Abstract
Purpose
The purpose of this study was to determine the prevalence of ocular surface neoplasia (OSSN) coexistent with pterygia in South Florida and to study the treatment and related outcomes.
Design
Non-interventional retrospective study.
Participants
Two thousand and five patients with surgically excised pterygia at the Bascom Palmer Eye Institute from 2000 – 2010.
Methods
Pathology reports of patients with pterygia were reviewed for evidence of OSSN. Patients were divided into the following groups: pterygium and no OSSN (group1), clinically suspected OSSN with pterygium (group 2) and unexpected OSSN with pterygium found on histopathology (group 3). Clinical charts of patients in group 2 and 3 were reviewed.
Main outcome measures
Period prevalence, treatment and outcome.
Results
In surgically excised pterygia, we found the prevalence of coexistent OSSN to be 1.7% (n=34), of which 41% (n=14) were clinically suspected preoperatively (group 2) and 59% (n=20) were unexpectedly found on histopathology (group 3). Clinically suspected OSSN with pterygia was generally treated with wide surgical margins and cryotherapy, whereas unexpected OSSN with pterygia was treated with simple excision, followed by adjuvant interferon treatment in 30% (n=6). After a mean follow up of 2 years, there were no recurrences in the suspected OSSN group and 2 recurrences in the unexpected OSSN group. The recurrence rate in the latter group was 11% at 1 year and 24% at 2 years.
Conclusion
OSSN is uncommonly found to coexist with pterygium. The prognosis in suspected OSSN cases is excellent with no recurrences noted despite positive margins in 50% of cases. The recurrence rates of unexpected OSSN mirrors that of OSSN not associated with pterygium, and thus vigilance for recurrence is important.
Keywords: pterygium, OSSN, CIN, intraepithelial carcinoma, conjunctiva, tumor
Introduction
Ocular surface squamous neoplasia (OSSN) represents a spectrum of corneal and/or conjunctival epithelial neoplasias that range from mild dysplasia to squamous cell carcinoma (SCC).1–3 Several studies have evaluated the frequency of OSSN in various locations. Lee et al found that the 10 year period prevalence of OSSN was 1.9% in Australia4 and Newton et al estimated the incidence of disease to be 0.10 in the United Kingdom (UK).5 While the exact etiology of OSSN is unknown, the most common risk factors are older age, ultraviolet light exposure, human papilloma virus (HPV), human immunodeficiency virus (HIV) and fair skin pigmentation.1
Pterygium is a common ocular surface degenerative disorder with a prevalence ranging from 1,000 to 33,000 per 100,000 depending on geographic location, with higher prevalence in regions closer to the equator.6, 7 The common risk factors for pterygia are similar to those of OSSN, namely, exposure to ultraviolet (UV) light. Chronic ocular surface irritation such as from a dusty, dry environment also may play an important role.6, 7 Another possible risk factor between the two may be human papilloma virus (HPV) infection. While the role of HPV is still unclear in OSSN, Scott et al. found high risk HPV16/18 mRNA (indicative of active HPV infection) in 10 out of 10 conjunctival/corneal intraepithelial neoplasia (CIN) samples using reverse-transcriptase PCR.8 A study from Taiwan detected HPV16/18 E6 oncoprotein (also indicative of active HPV infection) in 15 of 131 pterygia samples by immunohistochemistry.9 In a meta-analysis, Di Giralomo reported an overall prevalence of 18.6% (range 0 – 100%) of HPV infection in pterygia and 33.8% in OSSN (range 0 – 100%) and noted that the inconsistency in results may be in part explainable by methodological difference and racial and geographical factors.10
As OSSN and pterygia have shared risk factors, it is not surprising that they can exist concomitantly. OSSN with pterygia have been found in 9.8% of all pterygia cases in a single surgeon practice in Brisbane, Australia, and in this series, 94% of cases were an unexpected finding on pathologic review.11 In a subsequent study from Sidney, Australia, 5% of pterygia were found to have foci of OSSN on pathologic review.12 A study in Canada, a region with much lower UV light exposure than Australia, found that within 1127 consecutive pterygia specimen over a period of 8 years, no cases of OSSN were identified.13 The presence of an unexpected OSSN with pterygium poses a management dilemma since routine surgeries for pterygium and OSSN differ. OSSN is generally removed utilizing a no touch technique, with 4 – 5 mm margins, alcohol epitheliectomy to the cornea and application of cryotherapy to the surgical margins.1 For pterygium, most surgeons prefer simple excision, with or without intraoperative application of mitomycin-C.14 The importance of positive margins in OSSN was demonstrated by Tabin et al., who showed a recurrence of 56% for tumors with positive margins compared to 33% for tumors with negative margins.15
Since the prevalence of OSSN with pterygia has been reported for geographic areas at the high (Australia, UV index 8–9)16 and low (Canada, UV index 2–3)17 end of the UV exposure spectrum, it is relevant to determine the prevalence of OSSN with pterygia in other geographic areas (South Florida, UV index 6–7).17 In addition, information about the best approach for the management of unexpected OSSN-associated pterygia is limited. Over the last decade, physicians at Bascom Palmer Eye Institute have treated a large number of patients with pterygia. The aim of the present study was to investigate the prevalence of OSSN in surgically excised pterygia in a multiple physician practice in South Florida and to report on outcomes of OSSN with pterygia in order to provide treatment guidelines for this situation.
Methods
Study population
The pathology records of the Florida Lions Ocular Pathology Laboratory at the Bascom Palmer Eye Institute (BPEI) were searched for diagnoses containing the word “pterygium”. A total of 2005 unique patient reports were identified from 01/01/2000 – 12/31/2010. All records were reviewed for patient demographic information and pathological characteristics of the lesions, specifically for evidence of OSSN with pterygia. Cases were divided into three groups: Group 1 consisted of patients with ptergyium and no OSSN, group 2 consisted of patients in which an OSSN with pterygium was suspected pre-operatively, and group 3 consisted of patients where OSSN was incidentally discovered in the epithelium overlying a ptergyium on pathologic review. Inclusion criteria for the groups were the following: group 1 had pterygium as a pathological diagnosis; group 2 had pterygium and OSSN as both a clinical and pathological diagnosis; group 3 had pterygium as a clinical diagnosis and pterygium/OSSN as a pathological diagnosis. Representative slit lamp images of each of the 3 groups are demonstrated in Figure 1.
Figure 1.
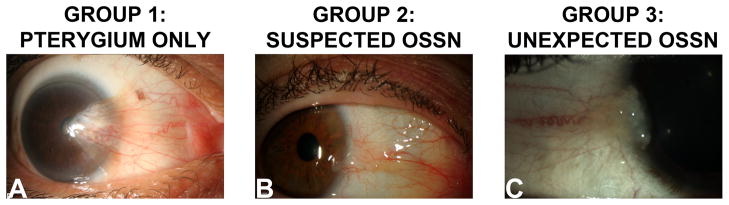
Representative slit lamp biomicroscopy photographs demonstrating the study groups. A: pterygium (group 1); B: pterygium with clinically suspected ocular surface squamous neoplasia (OSSN), C: pterygium with OSSN that was clinically unexpected.
As a part of our study we conducted a re-review of a select group of pathology slides. This involved re-reviewing the first and last 7 OSSN cases. For each OSSN case, we also re-reviewed two pterygia cases that were performed in the same time period. In total 42 cases were re-reviewed in a masked fashion by our ocular pathologist (SRD). On re-review, no changes were made to the original diagnosis. Approval was obtained from the University of Miami Institutional Review Board and the methods adhered to the tenets of the Declaration of Helsinki and were HIPAA-compliant.
Surgical approach
Surgical removal was performed by multiple surgeons. Suspected OSSN (group 2, n=14) was removed by excising the lesion (wide margins n=10 (71%), mean margin = 1.3 mm, range 1 – 3 mm). Cryotherapy was applied in 10 cases using a cryoprobe (Frigitronics, CE-200 cryosurgical system, Cooper Surgical, Trumbull, CT)) in a double-freeze slow thaw cycle. The cryotherapy temperature ranged from −40 to −80°C. Closure was performed as direct closure (n=4, 36%), placement of autograft (n=1, 9%) or amniotic membrane (n=6, 55%). Unexpected OSSN (group 3) was excised by simple excision and a conjunctival autograft (n=11) 69%) or amniotic membrane transplant (n=3, 19%) or primary closure (n=2, 13%) was then used to reconstruct the ocular surface. Intraoperative mitomycin C 0.02% was utilized for 30 seconds to 2 minutes in 4 patients (2 from each group). Postoperative adjuvant interferon eye drops (1 million international units (MIU), 4 times a day) were administered in 6 patients with unexpected OSSN (group 3) at a median of 49 days after surgery (range 28–87) for a median duration of 3 months (range 26 days – 5 months).
Data collection
All lesions had been previously examined by a single experienced ocular pathologist (SRD). Demographic information recorded from the pathology report included gender and age; information recorded on the OSSN included involved eye, tumor grade, location, and presence at surgical margins. Corresponding chart records for patients belonging to group 2 and 3 were obtained. Charts were reviewed for previous history of OSSN and lesion location. Treatment information including surgical technique and any adjuvant therapy was recorded as well as post-operative course including OSSN or pterygium recurrence and subsequent treatments.
Statistical analysis
All statistical analyses were performed using the SPSS 19.0 (SPSS Inc, Chicago, IL) statistical package. Means and frequencies of demographic and clinical variables were calculated for the groups. Analysis of variance and Chi square analysis were employed to look for differences in demographic and clinical variables between the groups. P value less than 0.05 were considered to be significant. Kaplan Meier survival analysis was used to calculate OSSN recurrence rates. Standard deviation (SD) was calculated.
Results
Table 1 shows the demographic and location information of pterygia and OSSN with pterygia. There were 2005 patients with surgically excised pterygia during the 11 year period. Among these, we found 34 cases of OSSN coexistent with pterygia (1.7%); which were further divided into 14 clinically suspected OSSN with pterygia (group 2; 0.7%) and 20 unexpected OSSN with pterygia (group 3; 1%). Patients in the pterygia only group were significantly younger (mean 51) than patients in the suspected OSSN with pterygia group (mean 62) (p=0.002). There was no significant difference between the groups in terms of gender or eye. Nasal location was the most frequent location for the lesions in all three groups. There was a significant predominance of inferior location in the suspected OSSN in pterygia group (p=0.009).
Table 1.
Demographic and location information for pterygium only (group 1), suspected OSSN with pterygium (group 2) and unexpected OSSN with pterygium (group 3).
| Group 1 | Group 2 | Group 3 | P value | |
|---|---|---|---|---|
| Total, n | 1971 | 14 | 20 | |
| Age, years, mean (SD) | 51 (14) | 62 (10) | 58 (17) | 0.002 |
| Male, n (%) | 1032 (53%) | 11 (79%) | 11 (55%) | 0.16 |
| Right eye, n % | 942 (49%) | 6 (43%) | 12 (60%) | 0.55 |
| Nasal location†; n (%) | 260 (82%) | 11 (85%) | 17 (85%) | 0.92 |
| Temporal location; n (%) | 69 (22%) | 2 (15%) | 3 (15%) | 0.66 |
| Inferior location; n (%) | 6 (2%) | 2 (15%) | 1 (5%) | 0.009 |
| Superior location; n (%) | 6 (2%) | 0 (0%) | 0 (0%) | 0.72 |
OSSN=ocular surface squamous neoplasia; n=Number in group; SD=standard deviation;
Post-hoc analysis reveals that difference in age significant between group 1 and 2 only.
Location information available for 357 pterygia specimen
The pathological grade of the tumors is listed in Table 2. Representative images of pathology grades are demonstrated in figure 2. Specific examples for histopathology of OSSN coexistent with pterygium can be seen in figure 3. Suspected OSSN with pterygium (group 2) displayed the whole pathologic spectrum from mild conjunctival intraepithelial neoplasia (CIN) to squamous cell carcinoma (SCC), although most cases were moderate in grade (43%). Most cases of unexpected OSSN with pterygium were also moderate in grade (40%) and no cases of SCC were seen. Pathologic tumor margins were found to be positive for tumor cells in 50% of group 2 and 23% of group 3. However, 35% of specimens from group 3 could not be evaluated due to inadequate tissue orientation/handling.
Table 2.
Pathological tumor grade and margins of suspected (group 2) and unexpected (group 3) OSSN with pterygium
| Group 2 | Group 3 | P value | |
|---|---|---|---|
| Mild dysplasia; n (%) | 1 (7%) | 4 (20%) | 0.56† |
| Moderate dysplasia; n (%) | 6 (43%) | 8 (40%) | |
| Severe dysplasia; n (%) | 3 (21%) | 1 (5%) | |
| Carcinoma in situ; n (%) | 3 (20%) | 7 (35%) | |
| Squamous cell carcinoma; n (%) | 1 (7%) | 0 (0%) | |
| Positive pathological margins; n (%) | 7 (50%) | 3 (23%)* | 0.15 |
OSSN= ocular surface squamous neoplasia
Non-assessable margins in 35 % (n=7) in group 3
Linear-by-linear association
Figure 2.
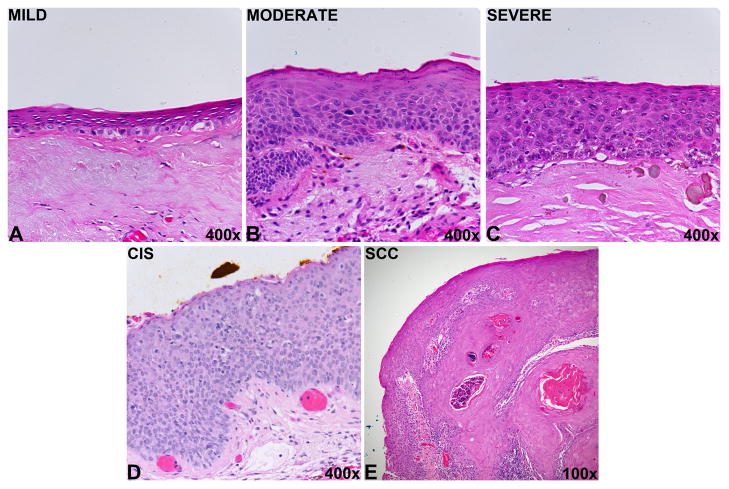
Representative histopathology images demonstrating ocular surface squamous neoplasia grades. A) Conjunctival intraepithelial neoplasia (CIN): dysplasia, mild. The conjunctival epithelium displays faulty epithelial maturational sequencing that extends to approximately 25% of the epithelial thickness. B) CIN: dysplasia, moderate. The conjunctival epithelium displays faulty epithelial maturational sequencing that extends to approximately 50% of the epithelial thickness. C) CIN: dysplasia, severe. The conjunctival epithelium displays faulty epithelial maturation sequencing that extends to near full thickness. D) CIN: Carcinoma in situ. The conjunctival epithelium displays faulty epithelial maturational sequencing that extends to full thickness. E) Conjunctival squamous cell carcinoma. The conjunctival epithelium displays faulty epithelial maturational sequencing that extends to full thickness. Foci of invasion of atypical epithelial cells into the underlying substantial propria are identified. All images stained with hematoxylin and eosin.
Figure 3.
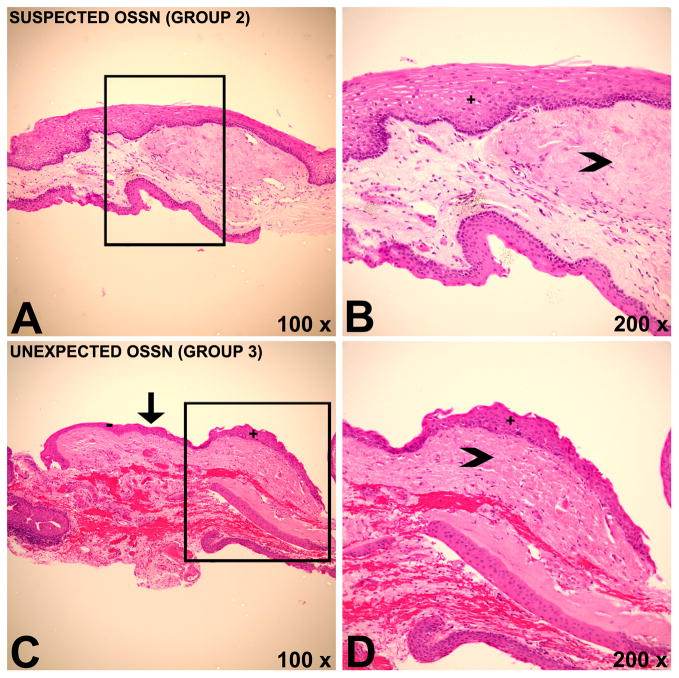
Histopathology images demonstrating coexistent pterygium and ocular surface squamous neoplasia (OSSN). A,B) Histopathology of a patient belonging to group 2 with marked solar elastosis (arrowhead) and overlying epithelium with faulty maturational sequencing (+), indicating OSSN with an underlying pterygium. In this case, OSSN was anticipated by the surgeon. C, D) Histopathology of a patient belonging to group 3 demonstrating focus of OSSN (+) with a clear transition (arrow) towards unremarkable epithelium (−). Solar elastosis (arrowhead) is present, indicating a pterygium. OSSN was found incidentally in pathology and was not suspected by the surgeon. Hematoxylin & Eosin (H&E).
Treatment information is shown in Table 3. Most cases of suspected OSSN with pterygia (group 2) were treated with excision utilizing wide surgical margins and cryotherapy (71%, n=10), a minority underwent additional treatment in the form of sclerectomy (n=2) and intra-operative mitomycin-C MMC (n=2). All cases of unexpected OSSN with pterygia (group 3) were treated with simple excision (n=20), 2 (10%) received intra-operative MMC and 6 (30%) received post-operative interferon eye drops (1 million international units, 4 times a day). One patient in group 3 underwent immediate repeat surgical excision utilizing wider margins after the OSSN diagnosis.
Table 3.
Treatment information for suspected OSSN with pterygium (group 2) and unexpected OSSN with pterygium (group 3).
| Group 2 | Group 3 | |
|---|---|---|
| Sclerectomy, n (%) | 2 (15%)* | 0 (0%) |
| Cryotherapy, n (%) | 10 (77%) | 0 (0%) |
| Intra-operative MMC, n (%) | 2 (15%) | 2 (10%) |
| Post-operative interferon drops, n (%) | 0 (0%) | 6 (30%) |
| Average time on interferon drops, mean (SD), months | 0 | 3.2 (1.3) |
| Immediate repeat surgical excision, n (%) | 0 | 1 (5%) |
one treatment information missing in group 2
OSSN=ocular surface squamous neoplasia; n=number in group; MMC=mitomycin-C
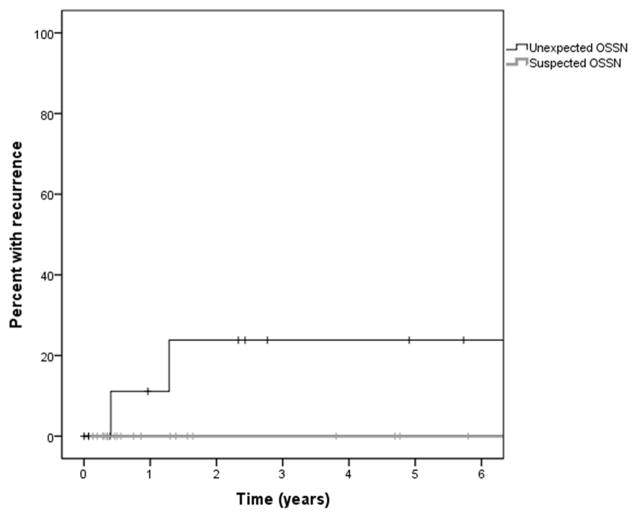
Figure 4 displays the time to recurrence in both groups. In group 2, no recurrences were noted after a mean follow up of 815 days (standard deviation; SD=871). In group 3, after a mean follow up of 639 days (SD=768), two recurrences were recognized after 148 (patient 1) and 468 days (patient 2), respectively. Kaplan Meier survival analysis indicate that the estimated recurrence rate was 11% after one year and 24% after two years in group 3.
Figure 4.
Kaplan Meier survival curve demonstrating the recurrence rate of ocular surface squamous neoplasia (OSSN) over time in patients with suspected and unexpected OSSN with pterygium.
In the 2 patients with recurrences, patient 1 received intra-operative mitomycin-C at the time of initial pterygium removal and on pathology the OSSN was characterized as mild dysplasia with non-assessable margins. After recurrence was noted, the patient was lost to follow up. Patient 2 had a history of OSSN in the same eye 19 months earlier which had been treated with interferon eye drops (1 million international units/ml, 4 times a day) for four months. His pterygium was removed using simple excision with no adjuvant intra-operative therapy. On pathology, the OSSN was characterized as severe dysplasia with non-assessable margins and adjuvant interferon eye drops were prescribed for 5 months. The recurrence was treated with interferon injections (5 injections of 3 million international units/0.5cc within 6 months) which resulted in tumor resolution.
Discussion
This study demonstrated a period prevalence of 1.7% of OSSN within surgically excised pterygia in South Florida (UV-index 6–7). The prevalence of OSSN with pterygia was reported to be 9.8% in Brisbane11, 5% in Sidney12 (UV-index 8–9)16 and 0% in Canada13 (UV-index 2–3).17 Our finding of a 1.7% prevalence maybe fits with the theory that UV-radiation is involved in the pathophysiology of OSSN. This data suggests that surgeons in high UV radiation zones should perhaps have a higher index of suspicion for OSSN when evaluating and treating pterygia.
Analyzing the demographic data, patients with coexistent OSSN and pterygia were older than patients with simple pterygia. This correlates well with the general observation that OSSN occurs in older individuals.18 Although location data for our cohort was incomplete, inferiorly located lesions were seen more often in the suspected OSSN group. This finding highlights the need to perhaps increase the suspicion for malignancy when a lesion does not present at the nasal or temporal position.
We found that 2 recurrences occurred in the unexpected OSSN group and none occurred in the suspected group. Hirst et al, found no cases of OSSN recurrences 1 year after surgery; however, no further follow up information was available in this study.11 Our recurrence rate of 11% at one year in the unexpected group is similar to that of OSSN not associated to pterygium as Yousef et al found a recurrence rate of 12% at 1 year among 101 OSSN lesions.19 In that study, tumors were treated using oncologic standards of wide surgical margins, cryotherapy, and adjuvant therapy in some cases. While we treated a subset of unexpected OSSN with adjuvant interferon, the small number of patients in our study does not allow for evaluation of its effectiveness in decreasing OSSN recurrence. However, because of the favorable side effect profile20 and its effectiveness in treating primary and recurrent tumors21, 22 we favor using topical interferon in such cases, until conclusive data is available.
Our study findings must be considered bearing in mind its limitations which include its retrospective nature and variability in treatment algorithms. A limitation of our study is the lack of photographic documentation in the unsuspected OSSN group. In our center, patients with presumed pterygia are not routinely photographed prior to surgical excision. Its strength is that it is the first to systematically analyze demographics, treatment and outcomes for OSSN with pterygia, both clinically suspected and unexpectedly found in histopathology. In summary, this study revealed the coexistence of OSSN in surgically excised pterygia with a prevalence of 1.7%. In addition, we found that the prognosis of suspected OSSN with pterygia was excellent when treated with wide-surgical margins and cryotherapy. The recurrence rates for unexpected OSSN with pterygia were comparable to isolated OSSN, highlighting the need for vigilant and longtime follow up. Future investigations with larger patient numbers are needed to study whether adjuvant measures for unsuspected OSSN with pterygia can significantly lower the recurrence rate.
Acknowledgments
Financial support: Supported by NIH Center Core Grant P30EY014801, Research to Prevent Blindness Unrestricted Grant, Department of Defense (DOD-Grant#W81XWH-09-1-0675) (institutional grants) and Max Kade, New York, The Ronald and Alicia Lepke grant, The Claire and Lee Hager Grant.
Footnotes
Conflict of interest: None of the authors has a conflict of interest related to the study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Basti S, Macsai MS. Ocular surface squamous neoplasia: a review. Cornea. 2003;22:687–704. doi: 10.1097/00003226-200310000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Grossniklaus HE, Green WR, Luckenbach M, Chan CC. Conjunctival lesions in adults. A clinical and histopathologic review. Cornea. 1987;6:78–116. doi: 10.1097/00003226-198706020-00002. [DOI] [PubMed] [Google Scholar]
- 3.Pizzarello LD, Jakobiec FA. Bowen’s disease of the conjunctiva: a misnomer. In: Jakobiec FA, editor. Ocular and Adnexal Tumors. Birmingham, AL: Aesculapius Pub; 1978. pp. 553–71. [Google Scholar]
- 4.Lee GA, Hirst LW. Incidence of ocular surface epithelial dysplasia in metropolitan Brisbane. A 10-year survey. Arch Ophthalmol. 1992;110:525–7. doi: 10.1001/archopht.1992.01080160103042. [DOI] [PubMed] [Google Scholar]
- 5.Newton R, Ferlay J, Reeves G, et al. Effect of ambient solar ultraviolet radiation on incidence of squamous-cell carcinoma of the eye. Lancet. 1996;347:1450–1. doi: 10.1016/s0140-6736(96)91685-2. [DOI] [PubMed] [Google Scholar]
- 6.Norn MS. Prevalence of pinguecula in Greenland and in Copenhagen, and its relation to pterygium and spheroid degeneration. Acta Ophthalmol (Copenh) 1979;57:96–105. doi: 10.1111/j.1755-3768.1979.tb06664.x. [DOI] [PubMed] [Google Scholar]
- 7.Panchapakesan J, Hourihan F, Mitchell P. Prevalence of pterygium and pinguecula: the Blue Mountains Eye Study. Aust N Z J Ophthalmol. 1998;26(suppl):S2–5. doi: 10.1111/j.1442-9071.1998.tb01362.x. [DOI] [PubMed] [Google Scholar]
- 8.Scott IU, Karp CL, Nuovo GJ. Human papillomavirus 16 and 18 expression in conjunctival intraepithelial neoplasia. Ophthalmology. 2002;109:542–7. doi: 10.1016/s0161-6420(01)00991-5. [DOI] [PubMed] [Google Scholar]
- 9.Tsai YY, Chang CC, Chiang CC, et al. HPV infection and p53 inactivation in pterygium. [Accessed July 16, 2012];Mol Vis [serial online] 2009 15:1092–7. Available at: http://www.molvis.org/molvis/v15/a115/ [PMC free article] [PubMed] [Google Scholar]
- 10.Di Girolamo N. Association of human papilloma virus with pterygia and ocular-surface squamous neoplasia. Eye (Lond) 2012;26:202–11. doi: 10.1038/eye.2011.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirst LW, Axelsen RA, Schwab I. Pterygium and associated ocular surface squamous neoplasia. Arch Ophthalmol. 2009;127:31–2. doi: 10.1001/archophthalmol.2008.531. [DOI] [PubMed] [Google Scholar]
- 12.Chui J, Coroneo MT, Tat LT, et al. Ophthalmic pterygium: a stem cell disorder with premalignant features. Am J Pathol. 2011;178:817–27. doi: 10.1016/j.ajpath.2010.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeung SN, Kim P, Lichtinger A, et al. Incidence of ocular surface squamous neoplasia in pterygium specimens: an 8-year survey [letter] Br J Ophthalmol. 2011;95:592. doi: 10.1136/bjo.2010.197491. [DOI] [PubMed] [Google Scholar]
- 14.Hirst LW. The treatment of pterygium. Surv Ophthalmol. 2003;48:145–80. doi: 10.1016/s0039-6257(02)00463-0. [DOI] [PubMed] [Google Scholar]
- 15.Tabin G, Levin S, Snibson G, et al. Late recurrences and the necessity for long-term follow-up in corneal and conjunctival intraepithelial neoplasia. Ophthalmology. 1997;104:485–92. doi: 10.1016/s0161-6420(97)30287-5. [DOI] [PubMed] [Google Scholar]
- 16.Australian Radiation Protection and Nuclear Safety Agency. [Accessed February 27, 2012];Australian UV Index Models. Available at: http://www.arpansa.gov.au/uvindex/models/briuvmodel.htm.
- 17.United States Environmental Protection Agency. [Accessed February 27, 2012];Monthly Average UV Index. United States and Canada (link) Available at: http://www.epa.gov/sunwise/uvimonth.html.
- 18.Lee GA, Hirst LW. Ocular surface squamous neoplasia. Surv Ophthalmol. 1995;39:429–50. doi: 10.1016/s0039-6257(05)80054-2. [DOI] [PubMed] [Google Scholar]
- 19.Yousef YA, Finger PT. Squamous carcinoma and dysplasia of the conjunctiva and cornea: an analysis of 101 cases. Ophthalmology. 2012;119:233–40. doi: 10.1016/j.ophtha.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Galor A, Karp CL, Chhabra S, et al. Topical interferon alpha 2b eye-drops for treatment of ocular surface squamous neoplasia: a dose comparison study. Br J Ophthalmol. 2010;94:551–4. doi: 10.1136/bjo.2008.153197. [DOI] [PubMed] [Google Scholar]
- 21.Karp CL, Moore JK, Rosa RH., Jr Treatment of conjunctival and corneal intraepithelial neoplasia with topical interferon alpha-2b. Ophthalmology. 2001;108:1093–8. doi: 10.1016/s0161-6420(01)00577-2. [DOI] [PubMed] [Google Scholar]
- 22.Schechter BA, Koreishi AF, Karp CL, Feuer W. Long-term follow-up of conjunctival and corneal intraepithelial neoplasia treated with topical interferon alfa-2b. Ophthalmology. 2008;115:1291–6. doi: 10.1016/j.ophtha.2007.10.039. [DOI] [PubMed] [Google Scholar]