Abstract
Enrollment of US women with sufficient risk of HIV infection into HIV vaccine efficacy trials has proved challenging. A cohort of 799 HIV-negative women, aged 18-45, recruited from three US cities was enrolled to assess recruitment strategies based on geographic risk pockets, social and sexual networks and occurrence of sexual concurrency and to assess HIV seroincidence during follow-up (to be reported later). Among enrolled women, 90% lived or engaged in risk behaviors within a local risk pocket, 64% had a male partner who had concurrent partners and 50% had a male partner who had been recently incarcerated. Nearly half (46%) were recruited through peer referral. At enrollment, 86% of women said they were willing to participate in a vaccine efficacy trial. Results indicate that participant and partner risk behaviors combined with a peer referral recruitment strategy may best identify an at-risk cohort willing to participate in future trials.
Keywords: HIV vaccine trial preparedness, United States women, peer referral, respondent driven sampling, sexual concurrency, willingness to participate
Introduction
HIV vaccine efficacy trials require volunteers at high risk of HIV infection who express a willingness to participate and will maintain high adherence with the vaccination and follow-up visit schedules to ensure adequate assessment of vaccine efficacy(1-3). Inclusion of women in HIV vaccine efficacy trials is essential since women constitute more than half of HIV-infected adults worldwide(4). Gender may influence critical endpoints, such as HIV acquisition and viral load post-infection, thereby affecting vaccine efficacy, as has been observed with other vaccines(5-7). Although in the United States (US) women comprise a lower proportion of HIV-infected individuals than is the case worldwide, women account for about one quarter of all new US HIV diagnoses. The largest proportion of new infections among US women is among African American women, who have an estimated population rate of HIV infection 15 times higher than white women. The HIV infection rate among Hispanic/Latina women is over 4 times higher than white women(8).
Despite these rates, there have been significant challenges in identifying cohorts of women with HIV incidence sufficient to assess HIV vaccine efficacy. Past HIV vaccine trial preparedness studies among US women conducted in the 1990’s recruited women based predominately on the risk behaviors of women themselves, including injection drug use (IDU), crack cocaine use, exchanging sex for money or drugs, or having multiple partners and found HIV incidence rates of 0.8-1.2%(9;10). Women were also eligible for these studies if they had a male partner who was HIV infected, had sex with men, had a recent sexually transmitted infection or injected drugs. Most of the women enrolled based on partner criteria had male partners who injected drugs(9). Similar eligibility criteria were used in the VaxGen 004 phase 3 HIV vaccine efficacy trial, recruiting in 1998-1999, which had an HIV incidence of 0.8% among 308 women(11). The Step Study, a phase 2b HIV vaccine efficacy study, recruiting in 2005-2007, used similar eligibility criteria as these earlier trials(12). Despite high rates of unprotected vaginal/anal sex, exchange of sex, and crack cocaine use among the 512 women enrolled in North America, the HIV incidence was 0.5%, with all but one infection occurring after 1 year of follow-up (Statistical Center for HIV/AIDS Research and Prevention unpublished data). Unless enrollment of cohorts of US women with higher HIV incidence rates than those seen in these studies can be accomplished, the feasibility of conducting HIV vaccine efficacy trials among US women has been questioned(13).
Heterosexual contact is the primary mode of HIV transmission for US women(14). Empiric and modeling studies of the epidemiology of sexually transmitted infections (STIs) in the US, including HIV, emphasize a broader perspective on risk of infection that includes social and sexual networks and sexual concurrency (multiple sexual relationships that overlap in time)(15;16). Sexual networks share a compact geographical distribution(17-19), which accounts for the tight spacial grouping of some outbreaks of STIs(20-22). Venues where people meet offer a geographic nexus for bridging within or across sexual networks, and play an essential role in the spread of STIs(23-25). Sexual concurrency has been associated with more rapid transmission of STIs than sequential partnerships(26-28). Drug use, especially crack and cocaine, has been associated with sexual concurrency(29;30). Although a clear relationship between sexual concurrency and heterosexual transmission of HIV infection has not been established(19;31), sexual concurrency has been hypothesized to contribute, in part, to the racial disparities in HIV prevalence among US women(32), owing to a higher rate of engagement in sexual concurrency reported by African American women in the general US population compared to white women(30).
In HVTN 906, three US sites that enrolled women in the Step Study modified their previous recruitment strategies to assess the feasibility of enrolling a higher risk cohort of women and assessing HIV seroincidence and retention over 18 months of follow-up. The HVTN 906 recruitment strategies differed from those used for the Step Study by focusing on geographical risk pockets of HIV infection, sexual networks and the expansion of high-risk male partner criteria to include men with concurrent partners and recently incarcerated men. Secondary objectives included the assessment of HIV prevalence among those screened for the study, perceived willingness to participate in future HIV vaccine trials, and changes in risk behaviors over time among those enrolled. In this report of baseline data, we describe the recruitment methods evaluated to identify and enroll an at-risk cohort of urban US women, the HIV prevalence among women screened, and for women enrolled, their expressed willingness to participate in a future HIV vaccine trial as measured at study entry.
Methods
Study design
HVTN 906 was a prospective observational study conducted in Chicago, New York City (NYC) and Philadelphia to determine the feasibility of recruiting and retaining US women at high risk of HIV infection. HIV uninfected women were eligible if they were between the ages of 18 to 45, willing to receive HIV test results and risk reduction counseling, not pregnant or intending to become pregnant for 18 months, met high-risk behavioral criteria, and provided informed consent. High-risk behavior was defined as self-report of unprotected vaginal or anal sex with a male partner in the prior six months and either i) residing or engaging in risk behaviors (unprotected sex, exchange of sex, crack cocaine use) within a site-specific risk pocket or ii) having a male partner who had either been incarcerated in the last year, injected drugs in the last year or had concurrent sex with another partner (male or female) in the last six months. In addition, Chicago required that the woman be referred by an HIV-positive female peer and self-report crack cocaine use. Midway through the enrollment period, NYC added a requirement that all women meet both the risk pocket and the high-risk male partner criteria. The protocol and informed consent documents were approved by the Institutional Review Boards for each participating institution.
Study recruitment
Sites developed their own methods for recruitment based on the current literature highlighting the roles of geographic risk pockets, networks and sexual concurrency in the heterosexual transmission of HIV infection to women in the US(32-35). All sites implemented strategies not used in prior vaccine studies and used street outreach within local risk pockets and referral by peers to recruit women. NYC and Philadelphia also made limited use of referral by male sexual partners and others, and passive methods such as flyers, advertisements, and letters.
Chicago focused on identifying and recruiting from sexual networks with HIV-positive members through street outreach and use of a modified respondent driven sampling scheme. An indigenous outreach worker recruited women through street outreach who met the prescreening criteria and were located in six risk pockets, areas of the city with the highest number of new female HIV infections identified from Illinois Department of Public Health data. Twenty-three HIV-positive women identified during screening (seeds) were given between 4-15 (median 13) referral coupons. They were asked to give these coupons to women they knew who smoked crack and had sex with at least one of the seed’s male partners. HIV-negative women who met study criteria and received a coupon were enrolled in the study. The seeds were given $10 for each woman they referred and who qualified for screening. The outreach worker stopped recruiting seeds from a risk pocket when a steady stream of qualified referred women from the risk pocket was established.
NYC identified neighborhoods with the highest number of new HIV diagnoses among women based on data from the New York City Department of Health and Mental Hygiene. Within these neighborhoods, specific locations for potential recruitment were selected based on local reports and interviews with community-based organizations. Site recruiters then enumerated traffic flow and conducted brief interviews with women on the street to identify local risk pockets. Women were recruited through street outreach in these risk pockets and enrolled women were asked to refer other women (peer referral). The referring woman was given a $5 gift card for each referred woman who came in for a screening visit and 2 movie tickets if the participant enrolled. Other referrals came from local community agencies. In addition, NYC recruited women at bus stops for visitor transportation to upstate prisons and women in visitor waiting areas of jails and prisons. Finally, NYC recruited men in the identified risk pockets who reported being HIV-infected, an injection drug user, recently incarcerated or having concurrent partners and gave them cards with an identification number to give to their female sexual partners for referral to the study. Men were given $25 for their participation, did not receive any reimbursement for woman they referred, and had no further involvement in the study.
The Philadelphia site recruited within West and North Philadelphia neighborhoods with the greatest number of risk pockets that contain the highest prevalence of women living with AIDS according to Philadelphia Department of Health data. Within these areas, local ethnographers identified street locations where drugs were sold or exchanged for sex and where at-risk women could be approached for pre-screening evaluation. All pre-screening, screening, and follow-up evaluations were conducted on a mobile assessment unit parked within neighborhood risk pockets. The mobile unit was used so women did not have to travel to the study site for enrollment, a challenge identified in recruitment for the Step Study. Additionally, Philadelphia used peer referral, by which women reporting high-risk behaviors at prescreening referred their women friends, and partner referral, by which men with a history of HIV infection, IDU, recent incarceration, or sex with men recruited for other studies referred their female sexual partners.
Study visits
Figure 1 outlines the recruitment to enrollment process. Sites prescreened women, typically assessing age, protocol and site-specific behavioral risk criteria, self-report of HIV status and pregnancy status. Eligible and willing women were scheduled for a screening visit at the study site or mobile unit. After obtaining informed consent at the screening visit, staff confirmed that the woman self-reported she was not HIV positive or pregnant and met the protocol and any site-specific behavioral risk criteria (prescreening reassessment). Potentially eligible women then completed interviewer-administered demographics, behavioral risk, pregnancy history, and current contraception questionnaires, received risk reduction counseling, and were tested for pregnancy and HIV. Behavioral risk questions used the time interval of the six months prior to assessment. For HIV testing at screening and follow-up visits, sites followed their own testing procedures using FDA approved tests for initial and confirmatory testing on separate samples. Women were compensated $25 for the screening visit.
Figure 1.
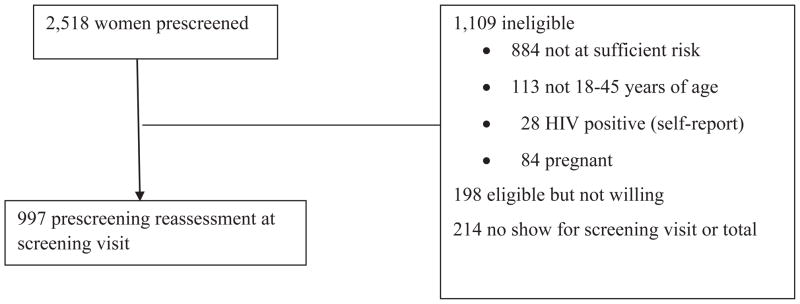
HVTN 906 Study Profile
By design the enrollment visit took place 7-28 days after screening to provide evidence of a woman’s adherence with a visit schedule. A questionnaire on attitudes regarding HIV/AIDS and future participation in HIV vaccine trials was administered, which included questions about the level of concern women had regarding issues related to vaccine trial participation (including safety, repeated HIV testing, possibility of testing HIV positive due to receipt of a vaccine, and reactions of others). Compensation for the enrollment visit was $25 for the Chicago and Philadelphia sites and $30 for NYC. Subsequent follow-up visits for HIV testing and risk assessment were conducted at 6, 12 and 18 months.
Statistical analysis
As a measure of recruitment efficiencies, the ratio of the number of women who had a prescreening reassessment at the screening visit (referred to as “screened”) to those enrolled was calculated by site and recruitment strategy. HIV prevalence was calculated among women who did not self-report HIV infection and who had an HIV test at screening. Two women who initially tested positive but refused confirmatory testing were excluded from the prevalence calculations. HIV prevalence by recruitment strategies was calculated to determine which recruitment approaches identified undiagnosed HIV infections. Data on prior HIV testing were not collected. HIV prevalence rates are presented with exact 95% binomial confidence intervals (CIs).
We used multivariable logistic regression modeling to evaluate factors predictive of willingness to participate in a future HIV vaccine trial. Willingness to participate was measured with four response levels, which were dichotomized as definitely or probably willing compared to definitely or probably not willing. Factors assessed were study site; eligibility based on risk pocket or partner characteristics or both; demographics [age, black non-Hispanic race/ethnicity, high school graduate, employed, annual household income (< $10,000 vs ≥ $10,000), had healthcare, living in own house/apartment, living with a main partner, homeless in last 6 months, in jail/prison in last 6 months]; number of male partners; had a main partner; sexual behaviors of the woman (unprotected anal sex, unprotected sex while drunk, unprotected sex while high, exchanged sex, forced to have sex); had an STI; heavy alcohol use (≥ 4 drinks everyday or consuming ≥ 6 drinks on a typical day); recreational drug use (marijuana, crack cocaine, cocaine, heroin use coded as any vs no use); male partner characteristics (HIV positive, incarcerated in past year, IDU in past year, had concurrent partners in last 6 months); perceived personal benefit of an HIV vaccine; perceived at risk of HIV infection in next 5 years; had family or friends who had or died from HIV/AIDS; recruited through street outreach, peer referral or other method; and a composite score of the level of concern regarding participation in an HIV vaccine trial (coded as tertiles of the sum of the 11 items of concern assessed on the Vaccine Participation Questionnaire, with responses very concerned=2, somewhat concerned=1, and not at all concerned=0). To evaluate these potential predictors of willingness, a best subset model selection approach was used to identify the best fit model based on the Aikaike information criterion limited to no more than 10 variables. Since this resulted in a model with several statistically non-significant variables (Wald Chi-square p-value > 0.05), we further examined the affect of eliminating the non-significant variables (including forward, backward and stepwise regression modeling which gave the same result) to obtain the final model containing only significant predictors.
Results
Recruitment
Between January 2009 and May 2010, 799 women were enrolled (319 from NYC, 243 from Philadelphia, and 237 from Chicago). The majority (71.3%) lived or engaged in risky behavior within a site-identified risk pocket and met at least 1 of the 3 partner-specific eligibility criteria; 18.3% met only the risk pocket criteria, and 10.4% met only partner criteria (Table I). At all three sites, more than 59% of women met both the risk pocket and partner eligibility criteria, although Philadelphia enrolled 40.3% based on risk pocket only and NYC enrolled 25.1% based on partner criteria only (Table I). For both NYC and Philadelphia women, the distributions by eligibility criteria did not differ between women recruited by street outreach or by peer referral and were similar to those shown in Table I (data not shown). In Chicago by design all women had to be referred by a peer.
Table I.
Protocol risk pocket and male partner eligibility criteria by site for enrolled womena
| Chicago (N=237) | New York (N=319) | Philadelphia (N=243) | All Sites (N=799) | |
|---|---|---|---|---|
| Criteria met | ||||
| Risk pocket (RP) + high risk (HR) partnerb | 210 (88.6%) | 216 (67.7%) | 144 (59.3%) | 570 (71.3%) |
| RP + HR partner with 1 partner criterion | 96 (40.5%) | 117 (36.7%) | 99 (40.7%) | 312 (39.0%) |
| RP + HR partner with 2 partner criteria | 85 (35.9%) | 67 (21.0%) | 39 (16.0%) | 191 (23.9%) |
| RP + HR partner with 3 partner criteria | 29 (12.2%) | 32 (10.0%) | 6 (2.5%) | 67 (8.4%) |
| High risk partner only | 2 (0.8%)) | 80 (25.1%) | 1 (0.4%) | 83 (10.4%) |
| HR partner with 1 partner criterion | 1 (0.4%) | 36 (11.3%) | 1 (0.4%) | 38 (4.8%) |
| HR partner only, with 2 partner criteria | 1 (0.4%) | 37 (11.6%) | 0 (0.0%) | 38 (4.8%) |
| HR partner only, with 3 partner criteria | 0 (0.0%) | 7 (2.2%) | 0 (0.0%) | 7 (0.9%) |
| Risk pocket only | 25 (10.5%) | 23 (7.2%) | 98 (40.3%) | 146 (18.3%) |
| Male partner who was Incarcerated in past year | ||||
| Yes | 127 (53.6%) | 189 (59.2%) | 82 (33.7%) | 398 (49.8%) |
| No | 59 (24.9%) | 119 (37.3%) | 148 (60.9%) | 326 (40.8%) |
| Don’t Know | 51 (21.5%) | 11 (3.4%) | 13 (5.3%) | 75 (9.4%) |
| Male partner who injected drugs in past year | ||||
| Yes | 46 (19.4%) | 64 (20.1%) | 11 (4.5%) | 121 (15.1%) |
| No | 105 (44.3%) | 228 (71.5%) | 204 (84.0%) | 537 (67.2%) |
| Don’t Know | 86 (36.3%) | 27 (8.5%) | 28 (11.5%) | 141 (17.6%) |
| Male partner who had concurrent partners in last 6 months | ||||
| Yes | 183 (77.2%) | 225 (70.5%) | 103 (42.4%) | 511 (64.0%) |
| No | 7 (3.0%) | 45 (14.1%) | 59 (24.3%) | 111 (13.9%) |
| Don’t Know | 47 (19.8%) | 49 (15.4%) | 81 (33.3%) | 177 (22.2%) |
All women had to have reported unprotected vaginal or anal sex with a male partner in the previous 6 months.
Risk pocket refers to currently residing or engaging in risk behavior (unprotected sex, exchange of sex, crack cocaine use) in a geographical local area which has high HIV incidence and/or prevalence. A high risk partner was defined as a male sexual partner who had either 1) been incarcerated in the past year, 2) had injected drugs in the past year, or 3) had concurrent partners (male or female) within the past 6 months. Mid-study, New York revised site-specific eligibility criteria to require women to meet both the risk pocket and the high-risk male partner criteria.
The characteristics of enrolled women varied substantially by site as expected due to the different site-specific eligibility criteria and recruitment strategies (Tables II and III). The Chicago cohort was primarily crack cocaine-using sex workers. Compared to the NYC and Philadelphia cohorts, they were older, of lower socio-economic status, more transient, more likely to have been incarcerated, reported more alcohol and heroin use, had more sex partners, and were more likely to be high during sex. The Chicago and Philadelphia cohorts were primarily African-American, compared to the NYC site which enrolled a sizable number of Hispanic women (33.5% of the NYC cohort). The NYC women were more likely to have attended college than women from Chicago and Philadelphia. Although women were engaging in high-risk behaviors, only 16% felt it was possible that they would become HIV infected in the next five years (Table IV).
Table II.
Demographic characteristics of enrolled women
| Chicago (N=237) | New York (N=319) | Philadelphia (N=243) | All Sites (N=799) | |
|---|---|---|---|---|
| Age | ||||
| 18 – 20 years | 0 (0.0%) | 37 (11.6%) | 24 (9.9%) | 61 (7.6%) |
| 21 – 30 years | 19 (8.0%) | 90 (28.2%) | 60 (24.7%) | 169 (21.2%) |
| 31 – 40 years | 117 (49.4%) | 101 (31.7%) | 95 (39.1%) | 313 (39.2%) |
| 41 – 45 years | 101 (42.6%) | 91 (28.5%) | 64 (26.3%) | 256 (32.0%) |
| Median (25th, 75th %tile) | 39 (36, 43) | 33 (25, 41) | 35 (27, 41) | 37(28, 42) |
| Race/ethnicity | ||||
| Black, non-Hispanic | 230 (97.0%) | 185 (58.0%) | 217 (89.3%) | 632 (79.1%) |
| Hispanic | 2 (0.8%) | 107 (33.5%) | 13 (5.3%) | 122 (15.3%) |
| Other | 5 (2.1%) | 27 (8.5%) | 13 (5.3%) | 45 (5.6%) |
| Education | ||||
| 8th grade or less | 14 (5.9%) | 15 (4.7%) | 6 (2.5%) | 35 (4.4%) |
| 9th–12th grade | 135 (57.0%) | 117 (36.7%) | 107 (44.0%) | 359 (44.9%) |
| HS grad or equivalent | 55 (23.2%) | 97 (30.4%) | 103 (42.4%) | 255 (31.9%) |
| Some college or graduate | 33 (13.9%) | 90 (28.2%) | 27 (11.1%) | 150 (18.8%) |
| Employed, other than sex work | ||||
| Yes | 11 (4.6%) | 55 (17.2%) | 56 (23.0%) | 122 (15.3%) |
| Household income | ||||
| Less than $10,000 | 220 (92.8%) | 243 (76.2%) | 169 (69.6%) | 632 (79.1%) |
| $10,000-$19,999 | 13 (5.5%) | 42 (13.2%) | 41 (16.9%) | 96 (12.0%) |
| $20,000 or greater | 2 (0.8%) | 29 (9.1%) | 25 (10.3%) | 56 (7.0%) |
| Don’t know | 2 (0.8%) | 5 (1.6%) | 7 (2.9%) | 14 (1.8%) |
| Refused | 0 (0%) | 0 (0%) | 1 (0.4%) | 1 (0.1%) |
| Has public or private health insurance | 60 (25.3%) | 281 (88.1%) | 186 (76.5%) | 527 (66.0%) |
| Current living situation | ||||
| Own house/apartment | 55 (23.2%) | 126 (39.5%) | 127 (52.3%) | 308 (38.5%) |
| Family member house/apt. | 80 (33.8%) | 83 (26.0%) | 57 (23.5%) | 220 (27.5%) |
| Someone else’s house/apt. | 94 (39.7%) | 19 (6.0%) | 33 (13.6%) | 146 (18.3%) |
| Other | 8 (3.4%) | 91 (28.5%) | 26 (10.7%) | 125 (15.6%) |
| Lives with a main partner | 101 (42.6%) | 105 (32.9%) | 94 (38.7%) | 300 (37.5%) |
| Homeless in last 6 months | 71 (30.0%) | 34 (10.7%) | 14 (5.8%) | 119 (14.9%) |
| Jail/prison in last 6 months | 86 (36.3%) | 32 (10.0%) | 20 (8.2%) | 138 (17.3%) |
Table III.
Sexual behaviors and alcohol/drug use of enrolled women
| Chicago (N=237) | New York (N=319) | Philadelphia (N=243) | All Sites (N=799) | |
|---|---|---|---|---|
| Sexual behaviorsa | ||||
| Total number of male partners | ||||
| Median (25th, 75th %tile) number unknown HIV status | 10 (3, 86) | 2 (1, 4) | 2 (1, 4) | 3 (2, 7) |
| Median (25th, 75th %tile) number HIV negative | 9 (2, 86) | 1 (0, 3) | 1 (0, 3) | 2 (0,5) |
| Median (25th, 75th %tile) | 1 (0, 1) | 1 (1, 2) | 1 (0, 1) | 1 (0, 1) |
| Had an HIV positive partner | 1 (0.4%) | 13 (4.1%) | 3 (1.2%) | 17 (2.1%) |
| Had a main partner | 168 (70.9%) | 257 (80.6%) | 198 (81.5%) | 623 (78.0%) |
| Had unprotected anal sex | 49 (20.7%) | 95 (29.8%) | 48 (19.8%) | 192 (24.0%) |
| Had unprotected sex while drunk | 130 (54.9%) | 158 (49.5%) | 126 (51.9%) | 414 (51.8%) |
| Had unprotected sex while high | 211 (89.0%) | 159 (49.8%) | 134 (55.1%) | 504 (63.1%) |
| Exchanged sex | 219 (92.4%) | 108 (33.9%) | 90 (37.0%) | 417 (52.2%) |
| Forced to have sex | 30 (12.7%) | 29 (9.1%) | 14 (5.8%) | 73 (9.1%) |
| Had a sexually transmitted disease | ||||
| Diagnosed/treated for STD | 32 (13.5%) | 31 (9.7%) | 20 (8.2%) | 83 (10.4%) |
| Symptoms only | 44 (18.6%) | 56 (17.6%) | 30 (12.3%) | 130 (16.3%) |
| Alcohol and drug usea | ||||
| Heavy alcohol useb | 95 (40.1%) | 85 (26.6%) | 68 (28.0%) | 248 (31.0%) |
| Injection drug use | 25 (10.5%) | 29 (9.1%) | 9 (3.7%) | 63 (7.9%) |
| Marijuana | 111 (46.8%) | 136 (42.6%) | 132 (54.3%) | 379 (47.4%) |
| Crack cocaine | 236 (99.6%) | 82 (25.7%) | 76 (31.3%) | 394 (49.3%) |
| Heroin use | 157 (66.2%) | 44 (13.8%) | 12 (4.9%) | 213 (26.7%) |
| Cocaine use | 23 (9.7%) | 36 (11.3%) | 34 (14.0%) | 93 (11.6%) |
Sexual behaviors and alcohol and drug use were assessed with reference to the six months prior to the screening visit.
Heavy alcohol use was defined as drinking ≥ 4 drinks everyday or drinking ≥ 6 drinks on a typical day that the woman consumed alcohol.
Table IV.
Personal attitude questions regarding HIV/AIDS
| Item | Chicago (N=237) | New York (N=319) | Philadelphia (N=243) | All Sites (N=799) |
|---|---|---|---|---|
| I will personally benefit from an HIV vaccine | ||||
| Agree Strongly | 108 (45.6%) | 87 (27.3%) | 57 (23.5%) | 252 (31.5%) |
| Agree | 99 (41.8%) | 120 (37.6%) | 95 (39.1%) | 314 (39.3%) |
| Neither Agree nor Disagree | 9 (3.8%) | 60 (18.8%) | 38 (15.6%) | 107 (13.4%) |
| Disagree | 18 (7.6%) | 41 (12.9%) | 40 (16.5%) | 99 (12.4%) |
| Disagree Strongly | 3 (1.3%) | 11 (3.4%) | 13 (5.3%) | 27 (3.4%) |
| It is possible I will become infected with HIV in the next 5 years | ||||
| Agree Strongly | 7 (3.0%) | 6 (1.9%) | 10 (4.1%) | 23 (2.9%) |
| Agree | 38 (16.0%) | 47 (14.7%) | 23 (9.5%) | 108 (13.5%) |
| Neither Agree nor Disagree | 49 (20.7%) | 54 (16.9%) | 26 (10.7%) | 129 (16.1%) |
| Disagree | 92 (38.8%) | 121 (37.9%) | 87 (35.8%) | 300 (37.5%) |
| Disagree Strongly | 51 (21.5%) | 91 (28.5%) | 97 (39.9%) | 239 (29.9%) |
| Have family members/friends with or died from HIV/AIDS | ||||
| Yes | 135 (57.0%) | 270 (84.6%) | 169 (69.5%) | 574 (71.8%) |
| No | 92 (38.8%) | 44 (13.8%) | 57 (23.5%) | 193 (24.2%) |
| Don’t Know | 10 (4.2%) | 5 (1.6%) | 17 (7.0%) | 32 (4.0%) |
The screening to enrollment ratio, a measure of recruitment efficiency, was highest for Chicago (1.40 screened per 1 enrolled) compared with NYC (1.15) and Philadelphia (1.23) (Table V). Peer referral had similar screening to enrollment ratios at the three sites (Chicago 1.21, NYC 1.22, Philadelphia 1.27; Table V). Street outreach by NYC (1.12) was slightly more efficient than at Philadelphia (1.25).
Table V.
Screening to enrollment ratio and HIV prevalence by site and recruitment strategy
| Screening and enrollment | HIV prevalence | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Site | Recruitment strategy | No. screened | No. enrolled | Screening to enrollment ratio | No. with screening HIV testa | No. infected | Percent (%) | 95% CI |
| All Sites | Any | 997 | 799 | 1.25 | 966 | 44 | 4.6 | (3.3,6.1) |
| Street outreach | 346 | 254 | 1.36 | 332 | 11 | 3.3 | (1.7, 5.9) | |
| Peer referral | 446 | 366 | 1.22 | 439 | 28 | 6.4 | (4.3,9.1) | |
| Other referralb | 79 | 72 | 1.10 | 77 | 0 | 0 | (0.0, 4.7) | |
| Jail/prison waiting areas | 39 | 36 | 1.08 | 36 | 0 | 0 | (0.0,9.7) | |
| Passive | 61 | 48 | 1.27 | 58 | 4 | 6.9 | (1.9,16.7) | |
| Multiple methodsc | 25 | 22 | 1.14 | 23 | 1 | 4.3 | (0.1, 21.9) | |
| Chicago | Any | 331 | 237 | 1.40 | 329 | 28 | 8.5 | (5.7,12.1) |
| Street outreachd | 45 | NA | NA | 43 | 7 | 16.3 | (6.8, 30.7) | |
| Peer referral | 286 | 237 | 1.21 | 286 | 21 | 7.3 | (4.6,11.0) | |
| New York | Any | 366 | 319 | 1.15 | 349 | 5 | 1.4 | (0.5,3.3) |
| Street outreach | 146 | 130 | 1.12 | 139 | 1 | 0.7 | (0.0,3.9) | |
| Peer referral | 79 | 65 | 1.22 | 75 | 3 | 4.0 | (0.8,11.2) | |
| Other referralb | 45 | 40 | 1.13 | 44 | 0 | 0 | (0.0, 8.0) | |
| Jail/prison waiting areas | 39 | 36 | 1.08 | 36 | 0 | 0 | (0.0,9.7) | |
| Passive | 49 | 40 | 1.23 | 47 | 1 | 2.1 | (0.1,11.3) | |
| Multiple methodsc | 8 | 8 | 1.00 | 8 | 0 | 0.0 | (0.0, 36.9) | |
| Philadelphiae | Any | 300 | 243 | 1.23 | 288 | 11 | 3.8 | (1.9,6.7) |
| Street outreach | 155 | 124 | 1.25 | 150 | 3 | 2.0 | (0.4, 5.7) | |
| Peer referral | 81 | 64 | 1.27 | 78 | 4 | 5.1 | (1.4, 12.6) | |
| Other referralb | 34 | 32 | 1.06 | 33 | 0 | 0 | (0.0,10.6) | |
| Passive | 12 | 8 | 1.50 | 11 | 3 | 27.3 | (6.0, 61.0) | |
| Multiple methodsc | 17 | 14 | 1.21 | 15 | 1 | 6.7 | (0.2, 31.9) | |
Among those who passed the prescreening reassessment at the screening visit, HIV test results were not available for 2 women who refused HIV testing, 9 ineligible women whom sites did not test, and 2 women who refused confirmatory testing for unknown reasons after a positive initial rapid blood test.
For New York, other referral sources included male partners, community based organizations, staff members, and other participants. For Philadelphia, other referral sources included male partners, an HPV study staff, relatives and friends.
In New York, 8 women were recruited through street outreach and another method (peer referral, other referral, jail/prison waiting, and passive method, n=2 for each). In Philadelphia, 12 women were recruited by street outreach and by peer referral, 3 by street outreach and another referral source, 1 by peer and other referral, and 1 by other referral and a passive method.
Women at Chicago who were not referred by a peer were not eligible.
1 Philadelphia woman is missing recruitment strategy data.
HIV testing during screening detected 44 confirmed HIV infections [overall prevalence = 4.6% (95% CI 3.3%, 6.1%)]. HIV prevalence was 8.5% (n=28) in Chicago, 3.8% (n=11) in Philadelphia, and 1.4% (n=5) in NYC (Table V). HIV infected women were not enrolled in the cohort. Only 4 of the 44 infected women reported having a known HIV-positive male partner in the six months prior to screening. At the Chicago site, the highest prevalence was among women approached on the street to be seeds (16.3%), although prevalence was also high among the women referred by seeds (7.3%). At the NYC and Philadelphia sites, women recruited by peer referral had higher HIV prevalence than those recruited by street outreach. No infections were observed among women recruited through other referral sources, including partners. HIV testing of women recruited by NYC in jail and prison waiting areas did not yield any infected women but all 5 infected women in NYC had a male partner who was incarcerated in the past year.
HIV vaccine trial participation questionnaire
In response to 11 specific items regarding future HIV vaccine trial participation, 98% of women responded “very concerned” to at least one item that might deter trial participation (Table VI). Safety issues and the potential of vaccine-induced seropositivity on a standard HIV antibody test were the items with the highest percentages of very concerned responses (84.6% permanent injury or death, 85.6% long-term side effects, 69.6% short-term side effects, and 78.5% testing positive on a standard HIV test). However, this high level of concern did not correspond with an unwillingness to participate in future vaccine trials. The majority of enrolled women reported that they would be definitely or probably willing to participate in a future HIV vaccine trial (Chicago 96.6%, NYC 79.7%, Philadelphia 83.1%). Variables that were identified as significant predictors of willingness to participate from multivariable logistic regression modeling were: perceived personal benefit from an HIV vaccine (OR=2.2; 95% CI=1.5, 3.4); household income <$10,000 (OR=2.0; 95% CI=1.3, 3.2); exchange of sex (OR=2.1; 95% CI=1.3, 3.4); and recruited through peer referral (OR=2.7; 95% CI=1.6, 4.8) (Table VII). The composite measure of concerns about participation was not significantly associated with willingness to participate.
Table VI.
Responses to the Vaccine Trial Participation Questionnairea
| Itemb | Chicago (N=237) | New York (N=319) | Philadelphia (N=243) | All Sites (N=799) |
|---|---|---|---|---|
| Items of concern regarding participating in an HIV vaccine trial | ||||
| Short-term side effects from the injection, such as fever, aches and pains, and pain or infection at the injection site | ||||
| Very concerned | 200 (84.4%) | 182 (57.1%) | 174 (71.6%) | 556 (69.6%) |
| Somewhat concerned | 31 (13.1%) | 105 (32.9%) | 57 (23.5%) | 193 (24.2%) |
| Not concerned at all | 6 (2.5%) | 32 (10.0%) | 12 (4.9%) | 50 (6.3%) |
| Long-term side effects | ||||
| Very concerned | 214 (90.3%) | 259 (81.2%) | 209 (86.0%) | 682 (85.4%) |
| Somewhat concerned | 20 (8.4%) | 43 (13.5%) | 24 (9.9%) | 87 (10.9%) |
| Not concerned at all | 3 (1.3%) | 15 (4.7%) | 10 (4.1%) | 28 (3.5%) |
| Missing | 0 (0%) | 2 (0.6%) | 0 (0%) | 2 (0.2%) |
| Having multiple HIV tests during the study | ||||
| Very concerned | 107 (45.1%) | 70 (21.9%) | 69(28.4%) | 246 (30.8%) |
| Somewhat concerned | 59 (24.9%) | 75 (23.5%) | 63(25.9%) | 197 (24.7%) |
| Not concerned at all | 71 (30.0%) | 174 (54.5%) | 110(45.3%) | 355 (44.4%) |
| Missing | 0 (0%) | 0 (0%) | 1 (0.4%) | 1 (0.1%) |
| Testing positive on a standard HIV test because you received the vaccine | ||||
| Very concerned | 198 (83.5%) | 215 (67.4%) | 214 (88.1%) | 627 (78.5%) |
| Somewhat concerned | 30 (12.7%) | 67 (21.0%) | 17 (7.0%) | 114 (14.3%) |
| Not concerned at all | 9 (3.8%) | 37 (11.6%) | 12 (4.9%) | 58 (7.3%) |
| Your spouse or partner not wanting to have sexual relations with you | ||||
| Very concerned | 149 (62.9%) | 152 (47.6%) | 139 (57.2%) | 440 (55.1%) |
| Somewhat concerned | 42 (17.7%) | 71 (22.3%) | 35 (14.4%) | 148 (18.5%) |
| Not concerned at all | 46 (19.4%) | 95 (29.8%) | 69 (28.4%) | 210 (26.3%) |
| Missing | 0 (0%) | 1 (0.3%) | 0 (0%) | 1(0.1%) |
| Your spouse or partner feels you are protected against HIV and insists on intercourse without a condom | ||||
| Very concerned | 150 (63.3%) | 176 (55.2%) | 158 (65.0%) | 484 (60.6%) |
| Somewhat concerned | 61 (25.7%) | 72 (22.6%) | 43 (17.7%) | 176 (22.0%) |
| Not concerned at all | 26 (11.0%) | 71 (22.3%) | 42 (17.3%) | 139 (17.4%) |
| Other negative reactions of family and friends | ||||
| Very concerned | 116 (48.9%) | 106 (33.2%) | 87 (35.8%) | 309 (38.7%) |
| Somewhat concerned | 63 (26.6%) | 80 (25.1%) | 60 (24.7%) | 203 (25.4%) |
| Not concerned at all | 58 (24.5%) | 133 (41.7%) | 96 (39.5%) | 287 (35.9%) |
| Not being able to donate or sell blood while in the study | ||||
| Very concerned | 89 (37.6%) | 89 (27.9%) | 68 (28.0%) | 246 (30.8%) |
| Somewhat concerned | 47 (19.8%) | 88 (27.6%) | 42 (17.3%) | 177 (22.2%) |
| Not concerned at all | 101 (42.6%) | 142 (44.5%) | 133 (54.7%) | 376 (47.1%) |
| Job discrimination | ||||
| Very concerned | 135 (57.0%) | 163 (51.1%) | 150 (61.7%) | 448 (56.1%) |
| Somewhat concerned | 41 (17.3%) | 65 (20.4%) | 30 (12.3%) | 136 (17.0%) |
| Not concerned at all | 61 (25.7%) | 91 (28.5%) | 63 (25.9%) | 215 (26.9%) |
| Permanent injury or death | ||||
| Very concerned | 217 (91.6%) | 245 (76.8%) | 214 (88.1%) | 676 (84.6%) |
| Somewhat concerned | 14 (5.9%) | 39 (12.2%) | 9 (3.7%) | 62 (7.8%) |
| Not concerned at all | 6 (2.5%) | 35 (11.0%) | 20 (8.2%) | 61 (7.6%) |
| Avoiding pregnancy during the study | ||||
| Very concerned | 112 (47.3%) | 82 (25.7%) | 99 (40.7%) | 293 (36.7%) |
| Somewhat concerned | 29 (12.2%) | 53 (16.6%) | 21 (8.6%) | 103 (12.9%) |
| Not concerned at all | 96 (40.5%) | 184 (57.7%) | 123 (50.6%) | 403 (50.4%) |
| Willingness to participate in an HIV vaccine trial | ||||
| Definitely willing | 166 (70.0%) | 123 (38.6%) | 116 (47.7%) | 405 (50.7%) |
| Probably willing | 63 (26.6%) | 131 (41.1%) | 86 (35.4%) | 280 (35.0%) |
| Probably not willing | 5 (2.1%) | 37 (11.6%) | 28 (11.5%) | 70 (8.8%) |
| Definitely not willing | 3 (1.3%) | 28 (8.8%) | 13 (5.3%) | 44 (5.5%) |
The questionnaire was interviewer-administered at the enrollment visit.
Items are listed in the order they were asked.
Table VII.
Factors associated with willingness to participate in a future HIV vaccine trial from multivariable logistic regression
| Predictorsa | Willing to participateb | Adjusted Odds Ratio (95%CI) |
|---|---|---|
| Referred by a peer | ||
| Yes | 341/362 (94.2%) | 2.7 (1.6, 4.8) |
| No | 326/418 (78.0%) | 1.0 |
| Perceived personal benefit from an HIV vaccine | ||
| Yes | 498/553 (90.1%) | 2.2 (1.5, 3.4) |
| No | 169/227 (74.4%) | 1.0 |
| Annual household income | ||
| < $10,000 | 557/629 (88.6%) | 2.0 (1.3, 3.2) |
| ≥ $10,000 | 110/151 (72.8%) | 1.0 |
| Exchanged sex for money, gifts, drugs, goods, shelter or services | ||
| Yes | 385/415 (92.8%) | 2.1 (1.3, 3.4) |
| No | 282/365 (77.3%) | 1.0 |
Variables assessed and found to be non-significant were site, recruited through street outreach, age, black, high school graduate, employed, had health care, living in own house/apartment, living with a main partner, homeless in last 6 months, in jail/prison in last 6 months, number of male partners, had a main partner, had an HIV positive male partner, had unprotected anal sex, had unprotected sex while drunk, had unprotected sex while high, forced to have sex, had an STD, heavy alcohol use, marijuana use, crack cocaine use, cocaine use, heroine use, currently lives or engages in risk behavior within a risk pocket, eligible based upon high-risk partner and the 3 individual partner characteristics, perceived likely to become infected with HIV in next 5 years, and had family members/friends with or who died from HIV/AIDS. Risk behaviors of the women were self-reported as occurring within the 6 months prior to screening. Drug use variables were dichotomized as any/no use. Heavy alcohol use was defined as drinking ≥ 4 drinks everyday or drinking ≥ 6 drinks on a typical day that the woman consumed alcohol.
19 women are excluded from the model due to missing data.
Discussion
Recognizing that previous recruitment strategies used for the Step Study did not result in a cohort with high HIV incidence, our research sites developed recruitment strategies based upon the literature linking HIV and other STIs to social and sexual network characteristics that place women at high risk of acquiring HIV, in addition to their aggregate risk behaviors. We enrolled women within geographical HIV risk pockets and targeted women in relationships with men engaged in sexual concurrency or recently incarcerated. About 90% of the enrolled cohort lived or engaged in risk behavior (unprotected sex, sex work or crack cocaine use) in locally identified HIV risk pockets within zip codes and neighborhoods with the greatest number of newly diagnosed HIV infections; 64% of the cohort reported their male partners had concurrent partners; and 50% reported a male partner who had been incarcerated in the last year. The low screening to enrollment ratios demonstrate the efficiency of this approach. The majority of participants at enrollment felt that they would personally benefit from a HIV vaccine (71%) and would probably or definitely be willing to participate in an HIV vaccine trial (86%).
Chicago’s recruitment strategy relied on peer referral of crack cocaine users by HIV positive women seeds who knew they shared male sexual partners in common with the women they referred. This method of tapping into sexual networks resulted in the cohort at the highest risk of HIV infection among the three study sites in terms of the characteristics of the women themselves, characteristics of their male partners, and the HIV prevalence among women screened.
NYC and Philadelphia recruited women primarily through street outreach and referral, and enrolled cohorts that were similar in terms of sexual behaviors of the women. Midstudy, NYC began requiring women to meet both risk pocket and a male partner eligibility criterion, which resulted in more NYC than Philadelphia women having high risk partners. Despite similarities in recruitment strategies, the HIV prevalence among women screened was lower in NYC. This observation is based on small numbers, but may be due to Philadelphia recruiting more women through street outreach and peer referral than NYC and Philadelphia’s use of the mobile van. With the mobile van parked in the same location for many days, recruiters may have developed a better understanding of the drug dealing and sexual exchange dynamics of the local geographical area allowing them to approach riskier women. Both sites attempted to recruit women based on partner referral but this strategy yielded a limited number of women for screening.
The NYC site was the only site to target women visiting incarcerated individuals. As many as 14% of the HIV-infected US male population has contact with the correctional system on a yearly basis(36), and community rates of incarceration correlate with the prevalence of gonorrhea and chlamydia(37). Incarceration has a significant effect on sexual networks with increased concurrency that follows partnership disruption(38), especially among inner city African Americans who experience a disproportionate rate of incarceration(39). The recruitment of women in jail/prison waiting rooms and prison visitor bus stops in NYC did not identify any women with undiagnosed infection, although the 5 infected NYC women recruited through other methods reported having a male partner who had been incarcerated in the past year. This suggests that multiple strategies may be useful to identify those women with partners that have experienced recent incarceration, and that women with partners who have been released from the correctional setting may have a greater risk of HIV acquisition compared to those with partners who are currently incarcerated(40;41).
We also assessed willingness to participate in a future HIV vaccine trial. Factors that influence HIV vaccine trial participation exist at the individual, social/organizational, and community level(42). Potential impediments to HIV vaccine trial participation identified by women in this study, including concerns about safety, vaccine-induced seropositivity, discrimination, and partner and family members’ views, have been expressed previously(43-51). However, in our study an aggregate measure of concerns did not predict willingness to participate in a future HIV vaccine trial. Factors predictive of willingness (low household income, exchange of sex, and perceived personal benefit from a HIV vaccine) suggest that personal gain would be a strong motivation to participate in an HIV vaccine trial among our cohort and this outweighs general concerns regarding safety or possible discrimination. Participants in HIV vaccine trials frequently cite altruistic reasons as their primary motivation for participation(49;51), although Colfax [2005] found women participants in the Vax004 trial more likely than men to cite personal benefit reasons. Interestingly, women referred by peers were more likely to express willingness, which suggests the importance of peer influence on the decision to participate in a trial.
Despite a high degree of willingness to participate in a future HIV vaccine trial expressed by the cohort, this may not necessarily correspond to subsequent enrollment in a vaccine trial(49). The decision to enroll in an HIV vaccine trial involves complex decision making and agreement to undergo study procedures including randomization. Perceived benefits of participation must be weighed against realistic consequences such as known side-effects of study products, the likelihood of vaccine-induced seropositivity, the time commitment, provision of childcare or forgoing pregnancy, and reactions of family, partners, and friends.
Our study has several limitations. Each of the three sites followed different recruitment strategies and Chicago and NYC used more stringent site-specific eligibility criteria. Therefore, we cannot be certain that an effective recruitment strategy in one city could be duplicated in another. Our data did not permit distinguishing living in a geographical risk pocket from engaging in risk behaviors therein. Although all women reported unprotected sex at screening, simply residing in a risk pocket may be less risky than engaging in risk behaviors in an area of high HIV incidence. Women enrolled in observational cohort studies may not be the same as those who enroll in an HIV vaccine trial, although our study employed many of the same eligibility criteria used in HIV vaccine efficacy trials (e.g, HIV negative, not pregnant and not intending to become pregnant). We have not performed a formal cost analysis, but the outreach component of our recruitment strategies was labor intensive. Chicago achieved a cohort with the highest risk based on behaviors of the women themselves and the highest HIV prevalence among women screened, but their recruitment strategy also had the highest screening to enrollment ratio. It is unknown whether their strategy is transferable to other US urban areas.
Conclusion
We employed geographic risk pockets and social and sexual network principles to recruit a cohort of women at risk of HIV infection. The HIV-uninfected women in the cohort are being followed longitudinally to estimate rates of HIV incidence and retention over 18 months, which will be the true indicator of recruitment of a high-risk cohort and ability of sites to retain these women. The Chicago cohort had the highest percent eligible based on risk pocket and high-risk partner criteria, were primarily crack using sex workers, and expressed the highest degree of willingness to participate in HIV vaccine trials. These characteristics suggest that use of a modified respondent driven sampling approach with HIV-positive women peers as seeds, combined with eligibility criteria based on participant and partner risk behaviors, may be necessary to recruit at-risk women for future HIV vaccine trials in the US. Further evaluation of this method in other US urban areas is needed.
Acknowledgments
The authors would like to thank the study participants, community advisory board members, and the study site staff, in particular the site recruiting staff, for their commitment to this study. This research was funded by the Division of AIDS, National Institute of Allergy and Infectious Disease, National Institutes of Health, grants 1UM1AI068614, 1UM1A1069470, 1UM1A1069554, 1UM1A1069534, , and 1UM1AI068635.
Reference List
- 1.Djomand G, Metch B, Zorrilla CD, et al. The HVTN protocol 903 vaccine preparedness study: lessons learned in preparation for HIV vaccine efficacy trials. J Acquir Immune Defic Syndr. 2008;48(1):82–89. doi: 10.1097/QAI.0b013e31817236ab. [DOI] [PubMed] [Google Scholar]
- 2.Koblin BA, Taylor PE, Avrett S, Stevens CE. The feasibility of HIV-1 vaccine efficacy trials among gay/bisexual men in New York City: Project ACHIEVE. AIDS Community Health Initiative Enroute to the Vaccine EFfort. AIDS. 1996;10(13):1555–1561. doi: 10.1097/00002030-199611000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Buchbinder SP, Douglas JM, Jr, McKirnan DJ, Judson FN, Katz MH, MacQueen KM. Feasibility of human immunodeficiency virus vaccine trials in homosexual men in the United States: risk behavior, seroincidence, and willingness to participate. J Infect Dis. 1996;174(5):954–961. doi: 10.1093/infdis/174.5.954. [DOI] [PubMed] [Google Scholar]
- 4.UNAIDS. World AIDS Day Report. 2011. [Google Scholar]
- 5.Sterling TR, Vlahov D, Astemborski J, Hoover DR, Margolick JB, Quinn TC. Initial plasma HIV-1 RNA levels and progression to AIDS in women and men. N Engl J Med. 2001;344(10):720–725. doi: 10.1056/NEJM200103083441003. [DOI] [PubMed] [Google Scholar]
- 6.Stanberry LR, Spruance SL, Cunningham AL, et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med. 2002;347(21):1652–1661. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 7.Gandhi M, Bacchetti P, Miotti P, Quinn TC, Veronese F, Greenblatt RM. Does patient sex affect human immunodeficiency virus levels? Clin Infect Dis. 2002;35(3):313–322. doi: 10.1086/341249. [DOI] [PubMed] [Google Scholar]
- 8.Prejean J, Song R, Hernandez A, et al. Estimated HIV incidence in the United States, 2006–2009. PLoS One. 2011;6(8):e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seage GR, III, Holte SE, Metzger D, et al. Are US populations appropriate for trials of human immunodeficiency virus vaccine? The HIVNET Vaccine Preparedness Study. Am J Epidemiol. 2001;153(7):619–627. doi: 10.1093/aje/153.7.619. [DOI] [PubMed] [Google Scholar]
- 10.Seage GR, III, Holte S, Gross M, et al. Case-crossover study of partner and situational factors for unprotected sex. J Acquir Immune Defic Syndr. 2002;31(4):432–439. doi: 10.1097/00126334-200212010-00010. [DOI] [PubMed] [Google Scholar]
- 11.Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191(5):654–665. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 12.Buchbinder SP, Mehrotra DV, Duerr A, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372(9653):1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donnell D, Hughes JP, Fleming TR. Challenges in the design of HIV prevention trials in the United States. J Acquir Immune Defic Syndr. 2010;55 (Suppl 2):S136–S140. doi: 10.1097/QAI.0b013e3181fbcb61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. HIV among women fact sheet. 2011 http://www.cdc.gov/hiv/topics/women/index.htm. 6-15-0012.
- 15.Laumann EO, Youm Y. Racial/ethnic group differences in the prevalence of sexually transmitted diseases in the United States: a network explanation. Sex Transm Dis. 1999;26(5):250–261. doi: 10.1097/00007435-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Aral SO. Sexual network patterns as determinants of STD rates: paradigm shift in the behavioral epidemiology of STDs made visible. Sex Transm Dis. 1999;26(5):262–264. doi: 10.1097/00007435-199905000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Rothenberg R, Muth SQ, Malone S, Potterat JJ, Woodhouse DE. Social and geographic distance in HIV risk. Sex Transm Dis. 2005;32(8):506–512. doi: 10.1097/01.olq.0000161191.12026.ca. [DOI] [PubMed] [Google Scholar]
- 18.Friedman SR, Neaigus A, Jose B, et al. Sociometric risk networks and risk for HIV infection. Am J Public Health. 1997;87(8):1289–1296. doi: 10.2105/ajph.87.8.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothenberg RB, Long DM, Sterk CE, et al. The Atlanta Urban Networks Study: a blueprint for endemic transmission. AIDS. 2000;14(14):2191–2200. doi: 10.1097/00002030-200009290-00016. [DOI] [PubMed] [Google Scholar]
- 20.Becker KM, Glass GE, Brathwaite W, Zenilman JM. Geographic epidemiology of gonorrhea in Baltimore, Maryland, using a geographic information system. Am J Epidemiol. 1998;147(7):709–716. doi: 10.1093/oxfordjournals.aje.a009513. [DOI] [PubMed] [Google Scholar]
- 21.Bernstein KT, Curriero FC, Jennings JM, Olthoff G, Erbelding EJ, Zenilman J. Defining core gonorrhea transmission utilizing spatial data. Am J Epidemiol. 2004;160(1):51–58. doi: 10.1093/aje/kwh178. [DOI] [PubMed] [Google Scholar]
- 22.Zenilman JM, Ellish N, Fresia A, Glass G. The geography of sexual partnerships in Baltimore: applications of core theory dynamics using a geographic information system. Sex Transm Dis. 1999;26(2):75–81. doi: 10.1097/00007435-199902000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Potterat JJ, Rothenberg RB, Woodhouse DE, Muth JB, Pratts CI, Fogle JS. Gonorrhea as a social disease. Sex Transm Dis. 1985;12(1):25–32. doi: 10.1097/00007435-198501000-00006. [DOI] [PubMed] [Google Scholar]
- 24.De P, Singh AE, Wong T, Yacoub W, Jolly AM. Sexual network analysis of a gonorrhoea outbreak. Sex Transm Infect. 2004;80(4):280–285. doi: 10.1136/sti.2003.007187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Binson D, Woods WJ, Pollack L, Paul J, Stall R, Catania JA. Differential HIV risk in bathhouses and public cruising areas. Am J Public Health. 2001;91(9):1482–1486. doi: 10.2105/ajph.91.9.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watts CH, May RM. The influence of concurrent partnerships on the dynamics of HIV/AIDS. Math Biosci. 1992;108(1):89–104. doi: 10.1016/0025-5564(92)90006-i. [DOI] [PubMed] [Google Scholar]
- 27.Morris M, Zavisca J, Dean L. Social and sexual networks: their role in the spread of HIV/AIDS among young gay men. AIDS Educ Prev. 1995;7(5 Suppl):24–35. [PubMed] [Google Scholar]
- 28.Youm Y, Laumann EO. Social network effects on the transmission of sexually transmitted diseases. Sex Transm Dis. 2002;29(11):689–697. doi: 10.1097/00007435-200211000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Adimora AA, Schoenbach VJ, Martinson FE, Donaldson KH, Stancil TR, Fullilove RE. Concurrent partnerships among rural African Americans with recently reported heterosexually transmitted HIV infection. J Acquir Immune Defic Syndr. 2003;34(4):423–429. doi: 10.1097/00126334-200312010-00010. [DOI] [PubMed] [Google Scholar]
- 30.Adimora AA, Schoenbach VJ, Taylor EM, Khan MR, Schwartz RJ. Concurrent partnerships, nonmonogamous partners, and substance use among women in the United States. Am J Public Health. 2011;101(1):128–136. doi: 10.2105/AJPH.2009.174292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanser F, Barnighausen T, Hund L, Garnett GP, McGrath N, Newell ML. Effect of concurrent sexual partnerships on rate of new HIV infections in a high-prevalence, rural South African population: a cohort study. Lancet. 2011;378(9787):247–255. doi: 10.1016/S0140-6736(11)60779-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adimora AA, Schoenbach VJ, Floris-Moore MA. Ending the epidemic of heterosexual HIV transmission among African Americans. Am J Prev Med. 2009;37(5):468–471. doi: 10.1016/j.amepre.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adimora AA, Schoenbach VJ, Doherty IA. Concurrent sexual partnerships among men in the United States. Am J Public Health. 2007;97(12):2230–2237. doi: 10.2105/AJPH.2006.099069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grinstead OA, Faigeles B, Comfort M, et al. HIV, STD, and hepatitis risk to primary female partners of men being released from prison. Women Health. 2005;41(2):63–80. doi: 10.1300/J013v41n02_05. [DOI] [PubMed] [Google Scholar]
- 35.CDC. Characteristics associated with HIV infection among heterosexuals in urban areas with high AIDS prevalence --- 24 cities, United States, 2006–2007. MMWR Morb Mortal Wkly Rep. 2011;60(31):1045–1049. [PubMed] [Google Scholar]
- 36.Spaulding AC, Seals RM, Page MJ, Brzozowski AK, Rhodes W, Hammett TM. HIV/AIDS among inmates of and releasees from US correctional facilities, 2006: declining share of epidemic but persistent public health opportunity. PLoS One. 2009;4(11):e7558. doi: 10.1371/journal.pone.0007558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas JC, Sampson LA. High rates of incarceration as a social force associated with community rates of sexually transmitted infection. J Infect Dis. 2005;191 (Suppl 1):S55–S60. doi: 10.1086/425278. [DOI] [PubMed] [Google Scholar]
- 38.Gorbach PM, Drumright LN, Holmes KK. Discord, discordance, and concurrency: comparing individual and partnership-level analyses of new partnerships of young adults at risk of sexually transmitted infections. Sex Transm Dis. 2005;32(1):7–12. doi: 10.1097/01.olq.0000148302.81575.fc. [DOI] [PubMed] [Google Scholar]
- 39.Adimora AA, Schoenbach VJ. Social context, sexual networks, and racial disparities in rates of sexually transmitted infections. J Infect Dis. 2005;191 (Suppl 1):S115–S122. doi: 10.1086/425280. [DOI] [PubMed] [Google Scholar]
- 40.Spaulding A, Stephenson B, Macalino G, Ruby W, Clarke JG, Flanigan TP. Human immunodeficiency virus in correctional facilities: a review. Clin Infect Dis. 2002;35(3):305–312. doi: 10.1086/341418. [DOI] [PubMed] [Google Scholar]
- 41.Adams LM, Kendall S, Smith A, Quigley E, Stuewig JB, Tangney JP. HIV Risk Behaviors of Male and Female Jail Inmates Prior to Incarceration and One Year Post-Release. AIDS Behav. 2011 doi: 10.1007/s10461-011-9990-2. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frew PM, Archibald M, Hixson B, del Rio C. Socioecological influences on community involvement in HIV vaccine research. Vaccine. 2011;29(36):6136–6143. doi: 10.1016/j.vaccine.2011.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newman PA, Duan N, Roberts KJ, et al. HIV vaccine trial participation among ethnic minority communities: barriers, motivators, and implications for recruitment. J Acquir Immune Defic Syndr. 2006;41(2):210–217. doi: 10.1097/01.qai.0000179454.93443.60. [DOI] [PubMed] [Google Scholar]
- 44.Mills E, Cooper C, Guyatt G, et al. Barriers to participating in an HIV vaccine trial: a systematic review. AIDS. 2004;18(17):2235–2242. doi: 10.1097/00002030-200411190-00003. [DOI] [PubMed] [Google Scholar]
- 45.Strauss RP, Sengupta S, Kegeles S, et al. Willingness to volunteer in future preventive HIV vaccine trials: issues and perspectives from three U.S. communities. J Acquir Immune Defic Syndr. 2001;26(1):63–71. doi: 10.1097/00126334-200101010-00010. [DOI] [PubMed] [Google Scholar]
- 46.Rudy ET, Newman PA, Duan N, Kelly EM, Roberts KJ, Seiden DS. HIV vaccine acceptability among women at risk: perceived barriers and facilitators to future HIV vaccine uptake. AIDS Educ Prev. 2005;17(3):253–267. doi: 10.1521/aeap.17.4.253.66529. [DOI] [PubMed] [Google Scholar]
- 47.Koblin BA, Heagerty P, Sheon A, et al. Readiness of high-risk populations in the HIV Network for Prevention Trials to participate in HIV vaccine efficacy trials in the United States. AIDS. 1998;12(7):785–793. doi: 10.1097/00002030-199807000-00015. [DOI] [PubMed] [Google Scholar]
- 48.Voytek CD, Jones KT, Metzger DS. Selectively willing and conditionally able: HIV vaccine trial participation among women at “high risk” of HIV infection. Vaccine. 2011;29(36):6130–6135. doi: 10.1016/j.vaccine.2011.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buchbinder SP, Metch B, Holte Se, Scheer S, Coletti A, Vittinghoff E. Determinants of enrollment in a preventive HIV vaccine trial hypothetical versus actual willingness and barriers to participation. J Acquir Immune Defic Syndr. 2004;36:604–612. doi: 10.1097/00126334-200405010-00009. [DOI] [PubMed] [Google Scholar]
- 50.Dhalla S, Poole G. Barriers of enrolment in HIV vaccine trials: a review of HIV vaccine preparedness studies. Vaccine. 2011;29:5850–5859. doi: 10.1016/j.vaccine.2011.06.055. [DOI] [PubMed] [Google Scholar]
- 51.Colfax G, Buchbinder S, Vamshidar G, Celum C, McKirnan D, Neidig J, et al. Motivations for participating in an HIV vaccine efficacy trial. J Acquir Immune Defic Syndr. 2005;39:359–364. doi: 10.1097/01.qai.0000152039.88422.ec. [DOI] [PubMed] [Google Scholar]



