Abstract
Maturation of mesocorticolimbic dopamine systems occurs during adolescence, and exposure to social stress during this period results in behavioral dysfunction including substance abuse disorders. Adult male rats exposed to repeated social defeat in adolescence exhibit reduced basal dopamine tissue content in the medial prefrontal cortex, altered dopamine tissue content in corticoaccumbal dopamine regions following acute amphetamine, and increased amphetamine conditioned place preference following repeated amphetamine treatment. Such changes may reflect altered amphetamine-induced extracellular dopamine release in the corticoaccumbal regions. Therefore, we used in vivo microdialysis to measure extracellular dopamine simultaneously within the medial prefrontal cortex and nucleus accumbens core of previously defeated rats and controls, in response to either acute or repeated (7 daily injections) of amphetamine (1.0 mg/kg). Locomotion responses to acute / repeated amphetamine were also assessed the day prior to taking dopamine measurements. Adolescent defeat potentiated adult locomotion responses to acute amphetamine, which was negatively correlated with attenuated amphetamine-induced dopamine release in the medial prefrontal cortex, but there was no difference in amphetamine-induced accumbal dopamine release. However, both locomotion and corticoaccumbal dopamine responses to repeated amphetamine were equivalent between previously defeated rats and controls. These data suggest adolescent defeat enhances behavioral responses to initial amphetamine exposure as a function of diminished prefrontal cortex dopamine activity, which may be sufficient to promote subsequently enhanced seeking of drug-associated cues. Interestingly, repeated amphetamine treatment appears to normalize amphetamine-elicited locomotion and cortical dopamine responses observed in adult rats exposed to adolescent social defeat, providing implications for treating stress-induced dopamine dysfunction.
Keywords: medial prefrontal cortex, nucleus accumbens, psychostimulant, microdialysis
1. Introduction
Adolescent bullying victimization is associated with increased substance misuse (Nelson et al., 1995; Rossow & Lauritzen, 2001; Sullivan et al., 2006; Tharp-Taylor et al., 2009; Topper et al., 2010), which suggests alterations to reward pathways in the brain. Adolescent male rats exposed to repeated social defeat, as a model of adolecent bullying (Bjorkqvist, 2001; Watt et al., 2009), exhibit decreased basal medial prefrontal cortex (mPFC) dopamine (DA) tissue content as adults (Watt et al., 2009). Rats defeated in adolescence also show blunted mPFC DA tissue content in response to acute amphetamine in adulthood, while nucleus accumbens (NAc) core DA tissue content responses are enhanced (Burke et al., 2010). Adolescent defeat also increases adult amphetamine conditioned place preference (CPP) and locomotion in a novel environment (Watt et al., 2009; Burke et al., 2010; 2011). Rats that naturally exhibit high novelty-induced locomotion, which predicts psychostimulant self-administration and sensitization (Piazza et al., 1990; Hooks et al., 1991), show blunted basal mPFC DA activity and exaggerated NAc DA responses to cocaine (Piazza et al., 1991; Hooks et al., 1992). Collectively, this implies that enhanced adult behavioral responses to amphetamine following adolescent defeat may be a function of altered mPFC and NAc core DA activity.
Dopamine transmission in the NAc core during initial psychostimulant exposure is implicated in development of conditioned responses to associative cues (Ito et al., 2000; Sellings & Clarke, 2006; Everitt et al., 2008; Meredith et al., 2008). As such, enhanced CPP for amphetamine exhibited by adult rats exposed to adolescent defeat (Burke et al., 2011) suggests potentiated NAc core DA responses to drug associated cues. Furthermore, reward-elicited NAc DA release can be further enhanced by mPFC DA depletion (Mitchell & Gratton, 1992; Ventura et al., 2004), as increased mPFC DA activity functionally dampens NAc DA activity (Deutch et al., 1990; Doherty & Gratton, 1996; Pascucci et al., 2007; Del Arco & Mora, 2008). Given that rats defeated in adolescence show reduced mPFC DA tissue content (Watt et al., 2009), it is possible that heightened adult behavioral responses to amphetamine following adolescent defeat are a result of augmented NAc core DA levels in response to amphetamine via reduced DA release in the mPFC. In support of this, acute amphetamine-induced increases in ex vivo DA tissue content within the NAc core of adult rats are heightened following adolescent defeat exposure (Burke et al., 2010), but it is not known whether this reflects increased in vivo extracellular DA concentrations in response to either acute or repeated administration of amphetamine. Also, it is unknown whether naturally decreased mPFC DA activity, such as that seen following adolescent defeat, results in potentiated extracellular NAc DA to acute or repeated amphetamine as has been shown in rats with pharmcologially-induced mPFC DA lesions (Mitchell & Gratton, 1992; Ventura et al., 2004). Therefore, we measured amphetamine-induced locomotion and amphetamine induced DA release simultaneously in the mPFC and NAc core using in vivo microdialysis. Responses following either acute or repeated amphetamine were measured to determine whether adolescent defeat-induced alterations to amphetamine responses differ with increased amphetamine exposure.
2. Materials and Methods
2.1. Animals
Male Sprague-Dawley rats (Animal Resource Center, University of South Dakota, Vermillion, SD) used as experimental subjects were pair-housed according to treatment (social defeat or control) under a reverse light cycle (lights off from 10:00–22:00 hrs) from postnatal day (P) 21 onwards. Resident adult male Sprague-Dawley rats (300–400g) for social defeat experiments were housed singly prior to assessment of their aggressive behavior. Food and water were available ad libitum to all rats. All behavioral procedures were conducted between 11:00 and 15:00 in a dark room under red lighting. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of South Dakota, and were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Every effort was made to minimize the number of animals used and their suffering.
2.2. Adolescent Social Defeat
Social defeat of adolescent male rats followed previously established procedures (Watt et al., 2009; Burke et al., 2010). Briefly, an adolescent male rat (P35) was introduced into a resident adult male’s home cage. The adolescent subject was considered socially defeated when it exhibited a minimum of 3 submissive postures in response to separate resident attacks (Watt et al., 2009). Following this, a mesh barrier was used to separate the resident and the adolescent for 35 min to prevent further physical attacks, but still allowing further visual, auditory and olfactory intimidation from the resident (Watt et al., 2009; Burke et al., 2010). Adolescents (N = 71) were exposed to social defeat each day for 5 days (P35–39) and were confronted with a different resident male each day to control for individual variance in defeat intensity. Age-matched controls (N = 67) experienced no social defeat but instead were placed in empty novel cages at matched times to control for handling and novel environment stress (Watt et al., 2009; Burke et al., 2010). Rats were returned to their home cages (pair-housed according to treatment) after each daily trial. Following the final social defeat conditioning, subjects and controls were left in their original pairs in their home cages and allowed to mature undisturbed into early adulthood (P56) when adult behavioral testing commenced.
2.3. Adult Behavioral Testing
In early adulthood (P56+), equivalent numbers of previously defeated and control rats were assigned to either acute or repeated amphetamine (1.0 mg/kg, ip., N = 16 - 20 / group) or saline groups (N = 13–19 / group). The repeated amphetamine administration involved either amphetamine or saline injection once per day for five consecutive days prior to any behavioral testing, and mimicked the dosing regimen that revealed enhanced amphetamine conditioned place preference following adolescent social defeat (Burke et al., 2011). Locomotion responses to amphetamine in both acute (no previous amphetamine treatment) or repeated amphetamine groups were assessed as follows. Rats were first placed in an opaque plastic open field (45 cm x 30 cm x 39 cm) for 60 min to acclimate to the environment. Immediately following this, rats were administered either amphetamine or saline of equivalent volume and returned to the testing environment for 90 min. Rats already exposed to amphetamine or saline for the previous five days received the same injection on the behavioral test day. Distance moved (cm) in the open field was measured by Ethovision XT v5.1 (Noldus Information Technology, Inc., Leesburg, VA, USA).
2.4. Microdialysis Procedures
For the acute amphetamine experiment, three days were allowed between behavioral testing and microdialysis sampling to avoid any carry-over effect of the amphetamine administered on the behavioral testing day (Forster et al., 2002; Miller et al., 2002; Burke et al., 2010). For the repeated amphetamine experiment, rats were injected once daily for five consecutive days, followed 24 hr later by an injection for behavioral testing (day six), with a final injection upon microdialysis testing on day seven.
Consistent with our previous methodology (Forster et al., 2008; Lukkes et al., 2008; Scholl et al., 2010), microdialysis experiments were conducted under urethane anesthesia to allow implantation and recording from multiple microdialysis probes within the same rat for simultaneous recordings from the NAc and mPFC. Urethane is a long-lasting anesthetic that has minimal effects on neuronal firing rates and neurotransmitter release (Maggi and Meli, 1986), and we have previously shown that elicited monoamine responses are similar between urethane-anesthetized and awake rats (Forster et al., 2006; Forster et al., 2008; Mo et al., 2008; Scholl et al., 2010). Rats were anesthetized with urethane (1.8 g/kg ip.; Sigma) and placed in a stereotaxic frame (David Kopf Institute, Tujunga, CA, USA) with the incisor bar set at + 3.3 mm. Body temperature was maintained at 37 °C with a temperature regulated heating pad (Harvard Apparatus, Holliston, Massachusetts, USA). A laboratory-made concentric microdialysis probe (MW cutoff 5000) with a membrane length of 2 mm (Lukkes et al., 2009) was inserted into the right NAc core (AP: + 1.8 mm from bregma; ML: - 1.4 mm from midline; DV: - 8.1 mm from dura; Paxinos and Watson, 2007), and a second probe 4 mm in membrane length (Forster et al., 2008) was inserted into the ispilateral mPFC (AP: + 3.4 mm from bregma; ML: - 0.5 mm from midline; DV: - 5.4 mm from dura; Paxinos and Watson, 2007) to allow simultaneous sampling from both regions. The probes were attached to a 1.0 ml syringe by PE-20 tubing, and artificial cerebrospinal fluid was continuously perfused through the probes using a microinfusion pump (Harvard Apparatus) at a rate of 0.4 μl/min. Microdialysis sampling began 4 h after the implantation of the probes (Forster et al., 2008; Lukkes et al., 2008; Lukkes et al., 2009), with dialysates (8 μl) collected at 20 min intervals and analyzed for DA. Following at least three stable baseline DA samples, saline or amphetamine (1.0 mg/kg, ip.) was injected systemically and dialysates were collected for 200 min thereafter and analyzed for DA.
2.5. Dopamine Analysis
Analysis of DA in dialysates was accomplished using high-performance liquid chromatography (HPLC) with electrochemical detection. The mobile phase used for DA separation (0.34 g EDTA, 0.25 g 1-decanesulfonic acid, 4.70 g sodium phosphate monobasic, 85 ml methanol, 0.25 ml triethylamine in 500 ml nanopure water, pH 5.8; chemicals obtained from Sigma) was pumped through a UniJet 5 μm C18 silica column (Bioanalytical Systems, West Lafayette, IN, USA) under nitrogen gas pressure (2000 psi). Dialysate (8 μl) was injected using a rheodyne injector into a 5 μl loop to overfill the loop, and DA was detected by a glassy carbon electrode (Bioanalytical Systems) maintained at + 0.60 V with respect to the Ag/AgCl2 reference electrode using a LC-4C potentiostat (Bioanalytical Systems). The voltage output was recorded by Clarity v2.4. Chromatography Station for Windows (DataApex, Prague, Czech Republic). Dopamine peaks were identified by comparison to a DA standard (11.05 pg/5 μl DA). The 2:1 signal to noise detection limit for DA using this system for the mPFC was 0.09 ± 0.00 pg, and for the NAc core was 0.06 ± 0.00 pg. For the acute amphetamine experiment, the levels of DA prior to amphetamine injection (baseline; uncorrected for probe recovery) were 0.84 ± 0.13 pg/5 μl for social defeat rats and 0.90 ± 0.17 pg/5 μl for controls in the mPFC, and 3.51 ± 0.87 pg/5 μl for social defeat rats and 2.38 ± 0.53 pg/5 μl for controls in the NAc core. For the repeated amphetamine experiment, the levels of DA prior to amphetamine injection were 0.83 ± 0.13 pg/5 μl for social defeat rats and 0.68 ± 0.08 pg/5 μl for controls in the mPFC, and 1.98 ± 0.21 pg/5 μl for social defeat rats and 2.22 ± 0.39 pg/5 μl for controls in the NAc core. Baseline DA levels were not statistically different between the defeated and control groups within each brain region and within each experiment (P > 0.05).
2.6. Histology
Following each microdialysis experiment, rats were euthanized by a lethal dose of Fatal-plus (0.5 ml, ip., Vortech, Dearborn, MI, USA), and the brains were removed and fixed in 10% formalin. Sections (60 μm) were acquired using a sliding microtome, and microdialysis probe placement was confirmed under a light microscope by two experimenters, one blind to treatment and results. Only data from rats with correct probe placements were included in the DA data analyses (acute amphetamine experiment N = 11–12 / group, repeated amphetamine experiment N = 9–13 / group).
2.7. Statistical Analyses
All statistical tests were conducted with Sigma Stat for Windows v3.5 (Systat Software Inc., San Jose, California, USA) with the alpha level set at 0.05 throughout. Separate Grubb’s tests were applied to remove outliers from the behavioral data as described previously (Lowry et al., 2001; Burke et al., 2011), which resulted in removal of 2 from a possible 66 data points for the acute experiment and of 3 from a possible 77 data points for the repeated experiment. For both the acute and repeated amphetamine experiments, distance moved (cm) was collated into 30 min time bins. For microdialysis experiments, DA levels were expressed as a percent change from baseline levels (Lukkes et al., 2009). Separate Grubb’s tests were applied to the microdialysis data to remove outliers, which resulted in removal of 1 data point from the DA levels over time data and 1 data point from the area under the curve data in the repeated amphetamine experiment.
Separate two-way repeated measures ANOVA (adolescent stress x time) were then used to compare locomotion or DA levels across time between each adolescent stress group for saline and amphetamine treated rats. Significant main effects of stress and time or interactions between stress and time were followed by Student-Neuman-Keuls (SNK) tests for multiple comparisons at each time point. Significant main effects of time were followed by separate one-way repeated measures ANOVA for each adolescent stress group, with significant effects across time identified by post-hoc Holm-Sidak tests for multiple comparisons, with time point zero serving as the control comparison.
The magnitude of drug-induced changes in either locomotion or DA levels was calculated as area under the curve for each treatment group, with these values then compared across treatments using a two-way ANOVA (adolescent stress x drug) followed by SNK multiple comparison tests when significant main effects were revealed. To determine any potential relationship between amphetamine-induced DA responses in the mPFC and the NAc core, linear regressions were performed between the magnitude of DA release (measured as area under the curve) in the mPFC and NAc core using microdialysis data obtained from each animal. Locomotion following acute (but not repeated) amphetamine was found to be significantly different between rats defeated in adolescence and controls. Therefore, to examine possible relationships between amphetamine-induced behavior and DA levels within previously defeated subjects and controls, correlations between total distance moved and the magnitude of either mPFC or NAc core DA release (area under the curve) following acute amphetamine injection were performed using separate post hoc linear regressions.
3. Results
3.1. Adult Behavioral Testing
3.1.1. Acute Amphetamine Experiment
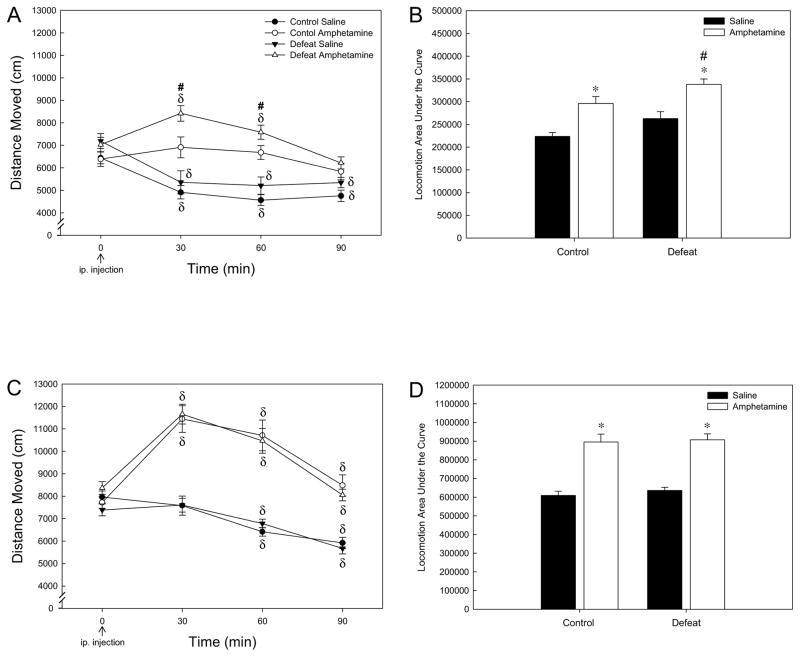
For amphetamine-injected rats, there was a significant effect of adolescent stress (F1,35 = 12.524; P < 0.01), a significant effect of time (F3,104 = 12.079; P < 0.001), and a significant interaction between stress and time (F3,104 = 2.953; P < 0.05; Fig. 1A). One-way repeated measures ANOVA did not reveal an effect of amphetamine over time for control rats (F3,43 = 1.383; P > 0.05) whereas an effect over time for socially-defeated rats was apparent (F3,59 = 15.858; P < 0.001) at both 30 and 60 mins post-amphetamine injection (Holm-Sidak; P < 0.001 and 0.05 respectively; Fig. 1A). Therefore, rats exposed to adolescent social defeat showed greater amphetamine-induced locomotion as compared to controls receiving amphetamine at 30 and 60 mins post-injection (SNK; P < 0.05; Fig. 1A). For saline-injected rats, there was an effect of time (F3,80 = 34.103; P < 0.001), but no significant effect of stress (F1,27 = 3.103; P > 0.05) nor an interaction between stress and time (F3,80 = 0.275; P > 0.05). Both adolescent stress groups injected with saline showed a decline in locomotion over time (F3,42 = 14.241; P < 0.001 for controls and F3,35 = 16.429; P < 0.001 for defeated rats), with all time post-injection time-points significantly lower than time point zero for both stress groups (Holm-Sidak; P < 0.001 Fig. 1A).
Figure 1.
Distance moved (mean ± SEM) in the open field following amphetamine (1.0 mg/kg, ip.) or saline injection. (A) Locomotion in adulthood in response to acute amphetamine was potentiated by experience of adolescent social defeat. (B) Overall locomotion (as measured by area under the curve) was increased by amphetamine in both control and adolescent defeat groups when compared to saline treatment, with adolescent stress increasing overall amphetamine-induced locomotion as compared to control rats receiving amphetamine. N = 16 for Control Saline, N = 16 for Control Amphetamine, N = 14 for Defeat Saline and N = 18 for Defeat Amphetamine groups. (C) Adult locomotion responses to amphetamine following repeated amphetamine treatment were not altered by adolescent defeat. (D) Overall locomotion (area under the curve) was equally increased by amphetamine in control and adolescent defeat groups when compared to saline treatment. N = 16 for Control Saline, N = 19 for Control Amphetamine, N = 19 for Defeat Saline and N = 20 for Defeat Amphetamine groups. δSignificant difference over time (compared to time point 0; P < 0.05). #Significantly different from amphetamine-treated control rats (P < 0.05). *Significantly different from saline groups (P < 0.05).
When the magnitude of locomotion induced by amphetamine was analyzed as area under the curve (Fig. 1B), there was a significant effect of adolescent stress (F1,57 = 9.061; P < 0.01) and a significant effect of drug (F1,57 = 30.517; P < 0.001) with no interaction between stress and drug (F1,57 = 0.001; P > 0.05). Both control and defeated rats showed increased overall locomotion following amphetamine injection as compared to saline-injected rats (SNK P < 0.05; Fig. 1B), but the total magnitude of the amphetamine response was greater in rats exposed to adolescent social defeat as compared to controls (SNK P < 0.05; Fig. 1B).
3.1.2. Repeated Amphetamine Experiment
In contrast to the acute experiment, there was no significant effect of adolescent stress (F1,40 = 0.001; P > 0.05) nor a significant interaction between stress and time (F3,118 = 0.708; P > 0.05), but a significant effect of time (F3,118 = 52.242; P < 0.001) was apparent for all amphetamine-treated rats (Fig. 1C). Both control and socially-defeated rats showed an increase in amphetamine-induced locomotion over time (F3,52 = 18.776; P < 0.001 for controls and F3,62 = 35.851; P < 0.001 for defeated rats), apparent at 30 and 60 mins post-amphetamine injection (Holm-Sidak P < 0.001; Fig. 1C). Similar to the acute experiment, there was an effect of time (F3,94 = 30.676; P < 0.001), but no significant effect of stress (F1,32 = 0.453; P > 0.05) nor an interaction between stress and time (F3,94 = 1.212; P > 0.05) for saline-treated rats. Again, both adolescent stress groups injected with saline showed a decline in locomotion over time (F3,40 = 10.339; P < 0.001 for controls and F3,54 = 23.583; P < 0.001 for defeated rats), with 60 and 90 min post-injection time-points significantly lower than time point zero for both stress groups (Holm-Sidak; P < 0.001 Fig. 1C).
For the magnitude of locomotion induced by amphetamine (area under the curve; Fig. 1D), there was a significant effect of drug (F1,70 = 77.673; P < 0.001) with no effect of adolescent stress (F1,70 = 0.364; P > 0.05) nor an interaction between stress and drug (F1,70 = 0.060; P > 0.05). Both control and defeated rats showed increased overall locomotion following amphetamine as compared to saline-injected rats (SNK P < 0.05; Fig. 1D.
3.2. Histology
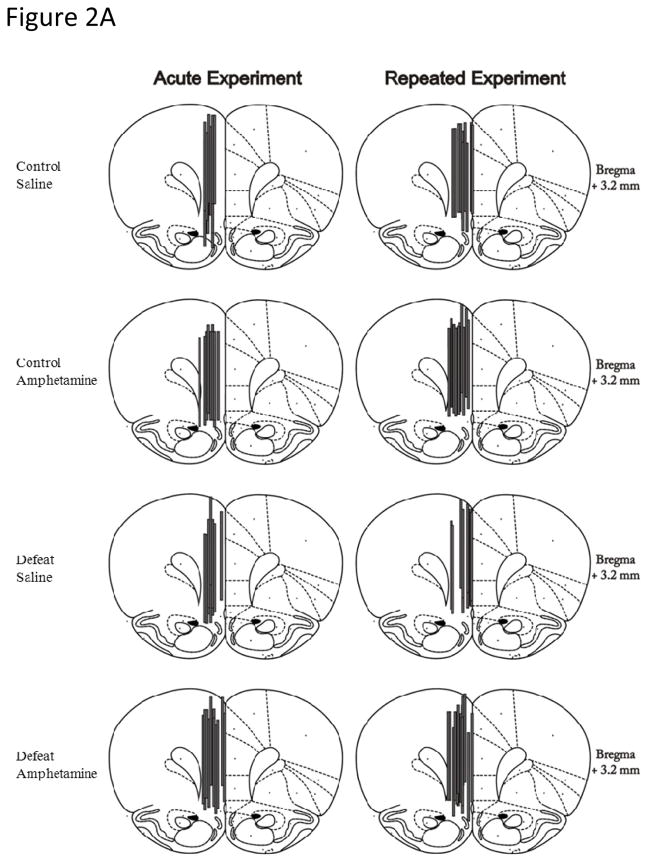
Microdialysis probe membranes within the mPFC were located 3.2 mm anterior from bregma (Paxinos & Watson, 2007), and encompassed the infralimbic, prelimbic and cingulate cortices (Fig. 2A). Probe membranes aimed at the NAc were located 1.2 to 1.6 mm anterior from bregma and 1.2 to 2.0 mm to the right of midline (Paxinos & Watson, 2007), and hence sampled predominantly from the core of the NAc (Fig. 2B). Both mPFC and NAc core probe placements were consistent across all treatment groups and experiments (Figs 2A & B).
Figure 2.
Representative coronal diagrams of microdialysis probe membrane (gray bars) placements in the (A) medial prefrontal cortex (mPFC) and (B) nucleus accumbens (NAc) core for experiments measuring DA release in response to either acute or repeated amphetamine (1.0 mg/kg, ip.) or saline. Figures adapted from Paxinos and Watson (1997).
3.3. Dopamine Analyses
3.3.1. Acute Amphetamine Experiment
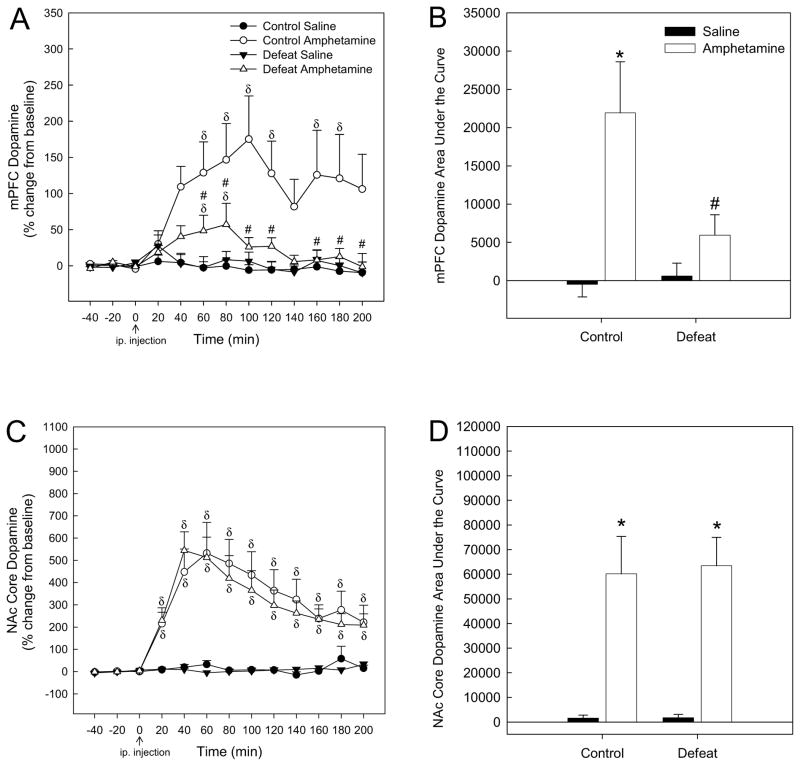
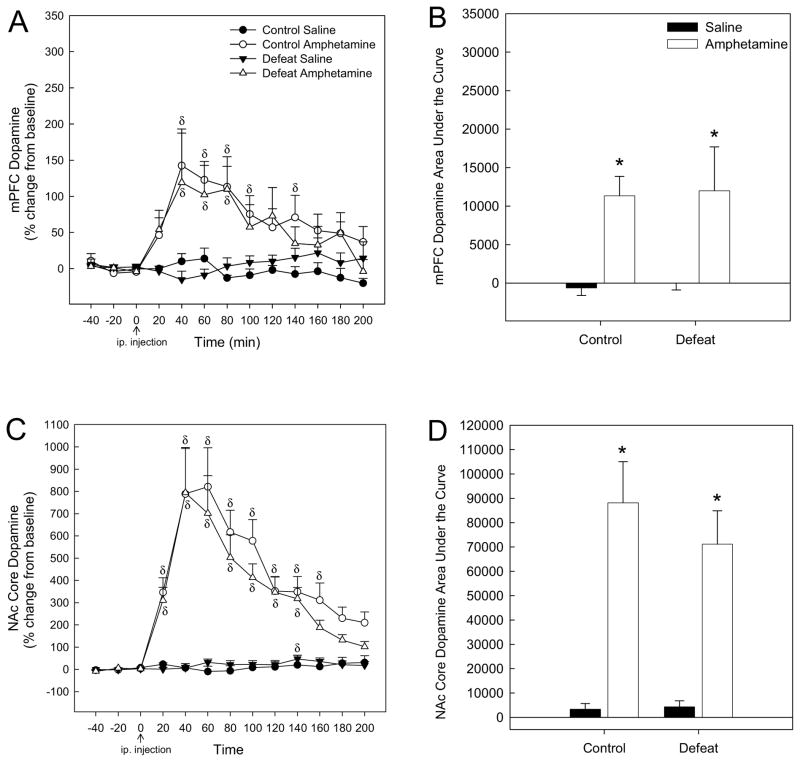
When mPFC DA levels were analyzed for amphetamine-injected rats, there was a significant effect of adolescent stress (F1,21 = 6.153; P < 0.05), a significant effect of time (F12,245 = 5.055; P < 0.001), and a significant interaction between stress and time (F12,245 = 2.991; P < 0.01; Fig. 3A). One-way repeated measures ANOVA revealed an effect of amphetamine over time for control rats (F12,100 = 2.481; P < 0.05), apparent at 60–120 min and 160–180 min post-injection (Holm-Sidak; P < 0.05; Fig. 3A). An effect of amphetamine on mPFC DA levels over time was also observed for socially-defeated rats (F12,104 = 2.885; P < 0.01), but only at 60 and 80 mins post-amphetamine injection (Holm-Sidak; P < 0.001 and 0.05 respectively; Fig. 3A). Thus, amphetamine-induced mPFC DA release in rats exposed to adolescent social defeat was lower than in controls receiving amphetamine from 60 to 200 mins post-injection (SNK; P < 0.05; Fig. 3A), with the exception of the 140 min time point (SNK; P > 0.05; Fig. 3A). For saline-injected rats, there was no effect of stress (F1,19 = 0.167; P > 0.05), no significant effect of time (F12,226 = 1.236; P > 0.05) nor an interaction between stress and time (F12,226 = 0.384; P > 0.05; Fig. 3A).
Figure 3.
(A) Adult DA release in the medial prefrontal cortex (mPFC) in response to acute amphetamine (1.0 mg/kg, ip.) was attenuated following adolescent social defeat. (B) The magnitude of adult mPFC DA release (expressed as area under the curve) in response to acute amphetamine was blunted in rats exposed to adolescent defeat compared to amphetamine-receiving controls. (C) Adult DA release in the NAc core in response to acute amphetamine was not altered by adolescent defeat exposure. (D) The magnitude of amphetamine-induced DA release in the NAc core (expressed as area under the curve) did not differ between previously defeated rats and controls. δSignificant difference over time (compared to time point 0; P < 0.05). #Significantly different from amphetamine-treated control rats (P < 0.05). *Significantly different from saline groups (P < 0.05). N = 11 for Control Saline, N = 11 for Control Amphetamine, N = 11 for Defeat Saline and N = 12 for Defeat Amphetamine groups.
Comparison of area under the curve of mPFC DA release for each treatment group revealed no main effect of stress (F1,40 = 3.762, P > 0.05), but a significant main effect of drug (F1,40 = 13.089, P < 0.001) and a significant interaction between adolescent stress and drug (F1,40 = 5.004, P < 0.05). In control rats, amphetamine elicited a significantly greater amount of mPFC DA release than saline (Fig. 3B, SNK P < 0.001). The magnitude of amphetamine-elicited DA release in the mPFC was significantly greater in controls than in rats that had experienced adolescent defeat (Fig. 3B, SNK P < 0.01), with previously defeated rats showing no difference in the overall amount of mPFC DA release in response to amphetamine when compared to saline-elicited DA (Fig. 3B, SNK P > 0.05).
In contrast to the mPFC, there was no significant effect of adolescent stress (F1,18 = 0.026; P > 0.05) nor a significant interaction between stress and time (F12,215 = 0.279; P > 0.05) on DA levels in the NAc core of amphetamine-treated rats, although a significant effect of time (F12,215 = 21.521; P < 0.001) was noted (Fig. 3C). One-way repeated measures ANOVA revealed an effect of amphetamine over time for both control (F12,100 = 6.403; P < 0.001) and previously defeated rats F12,79 = 13.509; P < 0.001), apparent at 20–200 min post-amphetamine injection (Holm-Sidak; P < 0.05; Fig. 3C). For saline-injected rats, there was no effect of stress (F1,18 = 0.114; P > 0.05), no significant effect of time (F12,205 = 1.567; P > 0.05) nor an interaction between stress and time (F12,205 = 1.396; P > 0.05; Fig. 3C).
When area under the curve of NAc core DA levels was compared across treatment groups (Fig. 3D), two-way ANOVA revealed a significant main effect of drug (F1,35 = 34.54, P < 0.001) with no effect of stress (F1,35 = 0.028, P > 0.05) nor an interaction between stress and drug (F1,35 = 0.023, P > 0.05). Both control and social defeat amphetamine-treated groups showed increased DA levels compared with saline-injected rats (Fig. 3D, SNK P < 0.001). Linear regression analysis of DA levels (area under the curve) from the mPFC and NAc suggested no relationship between amphetamine-induced mPFC and NAc extracellular DA levels for either control (R2 = 0.248; P > 0.05) or defeated (R2 =0.367; P > 0.05) rats.
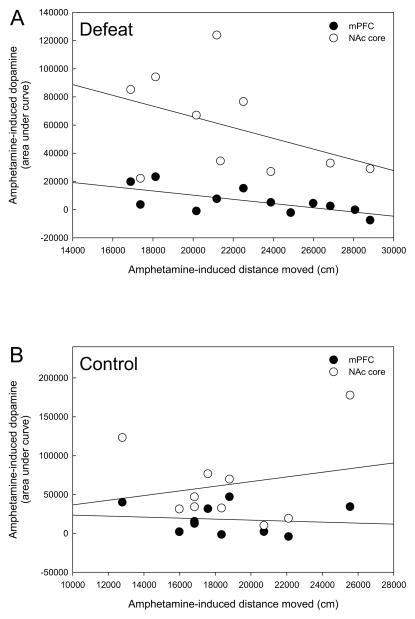
Post hoc linear regression analyses of behavioral and neurochemical data revealed a significant negative correlation between total acute amphetamine-induced locomotion and the amount of acute mPFC amphetamine-induced DA release (area under the curve) only within previously defeated rats (Table 1 and Fig. 4A, P < 0.05). This relationship was not apparent for control rats that received amphetamine (Table 1 and Fig. 4B, P > 0.05), nor were there any correlations between locomotion and mPFC DA within previously defeated rats and controls that received acute saline (Table 1, P > 0.05). No significant correlations were found between NAc core DA release in response to acute amphetamine and acute amphetamine-induced locomotion in either previously defeated or control rats (Table 1 and Figs 4A & B, P > 0.05). Similarly, there was no correlation between NAc core DA and locomotion following acute saline injection in either rats exposed to adolescent defeat or controls (Table 1, P > 0.05).
Table 1.
Correlations between total locomotion and the magnitude of dopamine release (area under the curve) in response to acute amphetamine (1.0 mg/kg, ip.).
| Treatment groups (N) | Medial prefrontal cortex (mPFC) dopamine | Nucleus accumbens (NAc) core dopamine |
|---|---|---|
| Control Saline (10 mPFC; 10 NAc) | R2 = 0.232 | R2 = 0.003 |
| F1,8 = 2.416 | F1,8 = 15.517 | |
| P > 0.05 | P > 0.05 | |
| Control Amphetamine (10 mPFC; 10 NAc) | R2 = 0.015 | R2 = 0.041 |
| F1,8 = 0.118 | F1,8 = 0.342 | |
| P > 0.05 | P > 0.05 | |
| Defeat Saline (9 mPFC, 8 NAc) | R2 = 0.043 | R2 = 0.005 |
| F1,7 = 0.315 | F1,6 = 0.0298 | |
| P > 0.05 | P > 0.05 | |
| Defeat Amphetamine (12 mPFC, 10 NAc) | R2 = 0.459 | R2 = 0.184 |
| F1,10 = 8.479 | F1,8 = 1.801 | |
| P < 0.05 | P > 0.05 |
Figure 4.
(A) Rats defeated in adolescence show a significant negative correlation between overall locomotion and the magnitude of mPFC DA release (both measured as area under the curve) in response to acute amphetamine in adulthood (black circles, P < 0.05), but not between locomotion and amphetamine-induced NAc DA release (open circles, P > 0.05). (B) No relationship between locomotion and either mPFC or NAc DA release was evident in controls rats that received acute amphetamine. N = 10 for Control mPFC and NAc, N = 12 for Defeat mPFC and N= 10 for Defeat NAc groups.
3.3.2. Repeated Amphetamine Experiment
In contrast to the findings from the mPFC in the acute amphetamine experiment, there was no significant effect of adolescent stress (F1,23 = 0.193; P > 0.05) and no significant interaction between stress and time (F12,268 = 0.299; P > 0.05) for amphetamine-induced mPFC DA levels in amphetamine pretreated rats, although a significant effect of time (F12,268 = 7.909; P < 0.001) was observed (Fig. 5A). One-way repeated measures ANOVA revealed an effect of amphetamine over time for control rats (F12,129 = 6.229; P < 0.001), apparent at 40–100 min and 120 min post-injection (Holm-Sidak; P < 0.05; Fig. 5A). An effect of amphetamine on mPFC DA levels over time was also observed for socially-defeated rats (F12,139 = 3.026; P < 0.001), at 40–80 min post-amphetamine injection (Holm-Sidak; P < 0.05, Fig. 5A). For saline-injected rats, there was no effect of stress (F1,17 = 1.598; P > 0.05), no significant effect of time (F12,199 = 0.346; P > 0.05) nor an interaction between stress and time (F12,199 = 1.767; P > 0.05; Fig. 5A).
Figure 5.
(A) Adult DA release in the mPFC in response to amphetamine (1.0 mg/kg, ip.) following 6 previous days of repeated amphetamine treatment was not altered by experience of social defeat in adolescence. (B) The magnitude of amphetamine-induced DA release in the adult mPFC (expressed as area under the curve) following repeated amphetamine administration was equivalent in rats exposed to adolescent defeat and controls. (C) Adult NAc core DA release evoked by amphetamine following repeated amphetamine treatment did not differ as a function of adolescent defeat exposure, (D) nor was the magnitude of amphetamine-induced NAc core DA release altered. δSignificant difference over time (compared to time point 0; P < 0.05). *Significantly different from saline groups (P < 0.05). N = 9 for Control Saline, 12 for Control Amphetamine, 10 for Defeat Saline and 13 for Defeat Amphetamine groups.
Comparison of area under the curve of mPFC DA release for each repeated treatment group (Fig. 5B) using a two-way ANOVA revealed a significant main effect of drug (F1,40 = 10.066, P < 0.01), but there was no effect of stress (F1,40 = 0.013, P > 0.05) and no interaction between drug and stress (F1,40 = 0.240, P > 0.05). Amphetamine elicited a significantly greater amount of mPFC DA release than saline in both control and previously defeated rats (Fig. 5B, SNK P < 0.05).
Similar to the acute amphetamine experiment, there was no significant effect of adolescent stress (F1,19 = 0.608; P > 0.05) nor a significant interaction between stress and time (F12,218 = 0.319; P > 0.05) on amphetamine-induced DA levels in the NAc core of amphetamine pre-treated rats, although a significant effect of time (F12,218 = 29.279; P < 0.001) was noted (Fig. 5C). One-way repeated measures ANOVA revealed an effect of amphetamine over time for control rats (F12,106 = 15.813; P < 0.001) apparent at 20–160 min post-amphetamine injection (Holm-Sidak; P < 0.05; Fig. 5C), and for previously defeated rats (F12,112 = 13.681; P < 0.001) at 20–140 min post-injection (Holm-Sidak; P < 0.05; Fig. 5C). For saline-injected rats, there was no effect of stress (F1,16 = 0.036; P > 0.05), nor an interaction between stress and time (F12,183 = 0.793; P > 0.05), but a significant effect of time was observed (F12,183 = 2.482; P < 0.01; Fig. 5C). The effect over time was not evident for saline pre-treated control rats (F12,96 = 1.143; P > 0.05) but was significant for rats exposed to social defeat (F12,87 = 2.468; P < 0.01), due to a slight increase in DA levels at 140 min post-saline injection (Holm-Sidak; P < 0.01; Fig. 5C).
When area under the curve of NAc core DA release was compared across repeated treatment groups, two-way ANOVA revealed a significant effect of drug (F1,34 = 38.559, P < 0.001), with both control and previously defeated rats showing a greater amount of DA release in response to amphetamine compared with saline (Fig. 5D, SNK P < 0.001). Similar to the acute experiment, there was no effect of adolescent stress (F1,34 = 0.516, P > 0.05) and no interaction between stress and drug (F1,34 = 0.540, P > 0.05).
4. Discussion
These experiments revealed specific alterations to adult amphetamine-induced behavior and DA release following adolescent social defeat, which were only apparent in response to acute amphetamine administration. In particular, previously defeated rats exhibited greater amphetamine-induced locomotion following a single injection in adulthood. In addition, DA release in the adult mPFC evoked by acute amphetamine was blunted in both duration and magnitude following adolescent defeat. Overall, these findings demonstrate that repeated social defeat during mid-adolescence can alter initial responses to a psychostimulant encountered well after the stress exposure. Interestingly, these behavioral and neural differences shown by previously defeated rats in response to acute amphetamine were no longer evident after repeated amphetamine administration, suggesting that repeated amphetamine reversed these effects of adolescent social defeat.
Amphetamine acts as a false substrate for the dopamine transporter (DAT) and increases DA release by reversing transport to expel intra-terminal DA stores (Fleckenstein et al., 2007). Of relevance, adult rats exposed to adolescent social defeat show increased DAT expression in the mPFC (Novick et al., 2011) potentially increasing the substrate for amphetamine in this region. However, blunted mPFC DA release in response to acute amphetamine was observed in previously defeated rats in the current study. As such, the attenuated extracellular DA response to amphetamine is most likely a result of decreased intracellular DA stores, as reflected by reduced basal mPFC DA tissue content following adolescent defeat exposure (Watt et al., 2009). Adolescent defeat had no effect on adult NAc core DA release in response to either acute or repeated amphetamine, which is in contrast to the increased amphetamine-induced DA tissue content observed in the NAc core of adult rats exposed to adolescent social defeat (Burke et al., 2010). This suggests the increased tissue content does not always equate to increased extracellular release of dopamine. Overall, the effects of adolescent defeat appear to be specific to mPFC DA, highlighting the vulnerability of the developing cortical DA system to stress-induced disruption and resulting in long-term consequences as manifested in adulthood.
The increased amphetamine-induced locomotion after adolescent social defeat observed here is congruent with many other studies demonstrating that adult social defeat results in cross-sensitization to the locomotor activating effects of acute amphetamine or cocaine (Nikulina et al., 2004; Covington & Miczek, 2005; de Jong et al., 2005; Yap et al., 2005; Miczek et al., 2011). Therefore, it appears that defeat in either adolescence or adulthood can increase subsequent psychostimulant-induced locomotion. However, the relationship between adult defeat, psychostimulant-induced locomotion and dopamine release in the prefrontal cortex has not been studied to date, so it is unclear whether blunted mPFC DA release in response to acute amphetamine shown by previously defeated rats in the current study is specific to social defeat being experienced in adolescence. While cross-sensitization to acute amphetamine following repeated social defeat in adulthood can persist for up to 60 days (Covington et al., 2005), it is currently unknown if the effects of adolescent defeat on amphetamine-induced locomotion are as persistent. Increased amphetamine-evoked locomotion can also occur after a single bout of adult defeat (de Jong et al., 2005), but whether this is also the case for adolescent defeat remains to be determined. Future experiments would be useful in elucidating these issues.
Here, we observed increased locomotion following adolescent social defeat in response to an acute low dose of amphetamine (1.0 mg/kg). This contrasts with results from a previous study, in which we administered a higher dose of amphetamine (2.5 mg/kg) and found that amphetamine-induced locomotion was decreased in adult rats that had experienced adolescent defeat (Burke et al., 2010). Other studies have shown that individual differences in behavioral responses to psychostimulants are revealed by low rather than high drug concentrations (Klebaur & Bardo, 1999; Klebaur et al., 2001; Cain et al., 2004; 2005). For example, rats that exhibit enhanced responses to novelty also show heightened amphetamine conditioned place preference (CPP), but this is only apparent at low (1.0 mg/kg) amphetamine concentrations (Klebaur & Bardo, 1999). Similarly, we recently found that rats defeated in adolescence display heightened CPP in adulthood to the same 1.0 mg/kg dose of amphetamine used in the current study (Burke et al., 2011). Beckmann et al. (2011) have suggested that use of higher psychostimulant concentrations in reward-related paradigms exceed reward thresholds, causing maximal behavioral responses that abolish individual differences. The current and previous (Burke et al., 2010; 2011) findings suggest that heightened amphetamine responses in adult rats exposed to adolescent social defeat appear to be detectable only at lower concentrations of amphetamine.
A significant negative correlation was observed between acute amphetamine-induced locomotion and mPFC DA release in previously defeated rats. Although these measurements were not taken simultaneously, they clearly suggest a relationship between amphetamine-induced behavior and mPFC DA in adulthood following experience of adolescent defeat. The specificity of alterations to mPFC DA activity in mediating acute amphetamine-induced locomotion in previously defeated rats is further supported by the absence of a correlation between NAc core DA and locomotion evoked by acute amphetamine. Excitotoxic lesions limited to the mPFC during adulthood result in increased acute amphetamine-induced locomotion without affecting NAc tissue DA or metabolite levels (Jaskiw et al., 1990). Furthermore, when mPFC lesions are administered pre-puberty (P7), rats display increased amphetamine-induced locomotion in adulthood (Flores et al., 1996). Similarly, mPFC DA depletion increases amphetamine or cocaine-induced locomotion, although these behavioral effects were accompanied by greater NAc DA release as sampled primarily from the NAc shell (Beyer and Steketee, 1999; Ventura et al., 2004). Combined with results from the current study, this implies that amphetamine-induced locomotion can be increased by perturbation to the mPFC DA system when experienced either during postnatal development or in adulthood. Our current findings add to this by suggesting that increased locomotion responses to acute amphetamine following adolescent defeat may be a function of decreased mPFC DA activity. This is in line with our previous findings in which we showed that rats defeated in adolescence display enhanced locomotion in novel environments (Watt et al., 2009; Burke et al., 2010; 2011), which is believed to reflect mPFC DA hypofunction (Bubser and Schmidt, 1990; Piazza et al., 1991). However, there is a possibility that other brain regions and neurotransmitter systems affected by adolescent social defeat, such as ventral hippocampus noradrenergic and serotonergic systems (Watt et al., 2009), play a role in mediating the novelty and amphetamine-induced hyperlocomotion observed in adult rats exposed to adolescent social defeat, given that the current study only recorded DA release from the mPFC and NAc core.
Other studies have demonstrated that dopaminergic lesions of the mPFC result in potentiated accumbal DA release and/or metabolism to either stressful or natural reward stimuli (Deutch et al., 1990; Mitchell & Gratton, 1992; Pascucci et al., 2007). Selective mPFC DA depletion also enhances accumbal DA release following acute amphetamine injection (Ventura et al., 2004). Moreover, Ventrua et al. (2004) showed that C57/BL6J mice, which are highly behaviorally responsive to amphetamine, show dampened mPFC DA release and high extracellular accumbal DA after systemic amphetamine administration. However, this inverse relationship between cortical and accumbal DA activity was not evident in response to acute amphetamine in adult rats that had been defeated in adolescence within the current study. Specifically, adolescent defeat resulted in diminished amphetamine-induced mPFC DA release, but this did not potentiate NAc core DA release in response to acute amphetamine, nor was there a signficant negative correlation between the two measures. The discrepancies between our findings and those of earlier studies may partly arise from differences in the functional effects of naturally reduced mPFC DA activity following adolescent stress exposure versus pharmacologically-induced DA depletion. In addition, Ventura et al. (2004) sampled mPFC and accumbal DA from separate groups of animals, as opposed to the concurrent sampling within single subjects that was employed in the current study. However, it should be noted that adult rats exposed to social defeat in adolescence show greater D2 receptor expression in the NAc core (Burke et al., 2011), suggesting potentially greater post-synaptic effects of DA release in this region that may contribute to differences in amphetamine-induced behaviors, despite the lack of potentiated accumbal DA release in response to amphetamine.
The heightened adult locomotion and blunted mPFC DA release in response to acute amphetamine following adolescent defeat exposure was effectively abolished by repeated low dose amphetamine treatment, with behavioral and cortical DA responses normalized to levels shown by amphetamine-receiving controls. Low dose administration of the psychostimulant methylphenidate reduces hyperactive behavior in spontaneously hypertensive rats (Arime et al., 2011), a strain which also shows mPFC DA hypofunction in adulthood (Russell et al., 1995; Russell, 2002; Vigianno et al., 2004, but see Heal et al., 2008). Performance in mPFC DA-dependent cognitive tasks is also improved by low dose methylphenidate (Arnsten, 2006), via preferentially increasing extracellular DA levels in the mPFC (Berridge et al., 2006). In addition, low doses of methylphenidate can restore pre-existing decreased DA fiber density in the mPFC when administered chronically in adolescence (Grund et al., 2007). Given that amphetamine and methylphenidate both act as indirect DA agonists by preventing DA uptake (Fleckenstein et al., 2007; Wilens, 2008), repeated low dose amphetamine treatment in the current study may have had similar actions in previously defeated rats to normalize the dampened mPFC DA and heightened locomotion responses to acute amphetamine. Therefore, future work should assess whether repeated low-dose stimulant treatment increases mPFC DA terminal fields and DA levels of adult rats exposed to adolescent social defeat and whether this relates to normalization of locomotor behavior and other mPFC DA-dependent tasks such as executive function (Robbins & Arnsten, 2009).
5. Conclusions
Our findings suggest that adolescent social defeat heightens initial behavioral responses to amphetamine in early adulthood as a function of defeat-induced reductions in mPFC DA release, which may be sufficient to promote subsequently enhanced seeking of drug-associated cues manifesting as increased amphetamine CPP (Burke et al., 2011). These data agree with clinical studies which report that adolescent bullying victimization is associated with the onset of substance abuse related behaviors (Nelson et al., 1995; Rossow & Lauritzen, 2001; Sullivan et al., 2006; Tharp-Taylor et al., 2009; Topper et al., 2010), and suggest that stress-induced disruption of the adolescent mPFC DA system plays a key role in increasing the vulnerability to addiction. Therefore, pharmacotherapies that restore mPFC DA function to dampen initial responses to psychostimulants may mitigate against the development of substance abuse.
Highlights.
Social defeat in adolescence heightens amphetamine-induced locomotion in adulthood
Adolescent social defeat attenuates acute amphetamine-evoked prefrontal dopamine
There is no effect of adolescent defeat on amphetamine-induced accumbal dopamine
Repeated amphetamine exposure reverses these effects of adolescent social defeat
Acknowledgments
This work was supported by NIDA RO1 DA019921 (GLF), Sigma Xi - G200803150251 (ARB), a USD Graduate Research Award (ARB), a Joseph F. Nelson and Martha P. Nelson Faculty Research Grant (MJW) and NIH P20 RR015567 which is designated a Center of Biomedical Research Excellence (COBRE). We also thank Dr. Kenneth Renner, Jamie L. Scholl, Emily D. Reinbold and Katie M. Oliver for their valuable technical assistance with these experiments. All authors state no conflict of interest.
Abbreviations
- CPP
Conditioned place preference
- DA
Dopamine
- DAT
Dopamine transporter
- HPLC
High-performance liquid chromatography
- ip
Intraperitoneal
- mPFC
Medial prefrontal cortex
- NAc
Nucleus accumbens
- P
Postnatal day
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arime Y, Kubo Y, Sora I. Animal models of attention-deficit/hyperactivity disorder. Biol Pharm Bull. 2011;34:1373–1376. doi: 10.1248/bpb.34.1373. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Stimulants: Therapeutic actions in ADHD. Neuropsychopharmacology. 2006;31:2376–2383. doi: 10.1038/sj.npp.1301164. [DOI] [PubMed] [Google Scholar]
- Beckmann JS, Marusich JA, Gipson CD, Bardo MT. Novelty seeking, incentive salience and acquisition of cocaine self-administration in the rat. Behavioural Brain Research. 2011;216:159–165. doi: 10.1016/j.bbr.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, Hamilton C, Spencer RC. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biological Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Beyer CE, Steketee JD. Dopamine depletion in the medial prefrontal cortex induces sensitized-like behavioral and neurochemical responses to cocaine. Brain Research. 1999;833:133–141. doi: 10.1016/s0006-8993(99)01485-7. [DOI] [PubMed] [Google Scholar]
- Bjorkqvist K. Social defeat as a stressor in humans. Physiology & Behavior. 2001;73:435–442. doi: 10.1016/s0031-9384(01)00490-5. [DOI] [PubMed] [Google Scholar]
- Bubser M, Schmidt WJ. 6-Hydroxydopamine lesion of the rat prefrontal cortex increases locomotor activity, impairs acquisition of delayed alternation tasks, but does not affect uninterrupted tasks in the radial maze. Behavioural Brain Research. 1990;37:157–168. doi: 10.1016/0166-4328(90)90091-r. [DOI] [PubMed] [Google Scholar]
- Burke AR, Renner KJ, Forster GL, Watt MJ. Adolescent social defeat alters neural, endocrine and behavioral responses to amphetamine in adult male rats. Brain Research. 2010;1352:147–156. doi: 10.1016/j.brainres.2010.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke AR, Watt MJ, Forster GL. Adolescent social defeat increases adult amphetamine conditioned place preference and alters D2 dopamine receptor expression. Neuroscience. 2011;197:269–279. doi: 10.1016/j.neuroscience.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain ME, Saucier DA, Bardo MT. Novelty seeking and drug use: contribution of an animal model. Experimental and Clinical Psychopharmacology. 2005;13:367–375. doi: 10.1037/1064-1297.13.4.367. [DOI] [PubMed] [Google Scholar]
- Cain ME, Smith CM, Bardo MT. The effect of novelty on amphetamine self-administration in rats classified as high and low responders. Psychopharmacology. 2004;176:129–138. doi: 10.1007/s00213-004-1870-2. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Kikusui T, Goodhue J, Nikulina EM, Hammer RP, Jr, Miczek KA. Brief social defeat stress: long lasting effects on cocaine taking during a binge and zif268 mRNA expression in the amygdala and prefrontal cortex. Neuropsychopharmacology. 2005;30:310–321. doi: 10.1038/sj.npp.1300587. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Miczek KA. Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology. 2005;183:331–340. doi: 10.1007/s00213-005-0190-5. [DOI] [PubMed] [Google Scholar]
- de Jong JG, Wasilewski M, van der Vegt BJ, Buwalda B, Koolhaas JM. A single social defeat induces short-lasting behavioral sensitization to amphetamine. Physiology & Behavior. 2005;83:805–811. doi: 10.1016/j.physbeh.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Mora F. Prefrontal cortex-nucleus accumbens interaction: in vivo modulation by dopamine and glutamate in the prefrontal cortex. Pharmacology, Biochemistry, and Behavior. 2008;90:226–235. doi: 10.1016/j.pbb.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Clark WA, Roth RH. Prefrontal cortical dopamine depletion enhances the responsiveness of mesolimbic dopamine neurons to stress. Brain Res. 1990;521(1–2):311–315. doi: 10.1016/0006-8993(90)91557-w. [DOI] [PubMed] [Google Scholar]
- Doherty MD, Gratton A. Medial prefrontal cortical D1 receptor modulation of the meso-accumbens dopamine response to stress: an electrochemical study in freely-behaving rats. Brain Research. 1996;715:86–97. doi: 10.1016/0006-8993(95)01557-4. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philosophical Transactions of the Royal Society of London. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Flores G, Wood GK, Liang JJ, Quirion R, Srivastava LK. Enhanced amphetamine sensitivity and increased expression of dopamine D2 receptors in postpubertal rats after neonatal excitotoxic lesions of the medial prefrontal cortex. J Neurosci. 1996;16:7366–7375. doi: 10.1523/JNEUROSCI.16-22-07366.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster GL, Falcon AJ, Miller AD, Heruc GA, Blaha CD. Effects of laterodorsal tegmentum excitotoxic lesions on behavioral and dopamine responses evoked by morphine and d-amphetamine. Neuroscience. 2002;114:817–823. doi: 10.1016/s0306-4522(02)00365-2. [DOI] [PubMed] [Google Scholar]
- Forster GL, Feng N, Watt MJ, Korzan WJ, Mouw NJ, Summers CH, Renner KJ. Corticotropin-releasing factor in the dorsal raphe elicits temporally distinct serotonergic responses in the limbic system in relation to fear behavior. Neuroscience. 2006;141:1047–1055. doi: 10.1016/j.neuroscience.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Forster GL, Pringle RB, Mouw NJ, Vuong SM, Watt MJ, Burke AR, Lowry CA, Summers CH, Renner KJ. Corticotropin-releasing factor in the dorsal raphe nucleus increases medial prefrontal cortical serotonin via type 2 receptors and median raphe nucleus activity. European Journal of Neuroscience. 2008;28:299–310. doi: 10.1111/j.1460-9568.2008.06333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grund T, Teuchert-Noodt G, Busche A, Neddens J, Brummelte S, Moll GH, Dawirs RR. Administration of oral methylphenidate during adolescence prevents suppressive development of dopamine projections into prefrontal cortex and amygdala after an early pharmacological challenge in gerbils. Brain Research. 2007;1176C:124–132. doi: 10.1016/j.brainres.2007.06.107. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Smith SL, Kulkarni RS, Rowley HL. New perspectives from microdialysis studies in freely-moving, spontaneously hypertensive rats on the pharmacology of drugs for the treatment of ADHD. Pharmacology, Biochemistry, and Behavior. 2008;90:184–197. doi: 10.1016/j.pbb.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr Individual differences in locomotor activity and sensitization. Pharmacology, Biochemistry, and Behavior. 1991;38:467–470. doi: 10.1016/0091-3057(91)90308-o. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Colvin AC, Juncos JL, Justice JB., Jr Individual differences in basal and cocaine-stimulated extracellular dopamine in the nucleus accumbens using quantitative microdialysis. Brain Research. 1992;587:306–312. doi: 10.1016/0006-8993(92)91012-4. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20:7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskiw GE, Karoum F, Freed WJ, Phillips I, Kleinman JE, Weinberger DR. Effect of ibotenic acid lesions of the medial prefrontal cortex on amphetamine-induced locomotion and regional brain catecholamine concentrations in the rat. Brain Research. 1990;534:263–272. doi: 10.1016/0006-8993(90)90138-2. [DOI] [PubMed] [Google Scholar]
- Klebaur JE, Bardo MT. Individual differences in novelty seeking on the playground maze predict amphetamine conditioned place preference. Pharmacology, Biochemistry, and Behavior. 1999;63:131–136. doi: 10.1016/s0091-3057(98)00258-5. [DOI] [PubMed] [Google Scholar]
- Klebaur JE, Bevins RA, Segar TM, Bardo MT. Individual differences in behavioral responses to novelty and amphetamine self-administration in male and female rats. Behav Pharmacol. 2001;12:267–275. doi: 10.1097/00008877-200107000-00005. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Burke KA, Renner KJ, Moore FL, Orchinik M. Rapid changes in monoamine levels following administration of corticotropin-releasing factor or corticosterone are localized in the dorsomedial hypothalamus. Hormones and Behavior. 2001;39:195–205. doi: 10.1006/hbeh.2001.1646. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Forster GL, Renner KJ, Summers CH. Corticotropin-releasing factor 1 and 2 receptors in the dorsal raphe differentially affect serotonin release in the nucleus accumbens. European Journal of Pharmacology. 2008;578:185–193. doi: 10.1016/j.ejphar.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, Summers CH, Scholl JL, Renner KJ, Forster GL. Early life social isolation alters corticotropin-releasing factor responses in adult rats. Neuroscience. 2009;158:845–855. doi: 10.1016/j.neuroscience.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations in various systems. Part 1: General considerations. Experientia. 1986;42:109–114. doi: 10.1007/BF01952426. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Baldo BA, Andrezjewski ME, Kelley AE. The structural basis for mapping behavior onto the ventral striatum and its subdivisions. Brain Struct Funct. 2008;213:17–27. doi: 10.1007/s00429-008-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Nikulina EM, Shimamoto A, Covington HE., III Escalated or suppressed cocaine reward, tegmental BDNF and accumbal dopamine due to episodic vs. continuous social stress in rats. J Neurosci. 2011;31:9848–9857. doi: 10.1523/JNEUROSCI.0637-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AD, Forster GL, Metcalf KM, Blaha CD. Excitotoxic lesions of the pedunculopontine differentially mediate morphine- and d-amphetamine-evoked striatal dopamine efflux and behaviors. Neuroscience. 2002;111:351–362. doi: 10.1016/s0306-4522(01)00595-4. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Gratton A. Partial dopamine depletion of the prefrontal cortex leads to enhanced mesolimbic dopamine release elicited by repeated exposure to naturally reinforcing stimuli. J Neurosci. 1992;12:3609–3618. doi: 10.1523/JNEUROSCI.12-09-03609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo B, Feng N, Renner K, Forster G. Restraint stress increases serotonin release in the central nucleus of the amygdala via activation of corticotropin-releasing factor receptors. Brain Research Bulletin. 2008;76:493–498. doi: 10.1016/j.brainresbull.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DE, Higginson GK, Grant-Worley JA. Physical abuse among high school students. Prevalence and correlation with other health behaviors. Arch Pediatr Adolesc Med. 1995;149:1254–1258. doi: 10.1001/archpedi.1995.02170240072011. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Covington HE, 3rd, Ganschow L, Hammer RP, Jr, Miczek KA. Long-term behavioral and neuronal cross-sensitization to amphetamine induced by repeated brief social defeat stress: Fos in the ventral tegmental area and amygdala. Neuroscience. 2004;123:857–865. doi: 10.1016/j.neuroscience.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Novick AM, Forster GL, Tejani-Butt SM, Watt MJ. Adolescent social defeat alters markers of adult dopaminergic function. Brain Research Bulletin. 2011;86:123–128. doi: 10.1016/j.brainresbull.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascucci T, Ventura R, Latagliata EC, Cabib S, Puglisi-Allegra S. The medial prefrontal cortex determines the accumbens dopamine response to stress through the opposing influences of norepinephrine and dopamine. Cereb Cortex. 2007;17:2796–2804. doi: 10.1093/cercor/bhm008. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3. Academic Press; New York: 1997. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Maccari S, Mormede P, Le Moal M, Simon H. Individual reactivity to novelty predicts probability of amphetamine self-administration. Behavioural Pharmacology. 1990;1:339–345. doi: 10.1097/00008877-199000140-00007. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Rouge-Pont F, Deminiere JM, Kharoubi M, Le Moal M, Simon H. Dopaminergic activity is reduced in the prefrontal cortex and increased in the nucleus accumbens of rats predisposed to develop amphetamine self-administration. Brain Research. 1991;567:169–174. doi: 10.1016/0006-8993(91)91452-7. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossow I, Lauritzen G. Shattered childhood: a key issue in suicidal behavior among drug addicts? Addiction. 2001;96:227–240. doi: 10.1046/j.1360-0443.2001.9622275.x. [DOI] [PubMed] [Google Scholar]
- Russell V, de Villiers A, Sagvolden T, Lamm M, Taljaard J. Altered dopaminergic function in the prefrontal cortex, nucleus accumbens and caudate-putamen of an animal model of attention-deficit hyperactivity disorder--the spontaneously hypertensive rat. Brain Research. 1995;676:343–351. doi: 10.1016/0006-8993(95)00135-d. [DOI] [PubMed] [Google Scholar]
- Russell VA. Hypodopaminergic and hypernoradrenergic activity in prefrontal cortex slices of an animal model for attention-deficit hyperactivity disorder--the spontaneously hypertensive rat. Behavioural Brain Research. 2002;130:191–196. doi: 10.1016/s0166-4328(01)00425-9. [DOI] [PubMed] [Google Scholar]
- Scholl JL, Vuong SM, Forster GL. Chronic amphetamine treatment enhances corticotropin-releasing factor-induced serotonin release in the amygdala. European Journal of Pharmacology. 2010;644:80–87. doi: 10.1016/j.ejphar.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellings LH, Clarke PB. 6-Hydroxydopamine lesions of nucleus accumbens core abolish amphetamine-induced conditioned activity. Synapse. 2006;59:374–377. doi: 10.1002/syn.20247. [DOI] [PubMed] [Google Scholar]
- Sullivan TN, Farrell AD, Kliewer W. Peer victimization in early adolescence: association between physical and relational victimization and drug use, aggression, and delinquent behaviors among urban middle school students. Dev Psychopathol. 2006;18:119–137. doi: 10.1017/S095457940606007X. [DOI] [PubMed] [Google Scholar]
- Tharp-Taylor S, Haviland A, D'Amico EJ. Victimization from mental and physical bullying and substance use in early adolescence. Addictive behaviors. 2009;34(6–7):561–567. doi: 10.1016/j.addbeh.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topper LR, Castellanos-Ryan N, Mackie C, Conrod PJ. Adolescent bullying victimisation and alcohol-related problem behaviour mediated by coping drinking motives over a 12month period. Addictive behaviors. 2010;36(1–2):6–13. doi: 10.1016/j.addbeh.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Ventura R, Alcaro A, Cabib S, Conversi D, Mandolesi L, Puglisi-Allegra S. Dopamine in the medial prefrontal cortex controls genotype-dependent effects of amphetamine on mesoaccumbens dopamine release and locomotion. Neuropsychopharmacology. 2004;29:72–80. doi: 10.1038/sj.npp.1300300. [DOI] [PubMed] [Google Scholar]
- Viggiano D, Vallone D, Sadile A. Dysfunctions in dopamine systems and ADHD: evidence from animals and modeling. Neural Plast. 2004;11:97–114. doi: 10.1155/NP.2004.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt MJ, Burke AR, Renner KJ, Forster GL. Adolescent male rats exposed to social defeat exhibit altered anxiety behavior and limbic monoamines as adults. Behavioral Neuroscience. 2009;123:564–576. doi: 10.1037/a0015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE. Effects of methylphenidate on the catecholaminergic system in attention-deficit/hyperactivity disorder. Journal of Clinical Psychopharmacology. 2008;28:S46–53. doi: 10.1097/JCP.0b013e318173312f. [DOI] [PubMed] [Google Scholar]
- Yap JJ, Covington HE, 3rd, Gale MC, Datta R, Miczek KA. Behavioral sensitization due to social defeat stress in mice: antagonism at mGluR5 and NMDA receptors. Psychopharmacology. 2005;179:230–239. doi: 10.1007/s00213-004-2023-3. [DOI] [PubMed] [Google Scholar]