffeine. Finally, one more question is if paraxanthine could be used as a substitutive for classical psychostimulants, such as cocaine or amphetamine. Further preclinical studies with cGMP-preferring PDE inhibitors alone and in combination with A1R antagonists could also provide a new therapeutic approach for drug addiction.
Highlights: Paraxanthine as a psychostimulant.
Paraxanthine behaves as a stronger psychostimulant than caffeine in rats
Paraxanthine produces stronger motor-activating effects than caffeine
This depends on its selective ability to inhibit cGMP-preferring PDE
Paraxanthine produces dopamine release in the dorsal striatum
This profile of paraxanthine might contribute to the reinforcing effects of caffeine
Acknowledgments
Supported by fundings from NIDA Intramural Research Program
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antoniou K, Papadopoulou-Daifoti Z, Hyphantis T, Papathanasiou G, Bekris E, Marselos M, et al. A detailed behavioral analysis of the acute motor effects of caffeine in the rat: involvement of adenosine A1 and A2A receptors. Psychopharmacology. 2005;183:154–162. doi: 10.1007/s00213-005-0173-6. [DOI] [PubMed] [Google Scholar]
- Arnaud MJ. Comparative metabolic disposition of [1-Me14C] caffeine in rats, mice, and Chinese hamsters. Drug Metab Dispos. 1985;13:471–478. [PubMed] [Google Scholar]
- Arnaud MJ. Pharmacokinetics and metabolism of natural methylxanthines in animal and man. Handb Exp Pharmacol. 2011;200:33–91. doi: 10.1007/978-3-642-13443-2_3. [DOI] [PubMed] [Google Scholar]
- Baumgold J, Nikodijevic O, Jacobson KA. Penetration of adenosine antagonists into mouse brain as determined by ex vivo binding. Biochem Pharmacol. 1992;43:889–894. doi: 10.1016/0006-2952(92)90257-j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P, 3rd, Mayan H, Denaro C. Sympathomimetic effects of paraxanthine and caffeine in humans. Clin Pharmacol Ther. 1995;58:684–691. doi: 10.1016/0009-9236(95)90025-X. [DOI] [PubMed] [Google Scholar]
- Berthou F, Ratanasavanh D, Alix D, Carlhant D, Riche C, Guillouzo A. Caffeine and theophylline metabolism in newborn and adult human hepatocytes: comparison with adult rat hepatocytes. Biochem Pharmacol. 1988;37:3691–3700. doi: 10.1016/0006-2952(88)90402-9. [DOI] [PubMed] [Google Scholar]
- Berthou F, Guillois B, Riche C, Dreano Y, Jacqz-Aigrain E, Beaune PH. Interspecies variations in caffeine metabolism related to cytochrome P4501A enzymes. Xenobiotica. 1992;22:71–80. doi: 10.3109/00498259209053129. [DOI] [PubMed] [Google Scholar]
- Borycz J, Pereira MF, Melani A, Rodrigues RJ, Köfalvi A, Panlilio L, et al. Differential glutamate-dependent and glutamate-independent adenosine A1 receptor-mediated modulation of dopamine release in different striatal compartments. J Neurochem. 2007;101:355–363. doi: 10.1111/j.1471-4159.2006.04386.x. [DOI] [PubMed] [Google Scholar]
- Butcher RW, Sutherland EW. Adenosine 3′,5′-phosphate in biological materials. I Purification and properties of cyclic 3′,5′-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3′,5′-phosphate in human urine. J Biol Chem. 1962;237:1244–1250. [PubMed] [Google Scholar]
- Carney JM, Seale TW, Logan L, McMaster SB. Sensitivity of inbred mice to methylxanthines is not determined by plasma xanthine concentration. Neurosci Lett. 1985;56:27–31. doi: 10.1016/0304-3940(85)90435-5. [DOI] [PubMed] [Google Scholar]
- Casado V, Canti C, Mallol J, Canela EI, Lluis C, Franco R. Solubilization of A1 adenosine receptor from pig brain: characterization and evidence of the role of the cell membrane on the coexistence of high-and low-affinity states. J Neurosci Res. 1990;26:461–473. doi: 10.1002/jnr.490260409. [DOI] [PubMed] [Google Scholar]
- Collins LE, Galtieri DJ, Collins P, Jones SK, Port RG, Paul NE, et al. Interactions between adenosine and dopamine receptor antagonists with different selectivity profiles: Effects on locomotor activity. Behav Brain Res. 2010;211:148–155. doi: 10.1016/j.bbr.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, et al. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47(Suppl 1):227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Domek-Łopacińska KU, Strosznajder JB. Cyclic GMP and nitric oxide synthase in aging and Alzheimer’s disease. Mol Neurobiol. 2010;41:129–137. doi: 10.1007/s12035-010-8104-x. [DOI] [PubMed] [Google Scholar]
- El Yacoubi M, Ledent C, Ménard JF, Parmentier M, Costentin J, Vaugeois JM. The stimulant effects of caffeine on locomotor behaviour in mice are mediated through its blockade of adenosine A(2A) receptors. Br J Pharmacol. 2000;129:1465–1473. doi: 10.1038/sj.bjp.0703170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essayan DM. Cyclic nucleotide phosphodiesterases. J Allergy Clin Immunol. 2001;108:671–680. doi: 10.1067/mai.2001.119555. [DOI] [PubMed] [Google Scholar]
- Ferré S, Guix T, Sallés J, Badia A, Parra P, Jané F, et al. Paraxanthine displaces the binding of [3H]SCH 23390 from rat striatal membranes. Eur J Pharmacol. 1990;179:295–299. doi: 10.1016/0014-2999(90)90168-6. [DOI] [PubMed] [Google Scholar]
- Ferré S, Herrera-Marschitz M, Grabowska-Andén M, Ungerstedt U, Casas M, Andén NE. Postsynaptic dopamine/adenosine interaction: II. Postsynaptic dopamine agonism and adenosine antagonism of methylxanthines in short-term reserpinized mice. Eur J Pharmacol. 1991;192:36–42. doi: 10.1016/0014-2999(91)90065-x. [DOI] [PubMed] [Google Scholar]
- Ferré S. An update on the mechanisms of the psychostimulant effects of caffeine. J Neurochem. 2008;105:1067–1079. doi: 10.1111/j.1471-4159.2007.05196.x. [DOI] [PubMed] [Google Scholar]
- Fisone G, Borgkvist A, Usiello A. Caffeine as a psychomotor stimulant: mechanism of action. Cell Mol Life Sci. 2004;61:857–872. doi: 10.1007/s00018-003-3269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis SH, Blount MA, Corbin JD. Mammalian cyclic nucleotide phosphodiesterases: molecular mechanisms and physiological functions. Physiol Rev. 2011;91:651–690. doi: 10.1152/physrev.00030.2010. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Persson CG. Xanthine derivatives as adenosine receptor antagonists. Eur J Pharmacol. 1982;81:673–676. doi: 10.1016/0014-2999(82)90359-4. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Lindström K. Autoradiographic comparison of the potency of several structurally unrelated adenosine receptor antagonists at adenosine A1 and A(2A) receptors. Eur J Pharmacol. 1999;380:197–202. doi: 10.1016/s0014-2999(99)00533-6. [DOI] [PubMed] [Google Scholar]
- Garthwaite J. Concepts of neural nitric oxide-mediated transmission. Eur J Neurosci. 2008;27:2783–2802. doi: 10.1111/j.1460-9568.2008.06285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasior M, Jaszyna M, Munzar P, Witkin JM, Goldberg SR. Caffeine potentiates the discriminative-stimulus effects of nicotine in rats. Psychopharmacology. 2002;162:385–395. doi: 10.1007/s00213-002-1113-3. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Prada JA, Katz JL. Stereoselective behavioral effects of N6-phenylisopropyl-adenosine and antagonism by caffeine. Psychopharmacology. 1985;87:272–277. doi: 10.1007/BF00432706. [DOI] [PubMed] [Google Scholar]
- Hockemeyer J, Burbiel JC, Muller CE. Multigram-sacle syntheses, stability, and photoreactions of A2A adenosine receptor antagonists with 8-styrylxanthine structure: potential drugs for Parkinson’s disease. J Org Chem. 2004;69:3308–3318. doi: 10.1021/jo0358574. [DOI] [PubMed] [Google Scholar]
- Itzhak Y. Modulation of cocaine- and methamphetamine-induced behavioral sensitization by inhibition of brain nitric oxide synthase. J Pharmacol Exp Ther. 1997;282:521–527. [PubMed] [Google Scholar]
- Karcz-Kubicha M, Antoniou K, Terasmaa A, Quarta D, Solinas M, Justinova Z, et al. Involvement of adenosine A1 and A2A receptors in the motor effects of caffeine after its acute and chronic administration. Neuropsychopharmacology. 2003;28:1281–1291. doi: 10.1038/sj.npp.1300167. [DOI] [PubMed] [Google Scholar]
- Kayir H, Uzbay IT. Evidence for the role of nitric oxide in caffeine-induced locomotor activity in mice. Psychopharmacology. 2004;172:11–15. doi: 10.1007/s00213-003-1625-5. [DOI] [PubMed] [Google Scholar]
- Lelo A, Kjellen G, Birkett DJ, Miners JO. Paraxanthine metabolism in humans: determination of metabolic partial clearances and effects of allopurinol and cimetidine. J Pharmacol Exp Ther. 1989;248:315–319. [PubMed] [Google Scholar]
- Li SM, Yin LL, Shi J, Lin ZB, Zheng JW. The effect of 7-nitroindazole on the acquisition and expression of D-methamphetamine-induced place preference in rats. Eur J Pharmacol. 2002;435:217–223. doi: 10.1016/s0014-2999(01)01610-7. [DOI] [PubMed] [Google Scholar]
- Lindskog M, Svenningsson P, Pozzi L, Kim Y, Fienberg AA, Bibb JA, et al. Involvement of DARPP-32 phosphorylation in the stimulant action of caffeine. Nature. 2002;418:774–778. doi: 10.1038/nature00817. [DOI] [PubMed] [Google Scholar]
- Nehlig A, Daval JL, Debry G. Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res Rev. 1992;17:139–170. doi: 10.1016/0165-0173(92)90012-b. [DOI] [PubMed] [Google Scholar]
- Okuro M, Fujiki N, Kotorii N, Ishimaru Y, Sokoloff P, Nishino S. Effects of paraxanthine and caffeine on sleep, locomotor activity, and body temperature in orexin/ataxin-3 transgenic narcoleptic mice. Sleep. 2010;33:930–942. doi: 10.1093/sleep/33.7.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrú M, Bakešová J, Brugarolas M, Quiroz C, Beaumont V, Goldberg SR, et al. Striatal pre- and postsynaptic profile of adenosine A(2A) receptor antagonists. PLoS One. 2011a;6:e16088. doi: 10.1371/journal.pone.0016088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrú M, Quiroz C, Guitart X, Ferré S. Pharmacological evidence for different populations of postsynaptic adenosine A2A receptors in the rat striatum. Neuropharmacology. 2011b;61:967–974. doi: 10.1016/j.neuropharm.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6. 2006. [DOI] [PubMed] [Google Scholar]
- Przegaliński E, Filip M. Nitric oxide (NO) pathway and locomotor hyperactivity towards dopaminomimetics in rats. Pol J Pharmacol. 1997;49:291–298. [PubMed] [Google Scholar]
- Quarta D, Ferré S, Solinas M, You ZB, Hockemeyer J, Popoli P, et al. Opposite modulatory roles for adenosine A1 and A2A receptors on glutamate and dopamine release in the shell of the nucleus accumbens. Effects of chronic caffeine exposure. J Neurochem. 2004;88:1151–1158. doi: 10.1046/j.1471-4159.2003.02245.x. [DOI] [PubMed] [Google Scholar]
- Randall PA, Nunes EJ, Janniere SL, Stopper CM, Farrar AM, Sager TN, et al. Stimulant effects of adenosine antagonists on operant behavior: differential actions of selective A2A and A1 antagonists. Psychopharmacology. 2011;216:173–186. doi: 10.1007/s00213-011-2198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Irisarri E, Markerink-Van Ittersum M, Mengod G, de Vente J. Expression of the cGMP-specific phosphodiesterases 2 and 9 in normal and Alzheimer’s disease human brains. Eur J Neurosci. 2007;25:3332–3338. doi: 10.1111/j.1460-9568.2007.05589.x. [DOI] [PubMed] [Google Scholar]
- Sarrió S, Casadó V, Escriche M, Ciruela F, Mallol J, Canela EI, Lluis C, Franco R. The heat shock cognate protein hsc73 assembles with A(1) adenosine receptors to form functional modules in the cell membrane. Mol Cell Biol. 2000;20:5164–5174. doi: 10.1128/mcb.20.14.5164-5174.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale TW, Johnson P, Carney JM, Rennert OM. Interstrain variation in acute toxic response to caffeine among inbred mice. Pharmacol Biochem Behav. 1984;20:567–573. doi: 10.1016/0091-3057(84)90306-x. [DOI] [PubMed] [Google Scholar]
- Shi D, Daly JW. Chronic effects of xanthines on levels of central receptors in micew. Cell Mol Neurobiol. 1999;19:719–732. doi: 10.1023/A:1006901005925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder SH, Katims JJ, Annau Z, Bruns RF, Daly JW. Adenosine receptors and behavioral actions of methylxanthines. Proc Natl Acad Sci USA. 1981;78:3260–3264. doi: 10.1073/pnas.78.5.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder SH. Nitric oxide: first in a new class of neurotransmitters. Science. 1992;257:494–496. doi: 10.1126/science.1353273. [DOI] [PubMed] [Google Scholar]
- Solinas M, Ferré S, You ZB, Karcz-Kubicha M, Popoli P, Goldberg SR. Caffeine induces dopamine and glutamate release in the shell of the nucleus accumbens. J Neurosci. 2002;22:6321–6324. doi: 10.1523/JNEUROSCI.22-15-06321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavric B. Methylxanthines: toxicity to humans. 3 Theobromine, paraxanthine and the combined effects of methylxanthines. Food Chem Toxicol. 1988;26:725–733. doi: 10.1016/0278-6915(88)90073-7. [DOI] [PubMed] [Google Scholar]
- Stewart J, Deschamps SE, Amir S. Inhibition of nitric oxide synthase does not block the development of sensitization to the behavioral activating effects of amphetamine. Brain Res. 1994;641:141–144. doi: 10.1016/0006-8993(94)91827-9. [DOI] [PubMed] [Google Scholar]
- Sutherland EW, Rall TW. Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles. J Biol Chem. 1958;232:1077–1091. [PubMed] [Google Scholar]
- Svenningsson P, Nomikos GG, Fredholm BB. Biphasic changes in locomotor behavior and in expression of mRNA for NGFI-A and NGFI-B in rat striatum following acute caffeine administration. J Neurosci. 1995;15:7612–7624. doi: 10.1523/JNEUROSCI.15-11-07612.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Staay FJ, Rutten K, Bärfacker L, Devry J, Erb C, Heckroth H, et al. The novel selective PDE9 inhibitor BAY 73-6691 improves learning and memory in rodents. Neuropharmacology. 2008;55:908–918. doi: 10.1016/j.neuropharm.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Van Staveren WC, Markerink-van Ittersum M. Localization of cyclic guanosine 3′,5′-monophosphate-hydrolyzing phosphodiesterase type 9 in rat brain by nonradioactive in situ hydridization. Methods Mol Biol. 2005;307:75–84. doi: 10.1385/1-59259-839-0:075. [DOI] [PubMed] [Google Scholar]
- Vincent SR, Kimura H. Histochemical mapping of nitric oxide synthase in the rat brain. Neuroscience. 1992;46:755–784. doi: 10.1016/0306-4522(92)90184-4. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Karami M, Sepehri H, Sahraei H. Influence of nitric oxide on morphine-induced conditioned place preference in the rat central amygdala. Eur J Pharmacol. 2002;453:81–89. doi: 10.1016/s0014-2999(02)02328-2. [DOI] [PubMed] [Google Scholar]





