Abstract
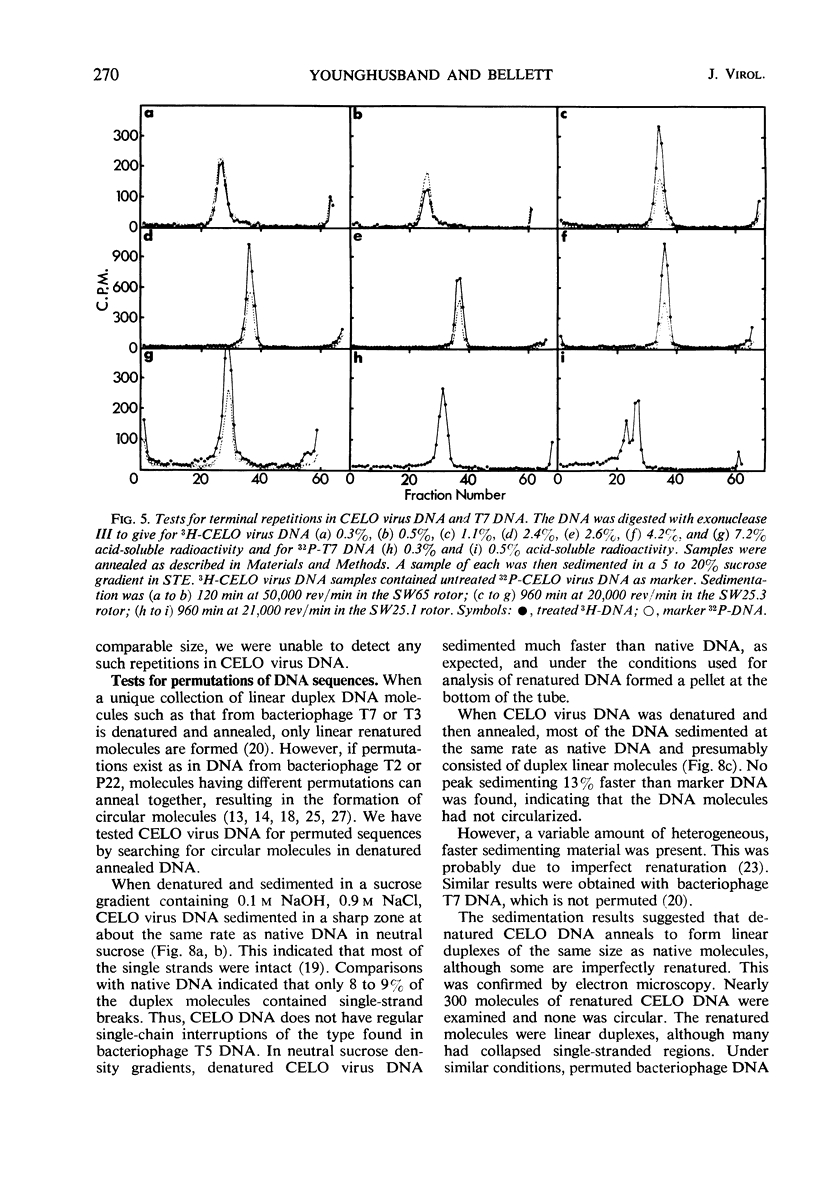
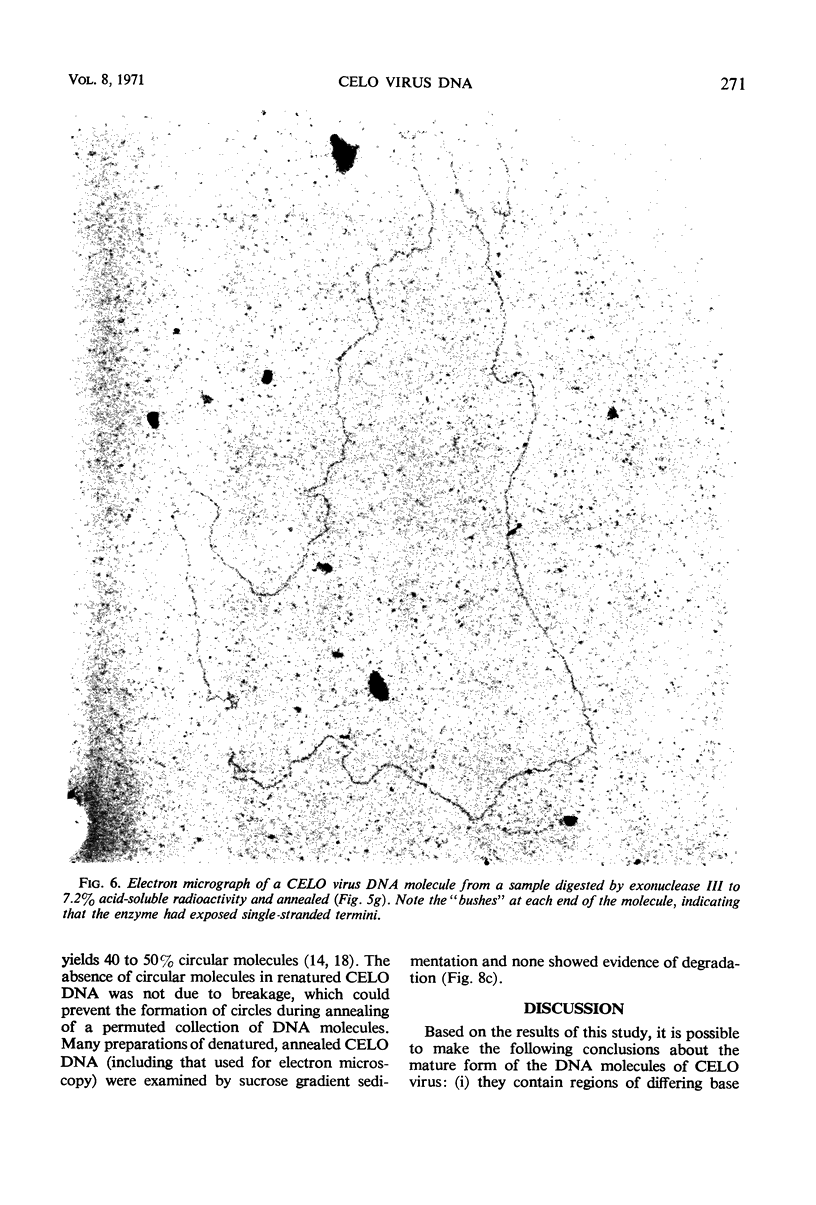
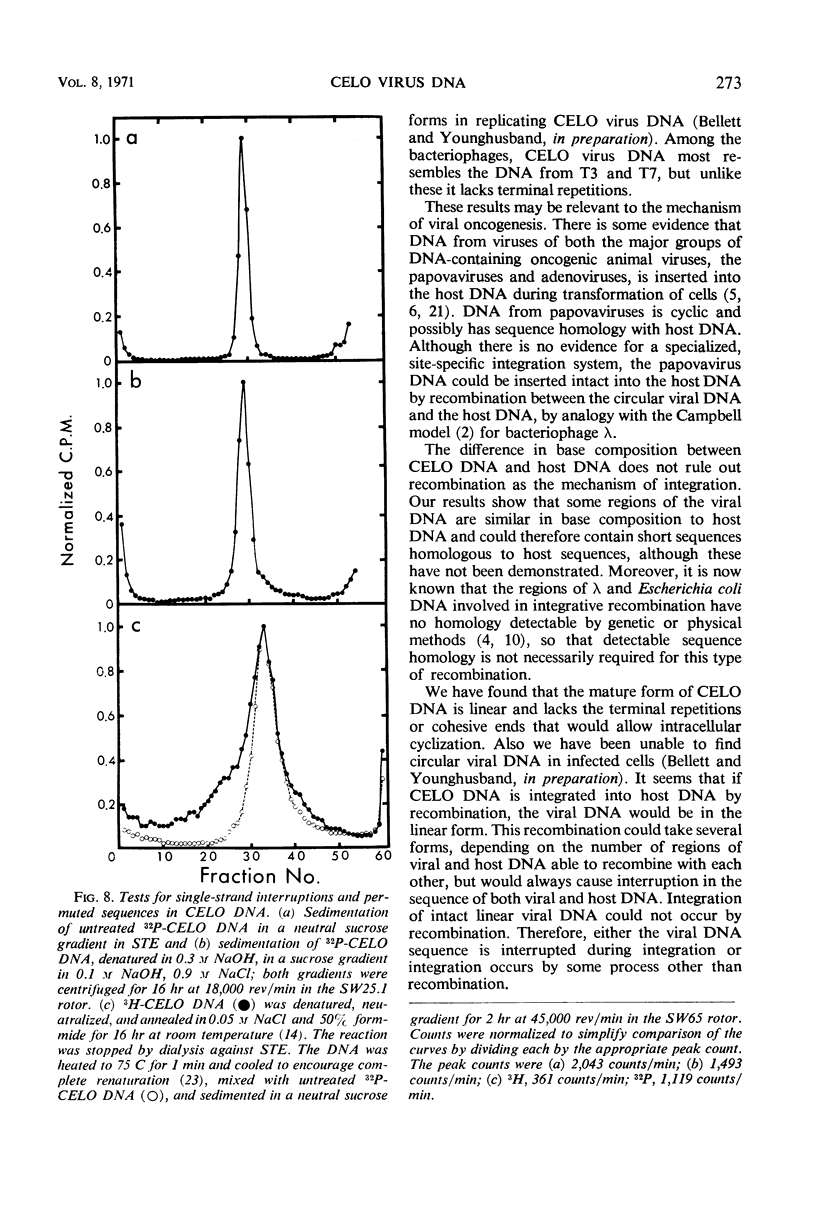
The deoxyribonucleic acid (DNA) of chick embryo lethal orphan (CELO) virus, an oncogenic avian adenovirus, had a biphasic denaturation profile indicating intramolecular base composition heterogeneity. This was confirmed by shearing the DNA and centrifuging it to equilibrium in Cs2SO4 in the presence of HgCl2 when two bands were formed. No circular molecules formed when CELO virus DNA was annealed, although λ DNA formed circles under the same conditions. No circular molecules were found by sedimentation or electron microscopy when the DNA was digested with exonuclease III and then annealed, but 30 to 40% of T7 DNA molecules became circular under similar conditions. The complementary strands of CELO virus DNA both appeared to be continuous, and, when CELO DNA was denatured and then annealed under appropriate conditions, all of the renatured molecules were linear. It is concluded that CELO virus DNA consists of a unique rather than permuted collection of linear molecules that lack exposed single-strand complementary ends or duplex terminal repetitions. These results are discussed in relation to the replication of viral DNA and the transformation of host cells.
Full text
PDF

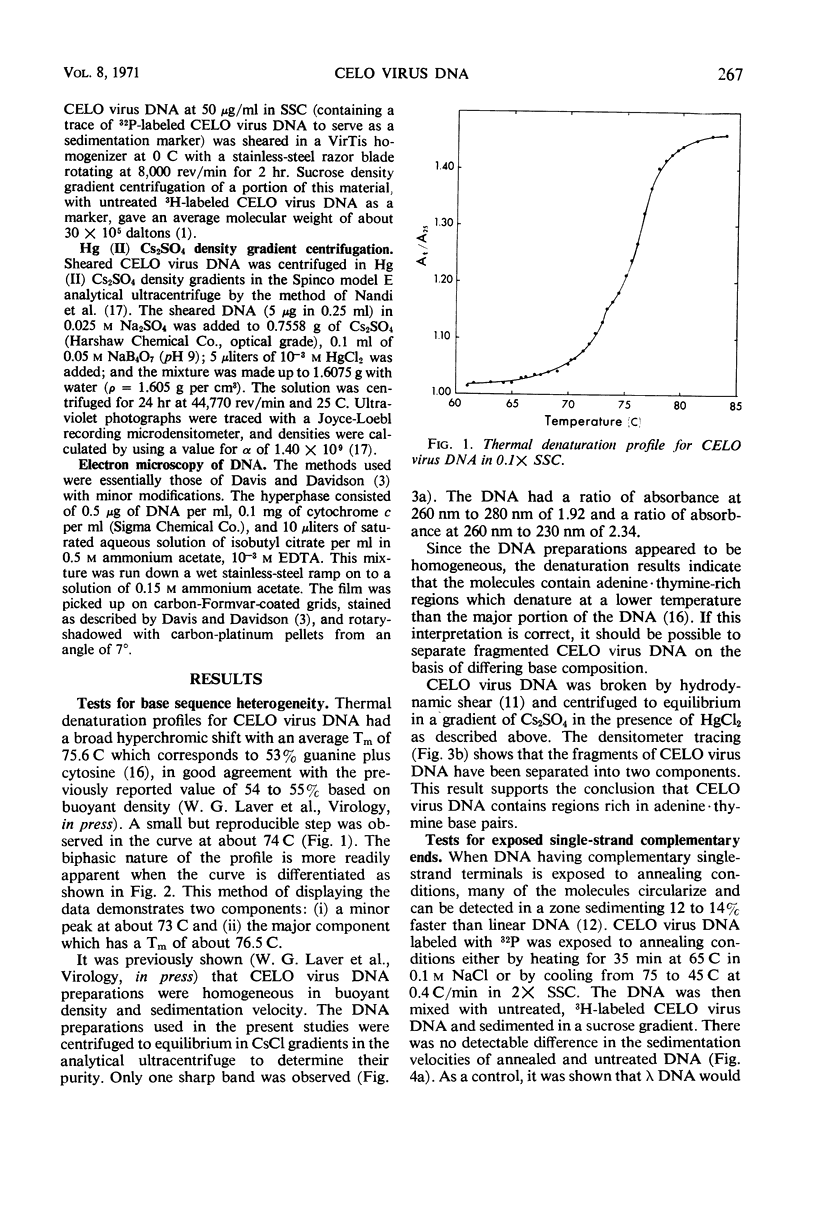


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. W., Davidson N. Electron-microscopic visualization of deletion mutations. Proc Natl Acad Sci U S A. 1968 May;60(1):243–250. doi: 10.1073/pnas.60.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. W., Parkinson J. S. Deletion mutants of bacteriophage lambda. 3. Physical structure of att-phi. J Mol Biol. 1971 Mar 14;56(2):403–423. doi: 10.1016/0022-2836(71)90473-6. [DOI] [PubMed] [Google Scholar]
- Doerfler W. Integration of the deoxyribonucleic acid of adenovirus type 12 into the deoxyribonucleic acid of baby hamster kidney cells. J Virol. 1970 Nov;6(5):652–666. doi: 10.1128/jvi.6.5.652-666.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W., Kleinschmidt A. K. Denaturation pattern of the DNA of adenovirus type 2 as determined by electron microscopy. J Mol Biol. 1970 Jun 28;50(3):579–593. doi: 10.1016/0022-2836(70)90086-0. [DOI] [PubMed] [Google Scholar]
- Doerfler W. The fate of the DNA of adenovirus type 12 in baby hamster kidney cells. Proc Natl Acad Sci U S A. 1968 Jun;60(2):636–643. doi: 10.1073/pnas.60.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAZEKAS DE ST GROTH S., GRAHAM D. M., JACK I. The serology of mumps infections. I. A new source of antigen and a simplified complement fixation test. J Lab Clin Med. 1958 Jun;51(6):883–896. [PubMed] [Google Scholar]
- Green M., Piña M., Kimes R., Wensink P. C., MacHattie L. A., Thomas C. A., Jr Adenovirus DNA. I. Molecular weight and conformation. Proc Natl Acad Sci U S A. 1967 May;57(5):1302–1309. doi: 10.1073/pnas.57.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrini F. On the asymmetry of lambda integration sites. J Mol Biol. 1969 Dec 28;46(3):523–542. doi: 10.1016/0022-2836(69)90194-6. [DOI] [PubMed] [Google Scholar]
- Hershey A. D., Burgi E., Ingraham L. COHESION OF DNA MOLECULES ISOLATED FROM PHAGE LAMBDA. Proc Natl Acad Sci U S A. 1963 May;49(5):748–755. doi: 10.1073/pnas.49.5.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey A. D., Burgi E., Ingraham L. Sedimentation Coefficient and Fragility under Hydrodynamic Shear as Measures of Molecular Weight of the DNA of Phage T5. Biophys J. 1962 Nov;2(6):423–431. doi: 10.1016/s0006-3495(62)86865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Tomizawa J. Prophage P1, and extrachromosomal replication unit. Cold Spring Harb Symp Quant Biol. 1968;33:791–798. doi: 10.1101/sqb.1968.033.01.091. [DOI] [PubMed] [Google Scholar]
- Lee C. S., Davis R. W., Davidson N. A physical study by electron microscopy of the terminally reptitious, circularly permuted DNA from the coliphage particles of Escherichia coli 15. J Mol Biol. 1970 Feb 28;48(1):1–22. doi: 10.1016/0022-2836(70)90215-9. [DOI] [PubMed] [Google Scholar]
- MacHattie L. A., Ritchie D. A., Thomas C. A., Jr, Richardson C. C. Terminal repetition in permuted T2 bacteriophage DNA molecules. J Mol Biol. 1967 Feb 14;23(3):355–363. doi: 10.1016/s0022-2836(67)80110-4. [DOI] [PubMed] [Google Scholar]
- Rhoades M., MacHattie L. A., Thomas C. A., Jr The P22 bacteriophage DNA molecule. I. The mature form. J Mol Biol. 1968 Oct 14;37(1):21–40. doi: 10.1016/0022-2836(68)90071-5. [DOI] [PubMed] [Google Scholar]
- Rhoades M., Thomas C. A., Jr The P22 bacteriophage DNA molecule. II. Circular intracellular forms. J Mol Biol. 1968 Oct 14;37(1):41–61. doi: 10.1016/0022-2836(68)90072-7. [DOI] [PubMed] [Google Scholar]
- Ritchie D. A., Thomas C. A., Jr, MacHattie L. A., Wensink P. C. Terminal repetition in non-permuted T3 and T7 bacteriophage DNA molecules. J Mol Biol. 1967 Feb 14;23(3):365–376. doi: 10.1016/s0022-2836(67)80111-6. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Westphal H., Srinivasan P. R., Dulbecco R. The integrated state of viral DNA in SV40-transformed cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1288–1295. doi: 10.1073/pnas.60.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Effects of the conformation of single-stranded DNA on renaturation and aggregation. J Mol Biol. 1969 Apr;41(2):199–209. doi: 10.1016/0022-2836(69)90385-4. [DOI] [PubMed] [Google Scholar]
- THOMAS C. A., Jr, MACHATTIE L. A. CIRCULAR T2 DNA MOLECULES. Proc Natl Acad Sci U S A. 1964 Nov;52:1297–1301. doi: 10.1073/pnas.52.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K. B., Sambrook J. F., Bellett A. J. Semliki forest virus temperature-sensitive mutants: isolation and characterization. Virology. 1969 Jul;38(3):427–439. doi: 10.1016/0042-6822(69)90155-x. [DOI] [PubMed] [Google Scholar]
- Thomas C. A., Jr, Kelly T. J., Jr, Rhoades M. The intracellular forms of T7 and P22 DNA molecules. Cold Spring Harb Symp Quant Biol. 1968;33:417–424. doi: 10.1101/sqb.1968.033.01.048. [DOI] [PubMed] [Google Scholar]
- Thomas C. A., Jr The rule of the ring. J Cell Physiol. 1967 Oct;70(2 Suppl):13–33. doi: 10.1002/jcp.1040700404. [DOI] [PubMed] [Google Scholar]
- Wu R., Taylor E. Nucleotide sequence analysis of DNA. II. Complete nucleotide sequence of the cohesive ends of bacteriophage lambda DNA. J Mol Biol. 1971 May 14;57(3):491–511. doi: 10.1016/0022-2836(71)90105-7. [DOI] [PubMed] [Google Scholar]
- YATES V. J., FRY D. E. Observations on a chicken embryo lethal orphan (CELO) virus. Am J Vet Res. 1957 Jul;18(68):657–660. [PubMed] [Google Scholar]