Abstract
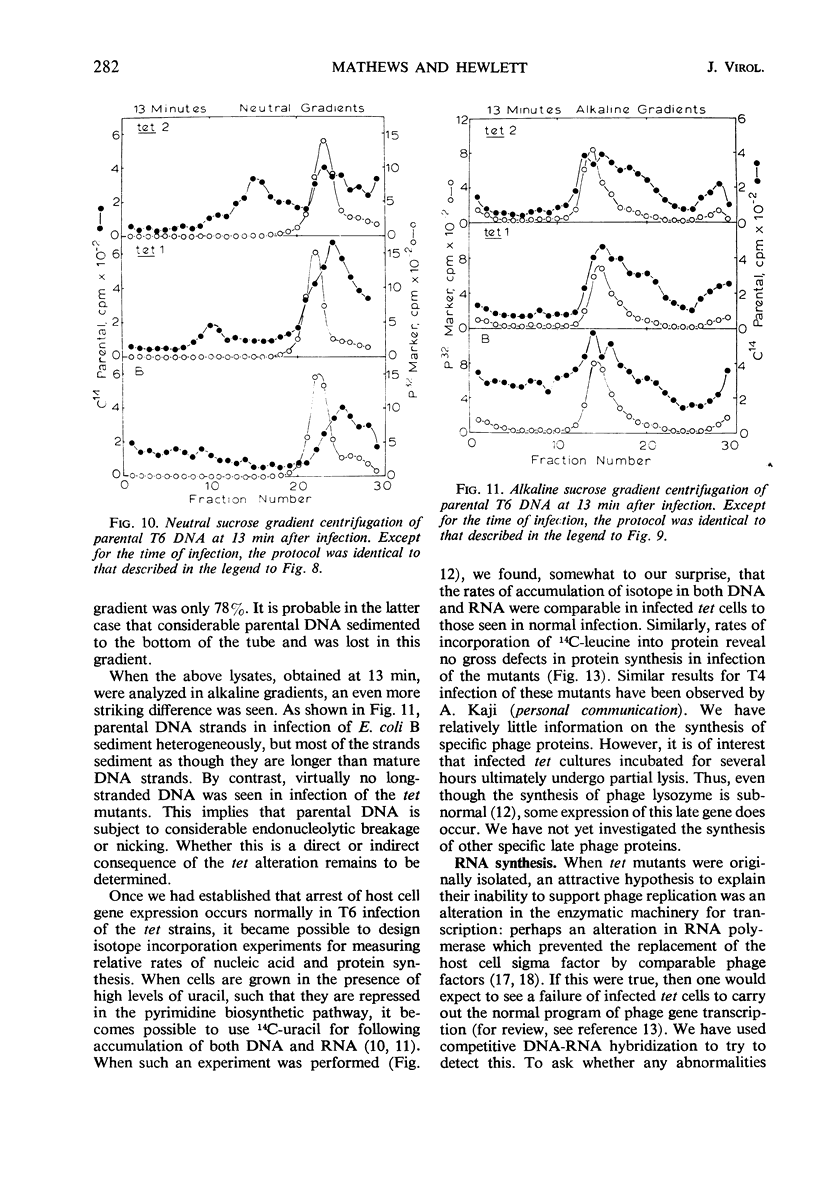
T-even bacteriophage-tolerant mutants are strains of Escherichia coli which can adsorb T-even phages but cannot support the growth of infective virus. Under some conditions, the infected cells are not killed. Mutant cells infected by phage T6 are able to carry out several metabolic functions associated with normal virus development, including arrest of bacterial nucleic acid and protein synthesis, incorporation of isotopic precursors into viral nucleic acids and proteins, synthesis of early enzymes of deoxyribonucleic acid (DNA) metabolism, formation of rapidly sedimenting DNA intermediates, and formation of normal levels of early and late messenger ribonucleic acid species. Phage are unable to mutate to forms capable of growth on these mutants. The nature of the biochemical alteration leading to tolerance is still unknown.
Full text
PDF
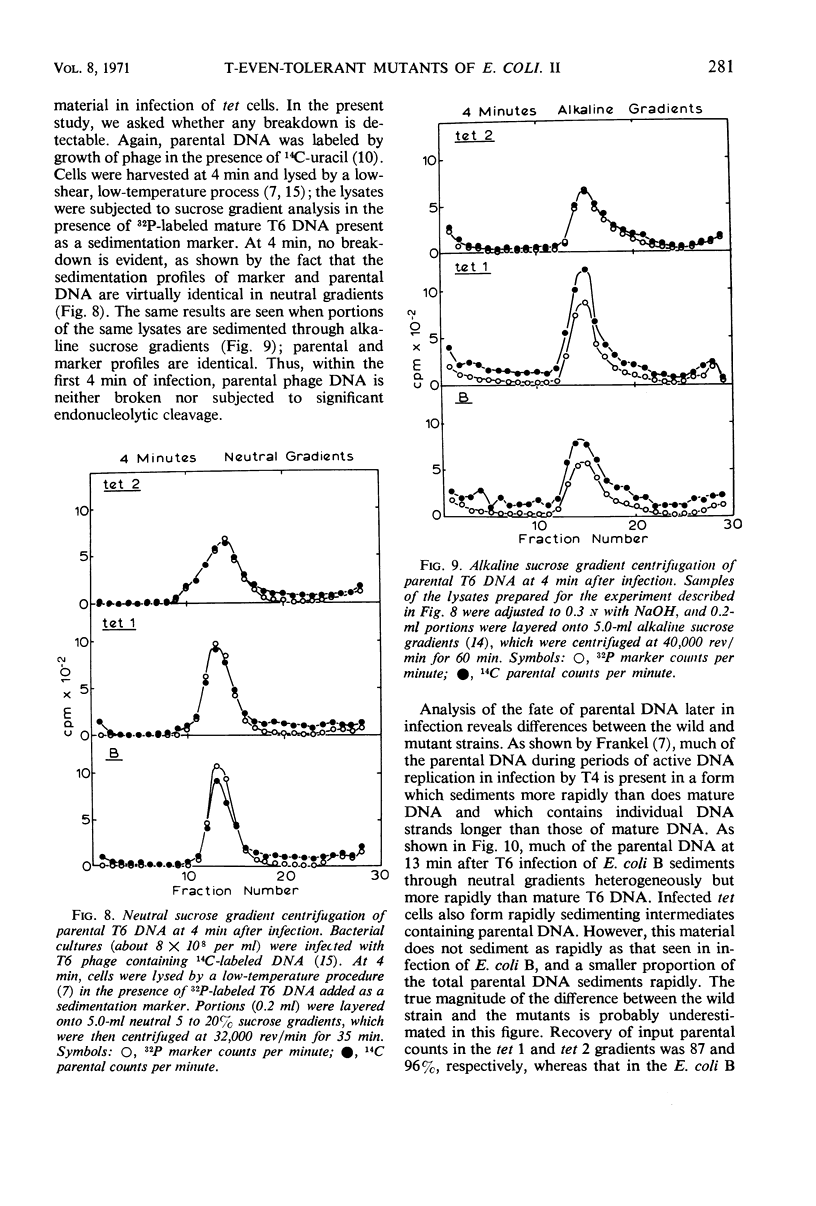
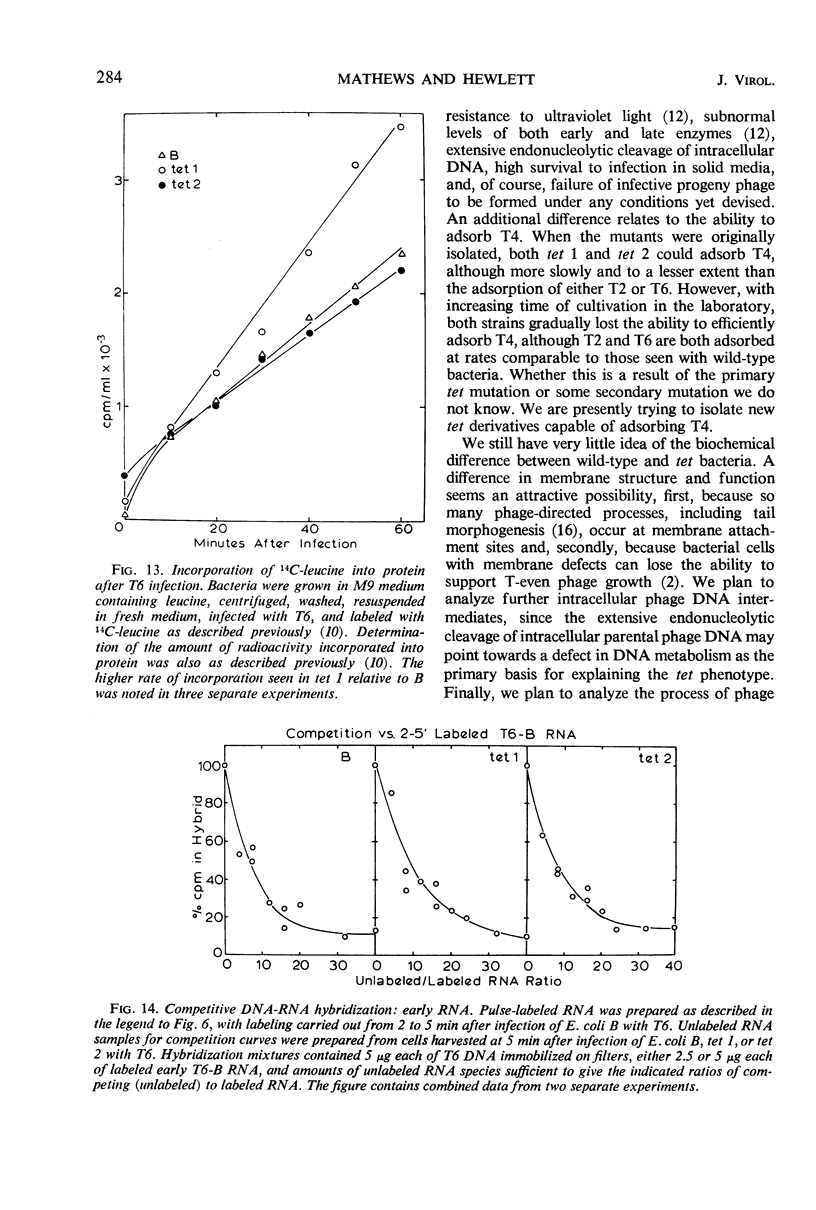
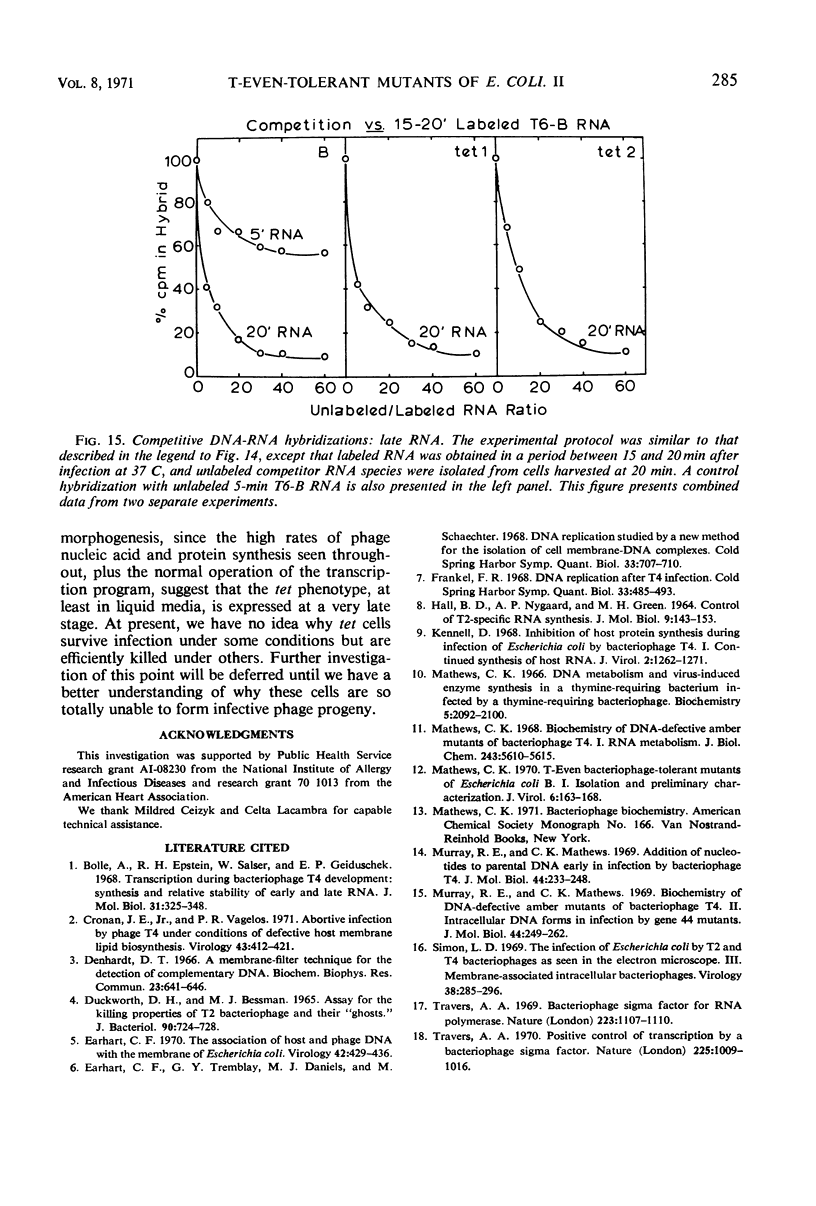
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: synthesis and relative stability of early and late RNA. J Mol Biol. 1968 Feb 14;31(3):325–348. doi: 10.1016/0022-2836(68)90413-0. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Vagelos P. R. Abortive infection by phage T4 under conditions of defective host membrane lipid biosynthesis. Virology. 1971 Feb;43(2):412–421. doi: 10.1016/0042-6822(71)90313-8. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Duckworth D. H., Bessman M. J. Assay for the Killing Properties of T2 Bacteriophage and Their "Ghosts". J Bacteriol. 1965 Sep;90(3):724–728. doi: 10.1128/jb.90.3.724-728.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earhart C. F. The association of host and phage DNA with the membrane of Escherichia coli. Virology. 1970 Oct;42(2):420–436. [PubMed] [Google Scholar]
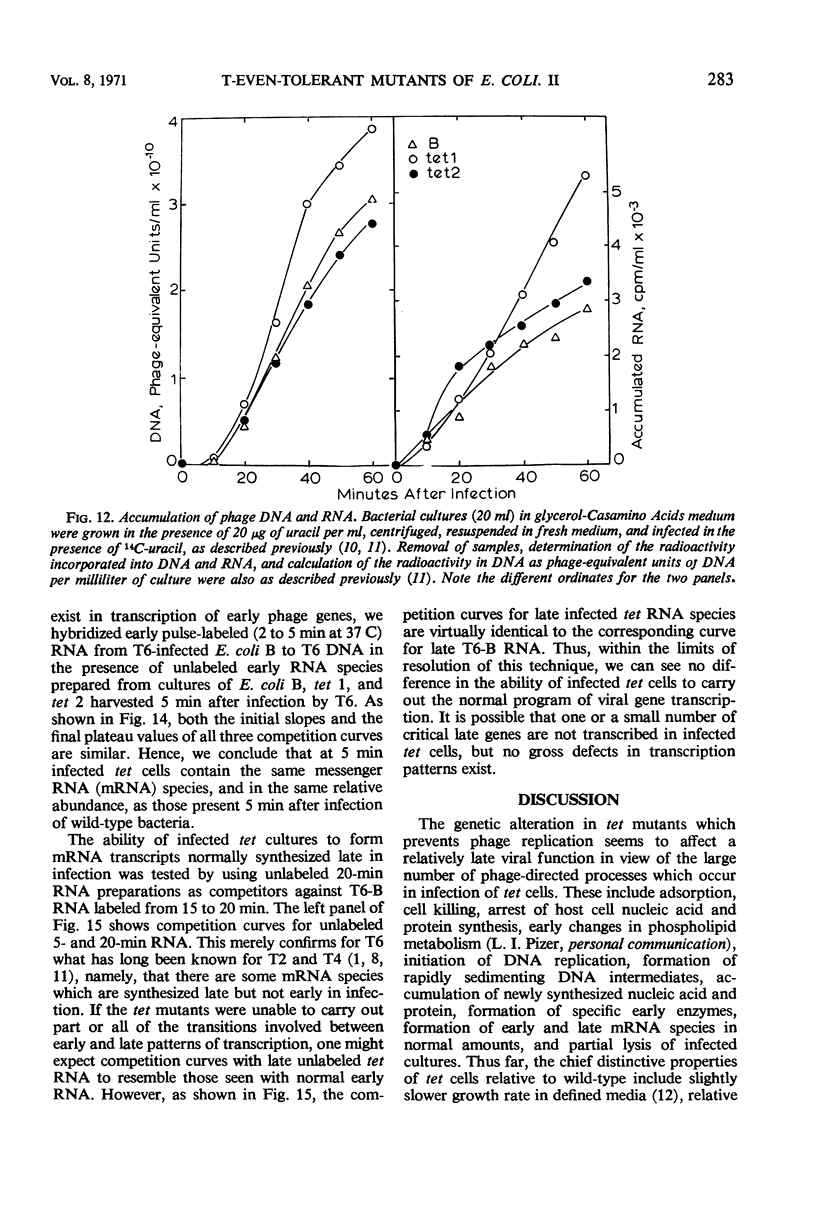
- Earhart C. F., Tremblay G. Y., Daniels M. J., Schaechter M. DNA replication studied by a new method for the isolation of cell membrane-DNA complexes. Cold Spring Harb Symp Quant Biol. 1968;33:707–710. doi: 10.1101/sqb.1968.033.01.079. [DOI] [PubMed] [Google Scholar]
- Frankel F. R. DNA replication after T4 infection. Cold Spring Harb Symp Quant Biol. 1968;33:485–493. doi: 10.1101/sqb.1968.033.01.056. [DOI] [PubMed] [Google Scholar]
- HALL B. D., NYGAARD A. P., GREEN M. H. CONTROL OF T2-SPECIFIC RNA SYNTHESIS. J Mol Biol. 1964 Jul;9:143–153. doi: 10.1016/s0022-2836(64)80096-6. [DOI] [PubMed] [Google Scholar]
- Kennell D. Inhibition of host protein synthesis during infection of Escherichia coli by bacteriophage T4. I. Continued synthesis of host ribonucleic acid. J Virol. 1968 Nov;2(11):1262–1271. doi: 10.1128/jvi.2.11.1262-1271.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews C. K. Biochemistry of deoxyribonucleic acid-defective amber mutant of bacteriophage T4. I. Ribonucleic acid metabolism. J Biol Chem. 1968 Nov 10;243(21):5610–5615. [PubMed] [Google Scholar]
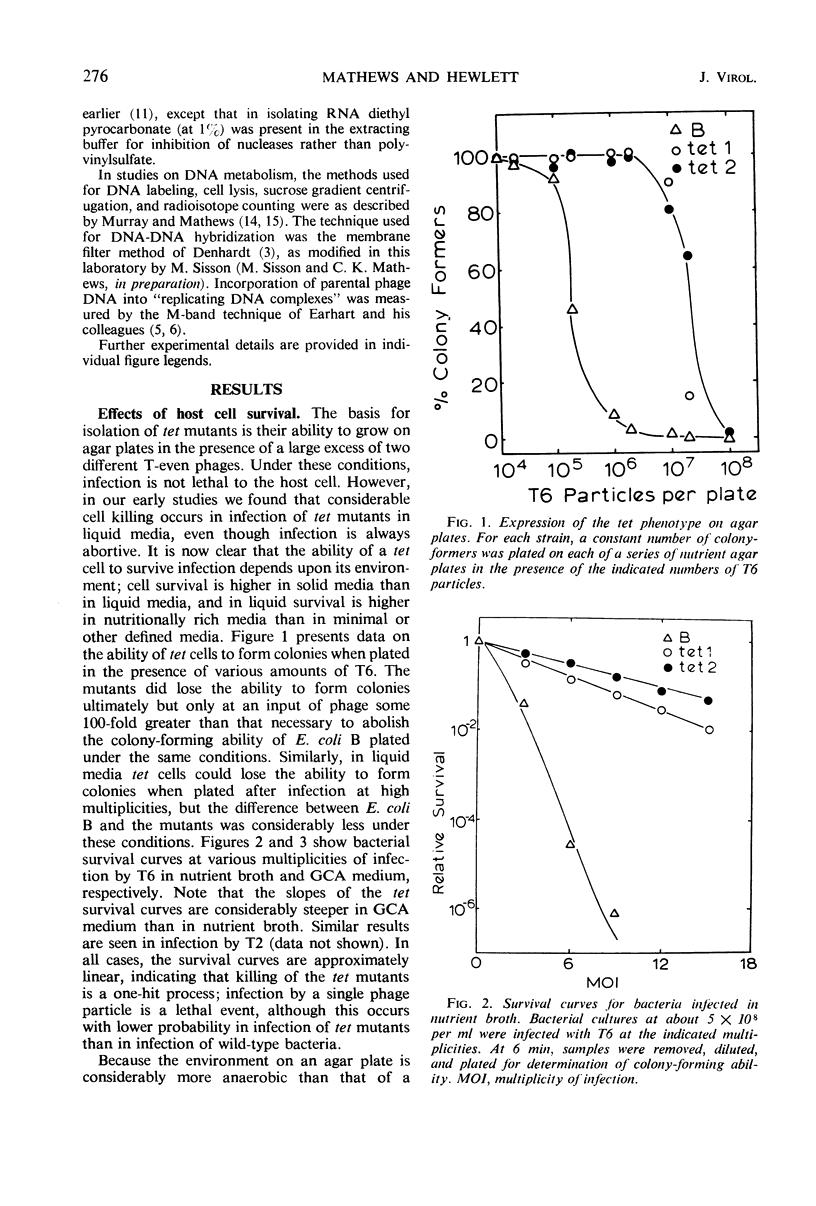
- Mathews C. K. T-even bacteriophage-tolerant mutants of Escherichia coli B. I. Isolation and preliminary characterization. J Virol. 1970 Aug;6(2):163–168. doi: 10.1128/jvi.6.2.163-168.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews C. K. Deoxyribonucleic acid metabolism and virus-induced enzyme synthesis in a thymine-requiring bacterium infected by a thymine-requiring bacteriophage. Biochemistry. 1966 Jun;5(6):2092–2100. doi: 10.1021/bi00870a042. [DOI] [PubMed] [Google Scholar]
- Murray R. E., Mathews C. K. Addition of nucleotides to parental DNA early in infection by bacteriophage T4. J Mol Biol. 1969 Sep 14;44(2):233–248. doi: 10.1016/0022-2836(69)90172-7. [DOI] [PubMed] [Google Scholar]
- Murray R. E., Mathews C. K. Biochemistry of DNA-defective amber mutants of bacteriophage T4. II. Intracellular DNA forms in infection by gene 44 mutants. J Mol Biol. 1969 Sep 14;44(2):249–262. doi: 10.1016/0022-2836(69)90173-9. [DOI] [PubMed] [Google Scholar]
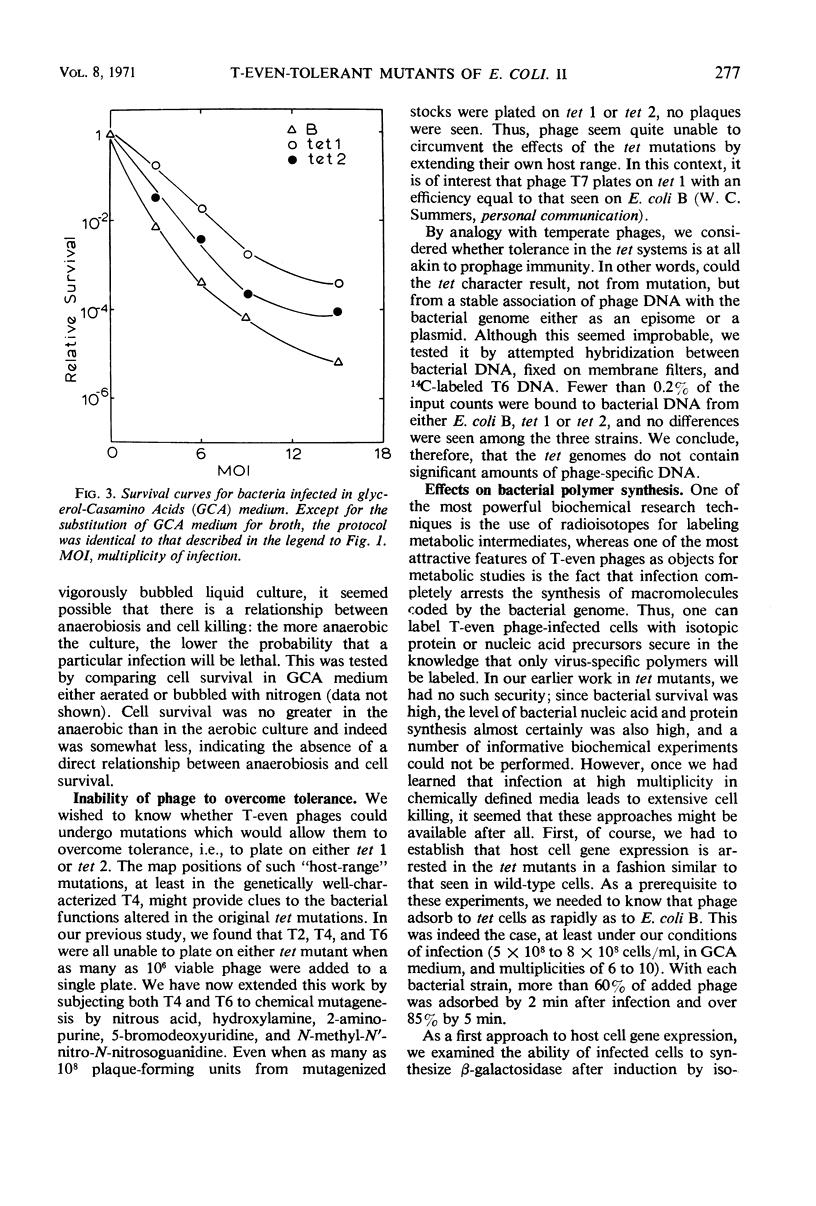
- Simon L. D. The infection of Escherichia coli by T2 and T4 bacteriophages as seen in the electron microscope. 3. Membrane-associated intracellular bacteriophages. Virology. 1969 Jun;38(2):285–296. doi: 10.1016/0042-6822(69)90370-5. [DOI] [PubMed] [Google Scholar]
- Travers A. A. Bacteriophage sigma factor for RNA polymerase. Nature. 1969 Sep 13;223(5211):1107–1110. doi: 10.1038/2231107a0. [DOI] [PubMed] [Google Scholar]
- Travers A. A. Positive control of transcription by a bacteriophage sigma factor. Nature. 1970 Mar 14;225(5237):1009–1012. doi: 10.1038/2251009a0. [DOI] [PubMed] [Google Scholar]



