Abstract
Purpose
To evaluate the reproducibility of regional apparent diffusion coefficient (ADC) measurements of the normal fetal brain in the second and third trimesters.
Materials & Methods
Fifty normal singleton fetuses between 19–37 weeks of gestation were studied without sedation from healthy pregnant women. Single-shot diffusion DWI of the fetal brain was obtained using a 1.5-Tesla MR scanner and a 6-channel body array coil. ADC maps were created using 0 and 1000 b-values along 3 orthogonal directions. Two examiners independently measured ADC values in the cerebellar hemispheres (CH), pons, thalamus, basal ganglia (BG), centrum semiovale (CSO), frontal (FWM), parietal (PWM), temporal (TWM), and occipital white matter (OWM). Correlation between ADC values and menstrual age was assessed by linear regression. The bias and agreement of ADC measurements were determined using Bland-Altman plots.
Results
ADC values either remained constant (BG, FWM, PWM, TWM, CSO) or decreased (CH, pons, thalamus) with advancing menstrual age. Mean intra-observer bias for ADC measurements was not significantly different from zero. Small differences in inter-observer bias were detected for CH (1.26 ± 0.20 vs. 1.20 ± 0.18, p = 0.006), PWM (1.37 ± 0.29 vs. 1.33 ± 0.26, p = 0.02), and CSO (1.36 ± 0.29 vs. 1.33 ± 0.28, p < 0.0001). Measurement agreement was acceptable.
Conclusions
ADC measurements in normal un-sedated fetuses in the second and third trimesters are reproducible except for small differences for PWM, CH, and CSO between examiners.
Keywords: pregnancy, fetus, MRI, ADC
INTRODUCTION
Although ultrasonography (US) is an important prenatal imaging technique, it can be limited in its ability to detect neurologic abnormalities such as cortical malformations and neurodevelopmental abnormalities.1 Magnetic resonance imaging (MRI) in fetuses can provide additional diagnostic information when the sonographic results are equivocal and has significantly contributed to the evaluation of neurologic maturation, gyration, and abnormalities of the cerebrospinal fluid spaces.2–5 While conventional T1- and T2-weighted MRI sequences are capable of detecting structural anomalies, like US, these sequences can be limited in the detection of subtle changes associated with early fetal brain injury.6–7
Diffusion-weighted imaging (DWI) permits objective measurement of the random microscopic diffusion of water, as measured by the apparent diffusion coefficient (ADC). The utility of this modality has been well described in the evaluation of adult patients with suspected acute cerebrovascular ischemia,8–13 traumatic brain injury,14 or primary brain malignancy.15–16 Previous studies detailing normal neurodevelopment in neonatal and infant populations have demonstrated an age-related progressive decline in water diffusivity.17–21 Imaging techniques, based on DWI, have greatly improved our understanding of neonatal hypoxicischemic injury,22–25 cortical malformations,25 infectious pathologies,26 and traumatic brain injury in the pediatric population.27–28 The same principles have been extended to prenatal imaging and recent reports have characterized subtle ADC changes associated with fetal hydrocephalus and ischemic lesions.29–30
Prior investigations have described the ADC values of white matter and gray matter regions from fetuses of varying menstrual ages.31–35 These studies have involved the use of DWI in fetuses with suspected or known abnormalities. Furthermore, the reproducibility of these manually traced ADC measurements has not been well described in fetuses. The main goal of our investigation was to examine the reproducibility of regional ADC measurements obtained in the normal fetal brain in second and third trimester un-sedated fetuses. Our secondary objective was to characterize the normative age-related changes in water diffusivity from various white and gray matter regions for un-sedated fetuses.
METHODS
Patient Selection
All patients were enrolled in research protocols approved by the HIC at WBHS and the Institutional Review Board of the National Institute of Child Health and Human Development. This retrospective, cross-sectional study included normal healthy pregnant women from August 2005 through September 2010 who had normal prenatal ultrasound and fetal MRI results.
MRI Acquisition
Fetal MRI was performed on pregnant women in the supine position using a 1.5T system (Sonata, Siemens Medical Solutions, Malvern, PA, USA) with gradient switching capabilities of 40 mT/m in 0.2ms (slew rate 200 T/m/s). A combined six-channel body array coil was positioned over the lower pelvic area of the pregnant women. All MRI studies were performed without using maternal or fetal sedation.
Conventional imaging was performed using non-breath-hold HASTE and TRUEFISP sequences to provide initial localization. Subsequently, rapid echo-planar DWI was acquired in the axial plane using a b-value of 0 and 1000 s/mm3 along 3 orthogonal directions. The following parameters were used: TR, 3400ms; TE, 125ms; section thickness, 4mm; field of view, 280 mm × 280 mm; matrix, 128 × 128; voxel size, 2.5 mm × 2.5mm × 4.0mm. Fat suppression was achieved using a frequency selective radio frequency pulse. Each DWI sequence was acquired over approximately 2 minutes. ADC maps were constructed using the assigned b-values.
Image Analysis and Measurement
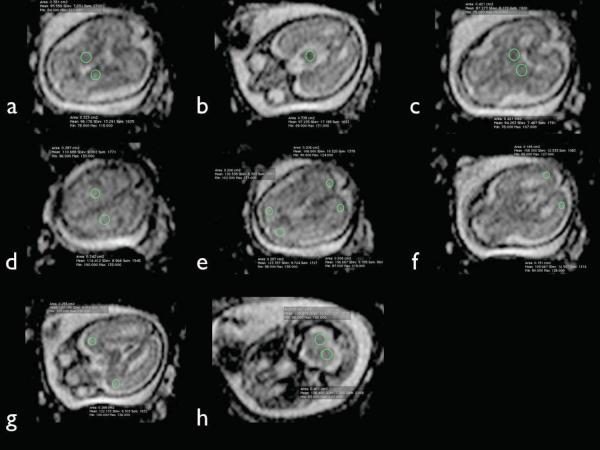
Offline image analysis software was used to analyze signal intensity within traced regions of interest on axial ADC maps (OsiriX DICOM software, http://www.osirixviewer.com, WACOM Intuous graphics tablet). Two examiners independently obtained bilateral ADC measurements by manually placing a region of interest (ROI) in the following areas: 1) basal ganglia (BG); 2) thalamus; 3) cerebellar hemisphere (CH); 4) frontal white matter (FWM); 5) parietal white matter (PWM); 6) occipital white matter (OWM); 7) temporal white matter (TWM); 8) centrum semiovale (CSO). The CSO does not develop until 30 menstrual weeks. Thus, in fetuses less than 30 week menstrual age, the CSO ROI was placed in the area where the future CSO would develop. A midline measurement was obtained in the pons (Figure 1). These measurements were based upon ROI placements previously reported in the literature.31–35 ROIs were manually traced and varied in shape and size depending on the brain region and size of the fetal brain. Approximately one month following the initial measurements, one observer repeated measurements for each case while blinded to the previously obtained data. A paired t-test was performed to examine for possible differences in ADC measurements between the left and right side for each of the regions measured bilaterally. Statistical significance was defined as P < 0.05. ADC values from each region were compared using ANOVA. Correlation between ADC values and menstrual age was assessed by linear regression analysis. The reproducibility of the measuring technique was analyzed among examiners using Bland-Altman plots for bias and agreement with their 95% limits of agreement (LOA).
Figure 1.
Regions of interest (ROI) for fetal brain ADC measurements. Multiple axial ADC images from a fetal brain at 22.7 gestational weeks demonstrating sample ROI placement within the basal ganglia (a), pons (b), thalamus (c), centrum semiovale (d), frontal and parietal white matter (e), occipital white matter (f), temporal white matter (g), and cerebellar hemispheres (h).
RESULTS
Fifty normal MRI examinations were performed. No fetus was imaged more than once. The mean menstrual age was 27.7 ± 4.3 weeks (range 19.3 to 37.1 weeks, MA). Ten fetuses were imaged before 24.0 weeks gestation, thirty-five between 24.0 and 34.0 weeks, and five after 34.0 weeks gestation.
Regional ADC Measurements
Regions of interest were successfully measured for the BG, pons, thalamus, FWM, PWM, and CSO in all 50 cases. Of the 50 total cases, regions of interest were successfully obtained in the CH in 42 cases (84%), OWM in 47 cases (94%) and TWM in 49 cases (98%).
No significant differences in ADC measurements were found between the left and right sides are of interest. Thus, all subsequent data analysis was performed on measurements obtained from the right side. The right side was selected randomly. ADC values (μm2/ms, mean ± SD) were: CH = 1.26 ± 0.20; pons = 1.05 ± 0.17; thalamus = 1.06 ± 0.19; BG = 1.07 ± 0.19; CSO = 1.36 ± 0.29; FWM = 1.37 ± 0.23; PWM = 1.37 ± 0.29; TWM = 1.36 ± 0.21; and OWM = 1.34 ± 0.25. Mean ADC values were similar in the deep gray matter regions (thalamus and BG) as well as the pons. ADC measurements were significantly higher in the CH and cortical white matter regions. No significant difference in ADC values was detected between the various white matter regions.
ADC and Menstrual Age
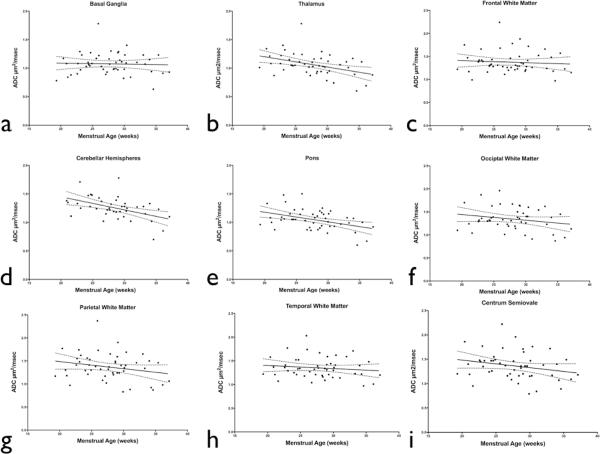
A statistically significant negative correlation was observed between advancing menstrual age and ADC measurements obtained in the pons, thalamus, and CH (P < 0.05). ADC measurements obtained in the BG and each cortical white matter region showed no statistically significant change with advancing menstrual age (Figure 2).
Figure 2.
Correlation between ADC values and menstrual age for different fetal brain regions: a) basal ganglia; R2=0.01; b) thalamus; R2=0.17; c) frontal white matter; R2=0.01; d) cerebellar hemispheres; R2=0.23; e) pons; R2=0.18; f) occipital white matter; R2=0.05, g) parietal white matter; R2=0.05, h) temporal white matter; R2=0.02, i) and centrum semiovale; R2=0.06. A significant menstrual agerelated decline in ADC values was observed in the pons, thalamus, and cerebellar hemispheres (p < 0.05).
Measurements of Reproducibility
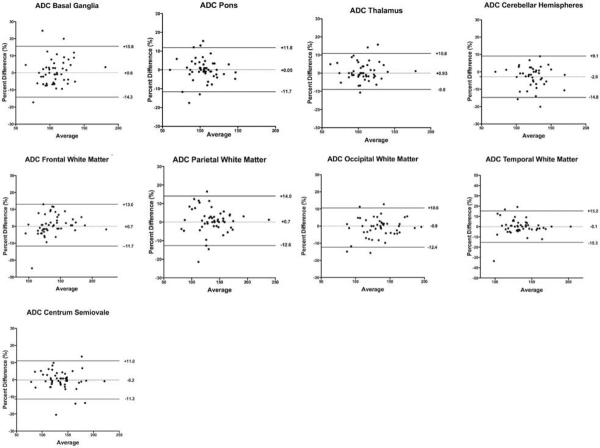
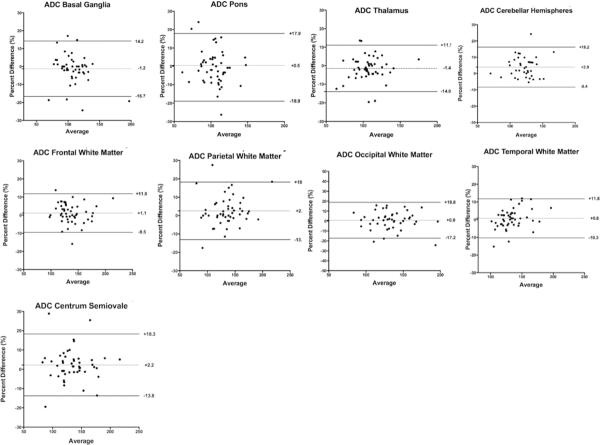
Mean intra-observer bias for ADC measurements was not significantly different from zero. Intra-observer LOA ranged from −9.0 to 15.6%. Small differences in inter-observer bias were detected for CH (1.26 ± 0.20 vs. 1.20 ± 0.18, p = 0.006), PWM (1.37 ± 0.29 vs. 1.33 ± 0.26, p = 0.02), and CSO (1.36 ± 0.29 vs. 1.33 ± 0.28, p < 0.0001). Inter-observer bias for all other regions in the fetal brain was not significantly different from zero. Inter-observer LOA ranged from −8.4 to 18.8% (Figures 3 and 4).
Figure 3.
Intra-observer bias and agreement for regional fetal brain ADC measurements. Intra-observer bias was not significantly different from zero. Intra-observer LOA ranged from −9.0 to 15.6%.
DISCUSSION
Diffusion weighted imaging has the potential to improve the detection of prenatal brain injury. The results of this study indicate that manually traced ADC measurements from normal fetal brain DWI are reproducible despite small differences in inter-observer bias for the CH, CSO, and PWM. Additionally, a significant menstrual age-related decrease in ADC values occurs in the pons, CH, and thalamus.
Prior Studies of Fetal Brain DWI
Clinical application of DWI has been mainly limited to adult and pediatric patients but recent investigations have demonstrated its ability to detect pathology for the fetal brain as well.29–30 Previous studies have described ADC values from fetuses with a normal brain MRI and/or a normal neonatal neurological examination.31–35 However, past investigators typically included subjects with known extra-cranial pathologies, twin-gestations, twin-to-twin transfusion syndrome, and fetal MR performed for suspected abnormal US findings. Structural anomalies may have altered fetal neurodevelopment and DWI measurements of the developing brain. For instance, abnormally high ADC values have been described in the thalamus and periatrial cortical white matter in fetuses with known congenital heart defects.36 Pharmacologically sedated fetuses could also alter findings on DWI33–34. Our results only included normal pregnancies and unsedated fetuses. Additionally, this is the first study to assess the reproducibility of this measurement technique. Table 1 compares the main features of the current study to other published investigations regarding the use of sedation, number of cases, b values used to obtain ADC values, menstrual ages, assessment of observer agreement, and inclusion criteria.
Table 1.
Characteristics of published studies describing normative ADC values in the fetal brain.
| Study | Subjects | b-values | Sedation | MA mean (range) | Observer Agreement | Inclusion Criteria |
|---|---|---|---|---|---|---|
| Righini 2003 | 15 | 0 and 600 s/mm2 | No | 27.6 weeks (22–35) | No | - Fetuses imaged for suspected clinical or US anomalies other than brain |
| - Normal neonatal neurological examination | ||||||
| - Includes cases of twin-to-twin transfusion syndrome | ||||||
| Schneider 2007 | 78 | 500 and 1000 s/mm2 | Yes | 31 (23–36) | No | - Fetuses imaged for known extra-cranial anomalies, family history of genetic disease or questionable neurological anomaly on US |
| - All included fetuses had a normal brain MRI and a normal neonatal neurological examination | ||||||
| Cannie 2007 | 66 | 0, 100, 250, 500, 750, and 1000 s/mm2 | Yes | not stated (17–37) | No | - Fetuses imaged for a variety of extra-cranial anomalies including (but not limited to) congenital diaphragmatic hernia, congenital cystic adenomatoid malformation, and polycystic kidney disease |
| Schneider 2009 | 38 | 0 and 600 s/mm2 | No | 24.3 (21–33.4) | No | - Fetuses imaged for suspected US anomalies, previous pregnancy with brain abnormalities, or normal healthy volunteers |
| - All included fetuses had a normal MRI and neonatal neurological examination | ||||||
| Boyer 2012 | 50 | 0 and 1000 s/mm2 | No | 27.7 (19.3–37) | Yes | - Fetuses imaged in healthy pregnant women |
| - All included fetuses had a normal US, MRI, and neonatal examination |
Factors Affecting Inter-Observer Bias
Several factors may explain small differences in the ADC values between observers. Due to the small size of the fetal brain and motion artifact, the posterior fossa including the CH was often difficult to clearly visualize on the axial ADC map. We found that accurate measurements of the CH could not be obtained in eight of the fifty cases. Thus, differences in inter-observer bias may be secondary to limited visualization of the CH, inclusion of surrounding structures in the ROI, or partial volume averaging which occurs when a single voxel or section contains multiple tissues, resulting in an averaged signal value. Additionally, there are local white matter inhomogeneities within the developing CSO and cerebral cortex, which represent developing fiber association tracts.37 These inhomogeneities and the small size of the fetal brain, when coupled with minor differences in image slice selection between investigators could also have contributed to the variability in these ADC measurements.
Regional Average ADC Values
Average ADC values of the pons and the deep gray matter of the thalamus and the BG were similar to each other. Significantly higher ADC values were obtained in the supratentorial white matter and the CH. No significant differences between the cortical white matter regions were noted. Our findings are similar to previously reported data.31, 35 An earlier study also found significantly higher ADC values in the cortical white matter compared to the CH and significantly lower ADC values in the thalamus and pons compared to the BG.35 Although we did observe the same trend, these differences did not reach statistical significance in our patient sample. Such variations may be due to differences in the shape and size of ROIs between the two studies. Our results and those from other studies also found no significant difference in ADC values between left and the right side regional measurements. In contrast to earlier investigations that averaged ADC measurements prior to performing additional analyses,31,34,35 we only evaluated right-sided brain structures for ADC measurements. This methodology was used to establish the reproducibility of a single, non-averaged ADC measurement.
Menstrual Age-Related Changes in Regional ADC Values
A significant decline in ADC values occurred with advancing menstrual age for the pons, CH, and thalamus. These findings are consistent with previously reported results.34–35 Earlier reports also describe a significant menstrual age- related decrease in the BG and periatrial white matter, which were not observed in the present study.35 An initial increase in cortical white matter ADC values from the 23rd-30th menstrual weeks followed by a decline following 30 menstrual weeks has also been reported.34 We did not observe such a pattern. Other investigators who reported this pattern utilized a fixed, single-sized circular ROI of 84 mm2. In contrast, we selectively examined individual brain regions by placing ROIs that varied both in size and shape depending upon the specific brain region as well as the size of the fetal brain. This approach minimized the amount of surrounding structures (i.e. cerebral cortex and germinal matrix) included in the ROI. These results are consistent with an earlier study that also varied the size and shape of ROIs in a similar fashion and did not find this initial increase in ADC values up to 30 menstrual weeks.35
Study Limitations
This investigation systematically describes the reproducibility of regional ADC brain measurements from a relatively large number of unsedated fetuses. Normal reference ranges are also provided for diffusion values of the fetal brain throughout the second and third trimesters. Our study also has the following limitations: 1) measurement variability associated with correlation between regional ADC values and menstrual age may have been exaggerated due to data analysis being performed only on right side, rather than on averaged measurements from both sides of the brain; and 2) fetal motion artifact may have degraded some of the brain ADC measurements, particularly when small regions of interest were being measured.
The regions of the cerebral cortex evaluated in this study as well as those previously reported do not specifically account for the laminar zones present in the fetal cerebrum. In fact, regions referred to as “white matter” are likely a combination of the subplate and intermediate (fetal white matter) zones. Additionally, the centrum semiovale does not develop until 30 weeks menstrual age and measurements performed in this region at younger ages likely reflect the transient subplate. The DWI signal characteristics of these laminar zones could be an area of future investigation.
CONCLUSION
We conclude that manually traced ADC measurements from fetal DWI are reproducible with an acceptable degree of inter-examiner variability. The data presented herein adds to the previously published data of ADC values at various menstrual ages but represents the first study to include only normal fetuses. Future research utilizing DWI in fetal neuroimaging should emphasize the early and accurate detection of perinatal brain pathology.
Figure 4.
Inter-observer bias and agreement for regional fetal brain ADC measurements. Inter-observer LOA ranged from −8.4 to 18.8% Small differences in inter-observer bias were detected for cerebellar hemispheres (1.26 ± 0.20 vs. 1.20 ± 0.18, p = 0.006), parietal white matter (1.37 ± 0.29 vs. 1.33 ± 0.26, p = 0.02), and centrum semiovale (1.36 ± 0.29 vs. 1.33 ± 0.28, p < 0.0001). Inter-observer bias for all other regions was not significantly different from zero.
ACKNOWLEDGMENTS
The Authors wish to acknowledge the technical assistance of Melissa Powell, RDMS and Beverley McNie, BS, CCRP. This research was supported (in part) by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS. R. Romero contributed to this work as part of his official duties as an employee of the United States Federal Government.
REFERENCES
- 1.Whitby E, Paley MN, Davies N, Sprigg A, Griffiths PD. Ultrafast magnetic resonance imaging of central nervous system abnormalities in utero in the second and third trimester of pregnancy: comparison with ultrasound. Br J Obstet Gynecol. 2001;108:519–526. doi: 10.1111/j.1471-0528.2001.00115.x. [DOI] [PubMed] [Google Scholar]
- 2.Girard N, Raybaud C, Poncet M. In vivo MR study of brain maturation in normal fetuses. AJNR. 1995;16:407–413. [PMC free article] [PubMed] [Google Scholar]
- 3.Girard N, Raybaud C, Gambarelli D, Figarella-Branger D. Fetal brain MR imaging. Magn Reson Imaging Clin N Am. 2001;9:19–56. [PubMed] [Google Scholar]
- 4.Fogliarini C, Chaumoitre K, Chapon F, Fernandez C, Levrier O, Figarella-Branger D, Girard N. Assessment of cortical maturation with prenatal MRI. Part I: normal cortical maturation. Eur Radiol. 2005;15:1671–1685. doi: 10.1007/s00330-005-2782-1. [DOI] [PubMed] [Google Scholar]
- 5.Fogliarini C, Chaumoitre K, Chapon F, Fernandez C, Levrier O, Figarella-Branger D, Girard N. Assessment of cortical maturation with prenatal MRI: part II: abnormalities of cortical maturation. Eur Radiol. 2005;15:1781–1789. doi: 10.1007/s00330-005-2779-9. [DOI] [PubMed] [Google Scholar]
- 6.Cannie M, Jani J, Dymarkowski S, Deprest J. Fetal magnetic resonance imaging: luxury or necessity? Ultrasound Obstet Gynecol. 2006;27:471–476. doi: 10.1002/uog.2776. [DOI] [PubMed] [Google Scholar]
- 7.Righini A, Kustermann A, Parazzini C, Fogliani R, Ceriani F, Triulzi F. Diffusion-weighted resonance imaging of acute hypoxic-ischemic cerebral lesions in the survivor of a monochorionic twin pregnancy: case report. Ultrasound Obstet Gynecol. 2007;29:453–456. doi: 10.1002/uog.3967. [DOI] [PubMed] [Google Scholar]
- 8.Warach S, Chien D, Li W, Ronthal M, Edelman RR. Fast magnetic resonance diffusion-weighted imaging of acute human stroke. Neurology. 1992;42:1717–1723. doi: 10.1212/wnl.42.9.1717. [DOI] [PubMed] [Google Scholar]
- 9.Olivot JM, Marks MP. Magnetic resonance imaging in the evaluation of acute stroke. Top Magn Reson Imaging. 2008;19:225–230. doi: 10.1097/RMR.0b013e3181aaf37c. [DOI] [PubMed] [Google Scholar]
- 10.Fiebach JB, Schellinger PD, Jansen O, Meyer M, Wilde P, Bender J, Schramm P, Juttler E, Oehler J, Hartmann M, Hahnel S, Knauth M, Hacke W, Sartor K. CT and diffusion-weighted MR imaging in randomized order: diffusion-weighted imaging results in higher accuracy and lower interrater variability in the diagnosis of hyperacute ischemic stroke. Stroke. 2002;33:2206–2210. doi: 10.1161/01.str.0000026864.20339.cb. [DOI] [PubMed] [Google Scholar]
- 11.Kucinski T, Vaterlein O, Glauche V, Fiehler J, Klotz E, Eckert B, Koch C, Rother J, Zeumer H. Correlation of apparent diffusion coefficient and computed tomography density in acute ischemic stroke. Stroke. 2002;33:1786–1791. doi: 10.1161/01.str.0000019125.80118.99. [DOI] [PubMed] [Google Scholar]
- 12.Mullins ME, Schaefer PW, Sorensen AG, Halpern EF, Ay H, He J, Koroshetz WJ, Gonzalez RG. CT and conventional and diffusion-weighted MR imaging in acute stroke: study in 691 patients at presentation to the emergency department. Radiology. 2002;224:353–360. doi: 10.1148/radiol.2242010873. [DOI] [PubMed] [Google Scholar]
- 13.Provenzale JM, Sorensen AG. Diffusion-weighted MR imaging in acute stroke: theoretic considerations and clinical applications. AJR. 1999;173:1459–1467. doi: 10.2214/ajr.173.6.10584783. [DOI] [PubMed] [Google Scholar]
- 14.Liu AY, Maldjian JA, Bagley LJ, Sinson GP, Grossman RI. Traumatic brain injury: diffusion-weighted MR imaging findings. AJNR. 1999;20:1636–164. [PMC free article] [PubMed] [Google Scholar]
- 15.Provenzale JM, Mukundan S, Barboriak DP. Diffusion-weighted and perfusion MR imaging for brain tumor characterization and assessment of treatment response. Radiology. 2006;239:632–649. doi: 10.1148/radiol.2393042031. [DOI] [PubMed] [Google Scholar]
- 16.Barboriak DP. Imaging of brain tumors with diffusion-weighted and diffusion tensor MR imaging. Magn Reson Imaging Clin N Am. 2003;11:379–401. doi: 10.1016/s1064-9689(03)00065-5. [DOI] [PubMed] [Google Scholar]
- 17.Neil J, Shiran SI, McKinstry RC, Schefft GL, Snyder AZ, Almli CR, Akbudak E, Aronovitz JA, Miller JP, Lee BC, Conturo TE. Normal brain in human newborns: apparent diffusion coefficient and diffusion anisotropy measured by using diffusion tensor MR imaging. Radiology. 1998;209:57–66. doi: 10.1148/radiology.209.1.9769812. [DOI] [PubMed] [Google Scholar]
- 18.Toft PB, Leth H, Peitersen B, Lou HC, Thomsen C. The apparent diffusion coefficient of water in gray and white matter of the infant brain. J Comput Assist Tomogr. 1996;20:1006–1011. doi: 10.1097/00004728-199611000-00029. [DOI] [PubMed] [Google Scholar]
- 19.Huppi PS, Maier SE, Peled S, Zientara GP, Barnes PD, Jolesz FA, Volpe JJ. Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatr Res. 1998;44:584–590. doi: 10.1203/00006450-199810000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Tanner SF, Ramenghi LA, Ridgway JP, Berry E, Saysell MA, Martinez D, Arthur RJ, Smith MA, Levene MI. Quantitative comparison of intrabrain diffusion in adults and preterm and term neonates and infants. AJR. 2000;174:1643–1649. doi: 10.2214/ajr.174.6.1741643. [DOI] [PubMed] [Google Scholar]
- 21.Girard N, Confort-Gouny S, Schneider J, Barberet M, Chapon, Viola A, Pineau S, Combaz X, Cozzone P. MR imaging of brain maturation. J Neuroradiology. 2007;34:290–310. doi: 10.1016/j.neurad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Cowan FM, Pennock JM, Hanrahan JD, Manji KP, Edwards AD. Early detection of cerebral infarction and hypoxic-ischemic encephalopathy in neonates using diffusion-weighted magnetic resonance imaging. Neuropediatrics. 1994;25:172–175. doi: 10.1055/s-2008-1073018. [DOI] [PubMed] [Google Scholar]
- 23.Johnson AJ, Lee BC, Lin WL. Echoplanar diffusion-weighted imaging in neonates and infants with suspected hypoxic-ischemic injury: correlation with patient outcome. AJR. 1999;172:219–226. doi: 10.2214/ajr.172.1.9888771. [DOI] [PubMed] [Google Scholar]
- 24.Inder T, Huppi PS, Zientara GP, Maier SE, Jolesz FA, di Salvo D, Robertson R, Barnes PD, Volpe JJ. Early detection of periventricular leukomalacia by diffusion-weighted magnetic resonance imaging techniques. J Pediatr. 1999;134:631–634. doi: 10.1016/s0022-3476(99)70251-9. [DOI] [PubMed] [Google Scholar]
- 25.Beitzke D, Simbrunner J, Riccabona M. MRI in paediatric hypoxicischemic disease, metabolic disorders and malformations--a review. Eur J Radiol. 2008;68:199–213. doi: 10.1016/j.ejrad.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 26.Gaudet LM, Flavin M, Islam O, Smith GN. Diffusion MRI brain findings in neonates exposed to chorioamnionitis: a case series. J Obstet Gynaecol Can. 2009;31:497–503. doi: 10.1016/s1701-2163(16)34211-6. [DOI] [PubMed] [Google Scholar]
- 27.Babikian T, Tong KA, Galloway NR, Freier-Randall M, Obenaus A, Ashwal S. Diffusion-weighted imaging predicts cognition in pediatric brain injury. Pediatr Neurol. 2009;41:425–431. doi: 10.1016/j.pediatrneurol.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Suskauer SJ, Huisman TA. Neuroimaging in pediatric traumatic brain injury: current and future predictors of functional outcome. Dev Disabil Res Rev. 2009;15:117–123. doi: 10.1002/ddrr.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erdem G, Celik O, Hascalik S, Karakas HM, Alkan A, Firat AK. Diffusion weighted imaging evaluation of subtle cerebral microstructural changes in intrauterine fetal hydrocephalus. Magn Reson Imaging. 2007;25:1417–1422. doi: 10.1016/j.mri.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 30.Guimiot F, Garel C, Fallet-Bianco C, Menez F, Khung-Savatovsky S, Oury JF, Sebag G, Delezoide AL. Contribution of diffusion-weighted imaging in the evaluation of diffuse white matter ischemic lesions in fetuses: correlations with fetopathologic findings. AJNR. 2008;29:110–115. doi: 10.3174/ajnr.A0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Righini A, Bianchini E, Parazzini C, Gementi P, Ramenghi L, Baldoli C, Nicolini U, Mosca F, Triulzi F. Apparent diffusion coefficient determination in normal fetal brain: A prenatal MR imaging study. AJNR. 2003;24:799–804. [PMC free article] [PubMed] [Google Scholar]
- 32.Manganaro L, Perrone A, Savelli S, Di Maurizio M, Maggi C, Ballesio L, Porfiri LM, De Felice C, Marinoni E, Marini M. Evaluation of normal brain development by prenatal MR imaging. Radiol Med. 2007;112:444–455. doi: 10.1007/s11547-007-0153-5. [DOI] [PubMed] [Google Scholar]
- 33.Cannie M, De Keyzer F, Meersschaert J, Jani J, Lewi L, Deprest J, Dymarkowski S, Demaerel P. A diffusion-weighted template for gestational age-related apparent diffusion coefficient values in the developing fetal brain. Ultrasound Obstet Gynecol. 2007;30:318–324. doi: 10.1002/uog.4078. [DOI] [PubMed] [Google Scholar]
- 34.Schneider JF, Confort-Gouny S, Le Fur Y, Viout P, Bennathan M, Chapon F, Fogliarini C, Cozzone P, Girard N. Diffusion-weighted imaging in normal fetal brain maturation. Eur Radiol. 2007;17:2422–2429. doi: 10.1007/s00330-007-0634-x. [DOI] [PubMed] [Google Scholar]
- 35.Schneider MM, Berman JI, Baumer FM, Glass HC, Jeng S, Jeremy RJ, Esch M, Biran V, Barkovich AJ, Studholme C, Xu D, Glenn OA. Normative apparent diffusion coefficient values in the developing fetal brain. AJNR. 2009;30:1799–1803. doi: 10.3174/ajnr.A1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berman JI, Hamrick SE, McQuillen PS, Studholme C, Xu D, Henry RG, Hornberger LK, Glenn OA. Diffusion-weighted imaging in fetuses with severe congenital heart defects. AJNR. 2011;32:E21–22. doi: 10.3174/ajnr.A1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kostovic I, Judas M, Rados M, Hrabac P. Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cerebral Cortex. 2002;12:536–544. doi: 10.1093/cercor/12.5.536. [DOI] [PubMed] [Google Scholar]